Pseudosubstrate Inhibition of the Anaphase-Promoting Complex by Acm1: Regulation by Proteolysis and Cdc28 Phosphorylation (original) (raw)
Abstract
The ubiquitin ligase activity of the anaphase-promoting complex (APC)/cyclosome needs to be tightly regulated for proper cell cycle progression. Substrates are recruited to the APC by the Cdc20 and Cdh1 accessory proteins. The Cdh1-APC interaction is inhibited through phosphorylation of Cdh1 by Cdc28, the major cyclin-dependent protein kinase in budding yeast. More recently, Acm1 was reported to be a Cdh1-binding and -inhibitory protein in budding yeast. We found that although Acm1 is an unstable protein and contains the KEN-box and D-box motifs typically found in APC substrates, Acm1 itself is not an APC substrate. Rather, it uses these motifs to compete with substrates for Cdh1 binding, thereby inhibiting their recruitment to the APC. Mutation of these motifs prevented Acm1-Cdh1 binding in vivo and rendered Acm1 inactive both in vitro and in vivo. Acm1 stability was critically dependent on phosphorylation by Cdc28, as Acm1 was destabilized following inhibition of Cdc28, mutation of consensus Cdc28 phosphorylation sites in Acm1, or deletion of the Bmh1 and Bmh2 phosphoprotein-binding proteins. Thus, Cdc28 serves dual roles in inhibiting Cdh1-dependent APC activity during the cell cycle: stabilization of the Cdh1 inhibitor Acm1 and direct phosphorylation of Cdh1 to prevent its association with the APC.
Ubiquitin-mediated proteolysis plays essential roles in cell cycle progression, targeting key proteins for degradation by the 26S proteasome. The RING-type ubiquitin ligases (E3s) participate in substrate recognition and, together with a cognate ubiquitin-conjugating enzyme (E2), mediate the formation of polyubiquitin chains on the substrate. Two major classes of conserved E3s play prominent roles in cell cycle progression: the anaphase-promoting complex (APC; also called the cyclosome) and SCF complexes (for reviews, see references 8, 29, 49, and 68). The APC functions during mitosis and G1. Among its key substrates are securin/Pds1, an inhibitor of anaphase initiation, and the mitotic cyclins, activators of the mitotic cyclin-dependent protein kinase (CDC2/CDK1, Cdc28) (10, 18, 59, 63, 74). The APC from yeast to humans consists of about 13 core proteins that interact with a substrate-binding regulatory protein, either Cdc20 or Cdh1 in vegetative cells (29, 49, 50, 66, 71). These related WD40-containing activators recognize substrates through one or both of the most common degradation motifs, typically a destruction box (D box; RXXLXXXXN/D/E) and the KEN box (22, 51).
APC activity is tightly regulated so that proteins are not degraded prematurely. For instance, Cdc20 activity is limited to mitosis via its degradation by APCCdh1 in G1 (15, 52, 61). APCCdc20 activity is further regulated by the spindle assembly checkpoint, which prevents the degradation of securin/Pds1 and the initiation of anaphase until all chromosomes are properly attached to the mitotic spindle (10, 31, 35). Two conserved spindle assembly checkpoint proteins, Mad2 and Mad3/BubR1, associate with Cdc20, leading to APCCdc20 inhibition (14, 16, 17, 28, 31, 39, 44, 64, 65). Recently, we and others found that budding yeast Mad3, which is a stable protein, binds to Cdc20 via conserved KEN boxes and a D box, suggesting that the spindle checkpoint functions via pseudosubstrate inhibition of APCCdc20 (6, 36).
In contrast to the expression of yeast Cdc20, that of Cdh1 changes little during the cell cycle, yet its activity is limited to late mitosis and G1 phase. At all other stages, phosphorylation of Cdh1 by Cdc28 excludes it from the nucleus and prevents its interaction with the core APC (32, 33, 72, 73). In the absence of these inhibitory phosphorylations of Cdh1, either by their mutation or due to low Cdc28 activity, the resulting depletion of Cdh1 substrates results in premature centrosome separation (11, 72). Another mode of Cdh1 regulation is present in higher eukaryotes, where Emi1 negatively regulates Cdh1 via pseudosubstrate inhibition to ensure accumulation of cyclin A, geminin, and other Cdh1 substrates that are required for the transition into S phase and for the prevention of DNA rereplication (13, 30, 40, 43, 55). Emi1 competes with substrates for binding to Cdh1 and the core APC through a conserved D box. Its inhibitory potential, in addition, requires a zinc-binding region that prevents substrates from binding APCCdh1 (43).
Two groups recently identified Acm1 (APCCdh1 modulator 1) as a potential new Cdh1 inhibitor in Saccharomyces cerevisiae. By Cdh1 affinity chromatography and mass spectrometry, Cdh1 was found to form a complex with two proteins, Acm1 and Bmh1/Bmh2 (two closely related 14-3-3 proteins) (12, 42). Several lines of evidence indicated that Acm1 inhibited Cdh1 activity. Overexpression of Acm1 rescued the toxicity associated with the expression of a constitutively active, nonphosphorylatable Cdh1-m11 mutant in vivo, and Acm1-Bmh1 inhibited APCCdh1-mediated ubiquitination in vitro. In addition, _acm1_Δ mutant cells exhibited higher rates of elongated buds (42), a phenotypic characteristic of low mitotic cyclin levels, possibly resulting from increased APCCdh1 activity (42, 59). Acm1 was found to be a phosphoprotein in vivo, and its phosphorylation was required for Bmh1/Bmh2 binding in vivo and in vitro, but not for Cdh1 binding (12, 42, 62).
Because Acm1 levels fluctuate during the cell cycle and Acm1 contains potential KEN and D boxes, we thought that Acm1 might actually be an APC substrate. Although we found that Acm1 was rapidly degraded in G1, it was not stabilized by APC mutations or alterations of its putative KEN and D boxes, nor was it ubiquitinated by purified APCCdh1 in vitro. However, the KEN and D boxes were essential for Acm1 inhibitory activity and required for Cdh1 binding, indicating that Acm1 acts as a pseudosubstrate inhibitor of Cdh1. Interestingly, we also found that Acm1 stability was critically dependent on phosphorylation by Cdc28, as inhibition of Cdc28 or mutation of putative phosphoacceptor sites within Acm1 resulted in rapid Acm1 degradation. Binding of Bmh1/Bmh2 to phosphorylated Acm1 was also required for stabilizing Acm1. The phosphorylation of Acm1 by Cdc28 ensures that Acm1 inhibition of Cdh1 is limited to cell cycle stages where Cdc28 activity is sufficiently high. Thus, Cdc28 inhibits Cdh1 in two ways, by direct phosphorylation of Cdh1 and through stabilization of the Cdh1 inhibitor Acm1.
MATERIALS AND METHODS
Yeast strains and plasmid construction.
Most of the yeast strains were derivatives of W303a (_ade2_-_1 trp1_-_1 leu2_-3,_112 his3_-11,_15 ura3_-1) (58); their relevant genotypes are listed in Table 1. The pdr5::Kan strain (54) was a gift from Mark Hochstrasser (Yale University, New Haven, CT). A conditional _cdc23_-1 strain was previously described (5). _cdh1_-m11 (72) was provided by Wolfgang Seufert (University of Stuttgart, Stuttgart, Germany). The _cdc28_-as analog-sensitive strain (3) was a generous gift of David Morgan (University of California, San Francisco). _cdc53_-1 and _cdc34_-2 temperature-sensitive strains (69) were provided by Mike Tyers (University of Toronto, Toronto, Canada). _cln1_Δ _cln2_Δ and _grr1_Δ _cln1_Δ _cln2_Δ double- and triple-deletion strains (60) were provided by Mark Goebl (Indiana University, Indianapolis). The _bmh1_Δ _bmh2_Δ double-mutant strain (57) was provided by Gerald Fink (Whitehead Institute, Cambridge, MA). Construction of _acm1_Δ (W303a acm1::NAT), _grr1_Δ (W303a grr1::NAT), and _cdh1_Δ (W303a cdh1::NAT) mutant strains was accomplished by a PCR-based method (23, 38). Gene disruptions were verified by PCR with a primer downstream of the deleted gene and a primer internal to NAT. The resulting _acm1_Δ mutant cells exhibited an elongated bud morphology that was complemented by the expression of exogenous _ACM1_-HA. The _grr1_Δ mutant strain exhibited slow growth and an elongated bud morphology.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| YJB14 | MATabar1 | 5 |
| YJB115 | MATa_cdc23_-1 bar1::URA3 | 5 |
| KS481 | MATacln1::URA3 cln2::KanMX4 | 60 |
| KS489 | MATacln1::URA3 cln2::KanMX4 grr1::LEU2 | 60 |
| MHY3910 | MATapdr5::KanMX4 | 54 |
| MTY670 | MATa_cdc34_-2 | 69 |
| MTY740 | MATa_cdc53_-1 | 69 |
| CDC28-as1 | MATa_cdc28_-as | 3 |
| DOY750 | YJB14/pRS306-_GALL_-_cdh1_-m11 | This study |
| DOY763 | MTY670/YCplac22-_GAL_-_ACM1_-TAP | This study |
| DOY764 | MTY740/YCplac22-_GAL_-_ACM1_-TAP | This study |
| DOY765 | _CDC28_-as1/YCplac22-_GAL_-_ACM1_-TAP | This study |
| DOY767 | YJB14 acm1::NAT | This study |
| DOY768 | YJB14 cdh1::NAT | This study |
| DOY772 | DOY768/YCplac22-_GAL_-_ACM1_-HA | This study |
| DOY783 | YJB14/YCplac22-_GAL_-_ACM1_-HA | This study |
| DOY784 | YJB14/YCplac22-_GAL_-_acm1_-_mkb_-HA | This study |
| DOY785 | YJB14/YCplac22-_GAL_-_acm1_-_mdb_-HA | This study |
| DOY786 | YJB14/YCplac22-_GAL_-_acm1_-mkm/_mdb_-HA | This study |
| DOY794 | YJB14 _MPS1_-TAP HIS3MX6 URA:_GALL_-_cdh1_-m11 | This study |
| DOY798 | YJB14/YCplac22-_GAL_-_ACM1_-TAP | This study |
| DOY799 | YJB14/YCplac22-_GAL_-_acm1_-mkb/_mdb_-TAP | This study |
| DOY805 | YJB14 grr1::NAT | This study |
| DOY806 | DOY805/YCplac22-_GAL_-_ACM1_-TAP | This study |
| DOY809 | YJB115/YCplac22-_GAL_-_ACM1_-TAP | This study |
| DOY820 | DOY750 LEU2:_GAL_-_ACM1_-HA | This study |
| DOY821 | DOY750 LEU2:_GAL_-_acm1_-_mkb_-HA | This study |
| DOY822 | DOY750 LEU2:_GAL_-_acm1_-_mdb_-HA | This study |
| DOY823 | DOY750 LEU2:GAL acm1 mkb/_mdb_-HA | This study |
| DOY834 | DOY767 URA3:_GAL CLB2_-HA LEU2:_ACM_-_ACM1_-HA | This study |
| DOY835 | DOY767 URA3:_GAL CLB2_-HA LEU2:_ACM_-_acm1_-_mkb_-HA | This study |
| DOY836 | DOY767 URA3:_GAL_-_CLB2_-HA LEU2:_ACM_-_acm1_-_mdb_-HA | This study |
| DOY837 | DOY767 URA3:_GAL_-_CLB2_-HA LEU2:_ACM_-_acm1_-mkb/_mdb_-HA | This study |
| DOY857 | KS481/YCp22-_GAL_-_ACM1_-TAP | This study |
| DOY858 | KS489/YCp33-_GAL_-_ACM1_-TAP | This study |
| DOY862 | MHY3910/YCp33-_GAL_-_ACM1_-TAP | This study |
| DOY873 | W9313/YCp22-_GAL_-_ACM1_-TAP | This study |
| DOY874 | DOY768/YCp22-_GAL_-_ACM1_-TAP | This study |
| DOY875 | DOY794/YCp22-_GAL_-_ACM1_-TAP | This study |
| DOY876 | DOY794/YCp22-_GAL_-_acm1_-mkb/_mdb_-TAP | This study |
| RRY1216 | MATabmh1::HIS3 bmh2::HIS3 | 57 |
| DOY890 | RRY1216/YCp33-_GAL_-_ACM1_-TAP | This study |
| DOY924 | RRY1216 LEU2:BMH1/YCp33-_GAL_-_ACM1_-TAP | This study |
_ACM1_-TAP was amplified from a TAP library (20) and cloned into the YCplac22-GAL vector (21). The resulting plasmid, YCplac22-_GAL_-_ACM1_-TAP, was used as a template to introduce mutations within putative degradation motifs such as 98KEN → 98AAA (mkb) and 119RIAL → 119AIAA (mdb) and phosphorylation sites such as 3SPSK → 3APSK, 31SPSK → 31APSK, 48SPIK → 48APIK, 102SPAK → 102APAK, and 161TPPR → 161APPR. All mutations were generated by QuikChange mutagenesis (Stratagene) and verified by sequencing of the entire coding region.
YCplac22-_GAL_-_ACM1_-HA expressed Acm1 with a C-terminal fusion of a single copy of the hemagglutinin (HA) epitope (GAYPYDVPDYASLG). YCplac22-_GAL_-_ACM1_-HA was used as a template to generate mkb, mdb, and mkb/mdb mutants by QuikChange mutagenesis. Integrating plasmid YIplac128-_ACM1_-HA contained the ACM1 promoter region (−490 to −1). Plasmids containing wild-type (WT) and mutant forms of ACM1 were cut with AflII and integrated within the LEU2 locus.
_CLB2_-HA was constructed similarly and cloned into YIplac211-GAL (21). The resulting plasmid was cut with NcoI and integrated within the URA3 locus. The BMH1 promoter and coding region (−390 to +804) was amplified from genomic DNA and integrated within the LEU2 locus of the _bmh1_Δ _bmh2_Δ double-mutant strain. The endogenous MPS1 locus was tagged with the TAP epitope by a PCR-based method (38). 3′ _MPS1_-TAP-HIS3MX6 PCR products were transformed into yeast, and the resulting HIS3+ clones were verified by PCR with primers corresponding to the upstream region of MPS1 and the internal region of the selectable marker, respectively.
All primer sequences and further details of the plasmids are available upon request.
Cell growth and arrest conditions.
Yeast cultures were grown in YPD and in complete minimal medium as previously described (1, 25). Cells of _bar1_Δ mutant strains were arrested in G1 phase with 100 ng/ml α-factor for 2 h at 30°C, in M phase with 20 μg/ml nocodazole for 2 h at 30°C, or in S phase with 100 mM hydroxyurea for 2 h at 30°C.
For analyses of protein stability, yeast cultures were grown in YP-raffinose to exponential phase (optical density at 600 nm [OD600] of ∼0.4) and induced with 2% galactose for 50 min at 30°C, and then cycloheximide (500 μg/ml; MP Biomedicals) and 2% dextrose were added. Subsequently, equal volumes of cells were collected at 15-min intervals by centrifugation at 5,000 rpm for 2 min and frozen in liquid nitrogen.
Yeast extracts and immunoblotting.
Cells from 50-ml cultures (OD600 of ∼0.4) were collected, washed with ice-cold TBS (10 mM Tris-Cl [pH 8.0], 150 mM NaCl), and suspended in 0.4 ml lysis buffer (83 mM Tris-Cl [pH 8.0], 6% sodium dodecyl sulfate [SDS], 29% glycerol, 100 mM dithiothreitol [DTT]). Denaturing cell lysis was achieved by shaking suspensions with 0.25 g glass beads (0.5-mm diameter; Sigma) in a bead beater (Biospec Products) for 2 min and then incubating them at 94°C for 10 min. Glass beads and cell debris were removed by centrifugation at 14,000 rpm in a Microfuge at 4°C for 5 min. The supernatant was clarified by centrifugation at 65,000 rpm in a TLA 100.2 rotor (Beckman) for 10 min at 15°C. Yeast protein extracts were separated on a protein gel containing 10% polyacrylamide and transferred to an Immobilon-P membrane (Millipore). The membranes were probed with peroxidase-antiperoxidase (PAP, 1.3 μg/ml; Sigma) overnight in Blotto (10 mM Tris-Cl [pH 7.5], 150 mM NaCl, 0.1% Tween 20, 5% dry milk) at 4°C, and proteins were visualized by chemiluminescence (SuperSignal; Pierce).
Gel filtration and immunoprecipitation.
Cells from 200-ml cultures were washed with ice-cold TBS, suspended in 0.4 ml immunoprecipitation (IP) buffer (50 mM HEPES [pH 8.0]; 1 mM EGTA; 40 mM EDTA; 20 mM NaF; 20 mM sodium pyrophosphate; 0.1% Tween; 10 μg/ml each leupeptin, chymostatin, and pepstatin [Chemicon]; 10% glycerol), and lysed by shaking with 0.4 g glass beads (0.5-mm diameter; Sigma) as previously described (46). For gel filtration analyses, 0.5-ml extract volumes containing 10 mg/ml protein were fractionated on Superdex 200 (GE Healthcare Life Sciences) in 50 mM HEPES (pH 8.0)-150 mM NaCl-1 mM DTT. Proteins from 1-ml fractions were precipitated with 10% trichloroacetic acid, washed with ice-cold acetone, and separated on a protein gel containing 10% polyacrylamide. For immunoprecipitation, 200-μl yeast extract volumes containing 10 mg/ml protein were incubated with 100 ng polyclonal rabbit anti-Cdh1 antibodies (7) in 300 μl IP buffer at 4°C for 2 h. A 50-μl volume of protein A-agarose (50% slurry; Invitrogen) was added, and the mixture was rotated for 90 min at 4°C. The beads were washed three times with 0.5 ml IP buffer for 5 min each time at 4°C; the precipitated proteins were separated on a gel containing 10% polyacrylamide and transferred to an Immobilon-P membrane (Millipore). For detection of Acm1-HA and Clb2-HA, the membranes were incubated with horseradish peroxidase-conjugated 12CA5 antibodies (1 μg/ml; Roche) overnight in Blotto at 4°C. Proteins were visualized by chemiluminescence (SuperSignal; Pierce).
Binding assays in vitro.
Recombinant His6-Cdh1 containing beads and MBP-Hsl1 were produced and purified from baculovirus-infected cells and Escherichia coli, respectively, as described previously (4, 7). MBP-Acm1 fusion proteins were produced and purified from E. coli strains containing WT, mdb, mkb, and mdb/mkb _ACM1_-pMALc-2 constructs by the same protocol as described for MBP-Hsl1. These constructs all encode full-length Acm1 proteins. MBP-Acm1 N128 (WT and mdb/mkb) and C81 contained only the first 128 or the last 81 amino acid residues of Acm1, respectively, fused to the C terminus of MBP and were produced and purified in the same fashion as their full-length counterparts. 35S-labeled Acm1 was prepared by translation in vitro with the TNT T7 quick-coupled transcription-translation system (Promega) according to the manufacturer's instructions in the presence of 20 μCi [35S]methionine (GE Healthcare). In all Cdh1-binding reaction mixtures, approximately 4.0 μg of His6-Cdh1 was on the beads. For 35S-labeled Acm1 binding, 15 μl of the in vitro-translated reaction mixture was used to bind to Cdh1-beads and bound Acm1 was detected by autoradiography and quantitated by PhosphorImager analysis. The binding reactions of the full-length, N128, and C81 MBP-Acm1 proteins to Cdh1 beads were carried out as previously described for MBP-Hsl1 (7), with approximately 1.0 μg of Acm1, with bound protein being detected by immunoblotting with anti-MBP antibodies (New England BioLabs). D-box and KEN-box peptide competition assays were performed by preincubation of Cdh1 beads with the indicated peptides at 500 μM, followed by the addition of approximately 0.3 μg of each recombinant protein as previously described (7). Competition experiments between MBP-Acm1 and MBP-Hsl1 for binding to Cdh1 beads were conducted by preincubation of Cdh1 beads with 5.0 μg of either WT or MBP-Acm1-mdb/mkb or no Acm1 for 15 min prior the addition of 0.5 μg MBP-Hsl1 and a subsequent 30-min incubation. Bound proteins were detected by immunoblotting with anti-MBP antibodies.
Ubiquitination assays.
The APC was purified from YJB910 (MATa _pep4_-_3 his3_-Δ_1 leu2_-3,_112 CDC16_-TAP::_CDC16 APC4_-HA::APC4) by the tandem affinity purification procedure (53). The methodology used for APC purification was virtually the same as that described by Passmore et al. (48). A yield of approximately 45 ng of purified APC/g of yeast cells was achieved. A yeast strain containing His6-UBA1 from yeast behind a copper-inducible promoter (DOMO907) was a kind gift from David Morgan (University of California San Francisco). The DOMO907 strain was grown in 2 liters of YP-dextrose to an OD600 of 0.35, upon which 1 mM CuSO4 was added to the culture for 24 h of incubation to induce the expression of His6-Uba1. Induced cells were lysed in 15 ml of lysis buffer (50 mM Tris-Cl [pH 8.0], 300 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg each of leupeptin, chymostatin, and pepstatin [Chemicon]). Subsequent to cell lysis, Triton X-100 was added to a final concentration of 0.2% and the cell extract was clarified by ultracentrifugation. His6-Uba1 was purified from these extracts with a 1-ml bed volume of Talon resin (BD Biosciences). Bound protein was eluted with lysis buffer containing 150 mM imidazole, and the purified protein was dialyzed against storage buffer (20 mM HEPES [pH 7.5], 100 mM NaCl, 1 mM MgCl2, 10% glycerol), aliquoted, and stored at −80°C at a concentration of 3.1 mg/ml. The total yield of Uba1 was 1.55 mg. The _UBC4_-pET28c construct expressing mammalian Ubc4 was a kind gift from J. Wade Harper (Harvard University, Boston, MA) (48). Expression of His6-Ubc4 was induced in BL21(DE3) cells by the addition of 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C in LB medium containing kanamycin (30 μg/ml). Expressed protein was purified on Talon resin essentially as previously described, with a yield of 10 mg/liter of culture (6). The protein was stored at 8.4 mg/ml. 35S-labeled MBP-Hsl1 was used as the APC substrate and was prepared by translation in vitro as described above. APC assays were conducted in the presence or absence of 2.5 μg His6-Cdh1 plus 3.0 μg His6-Uba1, 5.0 μg His6-Ubc4, 100 ng purified APC, 150 μM bovine ubiquitin (Sigma), 1 mM ATP, and 2 μl 35S-labeled MBP-Hsl1 in a 15-μl volume containing 1× ubiquitination buffer (40 nM Tris-Cl [pH 7.6], 10 mM MgCl2, 0.6 mM DTT) essentially as previously described (9). Approximately 1 μg of Acm1 was added to inhibit ubiquitination reactions. The ubiquitination reaction products were separated by SDS-polyacrylamide gel electrophoresis. The level of ubiquitinated, 35S-labeled MBP-Hsl1 was visualized by autoradiography and quantitated by PhosphorImager analysis.
RESULTS
Acm1 is an unstable protein but not an APC substrate.
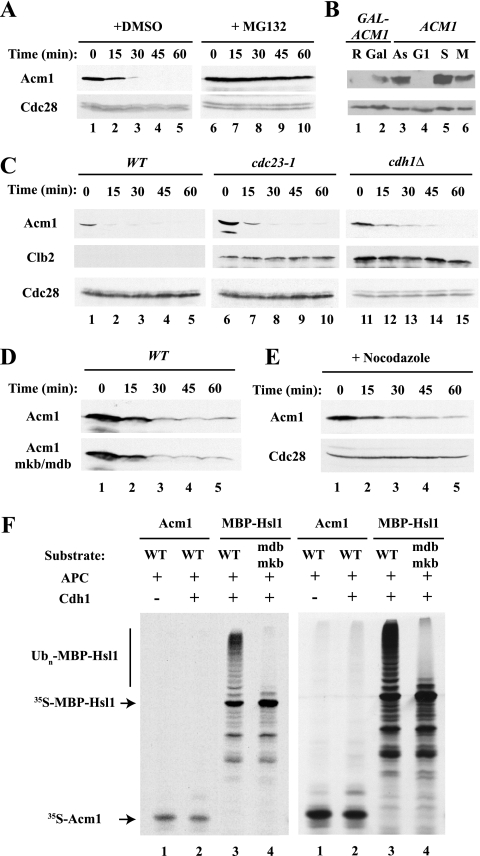
Because Acm1 levels fluctuate during the cell cycle and the protein binds to Cdh1 and contains potential D-box and KEN-box motifs, we hypothesized that Acm1 might be an APC substrate in addition to being a Cdh1 regulator. To examine this possibility, we first assessed whether Acm1 is unstable following the inhibition of its synthesis in asynchronous cells. We found that Acm1 was constitutively unstable but that it was stabilized in the presence of the proteasome inhibitor MG132, indicating that Acm1 degradation was proteasome dependent (Fig. 1A). For this and other degradation experiments, Acm1 was expressed from the GAL promoter to less than the level of endogenous Acm1 in asynchronous cells (Fig. 1B). Since Acm1 physically associates with Cdh1 (12, 42), we asked whether Acm1 degradation is APCCdh1 dependent. Acm1 was barely detectable and was rapidly degraded in cells arrested in G1 phase by treatment with the mating pheromone α-factor (Fig. 1C, lanes 1 to 5). Acm1 remained highly unstable following inactivation of APCCdh1 with a temperature-sensitive allele of CDC23 (which encodes a core subunit of the APC) or upon deletion of CDH1 (Fig. 1C, lanes 6 to 15), conditions that fully stabilize other APCCdh1 substrates, such as Clb2 (Fig. 1C, lanes 1 to 15) (59, 61, 67, 70). There was also no stabilization of Acm1 following the mutation of its putative D box and KEN box (Fig. 1D) or of two weaker D-box-like sequences (8RTIL and 111RAFL) (see Fig. 2A; data not shown). Thus, APCCdh1 is not involved in Acm1 degradation.
FIG. 1.
Acm1 is an unstable protein but is not an APC substrate. (A) _pdr5_Δ mutant cells (which respond to MG132) were treated with 25 μg/ml MG132 (from a 2-mg/ml stock solution in dimethyl sulfoxide [DMSO]) for 90 min to inhibit the proteasome. _GAL_-ACM1 expression was induced by 2% galactose for 50 min and terminated by the addition of 2% dextrose and 500 μg/ml cycloheximide at time zero. Samples were withdrawn at the indicated times and processed for immunoblot analysis with PAP antibodies to detect Acm1-TAP. Anti-Cdc28 was used as a loading control. (B) The expression level of _GAL_-ACM1 is comparable to that of endogenous Acm1. WT cells carrying a _GAL_-ACM1 vector were grown in raffinose (lane 1) or induced by 2% galactose for 35 min (lane 2). The resulting levels of Acm1-TAP were compared to that of endogenous Acm1 in asynchronous cells (lane 3) and in cells arrested in G1 with 100 ng/ml α-factor (lane 4), in S phase with 100 mM HU (lane 5), or in M phase with 50 μg/ml benomyl (lane 6). (C) WT and _cdc23_-1 mutant cells were arrested in G1 phase by treatment with 100 ng/ml α-factor and transferred to 37°C for 2 h to inactivate _cdc23_-1. _cdh1_Δ mutant cells were asynchronous, as they fail to arrest with α-factor. _GAL_-ACM1 and _GAL_-CLB2 expression was induced with galactose and terminated with cycloheximide as in panel A. Acm1-TAP and Clb2-TAP were detected as in panel A. (D) WT Acm1 and Acm1-mdb/mkb containing mutations in the D box and KEN box of Acm1 were expressed and detected as in panel A. (E) WT cells were arrested in mitosis with 20 μg/ml nocodazole. Acm1 was expressed and detected as in panel A. (F) Acm1 is not ubiquitinated by APCCdh1 in vitro. Acm1, MBP-Hsl1, and MBP-Hsl1-mdb/mkb were translated in vitro in the presence of [35S]methionine and incubated with yeast TAP tag-purified APC, with other components of an in vitro ubiquitination system, and with or without His6-Cdh1 purified from baculovirus-infected cells. MBP-Hsl1 was ubiquitinated in a D-box- and KEN-box-dependent manner, while no appreciable ubiquitination of Acm1 was detected. Two exposures of the same gel are shown.
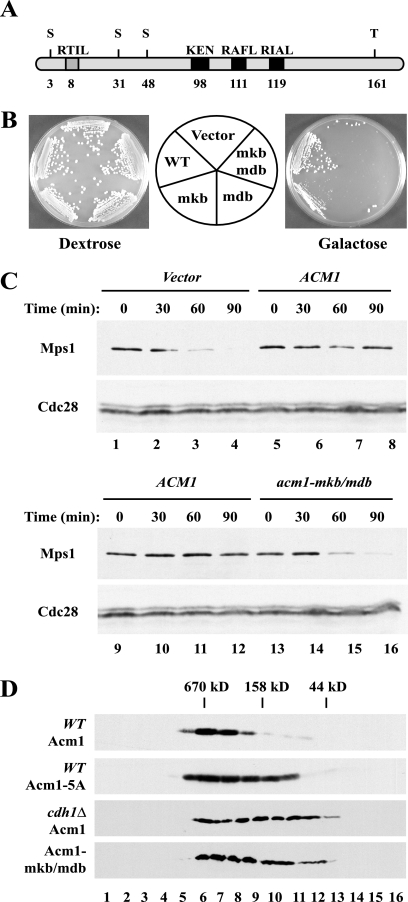
FIG. 2.
Acm1's KEN box and D box are required for its function in vivo. (A) Schematic representation of Acm1 motifs where the number below each motif corresponds to its first amino acid. (B) A yeast strain carrying _GALL_-_cdh1_-m11 was transformed with _GAL_-ACM1, _GAL_-_acm1_-mkb, _GAL_-_acm1_-mdb, _GAL_-_acm1_-mkb/mdb, or an empty vector. The resulting strains were tested for the ability to grow under repressing (dextrose) and inducing (galactose) conditions. Cdh1-m11 lacks inhibitory phosphorylation sites and is toxic when overexpressed. (C) Strains carried endogenous _MPS1_-TAP and _GALL_-_cdh1_-m11, as well as _GAL_-ACM1, _GAL_-_acm1_-mkb/mdb, or an empty vector. Samples were withdrawn at the indicated times after galactose induction of Cdh1-m11 and processed for immunoblotting. Mps1-TAP was enriched by precipitation with immunoglobulin G-Sepharose, resolved by SDS-polyacrylamide gel electrophoresis, and immunoblotted with PAP antibodies. Cdc28 was used as a loading control. (D) Protein extracts from WT cells expressing endogenous _ACM1_-TAP (panel 1 [top]) and _GAL_-_acm1_-5A (panel 2) and _cdh1_Δ mutant cells expressing _ACM1_-TAP (panel 3) and _acm1_-mkb/_mdb_-TAP (panel 4) were fractionated on a Superdex 200 gel filtration column. Proteins from individual fractions were analyzed by immunoblotting with PAP antibodies. Numbers at the bottom correspond to the fraction numbers; the positions of molecular mass standards are indicated at the top.
We tested a possible role for APCCdc20 in Acm1 degradation by extending our analysis to mitosis and assessed Acm1 stability in WT cells treated with the microtubule-depolymerizing drug nocodazole, which activates the spindle assembly checkpoint, thereby inhibiting APCCdc20 and stabilizing its substrates. Under these conditions, Acm1 turnover was slightly delayed but still significant, so that most of the protein was degraded within 30 min (Fig. 1E). This degradation of Acm1 was in sharp contrast to the complete stabilization of other APCCdc20 substrates such as Clb2 (2; data not shown) and indicates that Acm1 is not ubiquitinated by APCCdc20.
Finally, we examined whether Acm1 could be ubiquitinated by APCCdh1 in vitro. We found no significant ubiquitination of Acm1, whereas Hsl1 was efficiently ubiquitinated in a D-box- and KEN-box-dependent manner (Fig. 1F). Similar results were obtained with APCCdc20 in these assays (data not shown). Therefore, we conclude that neither APCCdh1 nor APCCdc20 plays a significant role in Acm1 degradation. Of course, we cannot exclude the possibility that the APC plays a minor role in Acm1 degradation or that it acts redundantly with another ubiquitin ligase.
Acm1's KEN box and D box are required for Acm1 function in vivo.
Although Acm1 is not an APC substrate, it nevertheless contains consensus destruction boxes and a KEN box (Fig. 2A). These motifs, which are not required for Acm1 degradation (Fig. 1D), are conserved in the putative Acm1 homologues in the yeasts Ashbya gossypii and Candida albicans (data not shown). Because Cdh1 recognizes APC substrates via KEN boxes and D boxes, we hypothesized that Acm1 might bind to and inhibit Cdh1 through one or both of these motifs.
We first examined whether these motifs are necessary for Acm1 function by using the previously described ability of overexpressed ACM1 to restore growth to a strain that overexpresses the nonphosphorylatable Cdh1-m11 protein, which is active and degrades APC substrates prematurely during mitosis (12, 42, 72). In agreement with previous reports, cells expressing Cdh1-m11 from a GALL promoter were inviable but the simultaneous overexpression of WT Acm1 prevented this toxicity and restored growth in the presence of galactose (12, 42). In contrast, mutation of the Acm1 KEN box compromised the rescuing ability of Acm1 and mutation of one of the putative destruction boxes, 119RIAL, or of both this D box and the KEN box completely abolished rescue by Acm1 (Fig. 2B), even though the double-mutant protein was expressed at levels comparable to that of the WT protein (Fig. 1D) and, according to gel filtration analysis, exhibited normal hydrodynamic properties (Fig. 2D). Mutations within two other putative destruction boxes, 8RTIL and 111RAFL, had no effect on the rescue by Acm1 (data not shown). Therefore, we conclude that the 98KEN and 119RIAL motifs are important for the ability of Acm1 to inhibit Cdh1 in vivo.
We extended this analysis by asking whether these motifs are important for the ability of Acm1 to protect APC substrates from degradation in vivo. Induced expression of Cdh1-m11 caused premature degradation of the APC substrate Mps1 (Fig. 2C, lanes 1 to 4; data not shown) (47). In contrast, cells that simultaneously overexpressed Acm1 maintained their Mps1 levels (Fig. 2C, lanes 5 to 8), indicating that Acm1 was able to prevent Mps1 degradation. Importantly, overexpression of Acm1-mkb/mdb could not protect Mps1 from degradation (Fig. 2C, lanes 13 to 16), indicating that the KEN box and D box of Acm1 are required to block Mps1 accessibility to Cdh1 and protect it from subsequent degradation.
Cdh1 recognizes Acm1's KEN box and D box and an additional sequence in the N-terminal portion of the protein.
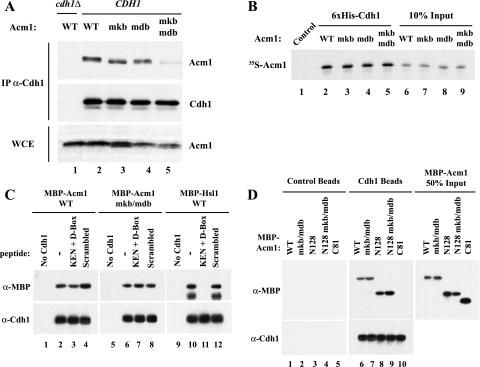
Given the requirement of the KEN box and D box for Acm1 function, we asked whether Acm1 might use these motifs to interact with Cdh1. To investigate this possibility, we tested for the ability of Acm1 mutants to associate with Cdh1 in coimmunoprecipitation assays of yeast extracts. We found that Cdh1 associated with WT Acm1 but that the recovery of Acm1-mkb and Acm1-mdb was reduced (Fig. 3A, lanes 2 to 4) and that Acm1-mkb/mdb showed almost no association with Cdh1 (Fig. 3A, lane 5), indicating that both motifs are important for efficient Acm1-Cdh1 association. Interestingly, Acm1 requires both motifs for function but only one for binding Cdh1, just as the APC substrate Hsl1 requires both a KEN box and a D box for efficient degradation but only one motif for binding to Cdh1 (albeit with reduced efficiency) (4, 7).
FIG. 3.
Acm1's KEN box and D box participate in binding Cdh1. (A) Acm1 binds to Cdh1 in vivo via its KEN box and D box. Expression of WT and mutant forms of Acm1-HA were induced by galactose for 2 h. Cdh1 was immunoprecipitated (IP) from cell extracts with anti-Cdh1 antibodies, and bound proteins were analyzed by immunoblotting. The levels of Acm1-HA in the whole-cell extracts (WCE) are shown in the lower panel. (B) Sequences outside of the Acm1 KEN box and D box contribute to association with Cdh1 in vitro. The indicated forms of [35S]methionine-labeled Acm1 were incubated with control or His6-Cdh1 beads. Bound Acm1 was detected by autoradiography (lanes 1 to 5). Ten percent of the input 35S-labeled Acm1 is shown in lanes 6 to 9. (C) MBP-Acm1 or MBP-Acm1-mdb/mkb was incubated with control or His6-Cdh1 beads in the absence or presence of D-box- and KEN-box-containing peptides (lanes 1 to 8). Acm1 bound specifically to Cdh1 beads under all conditions, whereas the D-box and KEN-box peptides completely blocked the association of MBP-Hsl1 with the Cdh1 beads (lanes 10 to 12). (D) The Cdh1-binding sequences are contained within the first 128 amino acids of Acm1. Full-length, N128, and C81 MBP-Acm1 proteins were incubated with control or His6-Cdh1 beads and assayed for binding by immunoblot analysis with anti-MBP antibodies (top panels, lanes 1 to 10). WT and mdb/mkb isoforms of either full-length or N128 bound equally well to Cdh1 (lanes 6 to 9), whereas no detectable binding to Cdh1 was observed for the last 81 residues of Acm1 (C81, lane 10). Fifty percent of the input in the binding assays for the indicated MBP-Acm1 proteins is shown in the panel on the right. Cdh1 present on the beads was detected by immunoblotting with anti-Cdh1 antibodies (lower panel).
We next examined whether Acm1 and Cdh1 could bind directly in vitro in the absence of other yeast proteins. Control beads or beads with bound recombinant His6-Cdh1 were incubated with 35S-labeled WT, mdb, mkb, or mdb/mkb Acm1 produced by translation in vitro. As expected, WT Acm1 bound very efficiently to the Cdh1 beads. Interestingly, the D-box and KEN-box mutants also bound efficiently to Cdh1 (Fig. 3B, lanes 2 to 5). In all cases, more than 40% of the input Acm1 bound to Cdh1 and this binding was specific as there was no binding to control beads (Fig. 3B, lane 1). These results suggested that an additional motif participates in Acm1-Cdh1 binding.
One possibility for this additional motif could be a cryptic D-box- or KEN-box-like sequence within Acm1. To test this idea, we attempted to block the Acm1-Cdh1 interaction with D-box and KEN-box peptides derived from the APC substrate Hsl1. As shown previously (7), these peptides could completely and specifically block MBP-Hsl1 binding to Cdh1 beads (Fig. 3C, lanes 9 to 12). However, they had no effect on the binding of either WT or mdb/mkb MBP-Acm1 to Cdh1 (Fig. 3C, lanes 1 to 8), suggesting that the additional binding sequence is not a cryptic KEN box or D box.
To further delineate what portion of Acm1 binds Cdh1, we made amino-terminal and carboxyl-terminal forms of Acm1 containing the first 128 residues (N128) or the last 81 residues (C81) of Acm1, respectively. Both full-length and N128 forms of Acm1 bound efficiently to Cdh1, and these interactions were independent of the D-box and KEN-box motifs of Acm1 (Fig. 3D, lanes 6 to 9). In contrast, the carboxyl-terminal portion of Acm1 (C81) was unable to bind to Cdh1 (lane 10) and no binding of any of the Acm1 proteins to control beads was detected (lanes 1 to 5). Thus, like the KEN box and the D box, the additional Cdh1-binding sequences are located in the N-terminal portion of Acm1. We are currently trying to identify the additional binding determinant that is critical for the observed Cdh1 association. We do not know why this binding determinant is critical for the observed Cdh1 association in vitro but appears to play less of a role in vivo. We speculate that Acm1 phosphorylation, Bmh1/Bmh2 binding, or an unknown factor may influence the interaction in vivo.
Acm1 competes with APC substrates for binding to Cdh1.
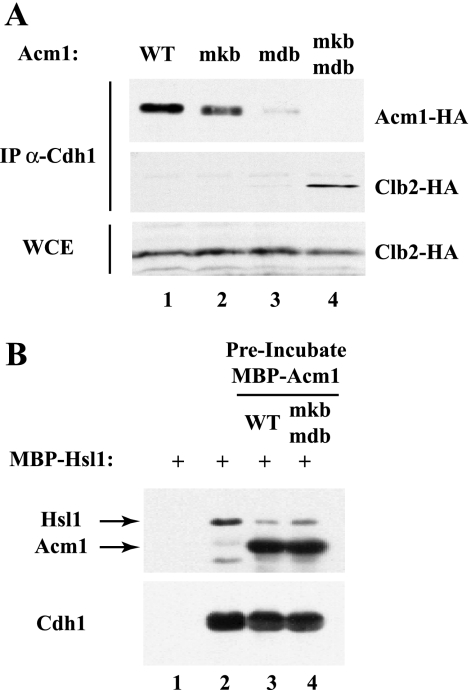
Given that Acm1's KEN box and D box are required for both Cdh1 inhibition and binding in vivo, we hypothesized that they might function to prevent substrates from gaining access to Cdh1. To address this possibility, we examined Cdh1 binding to Clb2 in the presence of WT and mutant forms of Acm1. A previous study demonstrated that deletion of ACM1 significantly increased Clb2 binding to Cdh1 in S-phase-arrested cells (42). Under similar conditions, we found that expression of exogenous Acm1, but not Acm1-mdb/mkb, could abrogate the Clb2-Cdh1 interaction in _acm1_Δ mutant cells (Fig. 4A, compare lanes 1 and 4). Thus, Acm1 can prevent the interaction between Cdh1 and its substrates by blocking Cdh1's KEN-box- and D-box-binding domains.
FIG. 4.
Acm1 competes with APC substrates for Cdh1 binding. (A) _acm1_Δ mutant strains carrying _GAL_-_CLB2_-HA and exogenous _ACM1_-HA or its mutant derivatives were arrested in S phase by treatment with 100 mM hydroxyurea. The expression of _CLB2_-HA was induced for 2 h. Cdh1 was immunoprecipitated (IP) from cell extracts, and associated proteins were detected by immunoblotting. The input levels of Clb2-HA in the whole-cell extracts (WCE) are shown in the lower panel. (B) Acm1 can block the association of MBP-Hsl1 with Cdh1 in vitro. Control or Cdh1-containing beads were incubated with MBP-Hsl1 in the absence of MBP-Acm1 or following preincubation with WT MBP-Acm1 or MBP-Acm1-mdb/mkb.
We extended this analysis by examining whether Acm1 could also block Cdh1-substrate association in vitro. Cdh1-containing beads were preincubated with buffer only, Acm1, or Acm1-mdb/mkb prior to addition of the APC substrate Hsl1. In the presence of Acm1, Hsl1 binding to Cdh1 was substantially reduced, with WT Acm1 being slightly more effective than Acm1-mdb/mkb (Fig. 4B, lanes 2 to 4). The ability of Acm1-mdb/mkb to block Hsl1 binding to Cdh1 presumably reflects the additional Cdh1-interacting sequences in Acm1 (see above and Fig. 3). These results suggest that the molecular mechanism by which Acm1 inhibits APCCdh1 in vitro and in vivo is blocking of substrate access to Cdh1.
Acm1 inhibits APCCdh1 ubiquitination of Hsl1.
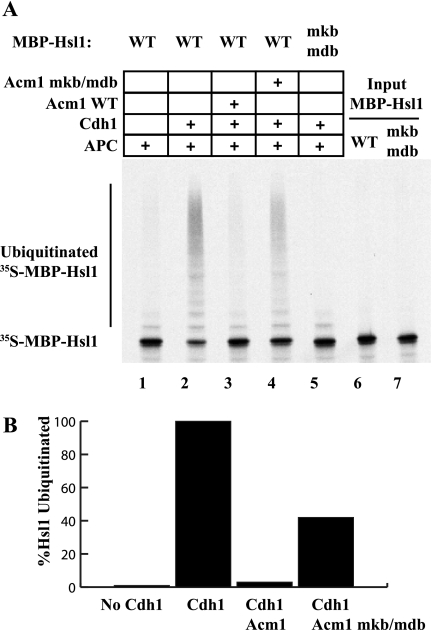
Previous work demonstrated that Acm1 could inhibit the ubiquitination of the B-type cyclin Clb2 in vitro (12). To determine the role of the Acm1 KEN box and D box in this inhibition, we analyzed the effects of Acm1 on the ubiquitination of Hsl1 in vitro. We performed the ubiquitination reactions as described in the legend to Fig. 1, with either WT or mdb/mkb 35S-labeled MBP-Hsl1 as the substrate. As expected, very little ubiquitination of Hsl1 was detected in the absence of added Cdh1 or when using MBP-Hsl1-mdb/mkb, a substrate that cannot bind Cdh1 (Fig. 5A, lanes 1 and 5). Addition of Cdh1 promoted robust ubiquitination of WT MBP-Hsl1 (Fig. 5A, lane 2). Addition of WT Acm1 effectively blocked the ubiquitination of Hsl1 to a level less than 3% of what was observed in the absence of Acm1 (Fig. 5A, compare lane 2 with lane 3, and B). Acm1-mdb/mkb was much less effective at inhibiting APCCdh1 (Fig. 5, lane 4). These results, together with the in vivo data, indicate that Acm1 blocks APC substrate ubiquitination by utilizing its D-box and KEN-box motifs to inhibit Cdh1-substrate association.
FIG. 5.
Acm1 requires its KEN box and D box for efficient inhibition of MBP-Hsl1 ubiquitination in vitro. 35S-labeled MBP-Hsl1 was monitored for APCCdh1-mediated ubiquitination by autoradiography as described in the legend to Fig. 1. Where indicated, Cdh1 and the APC were preincubated with WT or mdb/mkb MBP-Acm1 prior to the addition of 35S-labeled MBP-Hsl1. Hsl1 ubiquitination was reduced by 97% in the presence of WT Acm1 but by only 58% in the presence of Acm1-mdb/mkb (compare lanes 3 and 4 with lane 2). No ubiquitination of Hsl1 lacking its D-box and KEN-box motifs was detected (lane 5). Quantitation of Hsl1 ubiquitination for lanes 1 to 4 was performed with a PhosphorImager and is shown in the bottom panel with the level of APCCdh1-mediated Hsl1 ubiquitination in the absence of Acm1 defined as 100%.
The SCF ubiquitin ligase is not directly involved in promoting Acm1 degradation.
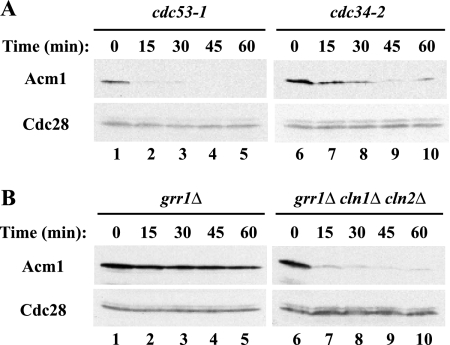
Because Acm1 is unstable in G1 but is not an APC substrate (see above), we investigated whether it might be targeted for ubiquitination by another cell cycle ubiquitin ligase, the SCF complex. SCF complexes active in G1 contain, among other proteins, Cdc53 as a core subunit, the Cdc34 ubiquitin-conjugating enzyme, and either of two F-box-containing proteins, Grr1 or Cdc4, as a substrate-binding subunit (8). Growth of _cdc53_-1 cells at the nonpermissive temperature did not reveal any stabilization of Acm1 (Fig. 6A, lanes 1 to 5), and inactivation of _cdc34_-2 resulted in only partial stabilization of Acm1 (Fig. 6A, lanes 6 to 10). Surprisingly, given that Cdc53 and Cdc34 are present in all G1 SCF complexes, Acm1 abundance and stability increased significantly in a _grr1_Δ mutant strain (Fig. 6B, lanes 1 to 5). We questioned whether this effect was direct since SCFGrr1 also targets the G1 cyclins Cln1 and Cln2 for ubiquitin-mediated degradation. Cln1 and Cln2 activate Cdc28 in G1, and Cdc28 is thought to phosphorylate Acm1 (12, 42). Thus, the increased stability of Acm1 in _grr1_Δ mutant cells might be due to elevated Cdc28 activity. Consistent with this view, we found that Acm1 was again highly unstable in a _grr1_Δ _cln1_Δ _cln2_Δ triple-mutant strain (Fig. 6B, lanes 6 to 10). Therefore, the effect of a _grr1_Δ deletion on Acm1 stability was indirect and was mediated by the increased abundance of Cln1 and Cln2.
FIG. 6.
Acm1 is not ubiquitinated by the SCF. (A) Cells containing the _cdc53_-1 and _cdc34_-2 temperature-sensitive alleles were incubated at 37°C for 2 h. _GAL_-ACM1 expression was induced by galactose for 50 min. Following dextrose and cycloheximide addition, samples were withdrawn at the indicated times and Acm1-TAP levels were determined by immunoblotting with PAP antibodies. Cdc28 was used as a loading control. (B) As in panel A, except that the asynchronous cultures contained deletions of GRR1 or of GRR1, CLN1, and CLN2 and there was no temperature shift.
It is interesting that while deletion of GRR1 promoted strong Acm1 stabilization, temperature inactivation of _cdc34_-2 and _cdc53_-1 strains did not, even though Cln1 and Cln2 should be stabilized in all of these mutant strains. A possible explanation for this discrepancy is that the conditional mutants may not completely disrupt SCFGrr1 function compared with the _grr1_Δ deletion. Alternatively, Cln1 and Cln2 may be free to associate with Cdc28 and thus phosphorylate and stabilize Acm1 in a _grr1_Δ mutant strain, whereas these cyclins may be bound to Grr1 in nonproductive complexes in the _cdc34_-2 and _cdc53_-1 mutant strains.
Cdc28-mediated phosphorylation protects Acm1 from degradation.
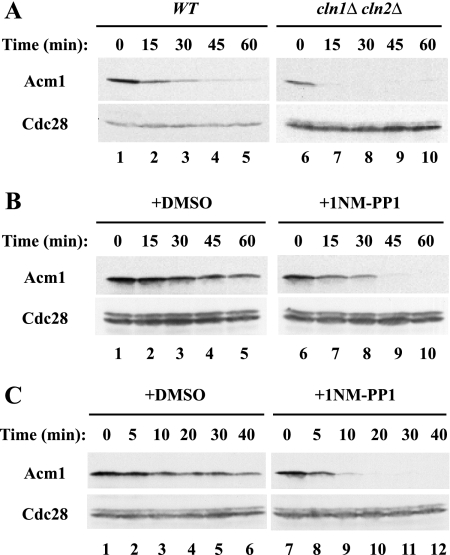
The stabilization of Acm1 in _grr1_Δ mutant cells and the abrogation of that stabilization following the deletion of CLN1 and CLN2 raised the possibility that phosphorylation by Cdc28 might stabilize Acm1. Indeed, Acm1 contains five consensus Cdc28 phosphorylation sites: 3SPSK, 31SPSK, 48SPIK, 102SPAK, and 161TPPR. Consistent with this idea, Acm1 was expressed at lower levels and was less stable in a _cln1_Δ _cln2_Δ mutant strain than in WT cells (Fig. 7A, lanes 1 to 10).
FIG. 7.
Cdc28-mediated phosphorylation regulates Acm1 stability. (A) Acm1 stability was assessed in asynchronous WT and _cln1_Δ _cln2_Δ double-mutant cells as in Fig. 1. Cdc28 was used as a loading control. (B) _GAL_-ACM1 expression was induced in asynchronous _cdc28_-as mutant cells by 2% galactose for 50 min and terminated by the addition of dextrose and cycloheximide in the presence of either dimethyl sulfoxide (DMSO) (lanes 1 to 5) or the ATP analog 1-NM-PP1 at 1 μM (from a 1 mM stock solution in dimethyl sulfoxide) (lanes 6 to 10) and incubation for 10 min. The stability of Acm1-TAP was determined as in Fig. 1. (C) As in panel B, except that cells were arrested in mitosis by treatment with 20 μM nocodazole for 2 h prior to the addition of 1-NM-PP1.
To test the role of Cdc28 in Acm1 degradation directly, we examined Acm1 degradation following inactivation of Cdc28 in a _cdc28_-as mutant strain. Cdc28-as contains a mutation allowing it to bind to a cell-permeable bulky ATP analog, 1-NM-PP1, leading to the rapid inactivation of Cdc28 activity (3). Cells were induced to express Acm1 by the addition of galactose, and the stability of the protein was monitored after the addition of glucose and cycloheximide in the presence or absence of 1-NM-PP1. Addition of 1-NM-PP1 led to an immediate and dramatic enhancement of Acm1 degradation compared with mock-treated cells (Fig. 7B). The half-life of Acm1 in treated _cdc28_-as mutant cells was just over 5 min, which was comparable to the rapid Acm1 degradation observed in G1-arrested cells (see, for example, Fig. 1C). Acm1 was similarly destabilized by 1-NM-PP1 in nocodazole-arrested _cdc28_-as mutant cells, indicating that Cdc28 activity was critical for Acm1 stability in mitosis, as well as in G1 phase (Fig. 7C).
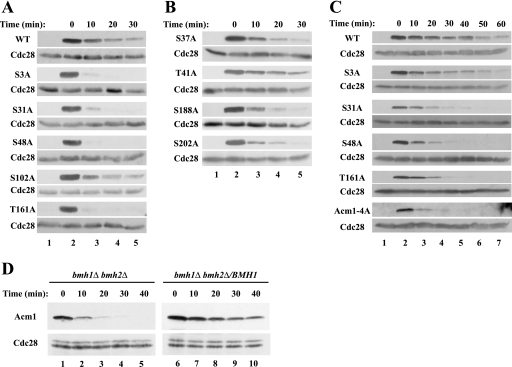
Acm1 phosphorylation at multiple sites controls its stability.
Given the apparent role of Cdc28 in stabilizing Acm1, we attempted to determine which potentially phosphorylatable residues of Acm1 are required for the increased stability. We systematically mutated the five consensus CDK phosphorylation sites in Acm1 and tested the stability of the resulting proteins. We found that mutations of Ser-3, Ser-31, Ser-48, and Thr-161—sites identified by mass spectrometry as phosphorylated in vivo (24, 62)—significantly shortened the half-life of Acm1 in asynchronous cells (Fig. 8A). The one CDK consensus mutation that showed only a weak effect on Acm1 stability was S102A. In contrast, mutations within non-CDK consensus sites known to be phosphorylated in vivo—Ser-37, Thr-41, Ser-188, and Ser-202 (24, 62)—did not significantly affect Acm1 stability (Fig. 8B). To examine the contributions of individual phosphorylation sites in more detail, we analyzed the stabilities of Acm1 mutant proteins in S-phase-arrested cells, when Acm1 is abundant and actively engaged in inhibiting Cdh1 (12, 42; data not shown). We found that WT Acm1 had a significantly longer half-life in HU-arrested S-phase cells than in other stages of the cell cycle. As in asynchronous cells, mutations of Ser-31, Ser-48, and Thr-161 shortened the Acm1 half-life whereas mutation of Ser-3 had a more subtle effect on Acm1 stability in S-phase cells than in asynchronous cells (Fig. 8C). The Acm1-4A quadruple mutant containing S3A, S31A, S48A, and T161A was the least stable and was rapidly degraded in HU-arrested cells (Fig. 8C), paralleling the rapid degradation of Acm1 following the inactivation of Cdc28-as (Fig. 7). The instability of the multiply mutated form of Acm1 does not seem to result from misfolding, as the hydrodynamic properties of an Acm1-5A mutant appeared normal (Fig. 2D). We conclude that Cdc28 likely regulates Acm1 stability by direct phosphorylation and that these four phosphorylation sites have additive effects on Acm1 stability in vivo. While this report was under review, Hall et al. also reported a role for Thr-161 phosphorylation in stabilizing Acm1 (26), though they did not examine the other single-point mutations.
FIG. 8.
Mutations within putative Cdc28 phosphorylation sites accelerate Acm1 degradation. The stabilities of WT Acm1-TAP and Acm1-TAP containing mutations of consensus Cdc28 phosphorylation sites (A) and of non-Cdc28 sites (B) were determined in exponentially growing cultures following galactose-induced expression of Acm1 as in Fig. 1. The samples for lanes 1 were taken before galactose addition. (C) As in panel A, except that cells were arrested in S phase with 0.1 M HU for 3 h prior to galactose-induced expression of Acm1 for 60 min. (D) The stabilities of Acm1-TAP were determined following S-phase arrest in a _bmh1_Δ _bmh2_Δ mutant strain and in its derivative expressing a single copy of BMH1. Cdc28 was used as a loading control. Note that four times as much protein was loaded in lanes 1 to 5 as in lanes 6 to 10 and that lanes 1 to 5 were also exposed to film 12 times as long as lanes 6 to 10, indicating that deletion of BMH1 and BMH2 greatly reduces Acm1 levels.
The Acm1-Cdh1 complex also contains Bmh1 and Bmh2, closely related 14-3-3 proteins (12, 19, 42). Since 14-3-3 proteins bind to phosphorylated proteins, it seemed possible that Bmh1 and Bmh2 might bind to phosphorylated Acm1 and protect it from degradation. Indeed, Hall et al. found that the T161A mutant form of Acm1 bound significantly less Bmh1 than did WT Acm1 (26). We tested this possibility by examining the stability of Acm1 in cells lacking Bmh1 and Bmh2. Because most yeast strains require at least one of these proteins for viability, this experiment was done with the Σ12778b strain, in which these proteins are not essential (57). As shown in Fig. 8D, Acm1 was rapidly degraded in S-phase-arrested cells lacking Bmh1 and Bmh2 but was significantly stabilized when BMH1 was reintroduced into these cells.
DISCUSSION
In this study, we have explored the regulation of Cdh1 activity by Acm1. Although Acm1 contains both a KEN box and a D box that are conserved in Acm1 orthologues in other yeasts, neither motif has any apparent role in promoting Acm1 instability. Rather, these motifs were required for Acm1-mediated inhibition of Cdh1, serving as binding sites for Cdh1 and blocking substrate access and subsequent ubiquitination by the APC. We also found that Acm1 degradation was proteasome dependent but independent of the two major classes of cell cycle ubiquitin ligases, APC and SCF. Instead, Acm1 stability was regulated via phosphorylation by Cdc28, which appeared to protect Acm1 from a constitutive degradation pathway. Thus, Acm1 is present when Cdc28 activity is high, preventing inappropriate Cdh1-substrate interactions during these phases of the cell cycle.
The inhibition of APCCdh1 by Acm1 has parallels in the inhibition of APCCdc20 by Mad3/BubR1 and of APCCdh1 by Emi1. Mad3 is a spindle assembly checkpoint protein conserved from yeast to humans (where it is called BubR1) that delays the metaphase-to-anaphase transition by inhibiting APCCdc20 activity and its ubiquitination of the anaphase inhibitor, Pds1/securin. Mad3 is a stable protein that nevertheless contains two conserved KEN boxes and a D box that promote its binding to Cdc20 (6, 36). These motifs are essential for Mad3's checkpoint function and its ability to act as a pseudosubstrate inhibitor of Cdc20. Emi1 functions in higher eukaryotes to inhibit Cdh1 activity outside of the G1 phase, thereby preventing unscheduled degradation of APCCdh1 substrates and DNA rereplication (13, 40, 56). Emi1 binds Cdh1 by using a conserved D box but, in addition, requires a distinct zinc-binding region to inhibit APC activity (43). Because Acm1 contains only one cysteine residue, which is not sufficient for zinc binding, the mechanism of Acm1-mediated inhibition must be different.
Although there are no yeast homologues of Emi1, Acm1 and Emi1 share important functional similarities. Both proteins are pseudosubstrate inhibitors of APCCdh1, functioning to suppress APCCdh1 activity throughout much of the cell cycle. Both proteins are cell cycle regulated: Emi1 is activated by the E2F transcription factor (30), whereas ACM1 transcription is likely to be controlled by SBF/MBF, as binding sites for these factors were found within the ACM1 promoter. Both are also highly unstable proteins whose degradation serves to terminate their activities and permit APCCdh1 activation. In mitosis, Emi1 is phosphorylated by Cdc2 and Plk1 within a conserved DSGXXS domain, which primes it for ubiquitination by SCFβTrCP (27, 41). We explored whether SCF might similarly ubiquitinate Acm1 in yeast and found that it did not.
In contrast to Emi1, we found that phosphorylation by Cdc28, rather than priming Acm1 for degradation, served the opposite function and protected Acm1 from degradation. Multiple phosphorylations appear to be required for Acm1 stabilization. Mutations within any of four Cdc28 consensus phosphorylation sites at Ser-3, Ser-31, Ser-48, and Thr-161 shortened the Acm1 half-life (and the quadruple mutant had an even greater effect), whereas mutations of a fifth consensus Cdc28 site at Ser-102 and of non-Cdc28 consensus sites at Ser-37, Thr-41, Ser-188, and Ser-202 (which are known to be phosphorylated in vivo [24, 62]) did not have significant effects on Acm1 stability. Interestingly, Cdc28 phosphorylation of another APC substrate, Mps1, on Thr-29 also leads to its stabilization (34). Since Cdc28 binds a number of cyclins in different phases of the cell cycle, we predicted that various cyclin-Cdc28 complexes would be able to phosphorylate Acm1. In agreement with this prediction, we found that Cln1-, Clb5-, and Clb2-associated Cdc28 complexes phosphorylated recombinant Acm1 in vitro (data not shown).
It remains unclear how phosphorylation by Cdc28 stabilizes Acm1. The role of phosphorylation in protecting Acm1 from degradation is intimately tied to its binding of Bmh1 and Bmh2 since we found that these proteins are also required for Acm1 stability. There are two attractive models for this interplay. First, binding of Bmh1 or Bmh2 may protect Acm1 from recognition by the ubiquitin system. In this model, phosphorylation would recruit Bmh1 and Bmh2 but would serve no direct stabilizing function. In the second model, phosphorylation would directly stabilize Acm1 and Bmh1/Bmh2 binding would serve to protect that phosphorylation against the action of phosphatases such as Cdc14 (26). While this report was under review, Hall et al. (26) came to similar conclusions regarding the role of phosphorylation in stabilizing Acm1 and speculated that Bmh1 and Bmh2 might exert a protective effect. Currently, we are trying to locate Acm1 residues responsible for its degradation and determine which ubiquitin-conjugating enzyme and ubiquitin ligase mediate Acm1 ubiquitination. We are also investigating what role these phosphorylations and Bmh1/Bmh2 binding might play in potentially masking the additional Cdh1-binding motif of Acm1 observed in vitro.
Our findings indicate that budding yeast employs dual mechanisms for inhibiting APCCdh1 via Cdc28. Cdc28 phosphorylation of Cdh1, particularly during mitosis, inhibits its interaction with the APC (33, 37, 72). In addition, as we have found, Cdc28 phosphorylation of Acm1 promotes its stabilization and allows it to inhibit Cdh1 by preventing substrate association. Why might budding yeast employ seemingly redundant mechanisms to inhibit APCCdh1? One possibility is that it may be critically important to prevent premature degradation of APC substrates, especially during S phase, just as there are multiple mechanisms to prevent DNA rereplication in budding yeast (45). It is also possible that Acm1 plays a preferential role in late G1 and S phases whereas phosphorylation of Cdh1 may play a preferential role in M phase.
Alternatively, these two modes of APCCdh1 inhibition may serve subtly different purposes, both of which are necessary for efficient cell cycle progression. Biochemically, Acm1 prevents substrate binding to Cdh1 whereas Cdc28-mediated phosphorylation of Cdh1 prevents Cdh1-APC association but has no reported effect on Cdh1-substrate interactions. Thus, Acm1 may be critical for blocking the formation of nonproductive Cdh1-Clb2 complexes. Both APCCdh1 activity and inappropriate Cdh1-substrate interactions may need to be inhibited for cells to proceed efficiently through S phase and into mitosis. Supporting a nonredundant role for Acm1 and a function in suppressing nonproductive Cdh1-substrate interactions are the observations that S phase proceeds more slowly in _acm1_Δ mutant cells than in WT cells and that there is an increase in Cdh1-Clb2 complexes observed in these cells (12, 42) (Fig. 4). This Cdc28-mediated two-pronged approach to APCCdh1 inhibition may also be important in M phase, where Acm1 may prevent Cdh1 from binding to Cdc20 (an APCCdh1 substrate), thereby allowing APCCdc20 activity to perform its necessary roles during mitosis. In addition, phosphorylation of Cdh1 during mitosis may prevent it from competing with Cdc20 for binding to the APC. Acm1 would be insufficient for this purpose and might even trap nonproductive Acm1-Cdh1 complexes on the APC. Thus, both modes of Cdh1 regulation may be needed for efficient APC regulation.
Acknowledgments
We thank Mark Goebl, J. Wade Harper, Mark Hochstrasser, David Morgan, Wolfgang Seufert, and Mike Tyers for plasmids and strains. We thank Aiyang Cheng for critical reading of the manuscript.
This work was supported by grants GM076200 from the National Institutes of Health and 1-FY05-114 from The March of Dimes Foundation awarded to M.J.S.
Footnotes
▿
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
- 2.Bäumer, M., G. H. Braus, and S. Irniger. 2000. Two different modes of cyclin Clb2 proteolysis during mitosis in Saccharomyces cerevisiae. FEBS Lett. 468142-148. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, A. C., J. A. Ubersax, D. T. Petsch, D. P. Matheos, N. S. Gray, J. Blethrow, E. Shimizu, J. Z. Tsien, P. G. Schultz, M. D. Rose, J. L. Wood, D. O. Morgan, and K. M. Shokat. 2000. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407395-401. [DOI] [PubMed] [Google Scholar]
- 4.Burton, J. L., and M. J. Solomon. 2001. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 152381-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, J. L., and M. J. Solomon. 2000. Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex. Mol. Cell. Biol. 204614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton, J. L., and M. J. Solomon. 2007. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 21655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton, J. L., V. Tsakraklides, and M. J. Solomon. 2005. Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol. Cell 18533-542. [DOI] [PubMed] [Google Scholar]
- 8.Cardozo, T., and M. Pagano. 2004. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 5739-751. [DOI] [PubMed] [Google Scholar]
- 9.Carroll, C. W., M. Enquist-Newman, and D. O. Morgan. 2005. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 1511-18. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Fix, O., J.-M. Peters, M. W. Kirschner, and D. Koshland. 1996. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 103081-3093. [DOI] [PubMed] [Google Scholar]
- 11.Crasta, K., P. Huang, G. Morgan, M. Winey, and U. Surana. 2006. Cdk1 regulates centrosome separation by restraining proteolysis of microtubule-associated proteins. EMBO J. 252551-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dial, J. M., E. V. Petrotchenko, and C. H. Borchers. 2007. Inhibition of APCCdh1 activity by Cdh1/Acm1/Bmh1 ternary complex formation. J. Biol. Chem. 2825237-5248. [DOI] [PubMed] [Google Scholar]
- 13.Di Fiore, B., and J. Pines. 2007. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J. Cell Biol. 177425-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang, G. 2002. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell 13755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang, G., H. Yu, and M. W. Kirschner. 1998. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2163-171. [DOI] [PubMed] [Google Scholar]
- 16.Fang, G., H. Yu, and M. W. Kirschner. 1998. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 121871-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraschini, R., A. Beretta, L. Sironi, A. Musacchio, G. Lucchini, and S. Piatti. 2001. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 206648-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funabiki, H., H. Yamano, K. Kumada, K. Nagano, T. Hunt, and M. Yanagida. 1996. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature 381438-441. [DOI] [PubMed] [Google Scholar]
- 19.Gelperin, D., J. Weigle, K. Nelson, P. Roseboom, K. Irie, K. Matsumoto, and S. Lemmon. 1995. 14-3-3 proteins: potential roles in vesicular transport and Ras signaling in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 9211539-11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaemmaghami, S., W. K. Huh, K. Bower, R. W. Howson, A. Belle, N. Dephoure, E. K. O'Shea, and J. S. Weissman. 2003. Global analysis of protein expression in yeast. Nature 425737-741. [DOI] [PubMed] [Google Scholar]
- 21.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74527-534. [DOI] [PubMed] [Google Scholar]
- 22.Glotzer, M., A. W. Murray, and M. W. Kirschner. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349132-138. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 151541-1553. [DOI] [PubMed] [Google Scholar]
- 24.Gruhler, A., J. V. Olsen, S. Mohammed, P. Mortensen, N. J. Faergeman, M. Mann, and O. N. Jensen. 2005. Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol. Cell. Proteomics 4310-327. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie, C., and G. R. Fink. 1991. Guide to yeast genetics and molecular biology. Methods Enzymol. 1941-863. [PubMed] [Google Scholar]
- 26.Hall, M. C., D. E. Jeong, J. T. Henderson, E. Choi, S. C. Bremmer, A. B. Iliuk, and H. Charbonneau. 2008. Cdc28 and Cdc14 control stability of the anaphase-promoting complex inhibitor Acm1. J. Biol. Chem. 28310396-10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen, D. V., A. V. Loktev, K. H. Ban, and P. K. Jackson. 2004. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFβTrCP-dependent destruction of the APC inhibitor Emi1. Mol. Biol. Cell 155623-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardwick, K. G., R. C. Johnston, D. L. Smith, and A. W. Murray. 2000. MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J. Cell Biol. 148871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper, J. W., J. L. Burton, and M. J. Solomon. 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 162179-2206. [DOI] [PubMed] [Google Scholar]
- 30.Hsu, J. Y., J. D. Reimann, C. S. Sorensen, J. Lukas, and P. K. Jackson. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nat. Cell Biol. 4358-366. [DOI] [PubMed] [Google Scholar]
- 31.Hwang, L. H., L. F. Lau, D. L. Smith, C. A. Mistrot, K. G. Hardwick, E. S. Hwang, A. Amon, and A. W. Murray. 1998. Budding yeast Cdc20: a target of the spindle checkpoint. Science 2791041-1044. [DOI] [PubMed] [Google Scholar]
- 32.Jaquenoud, M., F. van Drogen, and M. Peter. 2002. Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/CCdh1. EMBO J. 216515-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaspersen, S. L., J. F. Charles, and D. O. Morgan. 1999. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9227-236. [DOI] [PubMed] [Google Scholar]
- 34.Jaspersen, S. L., B. J. Huneycutt, T. H. Giddings, Jr., K. A. Resing, N. G. Ahn, and M. Winey. 2004. Cdc28/Cdk1 regulates spindle pole body duplication through phosphorylation of Spc42 and Mps1. Dev. Cell 7263-274. [DOI] [PubMed] [Google Scholar]
- 35.Kim, S. H., D. P. Lin, S. Matsumoto, A. Kitazono, and T. Matsumoto. 1998. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 2791045-1047. [DOI] [PubMed] [Google Scholar]
- 36.King, E., S. J. van der Sar, and K. Hardwick. 2007. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE 4e342-e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer, E. R., N. Scheuringer, A. V. Podtelejnikov, M. Mann, and J.-M. Peters. 2000. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 111555-11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14953-961. [DOI] [PubMed] [Google Scholar]
- 39.Luo, X., Z. Tang, J. Rizo, and H. Yu. 2002. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 959-71. [DOI] [PubMed] [Google Scholar]
- 40.Machida, Y. J., and A. Dutta. 2007. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 21184-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margottin-Goguet, F., J. Y. Hsu, A. Loktev, H. M. Hsieh, J. D. Reimann, and P. K. Jackson. 2003. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4813-826. [DOI] [PubMed] [Google Scholar]
- 42.Martinez, J. S., D. E. Jeong, E. Choi, B. M. Billings, and M. C. Hall. 2006. Acm1 is a negative regulator of the Cdh1-dependent anaphase-promoting complex/cyclosome in budding yeast. Mol. Cell. Biol. 269162-9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, J. J., M. K. Summers, D. V. Hansen, M. V. Nachury, N. L. Lehman, A. Loktev, and P. K. Jackson. 2006. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 202410-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nezi, L., G. Rancati, A. De Antoni, S. Pasqualato, S. Piatti, and A. Musacchio. 2006. Accumulation of Mad2-Cdc20 complex during spindle checkpoint activation requires binding of open and closed conformers of Mad2 in Saccharomyces cerevisiae. J. Cell Biol. 17439-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen, V. Q., C. Co, and J. J. Li. 2001. Cyclin-dependent kinases prevent DNA rereplication through multiple mechanisms. Nature 4111068-1073. [DOI] [PubMed] [Google Scholar]
- 46.Ostapenko, D., and M. J. Solomon. 2005. Phosphorylation by Cak1 regulates the C-terminal domain kinase Ctk1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 253906-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palframan, W. J., J. B. Meehl, S. L. Jaspersen, M. Winey, and A. W. Murray. 2006. Anaphase inactivation of the spindle checkpoint. Science 313680-684. [DOI] [PubMed] [Google Scholar]
- 48.Passmore, L. A., D. Barford, and J. W. Harper. 2005. Purification and assay of the budding yeast anaphase-promoting complex. Methods Enzymol. 398195-219. [DOI] [PubMed] [Google Scholar]
- 49.Peters, J.-M. 2006. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7644-656. [DOI] [PubMed] [Google Scholar]
- 50.Peters, J.-M. 2002. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9931-943. [DOI] [PubMed] [Google Scholar]
- 51.Pfleger, C. M., and M. W. Kirschner. 2000. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14655-665. [PMC free article] [PubMed] [Google Scholar]
- 52.Prinz, S., E. S. Hwang, R. Visintin, and A. Amon. 1998. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr. Biol. 8750-760. [DOI] [PubMed] [Google Scholar]
- 53.Puig, O., F. Caspary, G. Riguat, B. Rutz, E. Bouveret, E. Bragado-Nilsson, M. Wilm, and B. Seraphin. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24218-229. [DOI] [PubMed] [Google Scholar]
- 54.Ravid, T., and M. Hochstrasser. 2007. Autoregulation of an E2 enzyme by ubiquitin-chain assembly on its catalytic residue. Nat. Cell Biol. 9422-427. [DOI] [PubMed] [Google Scholar]
- 55.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J.-M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105645-655. [DOI] [PubMed] [Google Scholar]
- 56.Reimann, J. D., B. E. Gardner, F. Margottin-Goguet, and P. K. Jackson. 2001. Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev. 153278-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts, R. L., H. U. Mosch, and G. R. Fink. 1997. 14-3-3 proteins are essential for RAS/MAPK cascade signaling during pseudohyphal development in S. cerevisiae. Cell 891055-1065. [DOI] [PubMed] [Google Scholar]
- 58.Rothstein, R. 1991. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194281-301. [DOI] [PubMed] [Google Scholar]
- 59.Schwab, M., A. S. Lutum, and W. Seufert. 1997. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90683-693. [DOI] [PubMed] [Google Scholar]
- 60.Schweitzer, K., R. Cocklin, L. Garrett, F. Desai, and M. Goebl. 2005. The ubiquitin ligase SCFGrr1 is necessary for pheromone sensitivity in Saccharomyces cerevisiae. Yeast 22553-564. [DOI] [PubMed] [Google Scholar]
- 61.Shirayama, M., W. Zachariae, R. Ciosk, and K. Nasmyth. 1998. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 171336-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smolka, M. B., C. P. Albuquerque, S. H. Chen, and H. Zhou. 2007. Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. USA 10410364-10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stratmann, R., and C. F. Lehner. 1996. Separation of sister chromatids in mitosis requires the Drosophila pimples product, a protein degraded after the metaphase/anaphase transition. Cell 8425-35. [DOI] [PubMed] [Google Scholar]
- 64.Sudakin, V., G. K. Chan, and T. J. Yen. 2001. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154925-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang, Z., R. Bharadwaj, B. Li, and H. Yu. 2001. Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1227-237. [DOI] [PubMed] [Google Scholar]
- 66.Thornton, B. R., and D. P. Toczyski. 2006. Precise destruction: an emerging picture of the APC. Genes Dev. 203069-3078. [DOI] [PubMed] [Google Scholar]
- 67.Visintin, R., S. Prinz, and A. Amon. 1997. Cdc20 and Cdh1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278460-463. [DOI] [PubMed] [Google Scholar]
- 68.Vodermaier, H. C. 2004. APC/C and SCF: controlling each other and the cell cycle. Curr. Biol. 14R787-R796. [DOI] [PubMed] [Google Scholar]
- 69.Willems, A. R., S. Lanker, E. E. Patton, K. L. Craig, T. F. Nason, N. Mathias, R. Kobayashi, C. Wittenberg, and M. Tyers. 1996. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell 86453-463. [DOI] [PubMed] [Google Scholar]
- 70.Woodbury, E. L., and D. O. Morgan. 2007. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat. Cell Biol. 9106-112. [DOI] [PubMed] [Google Scholar]
- 71.Yu, H. 2007. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol. Cell 273-16. [DOI] [PubMed] [Google Scholar]
- 72.Zachariae, W., M. Schwab, K. Nasmyth, and W. Seufert. 1998. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 2821721-1724. [DOI] [PubMed] [Google Scholar]
- 73.Zhou, Y., Y. P. Ching, A. C. Chun, and D. Y. Jin. 2003. Nuclear localization of the cell cycle regulator Cdh1 and its regulation by phosphorylation. J. Biol. Chem. 27812530-12536. [DOI] [PubMed] [Google Scholar]
- 74.Zou, H., T. J. McGarry, T. Bernal, and M. J. Kirschner. 1999. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285418-422. [DOI] [PubMed] [Google Scholar]