Spike Timing-Dependent Long-Term Potentiation in Ventral Tegmental Area Dopamine Cells Requires PKC (original) (raw)
Abstract
Long-term potentiation (LTP) of excitatory synapses on ventral tegmental area (VTA) dopamine (DA) cells is thought to play an important role in mediating some of the behavioral effects of drugs of abuse yet little is known about its underlying mechanisms. We find that spike timing-dependent LTP (STD LTP) in VTA DA cells is absent in slices prepared from mice previously administered cocaine, suggesting that cocaine-induced LTP and STD LTP share underlying mechanisms. This form of STD LTP is dependent on NMDA receptor (NMDAR) activation and a rise in postsynaptic calcium but surprisingly was not affected by an inhibitor of calcium/calmodulin-dependent protein kinase II (CaMKII). It was blocked by antagonists of conventional isoforms of PKC, whereas activation of protein kinase C (PKC) using a phorbol ester enhanced synaptic strength. These results suggest that NMDAR-mediated activation of PKC, but not CaMKII, is a critical trigger for LTP in VTA DA cells.
INTRODUCTION
The projections of ventral tegmental area (VTA) dopamine (DA) cells to the nucleus accumbens (NAc) are a major component of the mesolimbic DA system, which is thought to play a key role in many aspects of reward related learning as well as addiction (Hyman et al. 2006; Kalivas and Volkow 2005; Schultz and Dickinson 2000). DA cell firing appears to encode salient aspects of novel reward by influencing the release of DA in target structures, most importantly the NAc (Schultz 2002; Schultz and Dickinson 2000). Excitatory inputs to VTA DA neurons arise from several brain regions including the prefrontal cortex, laterodorsal tegmentum, and bed nucleus of the stria terminalis and by influencing DA cell firing these presumably convey contextual information to the brains' reward circuitry (Kauer 2004; Wolf 2002). Thus changes in the efficacy of these synaptic inputs would be expected to have significant functional consequences.
Consistent with this prediction, in vivo administration of several different classes of drugs of abuse cause a robust potentiation of excitatory synapses on VTA DA cells that lasts for several days (Borgland et al. 2004; Saal et al. 2003; Ungless et al. 2001). This drug-induced “LTP” was reported to occlude LTP elicited by a pairing protocol (Ungless et al. 2001), suggesting that the two forms of plasticity share some underlying mechanisms. In support of this hypothesis, both cocaine-induced potentiation of excitatory strength in DA cells and LTP evoked by a pairing protocol are blocked by NMDAR antagonists (Bonci and Malenka 1999; Overton et al. 1999; Ungless et al. 2001). However, more recent work has suggested that a form of LTP elicited by a spike-timing protocol in VTA DA cells (that we term STD LTP) is enhanced not blocked by prior in vivo exposure to cocaine (Liu et al. 2005). These reported differences have important implications for the functional role of LTP in VTA DA cells, and thus we have re-examined this issue.
We find that STD LTP is absent in slices prepared from animals that were previously administered cocaine, suggesting that cocaine administration elicits a potentiation that does share some common underlying mechanisms with those involved in STD LTP. We therefore examined some of the basic properties of STD LTP in VTA DA cells and found that it was blocked by application of _N_-methyl-d-aspartate receptor (NMDAR) antagonists or by loading cells with the calcium chelator bis-(_o_-aminophenoxy)-N,N,_N_′,_N_′-tetraacetic acid (BAPTA). However, unlike other forms of NMDAR-dependent LTP, it was unaffected by an inhibitor of calcium/calmodulin-dependent protein kinase II (CaMKII). Instead we present evidence that conventional isoforms of protein kinase C (PKC) play a critical role in triggering STD LTP in VTA DA cells.
METHODS
Slice preparation
Horizontal midbrain slices (220 μm thick) were prepared from 3- to 4-wk-old C57BL6 female mice as previously described (Borgland et al. 2004; Ungless et al. 2001). Briefly, horizontal slices from the midbrain were cut in ice cold sucrose solution containing (in mM) 238 sucrose, 2.5 KCl, 1.3 NaH2PO4, 26.2 NaHCO3, 0.2 CaCl2, 1.3 MgSO4, and 11 d-glucose (saturated with 95% O2-5% CO2). Slices were transferred to a holding chamber containing artificial cerebrospinal fluid (ACSF) consisting (in mM) 119 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, 1.3 MgSO4, and 11 d-glucose (saturated with 95% O2-5% CO2). Slices were allowed to equilibrate at room temperature for ≥1 h before being transferred to a recording chamber and perfused (2 ml/min) with oxygenated artificial cerebropinal fluid (ACSF) containing picrotoxin (50 μM) warmed to 28–30°C. Hippocampal slices were prepared from 3- to 4-wk-old C57BL6 male mice using standard procedures. All experimental procedures were approved by the Stanford Institutional Animal Care and Use Committee (IACUC).
Electrophysiology
VTA neurons were identified morphologically under infrared DIC and by the presence of a large (>50 pA) _I_h current in response to a hyperpolarizing pulse to −110 mV on break-in (Johnson and North 1992; Lacey et al. 1990). Excitatory postsynaptic potentials (EPSPs) were collected in whole cell current-clamp configuration (using an Axopatch 1D, Axon Instruments) at a holding potential of −70 mV. Intracellular pipettes (2–4 MΩ) were filled with a solution containing (in mM) 140 K-methylsulfonate, 5 KCl, 10 HEPES, 2 MgCl2, 0.2 EGTA, 4 Mg-ATP, and 0.3 Na3-GTP, adjusted to pH 7.3 and 287–290 mOsm. Presynaptic stimulation was performed using a bipolar stainless steel electrode placed 200–300 μm immediately rostral to the recording site. Stimulation intensity was adjusted to evoke 50–70% of maximal responses, typically EPSPs with amplitudes of 3–6 mV. Bridge balance was monitored and adjusted continuously; cells were not included in the data analysis if input resistance varied by >20% throughout the experiment. STD LTP induction was performed within 10–12 min of break-in to avoid possible “wash out” of LTP. STD LTP induction was performed as described (Liu et al. 2005). Presynaptic stimulation was paired with a postsynaptic injection of current (1.5–2 nA) timed to elicit an action potential within 5 ms of the initial rise time of the EPSP. Five such prepost pairs of stimulation were performed at a spacing of 100 ms and repeated 10 times at 10 s intervals. LTP magnitude was assessed by measuring peak EPSP amplitude values from the average of 30 consecutive sweeps taken immediately before and 30 min after LTP induction. All summary graphs are binned at 90 s intervals. Data are presented as means ± SEM. Whole cell recordings were filtered at 2 kHz and digitized at 5 kHz with an A/D board (National Instruments) driven by custom acquisition software designed to run using IGOR Pro (version 5.04).
AMPAR/NMDAR ratios and AMPA receptor-mediated miniature excitatory postsynaptic current (mEPSC) recordings were performed under voltage clamp at −70 and −80 mV, respectively, using pipettes filled with a solution containing (in mM) 117 cesium methlysulfonate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 5 tetraethylammonium (TEA), 4 Mg-ATP, 0.3 Na3-GTP, and 5 QX-314 [5-_N_-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide], adjusted to pH 7.3 and 287–290 mOsm. mEPSC recordings were made in normal ACSF containing d-2-amino-5-phosphonovaleric acid (d-APV, 25 μM), TTX (500 nM), picrotoxin (50 μM), and sucrose (50 mM). mEPSC analysis was performed using MiniAnalysis (Synaptosoft) with a detection threshold of 5 pA. All mEPSCs included in data analyses were confirmed by visual inspection. Statistical significance was assessed by a two tailed, paired Student's _t_-test.
Drugs and inhibitors
Picrotoxin, phorbol-12-myristate-13-acetate (PMA) and cocaine hydrochloride were obtained from Sigma; all other chemicals and drugs were obtained from Tocris. Drugs that could not be dissolved in dH20 were dissolved in DMSO. Stock concentrations of drugs were prepared at 500–1,000 times the final concentrations used in the study. Cocaine hydrochloride was dissolved in sterile saline and injected intraperitoneally (i.p.) at a dose of 15 mg/kg. The same volume of saline was injected i.p. into a subset of control animals (n = 5), and slices from these animals expressed normal STD LTP (145.7 ± 8%), and therefore the data from saline injected and noninjected animals were combined.
RESULTS
In vivo cocaine administration occludes STD LTP
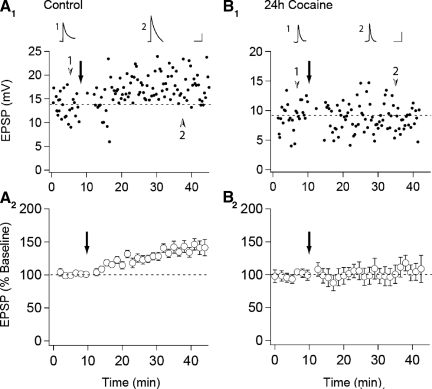
Previous work has demonstrated that in vivo administration of drugs of abuse potentiate excitatory synaptic strength in VTA DA neurons (Borgland et al. 2004; Faleiro et al. 2003; Saal et al. 2003; Ungless et al. 2001), but whether this occludes or enhances LTP may depend on the induction protocol used to elicit LTP (Liu et al. 2005; Ungless et al. 2001). In contrast to pairing-induced LTP, which was absent in slices prepared from animals that had received a single injection of cocaine the day before (Ungless et al. 2001), STD LTP was reported to be enhanced following 5–7 days of daily cocaine injections (Liu et al. 2005). This was suggested to be due to a cocaine-induced decrease in GABA-mediated inhibition as STD LTP was unaffected by prior cocaine administration when elicited in the presence of GABAA receptor antagonists (Liu et al. 2005). To determine whether under our experimental conditions, cocaine influenced STD LTP in the same manner as pairing-induced LTP, we prepared slices from naive animals (n = 22) or animals that had received injections of either saline (n = 5) or cocaine (n = 10, 15 mg/kg) 24 h earlier and bathed the slices in picrotoxin (50 μM) to maximize our ability to generate STD LTP (Liu et al. 2005). In slices prepared from control animals, LTP was, on average, reliably elicited (138 ± 2% of baseline, n = 27, Fig. 1 A). In contrast, in slices from cocaine-treated animals, the same LTP induction protocol did not elicit any potentiation (103 ± 3%, n = 10, Fig. 1_B_). This result is consistent with previous results using a pairing protocol to elicit LTP (Ungless et al. 2001) and suggests that the mechanisms underlying the cocaine-induced potentiation of synaptic strength in VTA DA cells may overlap with those underlying LTP elicited either by a spike-timing protocol or a pairing protocol.
FIG. 1.
In vivo cocaine administration occludes spike timing-dependent (STD) long-term potentiaion (LTP) in ventral tegmental area (VTA) dopamine neurons. A: representative experiment (A1) and summary graph (A2) of STD LTP in slices prepared from control animals (n = 27). B: representative experiment (B1) and summary graph (B2) from animals which received a single intraperitoneal injection of cocaine 24 h prior to slice preparation (n = 10). ↓, the application of the STD LTP induction protocol. Traces are averaged excitatory postsynaptic potentials (EPSPs) taken from the indicated time points. (Calibration bars: 5 mV/10 ms)
STD LTP depends on NMDARs and postsynaptic calcium
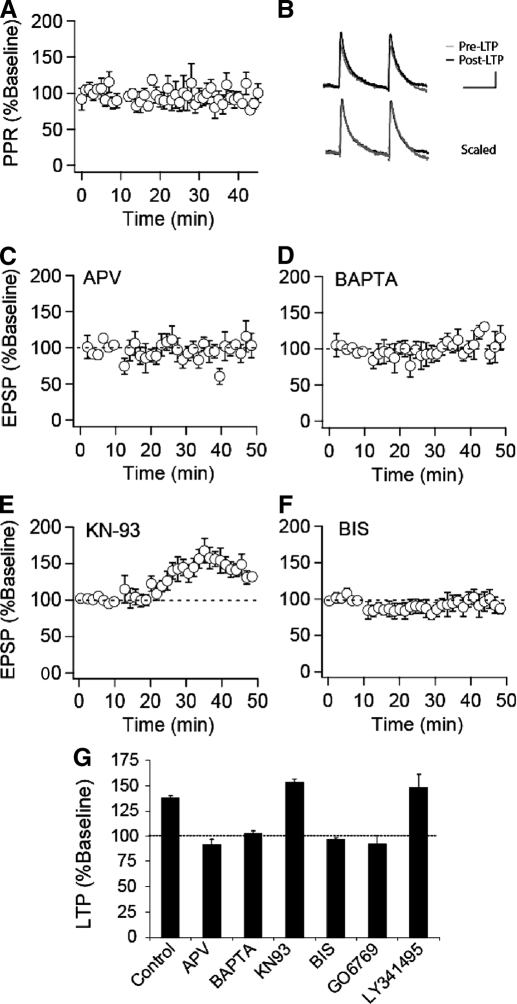
Little is known about the mechanisms underlying STD LTP in VTA DA cells, and thus we next performed straightforward experiments to examine some of its basic triggering and expression properties. Because some forms of STD plasticity have been associated with presynaptic changes in transmitter release, as assayed by changes in the paired-pulse ratio (PPR) (Bender et al. 2006; Markram et al. 1997; Sjostrom et al. 2003; Tzounopoulos et al. 2007), we monitored the PPR throughout a subset of our control LTP experiments. As shown in Fig. 2, A and B, robust STD LTP (171.9 ± 9%, n = 8) was not associated with any consistent change in the PPR (94 ± 4% of baseline). This result suggests that there is a minimal change in presynaptic release probability at these synapses after induction of STD LTP and that the primary locus of the LTP expression, like that of cocaine-induced potentiation (Bellone and Luscher 2006; Ungless et al. 2001), is likely postsynaptic.
FIG. 2.
STD LTP is _N_-methyl-d-aspartate receptor (NMDAER-dependent and requires activation of protein kinase C (PKC). A: paired-pulse ratios normalized to the pre-LTP baseline from control experiments in which LTP was elicited (n = 8). The interstimulus interval was 75 ms. B: representative averaged EPSPs (calibration bars: 5 mV/50 ms). C: summary graph of STD LTP in the presence of d-2-amino-5-phosphonovaleric acid (d-APV, 50 μM, n = 8), which was applied throughout the experiment. D: summary graph of STD LTP from experiments in which cells were loaded with bis-(_o_-aminophenoxy)-N,N,_N_′,_N_′-tetraacetic acid (BAPTA, 10 μM, n = 6). E: summary graph of STD LTP (n = 10) from experiments in which KN-93 (25 μM) was applied to slices prior to and throughout the experiment (n = 7) or in which KN-93 was included in the pipette solution (n = 3). F: summary graph of STD LTP (n = 10) from experiments in which bisindolylmaleimide (BIS, 1 μM) was applied to slices prior to and throughout the experiment (n = 6) or in which BIS was included in the pipette solution (n = 4). G: summary of STD LTP in the presence of various inhibitors. LTP values are taken as averages from 30–35 min post induction, expressed as a percentage of baseline values. (control, n = 27; d-APV, n = 8; BAPTA, n = 6; KN-93, n = 10; BIS, n = 10; GO6976, n = 4; LY341495, n = 4).
To examine some of the signaling events responsible for the triggering of this form of LTP, we used LTP in the CA1 region of the hippocampus as a guide and asked whether like this form of LTP, STD LTP in VTA DA cells requires NMDAR activation leading to an increase in postsynaptic calcium levels and activation of CaMKII (Malenka and Bear 2004). We found that STD LTP in VTA DA cells was also NMDAR dependent as it was not elicited in the presence of the NMDAR antagonist d-APV (50 μM; Fig. 2_C_, n = 8). Inclusion of the calcium chelator BAPTA (10 mM) in the recording pipette solution also prevented STD LTP (Fig. 2_D_, n = 6), suggesting that NMDAR-mediated rises in postsynaptic calcium are required. In contrast, bath application of the broad spectrum antagonist of metabotropic glutamate receptors (mGluRs) LY341495 (10 μM) did not affect STD LTP (148 ± 14%, n = 4, Fig. 2_G_), suggesting that mGluR activation is not required for triggering this form of plasticity.
PKC plays a role in STD LTP in VTA DA cells
Inhibiting the activity of CaMKII has been found to block LTP in hippocampal CA1 pyramidal cells (Malenka and Bear 2004; Malinow et al. 1989; Otmakhov et al. 1997; Yasuda et al. 2003). To examine the involvement of CaMKII in LTP in DA cells, we preincubated VTA slices with the specific CaMKII antagonist KN-93 (5 μM, n = 7) for 30–60 min prior to recording or included the drug in the pipette solution (10 μM, n = 3). Surprisingly, KN-93 had no effect on this form of LTP (153 ± 3%, n = 10, Fig. 2_E_). To test whether extracellular application of KN-93 in this manner was effective in inhibiting CaMKII activity in brain slices, we preincubated hippocampal slices with KN-93 (5 μM) and examined LTP in the CA1 region of the hippocampus using standard extracellular recording techniques. While a 100-Hz/1-s tetanus induced robust LTP in control slices (215.6 ± 4.9% of baseline measured 50–60 min after the tetanus; n = 6), LTP was markedly reduced in slices treated with KN-93 (130.4 ± 2.5% of baseline, n = 6), thus confirming its efficacy. In contrast to the lack of effect of KN-93 on STD LTP in DA cells, the PKC inhibitor bisindolylmaleimide (BIS) applied in the bath (1 μM, n = 6) or via the pipette solution (10 μM, n = 4) completely prevented STD LTP (97 ± 2%, n = 10, Fig. 2_F_).
PKCs are a large family of intracellular kinases composed of 10 isoforms classified on their localization and requirements for activation (Olive and Messing 2004). The conventional and novel isoforms differ in their expression patterns and sensitivity to activation by calcium with conventional but not novel PKCs being sensitive to activation by calcium. BIS inhibits both the conventional and novel PKCs. We therefore also examined the effects of GO6976, which specifically inhibits the conventional PKCs while having no detectable effect on the activity of novel PKC isoforms (Martiny-Baron et al. 1993). Intracellular application of GO6976 (1 μM) via the pipette solution blocked STD LTP (92 ± 8.9%, n = 4, Fig. 2_G_), suggesting that activation of one of the conventional isoforms of PKC, α, β, or γ, contributes to the induction of STD LTP in VTA DA cells. This result is also consistent with the role of postsynaptic calcium increases in triggering this form of LTP as only the conventional isoforms of PKC are sensitive to activation by calcium.
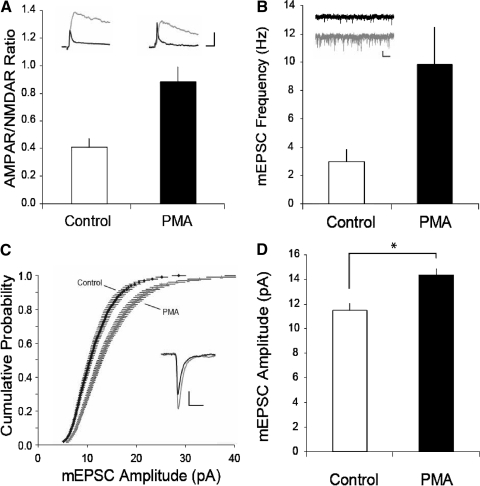
Both conventional and novel PKCs are activated by phorbol esters (Nishizuka 1988). If activation of PKC is critically involved in triggering the increase in synaptic strength in VTA DA cells following in vivo administration of drugs of abuse and during LTP, then application of a phorbol ester should mimic these increases in synaptic strength. Consistent with this prediction, pretreating slices for 30 min with PMA (0.5 μM) caused a robust increase in the ratio of AMPAR-mediated EPSCs to NMDAR-mediated EPSCs (the so-called AMPAR/NMDAR ratio; control 0.4 ± 0.06, n = 6; PMA 0.9 ± 0.1, n = 6, Fig. 3 A) that was comparable to that observed following in vivo drug administration (Saal et al. 2003; Ungless et al. 2001). Although phorbol esters are known to cause an enhancement of transmitter release (Malenka et al. 1986, 1987), the observed increase in the AMPAR/NMDAR ratio strongly suggested that PMA application caused some postsynaptic change in AMPAR function and/or number (Kauer and Malenka 2007). To further test this hypothesis, we examined the effects on mEPSCs of PMA application. As expected from previous work using phorbol esters (Carroll et al. 1998; Malenka et al. 1986, 1987), pretreatment of slices with PMA caused a large increase in the frequency of mEPSCs (control 3.0 ± 0.9 Hz, n = 11; PMA 9.8 ± 2.6 Hz, n = 12, Fig. 3_B_). It also caused a shift to the right in the cumulative probability amplitude distribution (Fig. 3_C_) and an increase in mean mEPSC amplitudes (control, 11.5 ± 0.6 pA; PMA 14.3 ± 0.6 pA, Fig. 3_D_). These results suggest that in addition to its known effects on transmitter release, activation of PKC in VTA DA neurons also leads to a postsynaptic enhancement of AMPAR function and/or number that likely contributes to LTP at these synapses.
FIG. 3.
Activation of PKC causes synaptic potentiation in VTA dopamine (DA) cells. A: summary graph of AMPAR/NMDAR ratios measured from control (n = 6) or phorbol-12-myristate-13-acetate (PMA, 0.5 μM, n = 6) treated slices. Insets: representative AMPAR- and NMDAR excitatory postsynaptic currents (EPSCs) recorded in voltage clamp at +40 mV (calibration bars: 50 pA/10 ms). NMDAR EPSCs (gray) were obtained by digitally subtracting AMPAR EPSCs (shown in black; obtained after application of d-APV) from the dual component traces. B: miniature EPSC (mEPSC) frequency in control (n = 11) and PMA-treated (n = 12) slices. Insets: representative sweeps from control (black) and PMA-treated (gray) cells (calibration bars: 10 pA/250 ms). C: cumulative probability plot of mEPSC amplitudes from control and PMA-treated DA neurons (200 consecutive mEPSCs from each cell are plotted). Inset: averaged mEPSC traces from representative control (black) and PMA treated (gray) cell (calibration bars: 4 pA/5 ms). D: summary graph of mEPSC amplitude from control and PMA-treated cells (asterisk, P < 0.01).
DISCUSSION
Synaptic plasticity at excitatory synapses on VTA DA neurons has received increased attention because of its possible role in reward-dependent learning and drug addiction (Hyman et al. 2006; Kalivas and Volkow 2005; Kauer and Malenka 2007; Wolf 2002). One finding used to support a critical role for synaptic plasticity in DA cells is that in vivo administration of drugs of abuse potentiates excitatory synaptic transmission in these neurons (Bellone and Luscher 2006; Borgland et al. 2004; Faleiro et al. 2003; Liu et al. 2005; Saal et al. 2003; Ungless et al. 2001). An important question is whether this drug-induced potentiation shares mechanisms with the LTP that can be elicited in VTA DA cells in slice preparations. If it does, then studying the mechanisms of LTP will provide insights into how drugs of abuse modify DA cell synaptic properties. One way of addressing this issue is to examine the effects of prior drug administration on LTP with the prediction that if the drug-induced potentiation and LTP share some underlying mechanisms, then LTP should be reduced in slices prepared from drug-treated animals. This result was obtained using a pairing protocol to elicit LTP (Ungless et al. 2001). However, subsequent work reported that a form of LTP elicited by a spike-timing protocol (STD LTP) was enhanced, not impaired, following in vivo cocaine administration (Liu et al. 2005).
Because of the profound difference in these results and their potential importance, we re-examined this issue. Consistent with the earlier work (Ungless et al. 2001), we found that STD LTP triggered using the published protocol (Liu et al. 2005) was absent in slices prepared from animals that had received cocaine 24 h earlier. This result is consistent with the hypothesis that the prior cocaine treatment “occluded” the generation of STD LTP because the cocaine-induced potentitation involves some triggering and/or expression mechanisms that are also used during STD LTP. In previous work (Ungless et al. 2001), the conclusion that cocaine administration occluded LTP was supported by the finding that long-term depression (LTD) was enhanced in slices prepared from cocaine-treated animals. Based on similar reasoning, we attempted to elicit LTD using a “post-pre” spike-timing protocol (spikes were elicited 3–5 ms prior to EPSP onset) but were unsuccessful in control VTA slices (n = 7 cells) as well as in slices prepared from animals administered cocaine 24 h earlier (n = 4 cells). Thus we cannot rule out that drug administration caused some adaptations in VTA DA cells which in turn caused an “inhibition” of LTP rather than its occlusion. We cannot provide an explanation for why our results differ from those previously published (Liu et al. 2005) except to note that rats rather than mice were used in this previous work and that it has been suggested that there may be some differences in synaptic properties between the two species (Bellone and Luscher 2005, 2006). Furthermore we examined STD LTP following a single dose of cocaine whereas the previous work administered cocaine daily for 5–7 days (Liu et al. 2005), although it should also be noted that chronic and single-dose administration of cocaine appear to have the same effects on VTA DA cell excitatory synapses (Borgland et al. 2004).
Examination of some of the basic mechanisms underlying STD LTP in VTA DA neurons revealed that it is likely triggered by an NMDAR-dependent rise in postsynaptic calcium. However, surprisingly, it was not affected by an inhibitor of CaMKII but instead was blocked by two different inhibitors of PKC when either bath applied or loaded into postsynaptic cells via the patch pipette. Furthermore, application of a phorbol ester to activate PKC caused an increase in the AMPAR/NMDAR ratio and an increase in both mEPSC amplitude and frequency. These phorbol ester induced changes in synaptic function are similar to those caused by in vivo cocaine administration (Ungless et al. 2001) and suggest that like the cocaine-induced potentiation, STD LTP involves postsynaptic modifications of AMPAR function and/or number. Consistent with this proposal, neither STD LTP nor the cocaine-induced potentiation (Ungless et al. 2001) were associated with changes in the PPR. The similarities in the synaptic effects of in vivo cocaine administration and PKC activation via either LTP induction or phorbol ester application also provide supportive evidence that the cocaine-induced potentiation occluded the subsequent generation of LTP because the two forms of synaptic plasticity share some common mechanisms.
PKC has also been suggested to be important for mediating the effects of orexin A on NMDAR-mediated synaptic responses in VTA DA neurons (Borgland et al. 2006). Thus in future work, it will be important to test the role of specific isoforms of PKC in VTA DA neurons in mediating the synaptic adaptations caused by different in vitro and in vivo manipulations including administration of drugs of abuse. The results of these experiments will in turn point the way to the types of in vivo molecular and pharmacological manipulations that can be performed to further explore the role of DA cell plasticity in adaptive and pathological forms of behavior.
GRANTS
This work was supported by National Institute on Drug Abuse Grants F32 DA-020220 and RO1 DA-009264.
Acknowledgments
The authors thank members of the Malenka lab, A. Bonci, and S. Borgland, for helpful discussions and technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bellone and Luscher 2005.Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci 21: 1280–1288, 2005. [DOI] [PubMed] [Google Scholar]
- Bellone and Luscher 2006.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature Neurosci 9: 636–641, 2006. [DOI] [PubMed] [Google Scholar]
- Bender et al. 2006.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci 26: 4166–4177, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci and Malenka 1999.Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci 19: 3723–3730, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland et al. 2004.Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 24: 7482–7490, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland et al. 2006.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49: 589–601, 2006. [DOI] [PubMed] [Google Scholar]
- Carroll et al. 1998.Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol 80: 2797–2800, 1998. [DOI] [PubMed] [Google Scholar]
- Faleiro et al. 2003.Faleiro LJ, Jones S, Kauer JA. Rapid AMPAR/NMDAR response to amphetamine: a detectable increase in AMPAR/NMDAR ratios in the ventral tegmental area is detectable after amphetamine injection. Ann NY Acad Sci 1003: 391–394, 2003. [DOI] [PubMed] [Google Scholar]
- Hyman et al. 2006.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598, 2006. [DOI] [PubMed] [Google Scholar]
- Johnson and North 1992.Johnson SW, North RA. Two types of neuron in the rat ventral tegmental area and their synaptic inputs. J Physiol 450: 455–468, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas and Volkow 2005.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Amer J Psychiat 162: 1403–1413, 2005. [DOI] [PubMed] [Google Scholar]
- Kauer 2004.Kauer JA Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol 66: 447–475, 2004. [DOI] [PubMed] [Google Scholar]
- Kauer and Malenka 2007.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858, 2007. [DOI] [PubMed] [Google Scholar]
- Lacey et al. 1990.Lacey MG, Mercuri NB, North RA. Actions of cocaine on rat dopaminergic neurons in vitro. Br J Pharmacol 99: 731–735, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. 2005.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437: 1027–1031, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka et al. 1987.Malenka RC, Ayoub GS, Nicoll RA. Phorbol esters enhance transmitter release in rat hippocampal slices. Brain Res 403: 198–203, 1987. [DOI] [PubMed] [Google Scholar]
- Malenka and Bear 2004.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004. [DOI] [PubMed] [Google Scholar]
- Malenka et al. 1986.Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature 321: 175–177, 1986. [DOI] [PubMed] [Google Scholar]
- Malinow et al. 1989.Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expressiono of LTP. Science 245: 862–866, 1989. [DOI] [PubMed] [Google Scholar]
- Markram et al. 1997.Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275: 213–215, 1997. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron et al. 1993.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go et al. 6976. J Biol Chem 268: 9194–9197, 1993. [PubMed] [Google Scholar]
- Nishizuka 1988.Nishizuka Y The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334: 661–665, 1988. [DOI] [PubMed] [Google Scholar]
- Olive and Messing 2004.Olive MF, Messing RO. Protein kinase C isozymes and addiction. Mol Neurobiol 29: 139–154, 2004. [DOI] [PubMed] [Google Scholar]
- Otmakhov et al. 1997.Otmakhov N, Griffith LC, Lisman JE. Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation. J Neurosci 17: 5357–5365, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton et al. 1999.Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport 10: 221–226, 1999. [DOI] [PubMed] [Google Scholar]
- Saal et al. 2003.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582, 2003. [DOI] [PubMed] [Google Scholar]
- Schultz 2002.Schultz W Getting formal with dopamine and reward. Neuron 36: 241–263, 2002. [DOI] [PubMed] [Google Scholar]
- Schultz and Dickinson 2000.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci 23: 473–500, 2000. [DOI] [PubMed] [Google Scholar]
- Sjostrom et al. 2003.Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39: 641–654, 2003. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos et al. 2007.Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron 54: 291–301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless et al. 2001.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587, 2001. [DOI] [PubMed] [Google Scholar]
- Wolf 2002.Wolf ME Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv 2: 146–157, 2002. [DOI] [PubMed] [Google Scholar]
- Yasuda et al. 2003.Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6: 15–16, 2003. [DOI] [PubMed] [Google Scholar]