Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice (original) (raw)
Abstract
Ascorbic acid is an essential nutrient commonly regarded as an antioxidant. In this study, we showed that ascorbate at pharmacologic concentrations was a prooxidant, generating hydrogen-peroxide-dependent cytotoxicity toward a variety of cancer cells in vitro without adversely affecting normal cells. To test this action in vivo, normal oral tight control was bypassed by parenteral ascorbate administration. Real-time microdialysis sampling in mice bearing glioblastoma xenografts showed that a single pharmacologic dose of ascorbate produced sustained ascorbate radical and hydrogen peroxide formation selectively within interstitial fluids of tumors but not in blood. Moreover, a regimen of daily pharmacologic ascorbate treatment significantly decreased growth rates of ovarian (P < 0.005), pancreatic (P < 0.05), and glioblastoma (P < 0.001) tumors established in mice. Similar pharmacologic concentrations were readily achieved in humans given ascorbate intravenously. These data suggest that ascorbate as a prodrug may have benefits in cancers with poor prognosis and limited therapeutic options.
Keywords: cancer, hydrogen peroxide, oxidation, free radical, vitamin C
Vitamin C (ascorbate) is an essential micronutrient used as a co-factor by numerous biosynthetic enzymes. An additional viewpoint is that ascorbate serves as an antioxidant and increased intake from either foods or dietary supplements might promote good health (1). Cancer chemoprevention studies have used this antioxidant rationale to examine a putative inverse association between tumor incidence and ascorbate ingestion (2, 3). In contrast to this line of investigation, we have tested the hypothesis that pharmacologic concentrations of ascorbate may engender a prooxidant cytotoxic state within tumors. In our initial in vitro experiments, we observed hydrogen peroxide (H2O2)-dependent cytotoxicity after ascorbate exposure (EC50 < 4 mM) in five cancer cell lines, whereas normal cells were resistant (4). The _in vivo_ pharmacokinetics of ascorbate treatment was subsequently determined in rats (5). These dosing and biodistribution data in rodents showed that oral ascorbate administration produced concentrations that cannot exceed 0.2 mM in plasma and extracellular fluids because of physiologic tight control, similar to mechanisms that exist in humans (6–8). Pharmacologic concentrations of ascorbate (>0.2 mM) in body fluids could be attained only when oral tight control mechanisms were bypassed by parenteral (i.v., i.p.) ascorbate administration routes. Pharmacologic ascorbate concentrations in plasma resulted in the formation of both ascorbate radical and H2O2 in extracellular fluid of the tissue parenchyma (5). On the basis of these data, the efficacy of parenteral ascorbate administration on tumor growth in vivo was examined by using the dose-toxicity relationships of ascorbate in numerous types of cancer cells in vitro.
Results
Range of Cancer Cell Sensitivity to Ascorbate-Derived Hydrogen Peroxide.
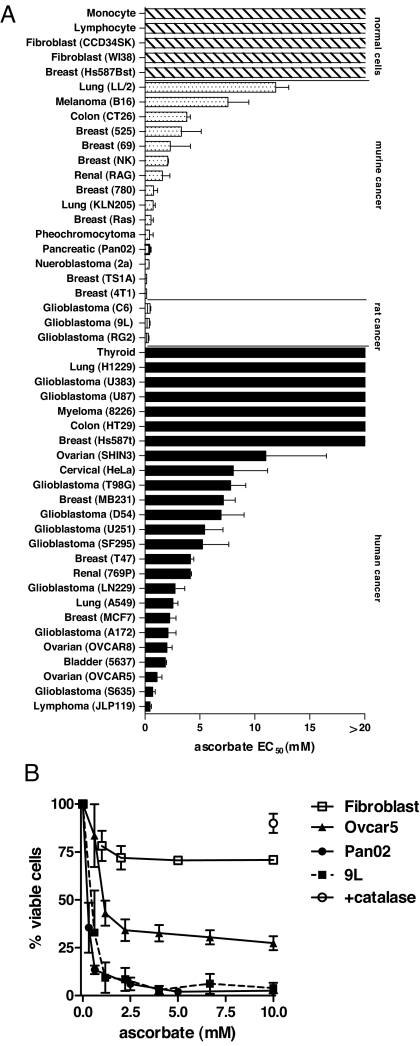
An extensive panel of 43 tumor and 5 normal cell lines were exposed to ascorbate in vitro for ≤2 h to mimic clinical pharmacokinetics, and the effective concentration that decreased survival 50% (EC50) was determined. EC50 was <10 mM for 75% of tumor cells tested, whereas cytotoxicity was not evident in normal cells with >20 mM ascorbate (Fig. 1A). The addition of catalase to the medium ameliorated death of ovarian carcinoma (Ovcar5), pancreatic carcinoma (Pan02), and glioblastoma (9L) cells exposed to 10 mM ascorbate (1 h), indicating cytotoxicity was mediated by H2O2 (Fig. 1B), which is consistent with previous work on a more limited sampling of cancer cell types (4, 9).
Fig. 1.
Relative cytotoxicity of ascorbate on cancer and normal cells. (A) Cells (1 × 104) in logarithmic growth phase were cultured in recommended growth media containing 10% FCS and exposed to serial dilutions of ascorbate (0–20 mM, pH 7) for 2 h and washed and cultured for an additional 24–48 h in growth medium in the absence of ascorbate. EC50 values indicate the concentration of ascorbate that reduced survival by 50% determined by viability assays as previously described (4, 9). EC50 values for 13 of 43 cells were previously shown (4). (B) Ovarian carcinoma-Ovcar5 (▴), pancreatic carcinoma-Pan02 (●), glioblastoma-9L (■), and normal human fibroblast-CCD34SK (□) were exposed to ascorbate (pH 7) for 1 h as described in A. Addition of catalase (600 units/ml) before ascorbate (10 mM) ameliorated cytoxicity equivalently for all cells tested (○). Data in A and B represent mean values of six determinations ± SD.
Pharmacological Ascorbate Treatment Decreases Tumor Growth.
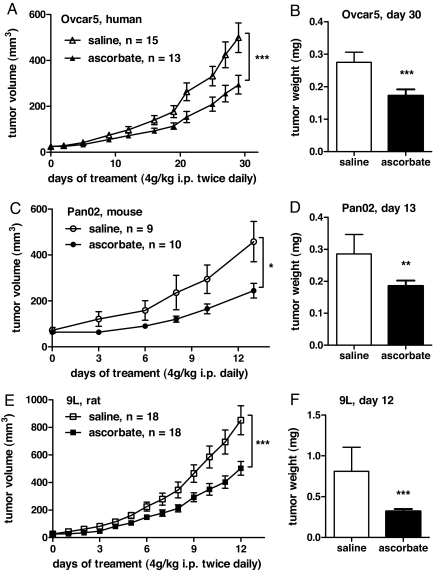
Given their relative sensitivity, the efficacy of pharmacologic ascorbate administration on the growth of Ovcar5, Pan02, and 9L tumors was examined in nude mice. The acidity of ascorbate solutions was neutralized to pH 7 with sodium hydroxide. A maximum tolerated dose for ascorbate was limited by potential stress from osmotic imbalance after injection into the peritoneal cavity. A treatment dose of 4 g ascorbate/kg body weight either once or twice daily did not produce any discernible adverse effects. Treatment commenced after tumors reached a palpable size of 5–7 mm in diameter.
Xenograft experiments showed that parenteral ascorbate as the only treatment significantly decreased both tumor growth and weight by 41–53% (P = 0.04–0.001) for Ovcar5, Pan02, and 9L tumors (Fig. 2 A–F). Metastases, present in ≈30% of 9L glioblastoma controls, were absent in ascorbate-treated animals (data not shown).
Fig. 2.
Impact of pharmacological ascorbate on tumor growth. Tumors were grown in the flanks of athymic mice to a volume of ≈50 ± 10 mm3, and treatment commenced with either ascorbate (4 g per kilogram of body weight) or osmotically equivalent saline by i.p. injection as indicated. Data (± SEM) show growth curves and final tumor weight with either saline (□) or ascorbate (■) treatments in mice bearing Ovcar5 (A and B), Pan02 (C and D) and 9L (E and F) tumors. P values were calculated by unpaired _t_-test: *, P < 0.01; **, P < 0.005; ***, P < 0.001.
In Situ Analysis Shows Prooxidant Metabolism of Ascorbate at Pharmacological Concentrations Is Achievable in Human Subjects.
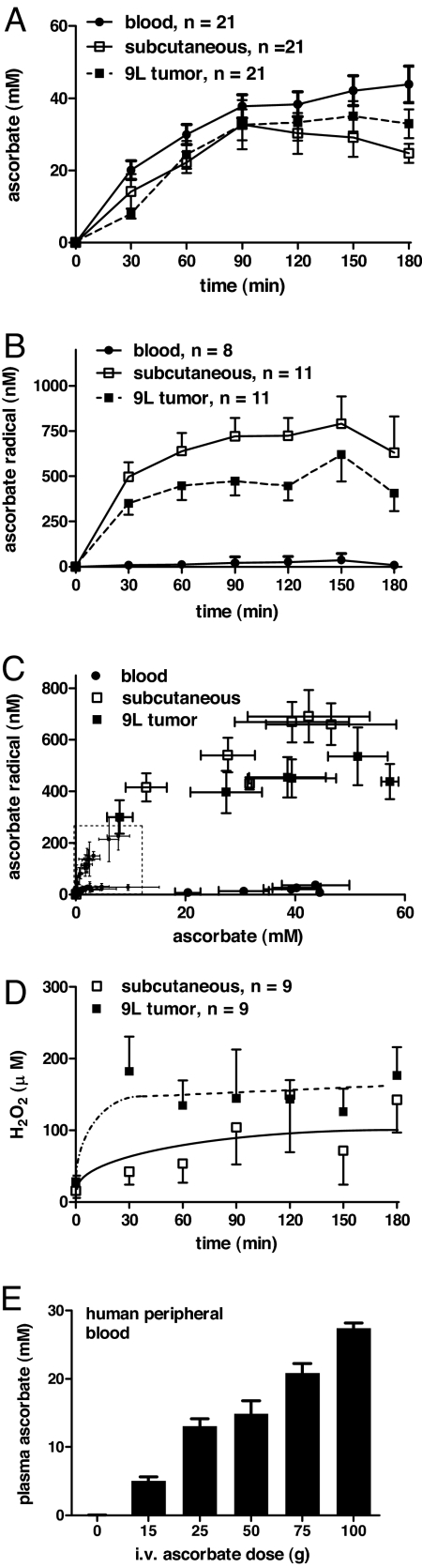
To explore potential mechanisms underlying ascorbate action in vivo, blood samples and interstitial fluids from s.c. and 9L tumor sites were obtained by microdialysis in athymic mice. Parenteral administration of a single ascorbate dose (4 g per kilogram of body weight) increased ascorbate in both blood and tissue sites >150-fold from a baseline of <0.2 mM to peak concentrations of >30 mm after 90–180 min (Fig. 3A). Treatment increased ascorbate radical in the extracellular fluid of both 9L tumors and the s.c. space from <10 nM to 500 nM and higher, but blood concentrations did not exceed 50 nM (Fig. 3B). Ascorbate radical concentration as a function of ascorbate concentration was displayed for all fluids and time points, and previous data were added from studies of rats given lower ascorbate doses (Fig. 3C) (5). The relationships were strikingly consistent in the two species and provide key evidence that with pharmacologic ascorbate concentrations, ascorbate radical increased selectively in extracellular fluids but not in blood. A rapid and sustained increase in H2O2 was detected within tumor extracellular fluids within 30 min of parenteral ascorbate administration, achieving a plateau of 150 μM (Fig. 3D). Clinical relevance of pharmacokinetics data were investigated in human subjects who received escalating doses of i.v. ascorbate as part of an exploratory treatment protocol. Peak plasma concentrations of ascorbate approached 30 mM (Fig. 3E), similar to concentrations observed in mice given parenteral ascorbate (Fig. 3A).
Fig. 3.
Real-time quantification of ascorbate prodrug metabolism in vivo. Mice were anesthetized and maintained for microdialysis as previously described in Materials and Methods. Separate probes implanted into either tumor tissue or s.c. spaces were perfused (1 μl/min) with sterile saline solution. A single dose of ascorbate (4 g per kilogram of body weight, pH 7) was given by i.p. injection at 0 min, and probe eluates were collected simultaneously from each site in 30-min intervals. Blood was collected from the tail vein into heparinized hematocrit tubes, and analytes were determined as single point measures every 30 min. (A and B) Ascorbate and ascorbate radical concentrations in blood (●), s.c. (□), and tumor (■) extracellular fluids. (C) Ascorbate radical in blood (●), s.c. (□), or tumor (■) extracellular fluid as a function of ascorbate concentrations for all time points (± SEM) are shown. (Inset) Previous data (dashed box) were added from studies of rat-administered ascorbate (either 0.25 or 0.5 g per kilogram of body weight) [Reproduced with permission from Chen Q, _et al._ (5) (Copyright 2007)]. (D) Formation of H2O2 in s.c. (□) or tumor (■) extracellular fluid. (E) Peak plasma concentrations of ascorbate in human subjects who received escalating doses of i.v. ascorbate.
Discussion
These preclinical data provide a firm basis for advancing pharmacologic ascorbate in cancer treatment to humans. The tumor xenograft results are especially noteworthy because ascorbate, considered a nutrient, was used here only as a single-agent drug. Pharmacologic concentrations of ascorbate decreased tumor volumes 41–53% in diverse cancer types known for both their aggressive growth and limited treatment options.
Ascorbate has a unique history in cancer treatment. Interest peaked over 30 years ago when retrospective data indicating possible benefit of high-dose ascorbate for patients with cancer were published (10, 11). Subsequent double-blind placebo-controlled trials showing no benefit were considered definitive, and ascorbate treatment was dismissed by conventional oncologists (12–14). We revisited the controversy of ascorbate in cancer therapy in light of several new observations. First, it was recognized that ascorbate was administered both intravenously and orally in the retrospective studies but only orally in the double-blind trials (15). Second, clinical and pharmacokinetics studies within the past 12 years indicate that oral ascorbate produces concentrations in plasma and tissue that are tightly controlled (<0.2 mM) (6–8). Our studies in rats demonstrated that pharmacologic concentrations of ascorbate in plasma (>0.2 mM) could be achieved only by circumventing oral tight control with parenteral administration (5). Third, complementary and alternative medicine practitioners continue to administer high-dose ascorbate off label, without apparent toxicity when patients are properly screened for normal renal function and absence of glucose-6-phosphate dehydrogenase deficiency, iron overload, and oxalate nephropathy (8, 14, 15). With such screening, data from a recent Phase I study show that i.v. ascorbate did not have adverse effects (16). These data coupled with the possibility of benefit (17) suggested that further rigorous studies were warranted.
Our findings showed that pharmacologic ascorbic acid concentrations were cytotoxic to many types of cancer cells in vitro (Fig. 1A) and significantly impeded tumor progression in vivo without toxicity to normal tissues (Fig. 3). The amelioration of ascorbate cytotoxicity in vitro by the addition of catalase was consistent among sensitive cancer cells (Fig. 1B) and points unambiguously to H2O2 generation in the extracellular medium (4). Hydrogen peroxide cytotoxicity is promiscuous in its action, compromising membranes, glucose metabolism, and DNA integrity (18). Variation in sensitivity among cancer cells may be related to the complex networks that H2O2 acts on combined with the range of functional mutations intrinsic to each cancer cell line, which are not present in normal cells (EC50 > 20 mM) (Fig. 1A). Although the molecular basis for the relative resistance of normal cells remains to be elucidated, the current in vivo data support that pharmacologic ascorbate concentrations, which can readily be achieved in humans (Fig. 3E), diminished growth of several aggressive cancer types in mice (Fig. 2) without causing apparent adverse effects.
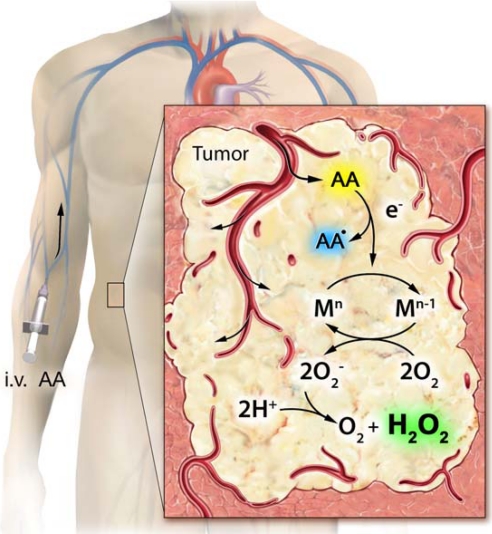
We observed that ascorbate radical was an essential intermediate in H2O2 generation from pharmacologic ascorbate (Fig. 4). Ascorbate radical concentrations in extracellular fluids of both mice and rats were evident over a wide dose range of ascorbate, reaching a steady-state plateau of >500 nM at tissue ascorbate concentrations of >20 mM. Despite corresponding ascorbate concentrations in blood, minimal ascorbate radical and no H2O2 were evident (5) (Fig. 3). These data suggest that the lifetimes of ascorbate radical and H2O2 in blood are limited to below the detection limit, likely because of the predominance of erythrocyte peroxidase capacity (18). Data generated using microdialysis technique show that the putative metallocatalyst(s) for the generation of ascorbate radical and H2O2 was present within extracellular fluids, including tumor interstitial space (Fig. 3) (5). Our previous work suggested that catalytic activity in serum was mediated by a protein (or proteins), because activity was heat-labile and between 10 and 30 kDa in size (4). Ascorbate is a reducing cofactor for a select small group of metal-centered enzymes (19, 20). Pharmacologic concentrations of ascorbate may react with a larger set of metallocatalysts with higher _K_Ms for ascorbate that otherwise are not engaged in normal biological conditions. This degeneration toward increased nonspecific reactions with pharmacologic ascorbate, with the subsequent formation of H2O2, may underlie the physiologic basis of tight control in ascorbate homeostasis.
Fig. 4.
Proposed mechanism for tumorcidal actions of pharmacological ascorbate. Ascorbate (AA) distributes from the blood to the tumor extracellular fluid compartments after i.v. administration. In the tumor interstitium, ascorbate is oxidized to ascorbate radical (AA•) by a metalloprotein catalyst (M), which donates an electron (e−) to oxygen forming superoxide radical (O2−) and ultimately the tumorcidal effector H2O2. In blood, these reactions are minimized (4, 5).
It was notable that the tumor parenchyma experienced an early and sustained increase in H2O2 after ascorbate treatment relative to s.c. sites (Fig. 3D). This finding may be because of either an enhanced formation or decreased destruction of H2O2 within tumor intersitium relative to normal extracellular fluid. These intratumoral H2O2 concentrations of >125 μM persisted for >3 h after ascorbate administration, similar to endogenous levels evident in dermal wound sites 2–5 d after injury (21). Although H2O2 formation may be a trigger for angiogenesis (22, 23), normal wound healing follows a defined temporal progression and resolution. In contrast to this healing process, our regimen of daily pharmacologic ascorbate treatment produced episodic and chronic H2O2 formation, which in the context of the tumor milieu manifested as an overall diminished tumor growth rate (Fig. 2). Of note, the tumoricidal effect of daily pharmacologic ascorbate treatment was mechanistically similar to the effects observed in other investigations that used adenoviral-driven overexpression of the extracellular isoform of superoxide dismutase in pancreatic tumors (24). Both approaches increased interstitial H2O2 concentrations, leading to a comparable suppression of cancer growth.
Pharmacologic ascorbate is readily available, inexpensive, and without apparent toxicity when used with proper screening. Moreover, substantial off-label administration of ascorbate already exists within the complementary medicine community. Figure 3E shows that pharmacologic plasma ascorbate concentrations similar to those showing efficacy in tumor-bearing mice can be attained in humans. Although our preclinical mouse data showed that tumor growth was significantly decreased (Fig. 2), the use of pharmacologic ascorbate as a single agent was not curative. As modalities in cancer are often combined, these data suggest that pharmacologic ascorbate in combination with other therapies deserves further exploration for treatment of cancers that otherwise have poor outcomes, such as pancreatic and ovarian carcinomas and glioblastoma.
Materials and Methods
Cells and Cytoxicity Assessment.
Cell lines were either purchased from American Type Culture Collection or donated by colleagues: Chuxia Deng (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health), William DeGraff (National Cancer Institute, National Institutes of Health), Peter Eck (National Cancer Institute, National Institutes of Health), Corinne Griguer (University of Alabama, Birmingham, AL), Lucia Martiniova (National Institute of Child Helath and Human Development, National Institutes of Health), Marsha Merrill (National Institute of Neurological Disorders and Stroke, National Institutes of Health), James Mitchell (National Cancer Institute, National Institutes of Health), Ana Robles (National Cancer Institute, National Institutes of Health), Anthony Sandler (Children's National Medical Center, Washington, DC), Emily Shacter (Center for Biologics Evaluation and Research, United States Food and Drug Administration), Lyuba Varticovski (National Cancer Institute, National Institutes of Health), and Lalage Wakefield (National Cancer Institute, National Institutes of Health). Cells (1 × 104) in logarithmic growth phase were cultured at 37°C in 5% CO2/95% air in recommended growth media containing 10% FCS and exposed to serial dilutions of ascorbate (0–20 mM, pH 7) for 2 h and washed and cultured for an additional 24–48 h in growth medium in the absence of ascorbate. Ascorbic acid was buffered to pH 7.0 with sodium hydroxide and prepared immediately before use. EC50 values indicate the concentration of ascorbate that reduced survival by 50% determined by viability assays as previously described (4, 25). Human lymphocyte and monocytes were freshly elutriated from peripheral blood donors. EC50 values for 13 of 43 cells in Fig. 1A were previously shown (4). Catalase (600 units/ml; Sigma) was prepared immediately before use.
Xenograft and Treatment Procedures.
Tumor cells (Ovcar5, 5 × 106; Pan02, 1 × 106; 9L, 2 × 106) suspended in normal saline solution were injected s.c. into the flanks of female athymic mice (Ncr-nu/nu aged 5–8 weeks). When tumor volume reached 25–50 mm3, treatment commenced with ascorbate (4 g per kilogram of body weight) by i.p. injection. Ascorbate was prepared as 1 M stock solution in sterile water adjusted to pH 7 with NaOH. Control mice received an identical regimen of osmotically equivalent saline solution. Longitudinal tumor volume was calculated from caliper measurements using volume = (length) × (width)2 × 0.5. At the end of the experiments, mice were killed with final tumor weight and metastases assessed by gross necropsy.
In Situ Sample Acquisition.
Mice were anesthetized and maintained for microdialysis as previously described (5) with the following modifications: separate probes (CMA/20 4 × 0.5 mm, 20 kDa cutoff) were implanted into tumor tissue (right flank) and s.c. spaces (left flank) and perfused (1 μl/min) with sterile 0.9% saline solution. After a 30-min baseline period, a single dose of ascorbate (4 g per kilogram of body weight, pH 7) was given by i.p. injection at 0 min and probe eluates were collected simultaneously from each site in 30-min intervals. Relative recovery of analytes through the probe membrane was: ascorbate 12%, ascorbate radical 65%, and H2O2 20%. Blood was collected from the tail vein into heparinized hematocrit tubes, and analytes were determined as single point measures every 30 min.
Analytical Chemistry.
Ascorbate and ascorbate radical concentrations in plasma and microdialysates were determined by HPLC separation with electrochemical detection and electron paramagnetic resonance, respectively, as previously described (5). Formation of H2O2 was determined by simultaneous collection of dialysate into tubes containing peroxyxanthone (20 μM) either with or without catalase (600 units/ml), followed by fluorescence spectroscopy as previously described (5). Nonspecific background signal and low probe relative recovery restricted the lower limit of sensitivity to 20 μM.
Human Studies.
Plasma ascorbic acid concentrations were measured in participants enrolled in two clinical trials using i.v. ascorbic acid as cancer therapy at the University of Kansas (ClinicalTrials.gov registration numbers: NCT00284427 and NCT0022831). The trials were approved by the University of Kansas Human Subjects Committee, and written informed consent was obtained from each participant. A single starting ascorbate dose of 15 g over 30 min at 0.5 g/min was infused with subsequent dose escalation of 25 g over 50 min, 50 g over 100 min, 75 g over 150 min, and 100 g over 200 min. Venous blood samples (n = 8, 4 at 100 g) were drawn at the completion of each infusion, and plasma was immediately prepared and frozen to −80°C until analyzed.
Acknowledgments.
We thank all those who kindly shared their cell lines with us. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11037.
References
- 1.Padayatty SJ, et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547–556. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 3.Hung HC, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:577–584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine M, et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padayatty SJ, et al. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Intern Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Menon M, Maramag C, Malhotra RK, Seethalakshmi L. Effect of vitamin C on androgen independent prostate cancer cells (PC3 and Mat-Ly-Lu) in vitro: Involvement of reactive oxygen species-effect on cell number, viability and DNA synthesis. Cancer Biochem Biophys. 1998;16:17–30. [PubMed] [Google Scholar]
- 10.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978;75:4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creagan ET, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301:687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 13.Moertel CG, et al. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 14.Wittes RE. Vitamin C and cancer. N Engl J Med. 1985;312:178–179. doi: 10.1056/NEJM198501173120310. [DOI] [PubMed] [Google Scholar]
- 15.Padayatty SJ, Levine M. Reevaluation of ascorbate in cancer treatment: Emerging evidence, open minds and serendipity. J Am Coll Nutr. 2000;19:423–425. doi: 10.1080/07315724.2000.10718941. [DOI] [PubMed] [Google Scholar]
- 16.Hoffer LJ, et al. Phase I clinical trial of intravenous ascorbate in advanced malignancy. Annal Oncol. 2008 doi: 10.1093/annonc/mdn377. in press. [DOI] [PubMed] [Google Scholar]
- 17.Padayatty SJ, et al. Intravenously administered vitamin C as cancer therapy: Three cases. Can Med Assoc J. 2006;174:937–942. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone JR, Yang S. Hydrogen peroxide: A signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 19.Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- 20.Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- 21.Tsantes AE, Bonovas S, Travlou A, Sitaras NM. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid Redox Signal. 2006;8:1205–1216. doi: 10.1089/ars.2006.8.1205. [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbiser JL, et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teoh ML, Sun W, Smith BJ, Oberley LW, Cullen JJ. Modulation of reactive oxygen species in pancreatic cancer. Clin Cancer Res. 2007;13:7441–7450. doi: 10.1158/1078-0432.CCR-07-0851. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed SA, Gogal RM, Jr., Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]