Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic (original) (raw)
Abstract
Vesicular traffic of the glucose transporter GLUT4 occurs in response to insulin, muscle contraction, and metabolic stimuli that lead to changes in the energy status of the cell. These stimuli are associated with linked kinase cascades that lead to changes in glucose uptake that meet the energy challenges imposed on the highly regulated cell types in insulin-responsive tissues. The need to mechanistically link these kinase-associated stimuli to identifiable intermediates in vesicular traffic has long been known but has been difficult to fulfill. The Rab-GTPase-activating proteins AS160 and TBC1D1 have now emerged as strong candidates to fill this void. Here we review the initial discovery of these proteins as phosphorylated substrates for Akt and the more recent emerging data that indicate that these proteins are substrates for additional kinases that are downstream of contraction and energy status signaling. The mechanism of coupling these phosphorylated proteins to vesicle traffic appears to be dependent on linking to small GTPase of the Rab family. We examine the current state of a hypothesis that suggests that phosphorylation of the Rab-GTPase-activating proteins leads to increased GTP loading of Rab proteins on GLUT4 vesicles and subsequently to increased interaction with Rab effectors that control GLUT4 vesicle translocation.
Keywords: glucose transport, insulin signaling, glucose transporter 4, type 2 diabetes, Akt
the insulin-regulated glucose transporter 4 (GLUT4) is expressed mainly in muscle and adipose tissue and plays an important role in whole body glucose homeostasis. Its translocation from vesicular intracellular compartments to the cell surface in response to insulin is a multiple-step process involving intracellular sorting, vesicular transport to the cell surface along cytoskeletal elements, and, finally, docking, priming, and fusion of the GLUT4 storage vesicles with the cell surface (reviewed in Refs. 17 and 60).
Studies on insulin regulation and GLUT4 traffic have historically been focused on either the beginning or the end of the signaling-to-traffic pathway. Much work has been carried out on early events in insulin signaling, such as the coupling of insulin receptor activity to downstream targets. However, equal focus has surrounded the defining of machinery and compartments involved in GLUT4 subcellular traffic. Emphasis has been placed on identification of targeting domains in GLUT4 that lead to its sequestration in its intracellular reservoir compartment. In addition, studies have revealed details of the translocation apparatus that facilitates GLUT4 vesicle movement from a perinuclear compartment (often associated with microtubules) outward and toward localized sites on the plasma membrane that promote docking and fusion of the vesicles with the plasma membrane. The ways in which these two nodes (the beginning and the end of the signaling-to-traffic pathway) are connected has been largely elusive. Now, however, a plausible link has emerged based on the discoveries of Kane et al. (24) and Roach et al. (47) that demonstrates that two closely related Rab-GTPase-activating proteins (GAPs), AS160 (also known as TBC1D4) and TBC1D1, are Akt substrates. The main aim of this review article is to provide a brief overview of the rapidly growing field of AS160 and TBC1D1 and their regulation and role in insulin-stimulated GLUT4 traffic. We will briefly discuss more recent studies that indicate that AS160 and TBC1D1 are also regulated by exercise/muscle contraction through protein kinases, including AMP-activated protein kinase (AMPK). This area has been reviewed extensively in a study on exercise and AMPK regulation of AS160 and glucose transport (6).
Established Role for the Phosphatidylinositol 3-Kinase/Akt Pathway in Insulin-Stimulated GLUT4 Translocation and Glucose Uptake
The insulin-signaling pathway to GLUT4 has been discussed extensively in recent review articles (17, 57, 60). The canonical insulin-signaling pathway is triggered by activation of the insulin receptor tyrosine kinase, leading to tyrosine phosphorylation of insulin receptor substrate proteins and their recruitment and activation of class IA phosphatidylinositol (PI) 3-kinase. This results in the generation of the critical second messenger PI-3,4,5-triphosphate, which in turn triggers the activation of Akt (also known as protein kinase B) through action of two distinct upstream mediators, 3-phosphoinositide-dependent protein kinase-1 (40) and the mammalian target of rapamycin (mTOR) complex 2 (33, 53). There is substantial evidence that Akt plays a key role in directing GLUT4 to the plasma membrane and thus promotes glucose transport. This has been supported by the following evidence: 1) Akt rendered constitutively active by membrane targeting mimics insulin in eliciting GLUT4 translocation and high levels of glucose transport in the absence of hormone (29), 2) depletion of Akt using small interfering RNA-mediated knockdown results in markedly reduced glucose transport in adipose and muscle cells (these studies also demonstrated that the Akt2 isoform plays a major role for insulin-stimulated glucose transport; Akt1 has little or no effect in cultured adipose and muscle cells but may compensate for the absence of Akt2 in experiments in which this isoform is depleted) (22, 26, 66), and 3) a major role of Akt, specifically the Akt2 isoform, is further supported by the fact that Akt2-deficient mice display a profound reduction of insulin-stimulated glucose transport in isolated skeletal muscle or primary adipocytes (9, 12, 37, 49, 63).
Although these studies provide compelling evidence that Akt is both necessary and sufficient for insulin-stimulated GLUT4 translocation and glucose transport, one major criticism of these findings is that they are limited by their dependence on long-term deficiency or exposure to the Akt signal. A recent study has addressed this problem and found that rapid and constitutive activation of Akt2 fully recapitulates the effects of insulin to GLUT4 translocation and glucose transport in the 3T3-L1 cell system (42). Furthermore, a highly selective non-ATP-competitive Akt inhibitor that potently inhibits Akt1 and Akt2 isoforms (thus termed Akt1/2) has been developed and characterized both in vitro and in intact cells (3, 36). “Short-term” inhibition of Akt with Akt1/2 resulted in a marked reduction of insulin-stimulated glucose transport in 3T3-L1 adipocytes (14). Taken together, molecular biological, genetic, and pharmacological evidence firmly establishes that Akt plays a pivotal role in the regulation of GLUT4 translocation and glucose transport in muscle and adipose cells in response to insulin.
AS160/TBC1D4, a New Player Beyond Akt: Identification of AS160 As a Novel Akt Substrate
One of the major challenges has been to identify the key substrate(s) of Akt and characterize the role that phosphorylation plays in regulating the function of these proteins. The challenge has been to provide critical links between signaling downstream of Akt and GLUT4 traffic. Alessi et al. (1) initially identified that the minimum motif in a peptide enabling Akt phosphorylation is Arg-Xaa-Arg-Yaa-Zaa-Ser/Thr-Hyd, where Xaa is any amino acid, Yaa and Zaa are preferably small residues other than Gly, and Hyd is a bulky hydrophobic residue. A subsequent study from Obata et al. (43) revealed that residues outside the originally proposed Akt phosphorylation motif strongly affect the ability of Akt to phosphorylate peptides. This has allowed Obenauer et al. (44) to use this information to generate a motif profile scoring algorithm, known as “Scansite” (http://scansite.mit.edu), that can compare all the Ser/Thr residues in the vertebrate genome that comprise this motif (of which there are >14,000 in ∼9,500 protein sequences) and rank each site according to how well it fits with the theoretical optimal Akt phosphorylation motif. This sequence motif information has allowed the generation of an antibody [the phospho (Ser/Thr)-Akt substrate (PAS) antibody] that can detect Akt substrates containing the generic Akt phosphomotif (Arg-Xaa-Arg-Xaa-Xaa pSer/Thr, Xaa, any amino acids).
In 2002, Kane et al. (24) used the PAS antibody to identify novel Akt substrates. They treated 3T3-L1 adipocytes with or without insulin, and cell extracts were then incubated with PAS antibody in an attempt to coimmunoprecipitate potential Akt substrates. Immunoprecipitated proteins were separated by SDS-PAGE and enzymatically digested with trypsin. They were then subjected to a tandem mass spectrometry analysis. This resulted in identification of five insulin-stimulated phosphoproteins that were sensitive to PI 3-kinase inhibitors wortmannin or LY-294004 but not to mTOR (mTOR complex 1) inhibitor rapamycin. One of these proteins was detected at ∼160 kDa and thus termed AS160 (Akt substrate of 160 kDa). AS160 is a protein of 1,298 amino acids [National Center for Biotechnology Information (NCBI), gi: 114688046] that was originally named TBC1D4 but whose function was not studied. It was also previously unknown as an Akt substrate. AS160/TBC1D4 was cloned (clone ID: KIAA0603) as a series of projects sequencing human cDNA by Nagase et al. (41) using human brain cDNA libraries. Interestingly, it has been reported that AS160 was also abundant in brain and pancreas as well as adipose and muscle tissues (24, 58). Motif scan analysis of the AS160 protein using the Scansite program found several potential Akt phosphorylation sites, and phosphopeptide analysis by mass spectrometry indeed identified six phosphorylation sites (Ser318, Ser341, Ser570, Ser588, Thr642, Thr751) in mouse AS160 that matched criteria for an Akt consensus motif (51). Quantification of the relative amount of phosphopeptides derived from extracts of 3T3-L1 adipocytes in the presence or absence of insulin revealed that levels of phosphorylation at five of these phosphorylation sites (Ser318, Ser570, Ser588, Thr642, and Thr751) was increased with insulin (51). Collectively, current data indicate that Kane et al. (24) and Sano et al. (51) have identified AS160 as an important new Akt substrate in adipocytes that contains multiple Akt phosphorylation sites that are regulated in response to insulin in a PI 3-kinase-dependent mechanism. Subsequent to this discovery, Bruss et al. (5) demonstrated that insulin stimulates AS160 phosphorylation in skeletal muscle in a PI 3-kinase-sensitive manner. Intriguingly, they also found that other stimuli known to stimulate GLUT4 translocation and glucose transport in muscle, such as contractile activity and the AMPK activator 5- aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, induced AS160 phosphorylation in isolated rat epitrochlearis muscle. Additional studies have reported that exercise-associated contraction and agents that activate AMPK in other muscle systems induce phosphorylation of AS160 (10, 13, 55, 59, 61). Collectively, these data provide strong evidence that AS160 is not only an Akt substrate but also a substrate for other kinases such as AMPK.
Evidence That AS160 is Involved in GLUT4 Trafficking
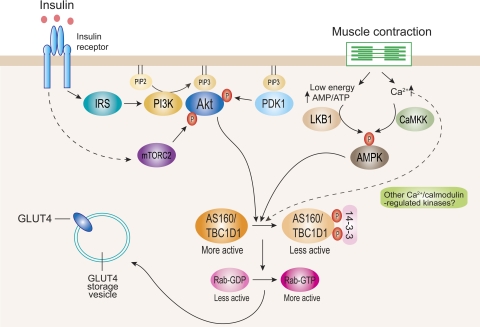
Domain structure analysis of AS160 using the Pfam program (http://www.sanger.ac.uk/) revealed that this protein contains two phosphotyrosine-binding (PTB) domains at the NH2 terminus and a Rab-GAP (GTPase-activating protein) domain at the COOH terminus (24). The presence of the GAP domain led Kane et al. (24) and Sano et al. (51) to hypothesize that Akt-mediated phosphorylation of AS160 may regulate GLUT4 traffic through the regulation of GAP activity toward particular Rab protein(s). It is proposed that the Rab-GAP activity promotes hydrolysis of GTP to GDP by Rab protein(s) on the GLUT4 storage vesicle (GSV). It is also proposed that, in their inactive GDP-bound form, the GSV-bound Rabs are unable to elicit GLUT4 translocation to the cell surface. Upon insulin stimulation, AS160 is phosphorylated, which leads to inactivation of the Rab-GAP activity. Thus GSV-associated Rabs are thought to become loaded with GTP and promote processes that lead to mobilization of GLUT4 at the plasma membrane (Fig. 1).
Fig. 1.
Currently proposed signaling pathways for insulin- and contraction-stimulated glucose transporter 4 (GLUT4) translocation in muscle. Insulin stimulates Akt through 2 distinct upstream mediators, phosphoinositide-dependent protein kinase-1 (PDK1) and mammalian target of rapamycin complex-2 (mTORC2). Activated Akt phosphorylates AS160 and TBC1D1 mainly at Thr642 and Thr596, respectively. This enhances 14-3-3 binding to these proteins, which is proposed to inhibit Rab-GTPase-activating protein (GAP) activity toward particular Rab isoform(s). Inhibition of GAP promotes conversion of less active GDP-loaded Rab to more active GTP-loaded Rab. The more active GTP-loaded Rab then allows GLUT4 storage vesicles to move to and fuse with the plasma membrane. Contraction through both energy depletion (i.e., an elevated AMP/ATP ratio) and elevated intracellular [Ca2+] leads to activation of AMP-activated protein kinase (AMPK) via LKB1 and Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), respectively (and possibly other Ca2+-regulated protein kinases). These regulators lead to AS160 and TBC1D1 phosphorylation at multiple phosphorylation sites. This is thought to regulate function of these proteins and GLUT4 trafficking by largely uncharacterized mechanism(s). IRS, insulin receptor substrate; PI3K, phosphatidylinositol 3-kinase.
This is an attractive hypothesis because small G protein Rabs are critical organizers of intracellular membrane traffic in many different cell systems (64). To test this hypothesis, wild-type and a mutant form of AS160 in which four of the phosphorylation sites (Ser318, Ser588, Thr642, Ser751) had been mutated to alanine (AS160-4P) were coexpressed with GLUT4 in 3T3-L1 adipocytes (51). Expression of AS160-4P, but not wild-type AS160, reduced insulin-stimulated GLUT4 translocation to the cell surface by ∼80%. This suggests that the high levels of AS160-4P compete with endogenous AS160 and that the mutant functions as a “dominant-negative” inhibitor of GLUT4 translocation. AS160-4P may have unrestrained GAP activity that cannot be suppressed by insulin-stimulated phosphorylation. The unrestrained GAP activity may lead to the conversion of the GLUT4 vesicle Rab to its inactive GDP-loaded form, with consequent blocking of the vesicle's ability to translocate and become incorporated into the plasma membrane. The inability of wild-type AS160 to act in a similar inhibitory manner has been attributed to the possibility that insulin may lead to complete phosphorylation of all the AS160 that is introduced in transfection experiments. It can be noted that, although many groups have now used the AS160-4P mutant that contains four mutated sites, a single mutation of Thr642 to Ala reduced insulin-stimulated GLUT4 translocation by ∼60%, and an additional Ser588 and Thr642 to Ala reduced this translocation by ≤80% when overexpressed in 3T3-L1 adipocytes. There was no further effect when other additional Akt phosphorylation sites were mutated (AS160-4P) (51). This indicates that Thr642 and Ser588 are the key residues regulating Akt-mediated GLUT4 translocation in response to insulin. Indeed, the Scansite program ranked Thr642 and Ser588 1st and 2nd, respectively, among other phosphorylation sites as optimal Akt phosphosites. It has also been shown that PAS antibody predominantly detects the Thr642 site (13, 46, 51). Consistent with the hypothesis that downstream signaling is mediated through the GAP activity of AS160, additional experiments by Sano et al. (51) provided evidence that the effect produced by AS160-4P is dependent on an intact Rab-GAP domain. They demonstrated that simultaneous disruption of the putative GAP domain in AS160-4P, by mutating a key arginine 973 residue (which is thought to disrupt GAP activity) to lysine, overcame this inhibitory effect on GLUT4 translocation. Although this and several additional studies suggest that an intact/functional GAP domain is required for blocking insulin-stimulated GLUT4 trafficking and that this blocking activity is regulated through phosphorylation, currently there is no direct evidence that the GAP activity is reduced by phosphorylation. It would be of interest to determine whether the GAP activity of full-length AS160 is reduced by phosphorylation. An alternative possibility is that phosphorylation indirectly reduces the GAP activity toward the Rab substrates that control GLUT4 translocation by altering the location of AS160 (this possibility is further discussed below in the section on AS160-interacting proteins).
RNA interference (RNAi)-mediated knockdown of AS160 has been shown to increase insulin-independent basal GLUT4 levels on the 3T3-L1 adipocyte cell surface, consistent with its proposed role in intracellular GLUT4 retention that is relieved upon insulin stimulation (11, 34). However, AS160 knockdown only partially releases the pool of intracellular GLUT4 mobilized by insulin. One of the technical problems associated with this experimental approach is the need to completely reduce levels of AS160. It appears that a threshold and minimal level of AS160 is sufficient to retain GLUT4 in its intracellular location. The Rab-GAP is likely to be in a stoichiometric excess of the levels of the Rab-GTP substrate on which it acts. It follows that insulin-induced phosphorylation of AS160 must be extremely efficient in reducing all AS160 activity. It would be of interest to determine whether any nonphosphorylated AS160 is present in the cell after an insulin treatment or whether the extremely effective insulin action on AS160 occurs on a discrete subpopulation of the cellular protein that is associated only with GLUT4 vesicles.
Identification of TBC1D1 As a Related Insulin-Regulated Rab-GAP That Regulates GLUT4 Traffic
The very large number of Rabs (∼60 in humans) in eukaryotic genomes is matched by a similarly large number of Rab-GAPs (∼50 in humans). This reflects the specificity that Rabs must exhibit to control the large number of membrane traffic processes and events occurring in specialized cells (15, 16). The closest relative of AS160 (or TBC1D4) is TBC1D1. Roach et al. (47) reported properties of TBC1D1 that revealed some similarities to those of AS160/TBC1D4 and some interesting differences (Fig. 2). Although there is only 50% identity between these two proteins, the GAP domains are 79% identical, and there are comparable predicted Akt phosphorylation sites at Thr596 and Ser507 corresponding to Thr642 and Ser570 on AS160, respectively. This prompted Roach et al. (47) to test whether TBC1D1 is also a substrate of Akt and a potential intermediate between the insulin-signaling cascade and GLUT4 translocation in 3T3-L1 adipocytes. They found that TBC1D1 is phosphorylated mainly at Thr596 (equivalent to Thr642 on AS160, which can be detected by PAS antibody) by insulin action through activated Akt and also demonstrated that the Rabs identified as substrates (from the panel of Rabs that they tested) are essentially identical between AS160 and TBC1D1.
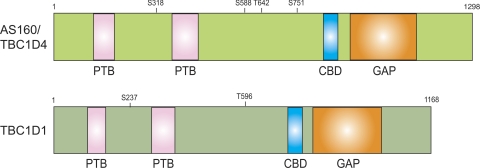
Fig. 2.
Schematic domain structures of human AS160/TBC1D4 and TBC1D1. The amino acid sequences used are National Center for Biotechnology Information (NCBI; gi: 114688046) for human AS160/TBC1D4 and NCBI (gi: 50658061) for human TBC1D1. The 2 phosphotyrosine-binding (PTB) domains and the GAP domains are those predicted by analysis with the Pfam program (http://www.sanger.ac.uk/). Sano et al. (51) originally found that insulin stimulates phosphorylation of AS160 at 5 sites via Akt, and mutation of AS160 in which 4 of phosphorylation sites (Ser318, Ser588, Thr642, Ser751) had been mutated to alanine (AS160-4P) abolished insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. Akt phosphorylates Thr596, and AMPK phosphorylates Ser237 of TBC1D1 in vitro and in intact cells. CBD, calmodulin-binding domain.
Both AS160/TBC1D4 and TBC1D1 have two NH2-terminally located PTB domains (Fig. 2). One of the notable differences between the two proteins is the presence of sequences in the PTB domains and in the occurrence of a predicted AMPK phosphorylation site between the two PTB domains of TBC1D1. Studies in cell culture systems have established that Ser237 becomes phosphorylated in response to treatments that elevate levels of active and phosphorylated AMPK (7, 8). In the 3T3-L1 system, AS160 phosphorylation appears to occur at higher levels than TBC1D1. In this system, wild-type AS160 is not inhibitory to insulin-stimulated GLUT4 translocation, whereas wild-type TBC1D1 is inhibitory. This difference has been attributed to an inability of insulin-signaling steps to phosphorylate and inactivate all the wild-type TBC1D1 that is introduced into the cells. The difference may also be due to a requirement for TBC1D1 GAP activity to be inactivated by phosphorylation at both the Akt and AMPK sites. Consistent with this notion, and in the 3T3-L1 system, addition of an AMPK activator leads to partial reversal of the inhibitory effect of the wild-type TBC1D1 protein (7).
It has been reported recently that TBC1D1 is expressed at higher levels in muscle than in fat (7, 58). However, Stone et al. (56) reported that, in a cohort of American subjects, TBC1D1 is a candidate severe obesity gene. A defect in this gene (leading to substitution of tryptophan for arginine at residue 125) is present in some cases of severe obesity in females. This genetic linkage has been confirmed in a cohort of French subjects (38). Therefore, a role for TBC1D1 in fat tissue in this human obesity disorder seems likely.
Regulation of AS160 and TBC1D1 in Muscle
As described above, many of the initial discoveries and characterizations of both AS160 and TBC1D1 were carried out by Kane et al. (24), Roach et al. (47), and Sano et al. (51) working on the 3T3-L1 adipocyte system. Similar inhibition of insulin-stimulated GLUT4 translocation by AS160-4P was reported by Thong et al. (59) using L6 skeletal muscle cells. To test whether the proposed mechanism functions in a more physiological context, Kramer et al. (32) overexpressed AS160-4P mutant by an electroporation method in mouse tibialis anterior skeletal muscle. They found that insulin-dependent stimulation of in vivo glucose uptake was reduced by ∼30% in muscle overexpressing AS160-4P compared with vector expression control. Interestingly, the 4P mutant also partially (∼40%) inhibits the contraction response to glucose uptake in intact skeletal muscle tissue (32). These inhibitory effects of the 4P mutant in response to either insulin or contraction were reversed when an Arg973 to Lys mutant was introduced in the AS160-4P mutant construct and expressed in muscle (32). Therefore, AS160 may function as a key signaling intermediate in mediating GLUT4 translocation to the cell surface not only with insulin but also with contractile activity in muscle. Whether the partial reduction of glucose uptake caused by overexpression of AS160-4P by the in vivo electroporation method is due to insufficient expression of the mutant proteins or to the presence and activity of other signaling molecule(s) apart from AS160, which may play an additional role in skeletal muscle, is unclear. Expression levels of transfected AS160 were ∼15-fold above endogenous protein in adipocytes (51), whereas six- to eightfold increases above endogenous levels were achieved in the muscle study (32).
Both AS160 and TBC1D1 have a calmodulin-binding domain (CBD; Fig. 2), and this domain may also be important in contraction-stimulated glucose transport. Studies in 3T3-L1 cells failed to reveal the functional role of this domain, possibly because any functional activity associated with this domain is not necessary for the insulin stimulation of AS160 function, and this domain is relevant only to muscle cells (23). However, control of contraction-stimulated GLUT4 translocation in muscle has long been known to be associated with increased calcium availability [reviewed in Rose and Richter (48) and Jessen and Goodyear (20)]. Kramer et al. (31) have studied the effects of AS160 mutated in the CBD by introducing this construct into tibialis anterior muscle using their in vivo electroporation technique. They found that a CBD mutant protein, in which critical Leu842 and Trp843 residues are both mutated to Gly, no longer bound to calmodulin in the presence of calcium and that it partly blocked increases in glucose transport that were associated with contraction but not with insulin stimulations. In addition, this inhibitory effect was found to be associated with the GAP activity of the mutant, since introduction of an additional mutation in the Rab-GAP domain (Arg973 to Lys) restored contraction-stimulated glucose transport. These data are important because they demonstrate a role for the CBD of AS160 and establish that AS160 may be an intermediate in activating GLUT4 vesicle translocation following either insulin signaling or contraction-associated calcium/calmodulin elevation.
Taken together with the data on the inhibitory effect of the AS160-4P mutant on contraction-stimulated glucose uptake, these data on the AS160-CBD mutant do, however, raise an associated complication. To account for the inhibition of the contraction-stimulated glucose transport caused by either mutation alone, it is necessary to assume that the suppression of the Rab-GAP activity of AS160 requires both phosphorylation and calcium/calmodulin binding. This is because the contraction-stimulated calcium/calmodulin effect does not appear to suppress the GAP activity of the AS160-4P mutant, whereas the contraction-stimulated phosphorylation effect does not suppress the GAP activity of the AS160-CBD mutant. However, since the AS160-CBD mutant does not inhibit the insulin response, insulin-induced phosphorylation alone seems sufficient to suppress the GAP activity of this construct.
The levels of phosphorylation of AS160 at its multiple phosphorylation sites may be such that insulin-induced phosphorylation alone is sufficient to inactivate the GAP, whereas contraction-induced phosphorylation alone is insufficient. More detailed quantification of the levels of phosphorylation of AS160 at its multiple sites and their functional significance would be required to address this issue in future. It can be noted that a recent study by Taylor et al. (58) has shown that TBC1D1 is present at high levels, especially in muscle with fast-twitch characteristics, whereas AS160 expression is relatively abundant in slow-twitch fibers. Therefore, it will be important in future to evaluate fiber type- and tissue-specific contributions of both AS160/TBC1D4 and TBC1D1 to insulin as well as to the contraction and energy status signaling. It appears from the above studies (32, 58) that the AS160-4P and AS160-CBD mutants are able to override the effects of both endogenous AS160 and TBC1D1.
Identification of Targets of AS160 and TBC1D1
Mîinea et al. (39) initially performed an immunoprecipitation study to identify specific Rab isoforms associated with GLUT4 vesicles. GLUT4 vesicles were isolated from the low-density microsome fraction of 3T3-L1 adipocytes with an antibody against GLUT4. These immunopurified vesicle proteins were analyzed by mass spectrometry. This technique identified isoforms of Rab1A, -1B, -2A, -3A, or -D; Rab4B, -5A, -5B, -5C, -6A, or -B; Rab7, -8A, or -B; Rab10, -11B, -14, -18, and -35. One caveat of this experiment was that they also identified the same Rabs in nonspecific rabbit immunoglobulin purified vesicles. It was unclear whether the presence of some of the identified Rabs was due to nonspecific binding, because only a small amount of GLUT4-containing vesicles were sufficient for a sensitive identification by mass spectrometry. Larance et al. (34) isolated a highly enriched population of GLUT4 vesicles and found that these were associated more specifically with Rab10, -11, and -14. Rab11 has also been identified on GLUT4 vesicles isolated from cardiac muscle cells (28).
Mîinea et al. (39) sought the target Rab of AS160 by expressing the isolated GAP domain. This is not ideal, because full-length AS160 might be expected to have somewhat different properties. Nevertheless, they found selective GAP activity toward Rab2A, -8A, -8B, -10, and -14. From these candidate substrates, Rab10 has emerged as currently the Rab most likely to be involved in GLUT4 vesicle translocation in 3T3-L1 cells. Evidence to support this possibility includes its presence on GLUT4 vesicles and RNAi-mediated knockdown data in which specific loss of Rab10 leads to ≤80% reduction in GLUT4 translocation (50, 52).
In L6 muscle cell lines, the AS160 substrate Rab8A appears to be the important Rab isoform regulating GLUT4 translocation (19). Although Rab11 does not appear to be an AS160 substrate, the Rab11 effector Rip11 has been shown to interact with AS160 (62). However, in one study knockdown of Rip11 led to inhibition of insulin-stimulated GLUT4 translocation (62), whereas in another study knockdown led only to an increase in basal GLUT4 translocation (54). Whether the different cell lines and tissues employed in these studies can account for the differences in the apparent Rab requirement for translocation remains to be established. As GLUT4 traverses several intracellular compartments, it will necessarily be associated with several different Rabs in different locations. The extent to which GLUT4 resides in these compartments (and is associated with a Rab specific for that compartment) may differ in the different cell lines used in these studies. A key question for future work will be the identification of the Rab that is most closely associated with the specialized GLUT4 vesicles that are involved in exocytosis to the cell surface in response to direct insulin stimulation.
To further explore the role of AS160 in insulin-regulated GLUT4 translocation and possibly find additional proteins involved in insulin-stimulated GLUT4 translocation, Ramm et al. (46) sought to identify new interacting partners for AS160. They overexpressed Flag-tagged AS160 in CHO IR/IRS-1 cells. AS160 binding proteins were isolated following affinity purification with anti-Flag resin, and their identities were determined by liquid chromatography-tandem mass spectrometry (46). This resulted in the identification of 14-3-3 isoforms as potential interacting partners for AS160. Interaction with 14-3-3 was shown to occur in an insulin- and Akt-dependent manner. Geraghty et al. (13) also reported that, in HEK-293 cells, IGF-I action enhances binding of 14-3-3 to AS160. This is direct and phosphorylation dependent. Mutagenesis studies revealed that insulin- or IGF-I-stimulated binding of 14-3-3 is mediated primarily through the phosphorylation of Thr642 (13, 46), with phosphorylation of Ser341 contributing to basal 14-3-3 binding in unstimulated cells (12). This correlates with the dominant-negative effect of both the AS160-Thr642 Ala and the AS160-4P mutants on insulin-stimulated GLUT4 translocation. Introduction of an engineered high-affinity 14-3-3-binding sequence termed R18 into the AS160-4P mutant adjacent to the Thr642 Ala residue restored 14-3-3 binding and reversed the inhibitory effect of AS160-4P on GLUT4 translocation (46). In contrast, this effect was abolished when the critical glutamate residue in R18 sequence (required for 14-3-3 binding) was mutated in the AS160-4P construct. These results indicate that the insulin-dependent interaction of 14-3-3 with AS160, through Thr642 phosphorylation, plays an important role in suppressing the inhibitory role of AS160 on GLUT4 traffic in adipocytes. TBC1D1 has also been shown to interact with 14-3-3 proteins, and in HEK-293 cells IGF-I promotes 14-3-3 binding through phosphorylated Thr596. In contrast, insulin failed to enhance 14-3-3 binding to TBC1D1 in the L6 muscle cell line (8). Interestingly, it has been demonstrated that pharmacological activation of AMPK increased Ser237 phosphorylation (7, 8) and enhanced the 14-3-3 interaction with TBC1D1 in L6 cells through phosphorylation of this residue. It would be interesting to determine whether 14-3-3 binding to TBC1D1 plays a key role for insulin- and/or AMPK-stimulated GLUT4 traffic in muscle cells.
The functions of the PTB domains in AS160 and in TBC1D1 are currently unknown. Since a mutation of TBC1D1, which is associated with metabolic dysregulation in humans (as described in the previous section), is located within one of PTB domains (Arg125 to Trp), this will be an important area of further investigation. Two reports (34, 45) have provided evidence for an interaction of these domains with the GLUT4 vesicle-associated protein insulin-regulated aminopeptidase (IRAP). This is probably not the only means by which AS160 is targeted to GLUT4 vesicles, since insulin-responsive GLUT4 vesicle translocation (and presumably GLUT4 vesicle Rab activity) can occur in the absence of IRAP (27). However, in the absence of IRAP, cellular GLUT4 levels are reduced.
It has not yet been fully established that nonphosphorylated AS160 needs to be associated with GLUT4 vesicles to block vesicle translocation. Some AS160 is found to be associated with GLUT4 vesicles (34), but large amounts are associated with the cytoplasmic fraction of cells and with vesicles other than GLUT4. Whether the CBD in AS160 and TBC1D1 can direct the localization is also currently unclear.
It seems particularly important for the future to establish how AS160 and TBC1D1 are targeted to appropriate cellular locations that can affect GLUT4 vesicle Rab loading and unloading and the associated changes in localization of GLUT4 vesicles. Some literature suggests that AS160 may affect the release of GLUT4 vesicles from intracellular locations (11), whereas other literature has suggested that AS160 may influence a docking or fusion step in exocytosis (2, 21). Total internal reflection of fluorescence (TIRF) microscopy is beginning to reveal details of the terminal steps in translocation of GLUT4 as it reaches a zone within from 100 to 250 nm of the plasma membrane (2, 14, 18, 21, 35). However, the cited studies differ in many respects and importantly in the difference between the number of GLUT4 vesicles that are located within the TIRF zone in the basal and insulin-stimulated states. In one of these studies, in which details of the docking and fusion of GLUT4 vesicles were kinetically analyzed, there appeared to be no difference between the number of vesicles in the TIRF zone in the basal, insulin-stimulated, and AS160-4P-treated conditions. However, this study also reported that the AS160-4P construct reduced the number of docked vesicles below those occurring in the basal state. This led to the suggestion that AS160 is required for vesicle docking and also that insulin influences vesicle fusion independently of AS160 (2). Other TIRF microscopy studies reach more uncertain conclusions on the site of action of insulin, Akt, and AS160 on the predocking, docking, and fusion steps. This may be partly related to the thickness of the TIRF zone that is observed and the number of vesicles within this zone in basal and insulin-stimulated conditions. Further exploration of these issues with TIRF microscopy and complementary cell-free studies on GLUT4 vesicle fusion (30) may help resolve where AS160 influences the overall process of GLUT4 exocytosis.
AS160 and TBC1D1 in the Whole Animal Context
As described in the previous sections, rapidly growing evidence supports the role for both AS160 and TBC1D1 in GLUT4 traffic. However, there are many mechanistic questions concerning how constructs derived from these proteins exert their inhibitory effects on GLUT4 translocation. Therefore, it is important to obtain more detailed analysis of the functional importance of these proteins in more physiological contexts and in particular in whole animals. There are currently no genetic mouse models that validate this hypothesis that are described in the literature. For example, it is currently unknown whether a lack of AS160 or TBC1D1 constitutively stimulates glucose transport in knockout mice. Also, it remains to be determined whether in AS160/TBC1D1 knockin mice, in which endogenous AS160 or TBC1D1 is replaced with an AS160-4P mutant or TBC1D1 mutant (e.g., Thr596 to Ala and/or Ser237 to Ala), there is abolition of insulin-stimulated glucose transport in adipose and insulin- and/or contraction-stimulated glucose transport muscle. Since AS160 and TBC1D1 are likely to play redundant roles, it might be necessary to generate double-knockout or knockin mice to fully understand the importance of these proteins in the regulation of GLUT4 traffic and glucose homeostasis in vivo. In addition, identification of whether a single specific Rab is downstream of the Rab-GAPs in all tissues needs to be determined. Associated with these efforts, a genetic approach to validate the role of the Rab or Rabs will be required. These comprehensive genetic approaches will allow us to assess whether pharmacological inhibitors for AS160 and/or TBC1D1 would be good drugs for the treatment of type 2 diabetes. However, it should be taken into account that inhibition of AS160 may not be always beneficial. For example, a recent study (4) has shown that AS160 is phosphorylated in response to glucose and that knockdown of AS160 resulted in reduced glucose-induced insulin release in pancreatic β-cells. Reduced AS160 levels were associated with increased apoptosis and reduced capacity to proliferate upon glucose stimulation. Zhou et al. (65) reported that although RNAi-mediated knockdown of TBC1D1 increases basal glucose uptake, it also stimulates the mTOR/p70S6k pathway and synthesis of GLUT1 glucose transporter in 3T3-L1 adipocytes. Moreover, both AS160 and TBC1D1 are phosphorylated at multiple sites by a variety of agonists that stimulate different protein kinases in intact cells (5, 8, 13, 25, 58, 59), indicating complex regulation and roles for these proteins. Further work is required to characterize the biochemical properties and physiological roles for AS160 and TBC1D1 in the regulation of GLUT4 traffic and how this specific function relates to other cellular functions of these Rab-GAPs.
GRANTS
K. Sakamoto is currently supported by Diabetes UK and the UK Medical Research Council and by the companies AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Merck KGaA, and Pfizer. G. D. Holman is supported by AstraZeneca, the Wellcome Trust, and by the the UK Medical Research Council.
Acknowledgments
We thank Carol MacKintosh for helpful suggestions and Stephan Wullschleger for technical help in preparing figures.
REFERENCES
- 1.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333–338, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab 5: 47–57, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, Malinowski J, McAvoy EM, Nahas DD, Robinson RG, Huber HE. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J 385: 399–408, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouzakri K, Ribaux P, Tomas A, Parnaud G, Rickenbach K, Halban PA. Rab GTPase-activating protein AS160 is a major downstream effector of protein kinase B/Akt signaling in pancreatic beta-cells. Diabetes 57: 1195–1204, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase activating protein abundant in skeletal muscle, is partially relieved by AMPK activation. J Biol Chem 283: 9187–9195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, MacKintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBbeta). Science 292: 1728–1731, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2: 263–272, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112: 197–208, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407: 231–241, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell 17: 4484–4493, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas AK, Fuchs E, Kopajtich R, Barr FA. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol 7: 887–893, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Haas AK, Yoshimura S, Stephens DJ, Preisinger C, Fuchs E, Barr FA. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J Cell Sci 120: 2997–3010, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Lifshitz LM, Jones C, Bellve KD, Standley C, Fonseca S, Corvera S, Fogarty KE, Czech MP. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol Cell Biol 27: 3456–3469, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikura S, Bilan PJ, Klip A. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem Biophys Res Commun 353: 1074–1079, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol 99: 330–337, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Jiang L, Fan J, Bai L, Wang Y, Chen Y, Yang L, Chen L, Xu T. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J Biol Chem 283: 8508–8516, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100: 7569–7574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane S, Lienhard GE. Calmodulin binds to the Rab GTPase activating protein required for insulin-stimulated GLUT4 translocation. Biochem Biophys Res Commun 335: 175–180, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54: 1692–1697, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Katome T, Obata T, Matsushima R, Masuyama N, Cantley LC, Gotoh Y, Kishi K, Shiota H, Ebina Y. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J Biol Chem 278: 28312–28323, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Keller SR, Davis AC, Clairmont KB. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J Biol Chem 277: 17677–17686, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kessler A, Tomas E, Immler D, Meyer HE, Zorzano A, Eckel J. Rab11 is associated with GLUT4-containing vesicles and redistributes in response to insulin. Diabetologia 43: 1518–1527, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271: 31372–31378, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Koumanov F, Jin B, Yang J, Holman GD. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab 2: 179–189, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes 56: 2854–2862, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC Jr. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol 28: 61–70, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larance M, Ramm G, Stockli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterisation of the role of the RabGAP AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 280: 37803–37813, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol 169: 481–489, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logie L, Ruiz-Alcaraz AJ, Keane M, Woods YL, Bain J, Marquez R, Alessi DR, Sutherland C. Characterization of a protein kinase B inhibitor in vitro and in insulin-treated liver cells. Diabetes 56: 2218–2227, 2007. [DOI] [PubMed] [Google Scholar]
- 37.McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54: 1349–1356, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Meyre D, Farge M, Lecoeur C, Proenca C, Durand E, Allegaert F, Tichet J, Marre M, Balkau B, Weill J, Delplanque J, Froguel P. R125W coding variant in TBC1D1 confers risk for familial obesity and contributes to linkage on chromosome 4p14 in the French population. Hum Mol Genet. In press. [DOI] [PubMed]
- 39.Mîinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391: 87–93, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Nagase T, Ishikawa K, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. Prediction of the coding sequences of unidentified human genes. IX. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res 5: 31–39, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab 7: 348–356, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem 275: 36108–36115, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peck GR, Ye S, Pham V, Fernando RN, Lance MS, Yeen CS, Albiston AL. Interaction of the Akt substrate, AS160, with the GLUT4 vesicle marker protein, insulin-regulated aminopeptidase. Mol Endocrinol 20: 2576–2583, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281: 29174–29180, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase activating protein Tbc1d1. Biochem J 403: 353–358, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20: 260–270, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J Physiol Endocrinol Metab 291: E1031–E1037, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, Lienhard GE, McGraw TE. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab 5: 293–303, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Sano H, Roach WG, Peck GR, Fukuda M, Lienhard GE. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J 411: 89–95, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Schwenk RW, Luiken JJ, Eckel J. FIP2 and Rip11 specify Rab11a-mediated cellular distribution of GLUT4 and FAT/CD36 in H9c2-hIR cells. Biochem Biophys Res Commun 363: 119–125, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone S, Abkevich V, Russell DL, Riley R, Timms K, Tran T, Trem D, Frank D, Jammulapati S, Neff CD, Iliev D, Gress R, He G, Frech GC, Adams TD, Skolnick MH, Lanchbury JS, Gutin A, Hunt SC, Shattuck D. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet 15: 2709–2720, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 56: 414–423, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 20: 271–284, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Welsh GI, Leney SE, Lloyd-Lewis B, Wherlock M, Lindsay AJ, McCaffrey MW, Tavare JM. Rip11 is a Rab11- and AS160-RabGAP-binding protein required for insulin-stimulated glucose uptake in adipocytes. J Cell Sci 120: 4197–4208, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 13: 444–451, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Zhou QL, Jiang ZY, Holik J, Chawla A, Hagan GN, Leszyk J, Czech MP. Akt substrate TBC1D1 regulates GLUT1 expression through the mTOR pathway in 3T3-L1 adipocytes. Biochem J 411: 647–655, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou QL, Park JG, Jiang ZY, Holik JJ, Mitra P, Semiz S, Guilherme A, Powelka AM, Tang X, Virbasius J, Czech MP. Analysis of insulin signalling by RNAi-based gene silencing. Biochem Soc Trans 32: 817–821, 2004. [DOI] [PubMed] [Google Scholar]