Identification of semaphorin E as a non-MDR drug resistance gene of human cancers (original) (raw)
Abstract
To improve cancer chemotherapy, a better understanding of the molecular mechanisms of drug resistance is essential. To identify the molecules responsible for drug resistance that is unrelated to MDR1 or MRP gene products, a eukaryotic expression cDNA library of _cis_-diamminedichloroplatinum(II) (CDDP)-resistant ovarian cancer TYKnuR cells was introduced into Cos-7 cells. After repeated CDDP selection, cDNA homologous to murine semaphorin E was isolated from surviving cells. Human semaphorin E (H-sema E) was overexpressed in CDDP-resistant cell lines and was readily induced not only by diverse chemotherapeutic drugs but also by x-ray and UV irradiation. Transfection of H-sema E conferred a drug-resistant phenotype to CDDP-sensitive cells. In addition, the aberrant expression of H-sema E protein was detected immunohistochemically in 14 of 42 (33.3%) recurrent squamous cell carcinomas removed at autopsy after extensive radiochemotherapy. Recently, another member of the semaphorin family, CD100, was shown to significantly improve the viability of B lymphocytes. These results suggest the involvement of semaphorins in diverse cell survival mechanisms.
In spite of a favorable response to the initial chemotherapy, the emergence of drug resistance in recurrent cancers often leads to chemotherapeutic failure. To improve cancer chemotherapy, a better understanding of the molecular mechanisms of drug resistance is essential. _cis_-diamminedichloroplatinum(II) (cisplatin; CDDP) is one of the most active chemotherapeutic agents used in the treatment of a variety of human solid tumors (1–3). Unlike cells showing typical multidrug resistance (MDR) that is induced by vinca alkaloids or anthoracyclines, CDDP-resistant cell lines selected in vitro do not overexpress MDR1 or MRP gene products (4–6). CDDP-resistant cells manifest crossresistance not only to chemically diverse drugs but also to x-ray irradiation, mimicking drug-refractory tumors in the clinic (7, 8). Several mechanisms for CDDP resistance have been postulated (4–6), including reduced drug accumulation by altered transmembrane efflux and uptake (9, 10), metabolic inactivation of drugs by glutathione and metallothionein (11–14), accelerated DNA repair (15–17), and inability to activate cell death/apoptosis pathways (18–20); however, the molecular mechanisms causing non-MDR drug resistance are largely unknown.
In this study, we undertook functional cDNA cloning to identify the molecules responsible for non-MDR drug resistance. The entire cDNA library of CDDP-resistant ovarian cancer cells was introduced into Cos-7 cells and CDDP resistance was selected for by adding a lethal concentration of CDDP. From the survivor transfectants, we isolated cDNA encoding the human homologue of mouse semaphorin E (21). We report here that semaphorin E is involved in non-MDR drug resistance of human cancers in vitro and in vivo.
MATERIALS AND METHODS
Cell Lines and Chemicals.
A CDDP-resistant human ovarian cancer cell line, TYKnuR, and its parental line, TYKnu (22), were obtained through the Japan Cancer Research Resources Bank (Tokyo). Two CDDP-resistant cell lines, Lu65/CDDP and MS-1/CDDP, were established from lung cancer cell line Lu65 and malignant mesothelioma cell line MS-1 (23) by increasing the concentration of CDDP stepwise to 1.0 μg/ml over a period of more than 1 year. Cells were cultured in drug-free media for at least 1 month before use. The IC50 values of TYKnuR, TYKnu, Lu65/CDDP, Lu65, MS-1/CDDP, and MS-1 cells, determined as described below, were 2.2, 0.12, 6.8, 0.91, 6.8, and 1.6 μg/ml CDDP, respectively. Cos-7 was obtained from the Riken Cell Bank (Tsukuba, Japan).
CDDP, _cis_-diammine (1,1-cyclobutane) dicarboxylatoplatinum(II) (CBDCA), etoposide (VP-16) (Bristol-Myers Squibb, Tokyo), mitomycin C (MMC), and doxorubicin (ADM) (Kyowa Hakko Kogyo, Tokyo) were prepared for clinical use. Cells were irradiated with a 60Co source or exposed to UV (254 nm) quantitatively by using a UV crosslinker (Stratalinker, Stratagene).
cDNA Library Construction and Screening.
A eukaryotic expression vector (pcDNA I, Invitrogen) cDNA library of TYKnuR cells, representing 1.8 × 106 clones, was introduced into Cos-7 cells by electroporation (23), and CDDP-resistant clones were selected by culturing the cells for 3 days with 2 μg/ml CDDP (IC95 for Cos-7 cells). The pool of cDNA clones was analyzed by PCR using a pair of primers flanking the polylinker site of pcDNA I: 5′-CCACTGCTTACTGGCTTATCG-3′ and 5′-CACACCACAGAAGTAAGGTTCC-3′.
To isolate the full-length human semaphorin E (H-sema E) cDNA, a ZAP express (Stratagene) cDNA library of MS-1/CDDP cells was screened by hybridizing with a radio-labeled probe (24). The cDNA insert of the clone was sequenced by the dideoxy chain termination method by using a Sequenase 7-deaza-dGTP kit (Amersham, UK) manually and by using a Model 373A autosequencer (Perkin–Elmer).
Northern Blot Analysis.
Total RNA (30 μg/lane) or mRNA (2 μg/lane) was fractionated by electrophoresis and transferred to Hybond N (Amersham). Human multiple tissue Northern blots I and II and Zoo-Blot were purchased from CLONTECH. Hybridization was performed by using a radio-labeled H-sema E cDNA fragment (nucleotides 1–2,965) (24). The quality and quantity of electrophoresed RNA was determined by rehybridization of the same blot with a β-actin cDNA (CLONTECH). Developed x-ray films were scanned with a model GS-700 imaging densitometer and quantified by using Molecular Analyst 2.1 software (Bio-Rad), as instructed by the supplier.
Transfection of H-Sema E.
H-sema E cDNA containing the entire coding region (nucleotides 44–5,186) was subcloned into the _Not_I site of the eukaryotic expression vector pcDNA3 (Invitrogen). Plasmid DNA was introduced into cells by Lipofectamine (GIBCO/BRL). Stable transfectants were selected by exposure to G418 (GIBCO/BRL) at 400 μg/ml and were cloned by limiting dilution. G418 was removed at least 7 days before drug sensitivity tests.
Drug Sensitivity Assay.
Drug sensitivity was determined by 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, as described previously (25). The fraction of the cells that survived was determined by dividing the absorbance of treated wells by that of untreated control wells. IC50 values were used as the measure of relative drug sensitivity.
In Vitro Translation Analysis.
H-sema E cDNA was transcribed and translated in vitro by using the TNT reticulocyte lysate system (Promega) as instructed by the supplier.
Immunoprecipitation Analysis, Immunofluorescence Microscopy, and Immunohistochemistry.
Rabbit polyclonal anti-H-sema E antibody was raised against a synthetic peptide, CYRKTKTGGKRLS, and affinity-purified.
Cells were labeled metabolically with 50 μCi/ml l-[35S]methionine (Amersham) for 16 hr at 37°C. Cell-free conditioned medium was isolated by 0.22-μm filtration. Immunoprecipitation analysis and immunofluorescence microscopy was performed as previously described (26, 27).
Dewaxed formalin-fixed and paraffin-embedded human tissue sections were treated with Target retrieval solution (Dako) as instructed by the supplier. Immunoperoxidase staining procedures using the avidin-biotin-complex method were performed as described previously (28).
RESULTS
Molecular Cloning of H-Sema E.
We undertook functional cDNA cloning to identify the molecules responsible for non-MDR resistance. The entire cDNA library of CDDP-resistant ovarian cancer TYKnuR cells was introduced into Cos-7 cells, and CDDP-resistant cells were selected by exposure to the minimal lethal dose of CDDP. Surviving cells remained attached to the plastic culture dishes and were separated easily from detached dead cells. Episomal plasmid DNA was recovered from adherent surviving cells (29) and reintroduced into Cos-7 cells for another round of selection. After five rounds of selection for CDDP-resistance, two cDNA clones were prominent among the surviving Cos-7 transfectants (Fig. 1a, lane 5). A 1.1-kb cDNA fragment hybridized with a gene that was overexpressed in the CDDP-resistant TYKnuR cells, compared with the parent TYKnu cells, as described below and was selected for further analyses.
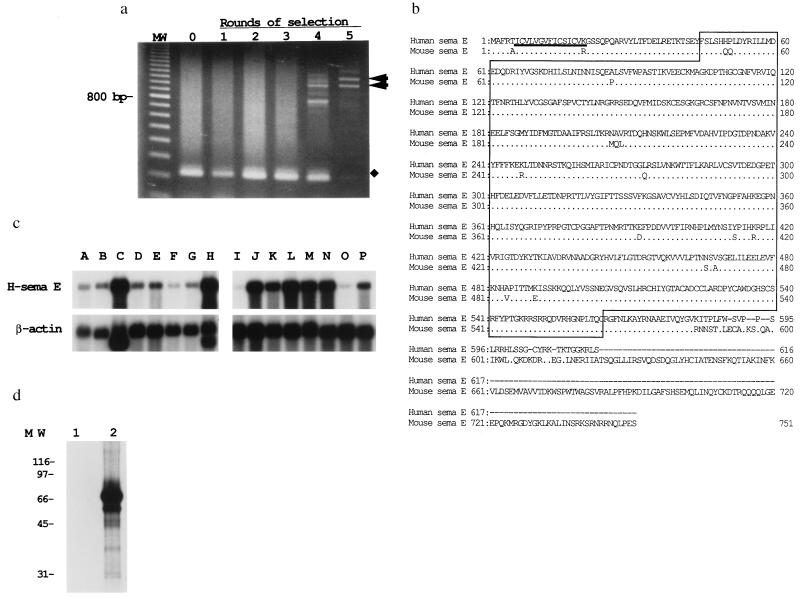
Figure 1.
Molecular cloning of H-sema E. (a) A cDNA library of CDDP-resistant TYKnuR cells was introduced into Cos-7 cells, which then were exposed to CDDP. Pooled cDNA inserts were amplified by PCR after each round of CDDP selection. After the fifth round of CDDP selection, two cDNA clones with inserts of 1.1 and 1.0 kb were prominent (arrowheads). Amplified fragments of approximately 200 bp were derived from plasmids without inserts (lanes 0–4, indicated by ⧫). MW, 100-bp ladder (Pharmacia Biotech). (b) Predicted amino acid sequence of H-sema E in alignment with mouse semaphorin E (21). The conserved semaphorin domain is given in a line box. The putative signal sequence is underlined. Identical amino acids are eliminated from the mouse semaphorin E sequence. Bars correspond to gaps introduced into the sequences to optimize alignment. (c) Expression of H-sema E mRNA in normal adult human tissues. Human multiple tissue Northern blots I and II (CLONTECH) were hybridized with H-sema E cDNA: lane A, pancreas; lane B, kidney; lane C, skeletal muscle; lane D, liver; lane E, lung; lane F, placenta; lane G, brain; lane H, heart; lane I, peripheral blood leukocytes; lane J, colon; lane K, small intestine; lane L, ovary; lane M, testis; lane N, prostate; lane O, thymus; lane P, spleen. (d) In vitro translation analysis of H-sema E. H-sema E cDNA (lane 2) and control plasmid DNA (lane 1) was transcribed and translated in vitro and analyzed by SDS/PAGE and autoradiography (23).
A 5,186-bp cDNA clone, almost full length as judged from the mRNA size in Northern blot analyses, was isolated and found to encode 616 amino acids. A database search revealed that this gene was homologous to mouse semaphorin E (21) (accession no. X85994; 87% nucleotide homology, 93% amino acid homology; Fig. 1b). The N-terminal signal sequence of 15 amino acids is followed by a conserved semaphorin domain (21, 30). Semaphorin E is likely to be secreted, as no potential transmembrane domain was revealed by hydrophobicity analyses.
Northern blot analyses revealed that 5.2-kb H-sema E transcripts were expressed intensely in the heart, skeletal muscle, colon, small intestine, ovary, testis, and prostate. Faint expression was observed ubiquitously among other organs, including the brain (Fig. 1c). Southern hybridization of Zoo-Blot with a H-sema E cDNA probe revealed sequence conservation among eukaryotic species, including monkey, rat, mouse, dog, cow, and rabbit (data not shown).
The calculated molecular mass of the H-sema E peptide was 69.6 kDa, and in vitro translation of H-sema E cDNA yielded a major product of approximately 70 kDa (Fig. 1d).
Overexpression of H-Sema E in CDDP-Resistant Cancer Cell Lines.
The CDDP-resistant cell lines TYKnuR, Lu65/CDDP, and MS-1/CDDP expressed 6.0, 4.0, and 3.2 times more H-sema E mRNA than the CDDP-sensitive parental cells, respectively, when the blot intensity was normalized to their β-actin expression (Fig. 2a). A protein of approximately 70 kDa, which was complete as judged from the above in vitro translation analyses, and a 65-kDa processed or degraded protein were secreted into the media by Cos-7 cells transiently transfected with H-sema E cDNA (Fig. 2b, lane 1) and by CDDP-resistant TYKnuR cells (lane 7). H-sema E protein was not detected in the conditioned media of a control transfectant (Fig. 2b, lane 3) or CDDP-sensitive TYKnu cells (lane 5). Immunofluorescence microscopy detected H-sema E protein in CDDP-resistant TYKnuR cells (Fig. 2c) but not in CDDP-sensitive TYKnu cells.
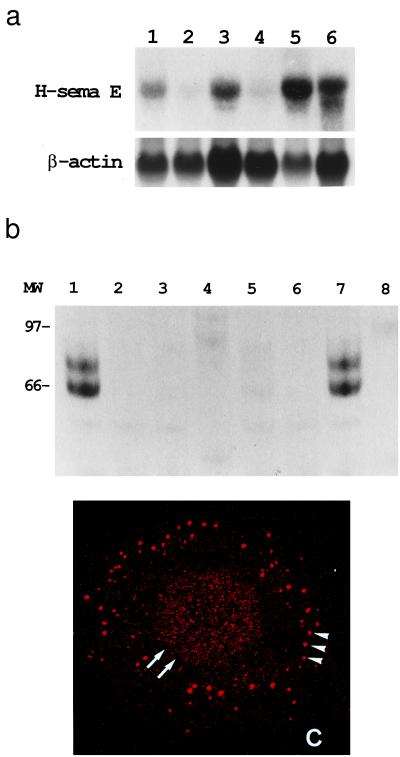
Figure 2.
Overexpression of H-sema E in CDDP-resistant cancer cell lines. (a) Northern blot analysis of H-sema E expression. H-sema E expression was stronger in CDDP-resistant cells (Upper), TYKnuR (lane 1), Lu65/CDDP (lane 3), and MS-1/CDDP (lane 5), than in the CDDP-sensitive parent cells, TYKnu (lane 2), Lu65 (lane 4), and MS-1 (lane 6). The quality and quantity of electrophoresed mRNA was determined by rehybridization of the same blot with β-actin cDNA (Lower). (b) Immunoprecipitation analysis of secreted H-sema E protein. Radio-labeled cell-free conditioned media from Cos-7 cells transfected with H-sema E cDNA (lanes 1 and 2), Cos-7 cells transfected with vector alone (lanes 3 and 4), CDDP-sensitive TYKnu cells (lanes 5 and 6), and CDDP-resistant TYKnuR cells (lanes 7 and 8) were immunoprecipitated with anti-H-sema E antibody (lanes 1, 3, 5, and 7) or normal rabbit IgG (lanes 2, 4, 6, and 8). (c) Laser scanning confocal immunofluorescence microscopy showing the subcellular localization of H-sema E protein in a CDDP-resistant TYKnuR cell. Dot-like staining of H-sema E in the periphery of the cytoplasm (arrowheads) and diffuse fine granular staining in the entire nucleus (arrows) are evident.
Induction of H-Sema E in CDDP-Sensitive Cells.
H-sema E expression was readily induced by exposure to CDDP in a dose- and time-dependent manner in CDDP-sensitive Lu65 and TYKnu cells (Fig. 3 a_–_c). Constitutive H-sema E expression in CDDP-resistant cell lines was not affected by similar treatment (Fig. 3a, lanes 1 and 2). In addition to CDDP, other chemotherapeutic drugs, including CBDCA, ADM, MMC and VP-16, (Fig. 3 c and d), as well as UV (Fig. 3e) and x-ray (Fig. 3f) irradiation, also were able to induce the expression of H-sema E.
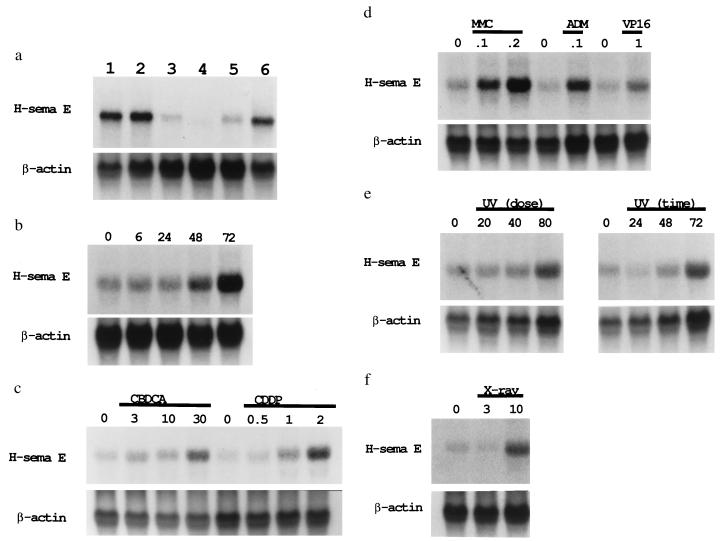
Figure 3.
Induction of H-sema E mRNA in CDDP-sensitive cells. (a) Induction of H-sema E in CDDP-sensitive TYKnu cells by CDDP treatment. TYKnu cells were treated for 3 days with 0.0 (untreated control, lane 3), 0.1 (lane 4), 0.3 (lane 5), or 1.0 (lane 6) μg/ml CDDP. The constitutive expression of H-sema E by CDDP-resistant TYKnuR cells (lane 1) was not affected by the 3-day treatment with 1.0 μg/ml CDDP (lane 2). The relative intensity of the blots compared with lane 3 was 5.4 (lane 1), 6.3 (lane 2), 0.2 (lane 4), 1.5 (lane 5), and 4.3 (lane 6). (b) Time-dependent induction of H-sema E in CDDP-sensitive Lu65 cells. Lu65 cells were untreated or treated with 2.0 μg/ml CDDP for 6 to 72 hr (hereafter, lanes marked “0” represent untreated controls). The relative intensity of the blots compared with the untreated control was 1.1 (6 hr), 1.0 (24 hr), 2.0 (48 hr), and 3.7 (72 hr). (c) Dose-dependent induction of H-sema E in CDDP-sensitive Lu65 cells by the platinum-containing compounds, CBDCA and CDDP. H-sema E was induced in a dose-dependent manner by 3-day treatment with 3–30 μg/ml CBDCA or 0.5–2.0 μg/ml CDDP. The relative intensity of the blots compared with the untreated controls was 1.8 (3 μg/ml CBDCA), 1.8 (10 μg/ml CBDCA), 3.4 (30 μg/ml CBDCA), 1.2 (0.5 μg/ml CDDP), 2.9 (1.0 μg/ml CDDP), and 5.7 (2.0 μg/ml CDDP). (d) Induction of H-sema E in CDDP-sensitive Lu65 cells by non-platinum-containing anti-cancer compounds. H-sema E was induced by 3-day treatment with 0.1 and 0.2 μg/ml MMC, 0.1 μg/ml ADM, and 1.0 μg/ml VP-16. The relative intensity of the blots compared with the untreated controls was 2.2 (0.1 μg/ml MMC), 2.7 (0.2 μg/ml MMC), 2.9 (0.1 μg/ml ADM), and 3.1 (1.0 μg/ml VP-16). (e) Time- and dose-dependent induction of H-sema E in CDDP-sensitive Lu65 cells by UV irradiation. Total RNA was extracted from Lu65 cells 3 days after UV irradiation at 20, 40, or 80 J/m2 (Left), or 24, 48, or 72 hr after UV irradiation at 80 J/m2 (Right). The relative intensity of the blots compared with the untreated controls was 1.1 (20 J/m2), 1.5 (40 J/m2), 2.5 (80 J/m2), 0.6 (24 hr), 2.0 (48 hr), and 3.2 (72 hr). (f) Induction of H-sema E in CDDP-sensitive Lu65 cells by x-ray irradiation. Total RNA was extracted from Lu65 cells 3 days after irradiation at a dose of 3 or 10 Gy. The relative intensity of the blots compared with the untreated control was 0.7 (3 Gy) and 3.9 (10 Gy).
Transfection of H-Sema E cDNA into CDDP-Sensitive Cells.
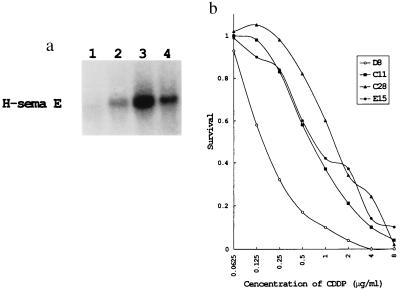
CDDP-sensitive TYKnu cells were transfected with H-sema E cDNA to determine the active involvement of H-sema E in a drug-resistant phenotype. Three clones with different expression levels of H-sema E (designated C11, C28, and E15) and one clone transfected with the vector alone (designated D8) were selected. Three H-sema E transfectants (C11, C28, and E15) expressed 3.3, 7.9, and 5.8 times more H-sema E mRNA than the mock transfectant, respectively (Fig. 4a). H-sema E tranfectants showed greater resistance to CDDP than the mock transfectant, in parallel to their expression level of H-sema E (Fig. 4b). Clone C28, which had the highest expression level of H-sema E, was 9.8 times more resistant to CDDP (calculated from the IC50 values) than the mock transfectant. The H-sema E transfectant C28 was also 7.2, 3.1, 10.0, and 3.4 times more resistant to CBDCA, VP-16, MMC, and ADM, respectively, than the mock transfectant.
Figure 4.
Stable transfection of H-sema E cDNA into CDDP-sensitive TYKnu cells. (a) Northern blotting of H-sema E in transfectants. Three stable G418-resistant clones with different expression levels of H-sema E (lane 2, C11; lane 3, C28; and lane 4, E15) and one clone transfected with vector alone (mock transfectant, lane 1, D8) were selected by Northern blotting. (b) Sensitivity of H-sema E transfectants (C11, C28, and E15) and a mock transfectant (D8) to CDDP.
Immunohistochemical Detection of H-Sema E Protein in Human Tissues.
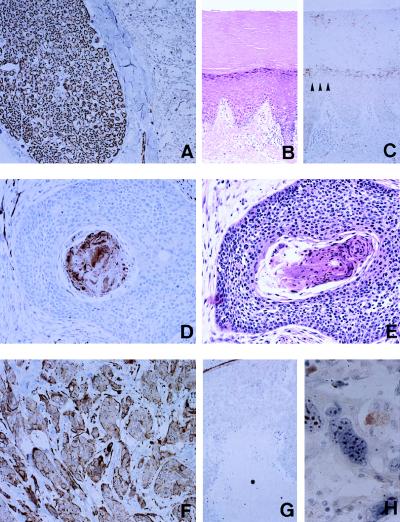
H-sema E protein was detected immunohistochemically in Schwann cells of a peripheral nerve (Fig. 5A), smooth muscle cells, myoepithelial cells, and vascular endothelial cells. To assess the involvement of H-sema E in drug-resistance mechanisms of human cancers in vivo, we examined the expression of H-sema E by 42 untreated squamous cell carcinomas (including 12 laryngeal, eight hypopharyngeal, seven esophageal, seven lung, five oral, and three uterine cervical cancers) that were removed surgically and by recurrent carcinomas removed at autopsy from the same patients after extensive radiotherapy and chemotherapy. We chose squamous cell carcinomas because untreated squamous cell carcinomas are generally radiosensitive and respond well to treatment (31). In non-neoplastic squamous epithelium, faint expression of H-sema E was observed in the granular layer (Fig. 5C, arrowheads). In most of the untreated squamous cell carcinomas, H-sema E expression was limited to parakeratotic cells in cancer pearls (Fig. 5D). Parakeratosis is a well-documented pathological condition characterized by the retention of the nucleus in spite of the terminally differentiated cytoplasm (32) (Fig. 5E). In two of 42 (4.7%) untreated tumors, and in 14 of 42 (33.3%) recurrent tumors, almost all the cells aberrantly overexpressed H-sema E irrespective of their histological phenotype (Fig. 5F). The expression of H-sema E in parakeratotic cells may indicate the failure of cell death, because denucleated necrotic cancer cells (Fig. 5G) and normally keratinized squamous epithelial cells (Fig. 5 B and C) did not express it. Bizarre multinucleated giant cells often were observed in drug-refractory cancers, probably reflecting the failure of proper cell division. Those cells demonstrated characteristic nucleolar staining (Fig. 5H).
Figure 5.
Immunohistochemical detection of H-sema E protein in human tissues. (A) Immunoperoxidase staining of H-sema E in Schwann cells of a peripheral nerve (Left) and the spinal cord (Right). (B) Hematoxylin and eosin staining of skin from the foot showing keratinization. (C) Immunoperoxidase staining of H-sema E in a section adjacent to the section shown in B. (D) Immunoperoxidase staining of H-sema E in a nest of an untreated pharyngeal cancer. Only parakeratotic cancer cells in the center of the nest are stained. (E) Hematoxylin and eosin staining of a section adjacent to the section shown in D. (F) Immunoperoxidase staining of H-sema E in a recurrent tumor taken from the same patient as the tissue in D. (G) Immunoperoxidase staining of H-sema E in untreated lung cancer with coagulation necrosis (∗). (H) Immunoperoxidase staining of H-sema E in bizarre giant cells in a recurrent lung cancer after treatment.
DISCUSSION
Semaphorins/collapsins make up a gene family characterized by the possession of a conserved semaphorin domain in the amino terminus (21, 30, 33). One of the best-characterized members of this family is chicken collapsin (33), a secreted protein that collapses neuronal growth cones. By repelling growth cone extension, collapsin contributes to axonal pathfinding during neural development. Püschel et al. (21) isolated five mouse semaphorins, A to E, by degenerative PCR techniques. Transfection with mouse semaphorin D, a homologue of chicken collapsin-1, inhibits neurite extension from ganglion cells, as does chicken collapsin; however, transfection with mouse semaphorin E in a similar experiment did not produce such activity (21), raising the possibility that mouse semaphorin E has other undetermined functions.
The function of semaphorins in non-neural systems is largely unknown. CD100 from T lymphocytes was identified as a cell surface transmembrane semaphorin (34). CD100 is thought to be essential for B cell development in the germinal center of lymph nodes. Co-culture with CD100-transfected T cells and a tumor necrosis factor family member, CD40L, significantly and synergistically enhanced the viability of B cells in vitro (34). These results suggest that semaphorins provide cell survival signals in various non-neural systems.
In this study, we demonstrated that H-sema E was actually secreted. Semaphorins/collapsins appear in two forms: transmembrane or secreted proteins (21, 30, 33, 34). Secreted semaphorin D induces axon growth cone collapse through mediation of intracellular protein CRMP-62 (35). CRMP-62 may be required for coupling a transmembrane semaphorin-binding receptor to a GTP-binding protein signaling cascade. Recently, neurophilin, a type I transmembrane protein, was identified as a semaphorin D receptor (36, 37). Secreted semaphorin E may act as an autocrine factor through binding to undetermined cell surface receptor(s). It is essential to identify the cell surface receptor and intracellular signaling system to elucidate the semaphorin E-mediated drug resistance mechanism.
Bcl-2 was originally identified at the chromosomal breakpoint in t(14;18)-bearing follicular lymphoma cells (38). Bcl-2 was reported to promote the regeneration of retinal axons in addition to its cell death-suppressing activity (39). There may be a link between the regulation of neurite growth and cell survival mechanisms. Mangasser-Stephan et al. (40) reported that a 493-bp cDNA fragment, identical to part of H-sema E, hybridized with a gene that was overexpressed in synovial cells from patients with rheumatoid arthritis. The cell death-resistant activity of semaphorin E may be involved in the irreversible destructive growth of synovial cells in this disease (41).
Finally, the genes encoding at least two other human semaphorins, A(V) and (IV), are clustered in the chromosome region 3p21.3 (42–44), where homozygous deletions frequently are observed in small-cell lung cancer, and where unidentified tumor suppressor gene(s) are thought to be located (45–47). Sekido et al. (42) reported mutations and down-regulation of the semaphorin A(V) gene in human lung cancers. Considering the possible role of semaphorins in cell survival mechanisms, it is feasible that abnormalities of genes in this family participate in the carcinogenesis and progression of certain human tumors.
Acknowledgments
This research was supported in part by a Grant-in-Aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CDDP, _cis_-diamminedichloroplatinum(II); H-sema E, human semaphorin E; MDR, multidrug resistance; CBDCA, _cis_-diammine (1,1-cyclobutane) dicarboxylatoplatinum(II); MMC, mitomycin C; ADM, doxorubicin; VP-16, etoposide.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB000220).
References
- 1.Lippard S J. Pure Appl Chem. 1987;59:731–742. [Google Scholar]
- 2.Loeherer P J, Einhorn L H. Ann Intern Med. 1984;100:704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg B. Cancer (Phila) 1985;55:2303–2316. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Andrew P A, Howell S B. Cancer Cells. 1990;2:35–43. [PubMed] [Google Scholar]
- 5.Doyle L A. Semin Oncol. 1993;20:326–337. [PubMed] [Google Scholar]
- 6.Johnson S W, Ozols R F, Hamilton T C. Cancer (Phila) 1993;71:644–649. doi: 10.1002/cncr.2820710224. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz J L, Rotmensch J, Beckett M A, Jaffe D R, Toohill M, Giovanazzi S M, McIntosh J, Weichselbaum R R. Cancer Res. 1988;48:5133–5135. [PubMed] [Google Scholar]
- 8.Chao C C, Huang S L, Huang H M, Lin-Chao S. Mol Cell Biol. 1991;11:2075–2080. doi: 10.1128/mcb.11.4.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waud W R. Cancer Res. 1987;47:6549–6555. [PubMed] [Google Scholar]
- 10.Ishikawa T, Ali-Osman F. J Biol Chem. 1993;25:20116–20125. [PubMed] [Google Scholar]
- 11.Godwin A K, Meister A, O’Dwyer P J, Huang C S, Hamilton T C, Anderson M E. Proc Natl Acad Sci USA. 1992;89:3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saburi Y, Nakagawa M, Ono M, Sasaki M, Muramatsu M, Kohno K, Kuwano M. Cancer Res. 1989;49:7020–7025. [PubMed] [Google Scholar]
- 13.Timmer-Bosscha H, Timmer A, Meijer C, de Vries G E, de Jong B, Oosterhuis J W, Mulder N H. Cancer Res. 1993;53:5707–5713. [PubMed] [Google Scholar]
- 14.Kelley S L, Basu A, Teicher B A, Hacker M P, Hamer D H, Lazo J S. Science. 1988;241:1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- 15.Masuda H, Tanaka T, Matsuda H, Kusaba I. Cancer Res. 1988;48:5713–5716. [Google Scholar]
- 16.Parker R J, Eastman A, Bostick-Bruton F, Reed E. J Clin Invest. 1991;87:772–777. doi: 10.1172/JCI115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen W, Link C J J, O’Connor P M, Reed E, Parker R, Howell S B, Bohr V A. Mol Cell Biol. 1992;12:3689–3698. doi: 10.1128/mcb.12.9.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal-Bendirdjian E, Jacquemin-Sablon A. Exp Cell Res. 1995;218:201–212. doi: 10.1006/excr.1995.1148. [DOI] [PubMed] [Google Scholar]
- 19.Perego P, Giarola M, Righetti S C, Supino R, Caserini C, Delia D, Pierotti M A, Miyashita T, Reed J C, Zunino F. Cancer Res. 1996;56:556–562. [PubMed] [Google Scholar]
- 20.Brown R, Clugston C, Burns P, Edlin A, Vasey P, Vojtesek B, Kaye S B. Int J Cancer. 1993;55:678–684. doi: 10.1002/ijc.2910550428. [DOI] [PubMed] [Google Scholar]
- 21.Püschel A W, Adams R H, Betz H. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 22.Yoshiya N, Adachi S, Misawa Y, Yuzawa H, Honda T, Nakazawa K, Takeuchi S, Tanaka K. Acta Obst Gynaec Jpn. 1989;41:7–14. [PubMed] [Google Scholar]
- 23.Yamada T, Jiping J, Endo R, Gotoh M, Shimosato Y, Hirohashi S. Br J Cancer. 1995;71:562–570. doi: 10.1038/bjc.1995.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Vol. 2. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 9.47–9.55. [Google Scholar]
- 25.Carmichael J, Mitchell J B, Degraff W G, Gamson J, Gazdar A F, Johnson B E, Glatstein E, Minna J D. Br J Cancer. 1985;57:540–547. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada T, Endo R, Tsukagoshi K, Fujita S, Honda K, Kinoshita M, Hasebe T, Hirohashi S. Lab Invest. 1996;75:539–600. [PubMed] [Google Scholar]
- 27.Sakamoto M, Ino Y, Ochiai A, Kanai Y, Akimoto S, Hirohashi S. Lab Inv. 1996;74:199–208. [PubMed] [Google Scholar]
- 28.Hsu S-M, Raine L, Fanger H. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 29.Hirt B. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 30.Kolodkin A L, Matthes D J, Goodman C S. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 31.Yu R, Chu A C. Treatment of Cancer. 3rd Ed. Vol. 1. London: Chapmann & Hall; 1995. pp. 742–744. [Google Scholar]
- 32.Rosai J. Ackerman’s Surgical Pathology. 6th ed. Vol. 1. St. Louis: Mosby; 1981. pp. 40–42. [Google Scholar]
- 33.Luo Y, Raible D, Raper J A. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 34.Hall K T, Boumsell L, Schultze J L, Boussiotis V A, Dorfman D M, Cardoso A A, Bensussan A, Nadler L M, Freeman G J. Proc Natl Acad Sci USA. 1996;93:11780–11785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goshima Y, Nakamura F, Strittmatter P, Strittmatter S. Nature (London) 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 36.He Z, Tessier-Lavigne M. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 37.Kolodkin A L, Levengood D V, Rowe E G, Tai Y-T, Giger R J, Ginty D D. Cell. 1997;90:752–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 38.Tsujimoto Y, Cossman J, Jaffe E, Croce C M. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 39.Chen D F, Schneider G E, Martinou J C, Tonegawa S. Nature (London) 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 40.Mangasser-Stephan K, Dooley S, Welter C, Mutschler W, Hanselmann R G. Biochem Biophys Res Commun. 1997;234:153–156. doi: 10.1006/bbrc.1997.6607. [DOI] [PubMed] [Google Scholar]
- 41.Fassbender H G. Coll Relat Res. 1983;3:141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- 42.Sekido Y, Bader S, Latif F, Chen J-Y, Duh F-M, Wei M-H, Albanesi J P, Lee C-C, Lerman M I, Minna J D. Proc Natl Acad Sci USA. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, Waggoner B, Fishel R, Franklin W, Gemmill R, Drabkin H. Oncogene. 1996;12:1289–1297. [PubMed] [Google Scholar]
- 44.Xiang R-H, Hensel C H, Garcia D K, Carlson H C, Kok K, Daly M C, Kerbacher K, van den Berg A, Veldhuis P, Buys C H C M, Naylor S L. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- 45.Yamakawa K, Takahashi T, Horio Y, Murata Y, Takahashi E. Oncogene. 1993;8:327–330. [PubMed] [Google Scholar]
- 46.Kok K, van den Berg A, Veldhuis P M J F, van der Veen A, Franke M, Schoenmakers E F P M, Hulsbeek M M F, van der Hout M, de Liej L, van de Ven W, Buys C H C M. Cancer Res. 1994;54:4183–4187. [PubMed] [Google Scholar]
- 47.Daly M C, Xiang R-H, Buchhagen D, Hensel C H, Garcia D K, Killary A M, Minna J D, Naylor S L. Oncogene. 1993;8:1721–1729. [PubMed] [Google Scholar]