Interleukin 1β facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 17.
Abstract
It has been shown that interleukin-1β (IL-1β) facilitates nociception during neuropathic and inflammatory pain, but its involvement in bone cancer pain and its mechanisms have not previously been established. This study is an investigation of IL-1β spinal expression and the NMDA receptor (NMDAR) NR1 subunit phosphorylation during cancer pain, co-localization of IL-1 receptor type I (IL-1RI) and NMDAR in the spinal cord, and the effects of IL-1 receptor antagonist (IL-1ra) on NR1 phosphorylation and hyperalgesia in a rat model of bone cancer pain. Cancer was induced by injecting AT-3.1 prostate cancer cells into the tibia of the male Copenhagen rat. Phosphorylation of NR1, an essential subunit of the NMDAR, is known to modulate NMDAR activity and facilitate pain. Mechanical hyperalgesia, established by a decrease in paw withdrawal pressure threshold (PWPT), was measured at baseline and 2 hr after IL-1ra treatment. IL-1ra was given (i.t.) daily for 7 days between days 13 and 19 after the cancer cell inoculation. Spinal cords were removed for western blot to measure IL-1β and NR1 phosphorylation and for double immunostaining of IL-1RI and NR1. The data showed that 1) spinal IL-1β was up-regulated and NR1 phosphorylation was increased, 2) IL-1ra at 0.1 mg/ rat significantly (P<0.05) inhibited mechanical hyperalgesia, increasing PWPT on day 14 from 71.1 ± 3.1 to 85.3 ± 4.6 grams and on day 19 from 73.5.0 ± 3.5 to 87.1 ± 3.7 grams, and inhibited NR1 phosphorylation compared to saline control, and 3) IL-1RI is localized in NR1-immunoreactive neurons within the spinal cord. The results suggest that spinal IL-1β enhances NR1 phosphorylation to facilitate bone cancer pain.
Keywords: IL-1β, NMDA receptor, Hyperalgesia, Phosphorylation, Spinal cord, Cancer pain
1. Introduction
Cancer pain is extremely disruptive to patient quality of life. It has been reported that 30-50% of all patients in the early stages of cancer and 70-90% of patients with advanced cancer experience substantial and intractable pain during their lifetimes (Foley, 1999, Portenoy and Lesage, 1999). Bone cancer pain is the most common cancer-related pain (Banning et al., 1991, Mercadante, 1997, Reale et al., 2001). Bone metastases have been identified at autopsy in up to 90% of patients dying from prostate cancer (Rana et al., 1993, Bubendorf et al., 2000) and 85% of those dying from breast or lung cancer (Nielsen et al., 1991). Our recent studies demonstrate that bone cancer, induced by injecting AT-3.1 prostate cancer cells into the tibia of the male Copenhagen rat, results in a significant up-regulation of spinal interleukin (IL)-1β and that intrathecal (i.t.) IL-1 receptor antagonist (IL-1ra) significantly inhibits bone cancer-caused hyperalgesia (Zhang et al., 2005, Zhang et al., 2007).
IL-1β is known as an inflammatory mediator of peripheral immune responses. Recent studies suggest that glial cell-produced IL-1β facilitates transmission and processing of noxious inputs at the spinal level (Watkins et al., 2003, Raghavendra et al., 2004). IL-1β is reportedly up-regulated in the spinal cord during inflammatory (Samad et al., 2001, Raghavendra et al., 2004) and neuropathic pain (Winkelstein et al., 2001, Raghavendra et al., 2003). IL-1ra produces anti-allodynic effects in rat models of neuropathic (Milligan et al., 2001, Sweitzer et al., 2001) and inflammatory pain (Zhang et al., 2008). IL-1β also reportedly produces increased activity in dorsal horn neurons (Reeve et al., 2000) and enhances NMDAR-mediated increase of inward current and intracellular Ca2+ (Viviani et al., 2003, Yang et al., 2005). Further, inflammation-induced IL-1β enhances phosphorylation of NR1, an essential subunit of NMDAR, to facilitate transmission of nociceptive inputs in inflammatory pain models (Guo et al., 2007, Zhang et al., 2008).
We hypothesized that IL-1β also increases NR1 phosphorylation in spinal cord neurons to facilitate bone cancer-induced pain. In the present study, we evaluated up-regulation of spinal IL-1β and spinal NR1 phosphorylation and the effects of IL-1ra on spinal NR1 phosphorylation and pain in a rat model of bone cancer pain. We also determined that IL-1 receptor type I (IL-1RI) is co-localized with NR1 in the spinal cord.
2. Experimental Procedures
2.1 Experimental design
Male Copenhagen rats weighing 200-220g (Harlan) were kept under controlled conditions (22°C±0.5°C, relative humidity 40-60%, 7 am to 7 pm alternate light-dark cycles, food and water ad libitum). The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
The present study consisted of the following three experiments. Experiment 1, Effects of bone cancer on IL-1β expression and NR1 phosphorylation: Rats were divided into bone cancer and sham cancer by injecting AT-3.1 prostate cancer cells or vehicle (Hank's solution) into the tibia (n=6 per group). On day 19, by which time cancer rats showed significant hyperalgesia in our previous study, the lumbar4-5 spinal cord was removed for measuring IL-1β and phosphorylated NR1 with western blot. Experiment 2, Effects of Anakinra (also called Kineret), a recombinant, non-glycosylated version of human IL-1ra, on spinal NR1 phosphorylation and mechanical hyperalgesia: Cancer rats, each surgically fitted with an i.t. catheter, were divided into IL-1ra treatment (n=6) and vehicle control (n=6) groups. Anakinra (Amgen Inc, Thousand Oaks, CA) blocks the biologic activity of naturally occurring IL-1 by competitively inhibiting the binding of IL-1 to the IL-1 type I receptor. Its plasma half-life is four to six hours. A single intrathecal injection of IL-1ra (100 μg/2 μl per rat) or saline was given daily for 7 days, beginning on day 13 and ending on day 19 after inoculation. Mechanical hyperalgesia, a decrease in paw withdrawal pressure threshold (PWPT), was assessed on day 0 for baseline and on day 12 to confirm the development of hyperalgesia. It was also assessed two hours post-drug on days 14 and 19 to determine the accumulated inhibitory effect of IL-1ra on IL-1beta. We did not test mechanical hyperalgesia daily to avoid potential confounding effect of daily testing. After the behavioral test on day 19, the spinal cord was removed to measure phosphorylated NR1 by western blot. The investigators performing the behavioral tests and western blot were blind to the treatment assignments. Experiment 3, Co-localization of IL-1RI with NR1 in the spinal cord. The spinal cord was removed from naive rats, sham rats, cancer rats, IL-1ra-treated cancer rats and vehicle-treated cancer rats (n=3 per group) for double staining of IL-1RI and NR1 to determine whether IL-1RI is localized in NMDAR-containing neurons and to determine whether bone cancer alters the expression profile of IL-1RI and NR1 on day 19 after induction of bone cancer and whether IL-1ra treatment changes the expression profile of IL-1RI and NR1.
2.2 Intrathecal Cannulation
Rats were prepared for i.t. injection under pentobarbital sodium anesthesia (50 mg/kg i.p.) (Zhang et al., 2004). The atlanto-occipital membrane was exposed, and a 7.0-cm length of PE-10 tubing was inserted into the subarachnoid space through a slit made in the membrane. The catheter was advanced to the level of the lumbar spinal cord and filled with saline (approximately 7-10 μl), and the outer end was plugged. At the end of the experiments, the location of the distal end of the catheter was verified when the spinal cord was removed.
The animals were allowed to recover for seven days after the operation prior to cancer cell implantation into the tibia. Ten percent of animals showed gross signs of motor impairment and were excluded from the study.
2.3 Cell culture and implantation
Detailed procedures for cell culture and cancer cell implantation into the tibia have been described previously (Zhang et al., 2005). The AT-3.1 prostate cancer cell line, obtained from American Type Culture Collection (ATCC, Rockville, MD), was maintained in T-75 plastic flasks (Corning Glass), grown in RPMI 1640 medium (Sigma) supplemented with 250 nM dexamethasone and 10% fetal bovine serum (Sigma), and cultured in a water-saturated incubator in 5% CO2:95% air. Cells were detached using a trypsin solution containing 0.05% trypsin and 0.02% EDTA and collected by centrifuging 10 ml of medium for 3 min at 1,200 rpm. The resulting pellet was washed twice with 10 ml of calcium- and magnesium-free Hank's solution and re-centrifuged for 3 min at 1,200 rpm. The final pellet was diluted to a final concentration of 3×105 cells/10 μl Hank's solution for injection and kept on ice until injection.
The cells were surgically implanted following induction of anesthesia with sodium pentobarbital (45 mg/kg, i.p.). One leg of each rat was shaved. The skin was disinfected with 7% tincture of iodine and 70% ethanol, and a 1-cm long rostrocaudal incision was made in the skin over the upper medial half of the tibia. The tibia was carefully exposed and pierced with a 23-gauge needle 5 mm below the knee joint medial to the tibial tuberosity. A 10 μl volume of prostate cancer cells (3×105 cells) or vehicle (Hank's solution only) was injected into the bone cavity with a 50 μl Hamilton syringe. After a 2-min delay while the cells filled the space in the bone cavity, the syringe was removed and the injection site was closed using bone wax (Ethicon). The muscle was stitched and the skin wound was closed using 3–0 silk thread. Each rat was monitored for general condition and changes in body weight during the 19-day experiment; none showed weight loss.
2.4 Mechanical hyperalgesia
The rats were tested for mechanical hyperalgesia by determining the nociceptive PWPT with a Paw Pressure Analgesia Instrument (Ugo Basile, Italy) (Zhang et al., 2005). The minimum paw pressure (in grams) that elicited paw withdrawal was defined as PWPT. A cut-off of 250 g was employed. Mean PWPT was established by averaging the values of four consecutive tests separated by intervals of 30 sec.
2.5 Western blot
Western blot was used to examine IL-1β and phosphorylated NR1. On day 19 post-cancer cell implantation, rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and immediately decapitated. After laminectomy, the lumbar4-5 spinal cord was separated along the midline posterior spinal vein into ipsilateral and contralateral portions and then removed. Since our previous study demonstrated that IL-1β is up-regulated ipsilaterally but not contralaterally (Zhang et al., 2005), we used only the ipsilateral portion. Tissues were was homogenized in protein extraction buffer containing: 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% NP40, 0.5% sodium dodecyl sulfate (SDS), 1% deoxycholic acid, 2.5 μg/ml aprotinin, 2 μg /ml leupetin, 2 μg /ml pepstatin A, 25 mM NaF, and 1 mM Na3VO4. After centrifuging at 14000 rpm for 10 min at 4°C, the supernatant containing the proteins was collected. Protein concentration was determined using the Bio-Rad Protein Assay. Equal amounts of proteins were mixed with loading buffer. After boiling for 10 min, the proteins were fractionated on a 4-20% (w/v) SDS-PAGE and transferred onto a polyvinylidine difluoride (PVDF) membrane (Bio-Rad) with a Trans-Blot Cell System (Bio-Rad). The membrane was blocked for 1 h at room temperature with 5% BSA in PBS containing 0.1% Tween 20 and then incubated overnight at 4°C with phosphor-NR1 antiserum (Serine 896, 1:1000, Upstate) or IL-1β antibody (1:1000, Endogen). After washing with TBS buffer (20 mM Tris, 150 mM NaCl, pH 7.4), membranes were incubated for 1 h at room temperature with goat anti-rabbit horseradish peroxidase-conjugated IgG (1:3000; Upstate) diluted in 3.0% (w/v) BSA in TBS buffer. The immunoreactivity of the proteins on the membrane was visualized using the chemilluminescence detection system (ECL, Amersham). Autoradiograms were digitized, and densitometric quantification of immunoreactive bands was carried out using Scion NIH Image 1.60. The membranes were then incubated in stripping buffer (100 μM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris [pH 6.7]) at 50°C for 30 min and re-probed with β-actin antibody (1:5000, Sigma) as a loading control.
2.6 Immunofluorescence
Rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and immediately perfused transcardially with 4% paraformaldehyde (Sigma) in 0.1 M phosphate buffer (PB) at pH 7.4. The lumbar4–5 spinal cord was removed, immersed in the same fixative for 2 h at 4 °C, and transferred to 30% sucrose (w/v) in PB saline (PBS) for overnight cryoprotection. Thirty micron-thick sections were cut on a cryostat, rinsed in PBS, blocked in PBS with 10% normal donkey serum for 60 min and incubated overnight at room temperature with a mixture of rabbit polyclonal IL-1RI (1:500, Santa Cruz) and goat polyclonal NR1 (1:100, Santa Cruz). After three 10-min washings in PBS, sections were incubated in a mixture of CY3-congugated donkey anti-rabbit (1:000, Jackson ImmunoResearch Laboratories) and CY2-conjugated donkey anti-goat (1:200) for 1 h at room temperature. The stained sections were mounted on gelatin-coated slides, coverslipped with aqueous mounting medium (Biomeda Corp., CA), and examined under a Nikon fluorescence microscope. Control sections were similarly processed, except that the primary antisera were omitted, which yielded no staining.
2.7 Statistical analyses
Data from the behavioral tests were presented as Mean ± SE and analyzed using repeated measures analysis of variance (ANOVA) followed by post-hoc Scheffé's multiple comparisons (Statistical Analysis System). Western blot data were analyzed with one-way ANOVA followed by the Scheffé's multiple comparison procedure. P<0.05 was set as the level of statistical significance.
3. Results
3.1 Bone cancer induced IL-1β up-regulation and NR1 phosphorylation
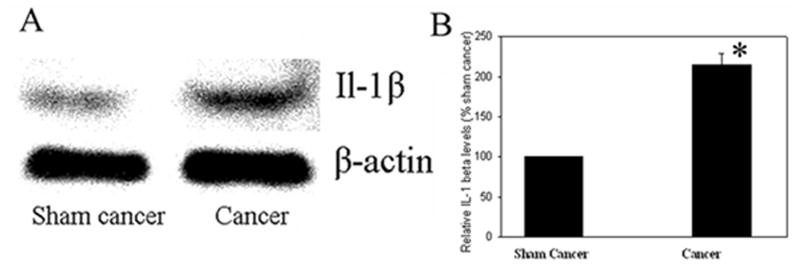
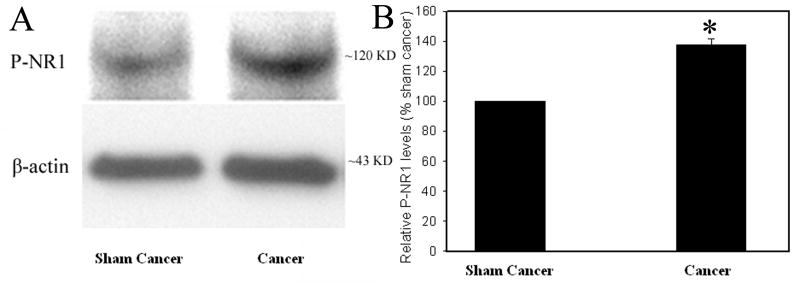
Fig. 1 and 2 show the relative levels of IL-1β and phosphorylated-NR1, respectively, in the spinal cord in bone cancer rats and sham control. IL-1β was significantly (P<0.05) up-regulated in cancer rats compared to that of sham control (Fig. 1). The levels of phosphorylated NR1 were also significantly (P<0.05) higher in cancer rats than in sham control (Fig. 2). This suggests that bone cancer induced increases of IL-1β expression and NR1 phosphorylation.
Fig. 1.
A: IL-1β up-regulation, revealed with western blot, 19 days after the cancer cell inoculation. β-actin was used as a loading control. B: Note that cancer cell inoculation of tibia significantly increased IL-1β level compared to vehicle injection (n=6 per group). *P<0.05 vs sham control, which was arbitrarily set as 100%.
Fig. 2.
A: NR1 phosphorylation during bone cancer pain. B: Note that intra-tibial cancer cell inoculation increased NR1 phosphorylation compared to vehicle injection (n=6 per group). P-NR1: phosphorylated NR1; *P<0.05 vs sham control, which was arbitrarily set as 100%.
3.2 IL-1ra attenuated mechanical hyperalgesia and NR1 phosphorylation
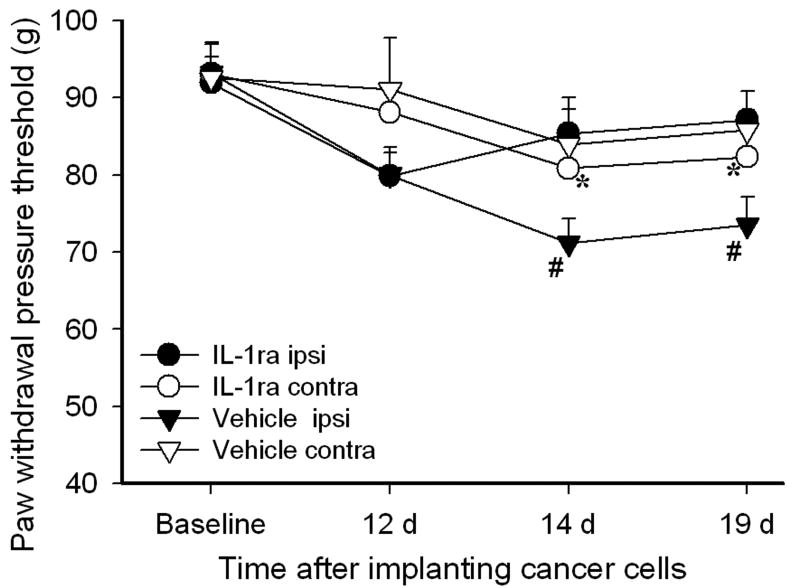
Figure 3 shows the effect of IL-1ra on PWPT in bone cancer rats. Before prostate cancer cell inoculation of the tibia, there were no significant differences in overall mean baseline PWPT to noxious mechanical stimuli between two groups of rats or between PWPT of the left and right hind paws. Statistical analysis revealed that cancer cell inoculation of the tibia induced a significant (P<0.05) PWPT decrease in ipsilateral hind paws compared to contralateral hind paws on days 14 (71.1 ± 3.1 vs 83.9 ± 4.5 grams) and 19 (73.5 ± 3.5 vs 85.7 ± 2.5 grams) after inoculation. The IL-1ra treatment significantly (P<0.05) increased PWPT of ipsilateral hind paws on day 14 (85.3 ± 4.6 vs 71.1 ± 3.1 grams) and 19 (87.1 ± 3.7 vs 73.5 ± 3.5 grams) compared to vehicle saline, but had no significant effect on the contralateral hind paws. These data demonstrated that bone cancer induced a significant and progressive mechanical hyperalgesia and that IL-1ra significantly alleviated the mechanical hyperalgesia of the ipsilateral paws (Fig. 3).
Fig. 3.
Effects of IL-1ra on bone cancer-induced mechanical hyperalgesia. IL-1ra (100 μg/2 μl per rat, i.t.) was given daily for 7 days on days 13-19 following cancer cell inoculation of the tibia. IL-1ra (n=6) significantly suppressed the cancer-induced mechanical hyperalgesia compared to vehicle control (n=6 per group). #P<0.05 compared to contralateral paws; *P<0.05 compared to vehicle ipsilateral paw; Ipsi: ipsilateral; contra:contralateral.
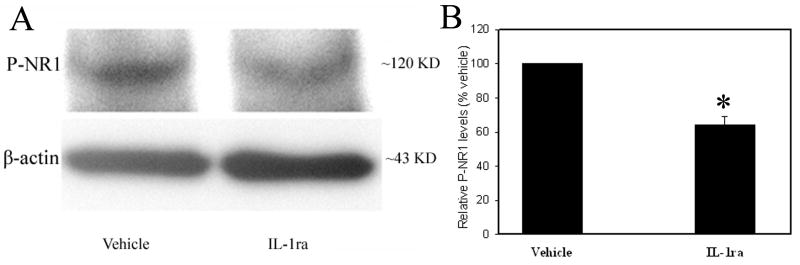
Fig. 4 shows the effects of IL-1ra (100 μg/2 μl per rat, i.t.) treatment on NR1 phosphorylation in the spinal cord. Phosphorylated NR1 levels were significantly (P<0.01) higher in vehicle-treated rats than in IL-1ra-treated rats, indicating that IL-1ra inhibited spinal cord NR1 phosphorylation during bone cancer pain. In other words, up-regulated endogenous IL-1β facilitated the phosphorylation of spinal NR1.
Fig. 4.
A: Effect of IL-1ra treatment on NR1 phosphorylation. B: Note that IL-1ra markedly decreased NR1 phosphorylation compared to vehicle (n=6 per group). *P<0.05 vs vehicle, which was arbitrarily set as 100%.
3.3 IL-1RI was localized in NMDAR-containing neurons
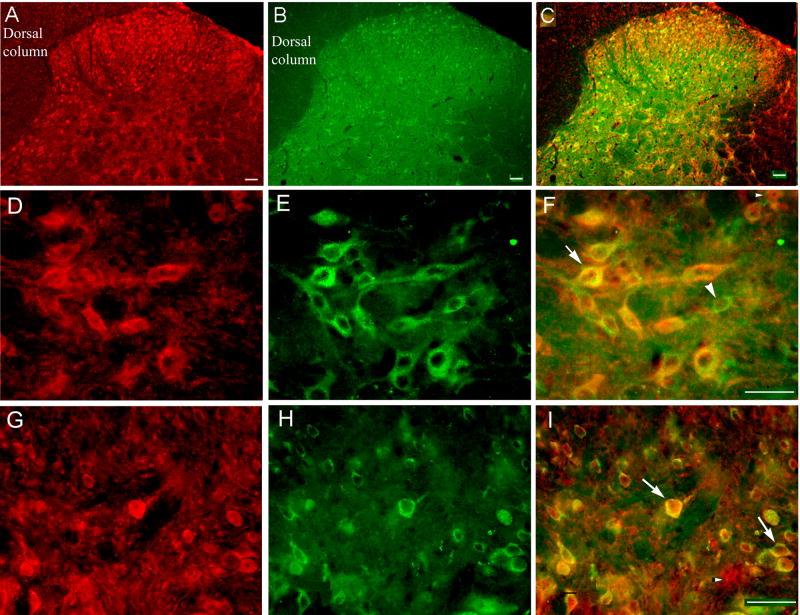
Double immunofluorescence labeling demonstrated that NR1- and IL-1RI-immunoreactive neurons are distributed in the spinal dorsal horn (laminae I-VI) and co-localized in some spinal cord neurons in these laminae. As shown in Fig. 5, some neurons are single labeled for either NR1 or IL-1R1 while some are labeled for both (Figs. 5F and 5I). Bone cancer and Il-1ra treatment did not result in any significant changes in NR1 and IL-1RI expression in the lumbar spinal cord.
Fig. 5.
Microphotographs showing distribution and co-localization of NR1 and IL-1R1 in lumbar spinal dorsal horn neurons. Sections were double-labeled with anti-IL-1R1 (red) and anti-NR1 (green). A: Localization of IL-1R1 in dorsal horn. B: Localization of NR1 in dorsal horn. C: Co-localization of NR1 and IL-1R1. D: Localization of IL-1R1 in lamina V. E: Localization of NR1 in lamina V. F: Co-localization of NR1 and IL-1R1 in lamina V neurons. G: Localization of IL-1R1 in superficial laminae. H: Localization of NR1 in superficial laminae. I: Co-localization of NR1 and IL-1R1 in superficial laminae neurons. Arrows indicate double-labeled NR1/IL-1R1 neurons (yellow); arrowheads indicate single-labeled NR1 (green) and IL-1R1 (red) neurons. Scale bars represent 40 μm.
4. Discussion
The present study shows that spinal IL-1β is significantly up-regulated in rats with bone cancer. The data are consistent with previous reports that spinal IL-1β is up-regulated during inflammatory (Sweitzer et al., 1999, Samad et al., 2001, Watkins et al., 2003, Raghavendra et al., 2004) and neuropathic pain (Raghavendra et al., 2003). It also shows that the levels of phosphorylated NR1 are significantly higher in bone cancer rats than in sham cancer rats. The data are consistent with previous reports that NR1 is phosphorylated during neuropathic pain (Gao et al., 2005, Ultenius et al., 2006) and inflammatory pain (Zou et al., 2000, Zhang et al., 2008). Because i.t. IL-1ra significantly inhibited neuropathic pain (Milligan et al., 2001, Sweitzer et al., 2001) and inflammatory pain (Zhang et al., 2008) as well as blockage of NR1 phosphorylation significantly reversed neuropathic pain (Gao et al., 2005) and inflammatory pain (Lee et al., 2004), our data suggest that both IL-1β and phosphorylated NR1 are involved in bone cancer-induced pain perception.
The present study further demonstrates that IL-1ra attenuates bone cancer-induced pain. The behavioral test is consistent with aforementioned reports on neuropathic and inflammatory pain. It demonstrates that IL-1β is involved in the spinal transmission and processing of noxious inputs from the peripheral bone cancer area and that it facilitates bone cancer-induced pain. Additionally, IL-1ra significantly inhibited the bone cancer-induced NR1 phosphorylation, indicating that up-regulated IL-1β enhances NR1 phosphorylation, which is consistent with previous reports that IL-1β enhances the NMDAR-mediated increase of inward current and intracellular Ca2+ (Viviani et al., 2003, Yang et al., 2005). Since NR1 phosphorylation plays a critical role in the transmission of noxious inputs in the spinal cord, IL-1β enhancement of NR1 phosphorylation may contribute to bone cancer-induced pain, and the ability of IL-1ra to attenuate pain may be linked to IL-1ra inhibition of NR1 phosphorylation. However, we do not exclude the possibility that IL-1β may facilitate pain through other signal transduction pathways. For instance, i.t. IL-1β also increases phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and results in hyperalgesia (Sung et al., 2005). IL-1β may facilitate pain through a variety of signal transduction pathways.
Double immunofluorescence labeling shows that IL-1RI and NR1 are co-localized in spinal neurons. This co-existence suggests that IL-1β may modulate NMDA receptors to influence pain transmission. A prior study demonstrated that chelerythrine chloride, a protein kinase C (PKC) inhibitor, blocked the capsaicin-enhanced NR1 phosphorylation of NR1 on serine 896 in the spinal cord, suggesting that NR1 phosphorylation is catalyzed by PKC (Zou et al., 2004). A recent in-vitro study demonstrated that IL-1β-induced NR1 phosphorylation was blocked by the PKC inhibitor, chelerythrine (Guo et al., 2007). It further demonstrated that phospholipase C (PLC) inhibitor, phospholipase A2 (PLA2) inhibitor and a membrane-permeable IP3 (inositol 1,4,5-trisphosphate) receptor antagonist blocked the IL-1β-induced NR1 phosphorylation (Guo et al., 2007). Since PLC and PLA2 are involved in PKC activation, this study suggests that the PLA2 and PLC downstream effectors, including IP3-induced-intracellular Ca2+ release, may activate PKC that in turn catalyze NR1 phosphorylation (Guo et al., 2007). Since activation of NMDA receptors located in presynaptic fibers also contributed to pain (Parada et al., 2003), the possibility that IL-1β directly or indirectly influences NR1 phosphorylation in presynaptic fibers to facilitate pain warrants further study.
In conclusion, the present study demonstrates that IL-1ra attenuates bone cancer pain and inhibits NR1 phosphorylation, suggesting that spinal IL-1β enhances NR1 phosphorylation to facilitate bone cancer pain.
Acknowledgments
We would like to thank Dr. Lyn Lowry for her editorial support. This work was funded by NIH grants R21 CA102383 and P01 AT002605.
List of abbreviations
IL-1β
interleukin-1β
NMDAR
NMDA receptor
IL-1RI
IL-1 receptor type I
IL-1ra
IL-1 receptor antagonist
PWPT
paw withdrawal pressure threshold
PB
phosphate buffer
PBS
PB saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banning A, Sjogren P, Henriksen H. Treatment outcome in a multidisciplinary cancer pain clinic. Pain. 1991;47:129–134. doi: 10.1016/0304-3959(91)90195-4. [DOI] [PubMed] [Google Scholar]
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathology. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- Foley KM. Advances in cancer pain. Archives of Neurology. 1999;56:413–417. doi: 10.1001/archneur.56.4.413. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. Journal of Neuroscience. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IO, Yukhananov R, Standaert DG, Crosby G. NMDA-R1 antisense oligodeoxynucleotides modify formalin-induced nociception and spinal c-Fos expression in rat spinal cord. Pharmacology Biochemistry and Behavior. 2004;79:183–188. doi: 10.1016/j.pbb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. Journal of Neuroscience. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. Journal Of Clinical Oncology. 1991;9:509–524. doi: 10.1200/JCO.1991.9.3.509. [DOI] [PubMed] [Google Scholar]
- Parada CA, Vivancos GG, Tambeli CH, Cunha FQ, Ferreira SH. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci U S A. 2003;100:2923–2928. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. Journal of Pharmacology & Experimental Therapeutics. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. European Journal of Neuroscience. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Rana A, Chisholm GD, Khan M, Sekharjit SS, Merrick MV, Elton RA. Patterns of bone metastasis and their prognostic significance in patients with carcinoma of the prostate. British Journal of Urology. 1993;72:933–936. doi: 10.1111/j.1464-410x.1993.tb16301.x. [DOI] [PubMed] [Google Scholar]
- Reale C, Turkiewicz AM, Reale CA. Antalgic treatment of pain associated with bone metastases. Critical Reviews in Oncology Hematology. 2001;37:1–11. doi: 10.1016/s1040-8428(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. European Journal of Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. Journal of Neurochemistry. 2005;94:742–752. doi: 10.1111/j.1471-4159.2005.03226.x. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Research. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neuroscience Letters. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. Journal of Neuroscience. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Advances in Experimental Medicine & Biology. 2003;521:1–21. [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. Journal of Comparative Neurology. 2001;439:127–139. [PubMed] [Google Scholar]
- Yang S, Liu ZW, Wen L, Qiao HF, Z WX, Zhang YX. Interleukin-1β enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Research. 2005;1034:172–179. doi: 10.1016/j.brainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Research. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang R, Li A, Liu B, Wang L, Ren K, Qiao J, Berman B, Lao L. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1β expression in a rat model. Anesthesia & Analgesia. 2007;105:1482–1488. doi: 10.1213/01.ane.0000284705.34629.c5. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced Phosphorylation of NMDA Receptor 1 Subunits in Spinal Cord Dorsal Horn and Spinothalamic Tract Neurons after Intradermal Injection of Capsaicin in Rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Research. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]