Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 1.
Abstract
Although parietal cortex is frequently activated during episodic memory retrieval, damage to this region does not markedly impair episodic memory. To account for these and other findings, a new dual attentional processes (DAP) hypothesis is proposed. According to this hypothesis, dorsal parietal cortex (DPC) contributes top-down attentional processes guided by retrieval goals, whereas ventral parietal cortex (VPC) contributes bottom-up attentional processes captured by the retrieval output. Consistent with this hypothesis, DPC activity increases with retrieval effort whereas VPC activity increases with confidence in old and new responses. The DAP hypothesis can also account for the overlap of parietal activations across different cognitive domains and for opposing effects of parietal activity on encoding vs. retrieval. Finally, the DAP hypothesis explains why VPC lesions yield a memory neglect syndrome: a deficit in spontaneously reporting relevant memory details but not in accessing the same details when guided by specific questions.
The role of lateral parietal regions in episodic memory retrieval is a very interesting cognitive neuroscience dilemma. On one hand, activations in these regions are among the most frequent in positron emission tomography (PET) and functional MRI (fMRI) studies of episodic retrieval (for a review, see Cabeza & Nyberg, 2000), whereas on the other hand, lesions in lateral parietal lesions do not typically yield severe episodic memory deficits, such as the ones associated with medial temporal lobe (MTL) damage. This inconsistency may be only apparent, as there is now evidence that certain parietal lesions do in fact impair some forms of episodic memory (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007). However, why episodic deficits following parietal damage are rare and why only certain forms of episodic memory are affected remain open questions. Moreover, functional neuroimaging evidence suggests that ventral and dorsal regions play different roles in episodic memory retrieval (Vilberg & Rugg, 2008; Wagner, Shannon, Kahn, & Buckner, 2005). To address these issues, the current paper proposes a dual attentional processes (DAP) hypothesis that links the role of dorsal parietal cortex (DPC) and ventral parietal cortex (VPC) in episodic retrieval to their presumed roles in attention (Corbetta & Shulman, 2002). DPC is defined here as lateral parietal regions in or above the intraparietal sulcus (Brodmann Area 7), whereas VPC is defined as the supramarginal and angular gyri (Areas 40 and 39). Although medial parietal regions are also associated with episodic retrieval, they may involve different processes than lateral parietal regions, and are not considered in the present article. The paper consists of five main sections. The first section describes the DAP hypothesis; the second and third sections review functional neuroimaging and lesion evidence supporting this hypothesis, the fourth section considers open questions regarding the hypothesis, and the final section provides some conclusions.
Role of ventral and dorsal parietal regions in episodic retrieval
Three hypotheses on the role of parietal cortex and episodic retrieval
The review by Wagner et al. (2005) considered three hypotheses on the role of parietal regions in episodic retrieval. First, the output buffer hypothesis postulates that parietal regions hold retrieved information in a form accessible to decision-making processes, similarly to one of Baddeley's working memory buffers. Second, the mnemonic accumulator hypothesis posits that parietal regions temporally integrate a memory-strength signal. Wagner et al. (1995) linked this idea to signal-detection models of recognition memory that postulate that old-new memory decisions are determined by a continuous memory magnitude. Finally, the attention to internal representation hypothesis states that parietal regions shift attention to, or maintains attention on, internally generated mnemonic representations.
As noted by Wagner et al. (2005), each of these hypotheses can account for some but not all available functional neuroimaging evidence. The output buffer hypothesis fits well with evidence that certain parietal regions are associated with recollection (vivid remembering of an event including specific contextual details) rather than with familiarity (vague feeling of oldness in the absence of specific details) (e.g., Daselaar, Fleck, & Cabeza, 2006; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Yonelinas, Otten, Shaw, & Rugg, 2005; Wheeler & Buckner, 2004). The idea is that these regions hold the qualitative content of retrieved information (e.g., mental images), which by definition are greater for recollection than for familiarity. However, the output buffer hypothesis cannot easily explain why activity in some parietal regions increases as a function of perceived oldness, which refers to the tendency to respond "old" regardless of the true nature of the stimuli (e.g., Kahn, Davachi, & Wagner, 2004; Wheeler & Buckner, 2003). This finding fits better with the mnemonic accumulator hypothesis, which assumes that parietal regions do not hold actual memories but rather a signal summarizing information coming from other brain regions, which is eventually used to make memory decisions. Thus, these regions show high activity not only for "old" responses to old items (hits) but also for "old" responses to new items (false alarms). Nevertheless, the mnemonic accumulator hypothesis cannot readily accommodate evidence that certain parietal regions show greater activity when participants attempt to recollect source information than when try to retrieve item information, regardless of responses and accuracy (Dobbins, Foley, Schacter, & Wagner, 2002; Dobbins, Rice, Wagner, & Schacter, 2003; Dobbins & Wagner, 2005). This recollective-orienting pattern suggests that these regions track the intention to remember, that is, voluntary attention to memory contents, and hence, it is fits well with the attention to internal representations hypothesis. Yet, voluntary attention cannot explain the aforementioned finding that activity in some parietal regions show greater activity for recollection than for familiarity.
Given that all three hypotheses are partly correct, one possible solution is to expand one of them so it can accommodate a larger set of findings. One of the hypotheses that can be expanded is the attention to internal representation hypothesis. Although this hypothesis primarily focuses on goal-driven voluntary attention processes, not all forms of attention are voluntary. In fact, a fundamental distinction in the attention literature contrasts top-down (or intentional) attention, which is guided by goals and expectations, and bottom-up (or reflexive) attention, which is guided by the saliency of incoming information (for a review, see Yantis, 2000). Thus, it is reasonable to expand the attention account of parietal contributions to episodic retrieval so that it includes not only top-down attention but also bottom-up attention. This new hypothesis depends on the assumption that top-down and bottom-up attention are mediated by different parietal regions, which, as reviewed below, is an idea supported by functional neuroimaging and lesion evidence.
Dual attentional processes in parietal cortex
According to Corbetta and Shulman (2002), top-down attention is supported by a dorsal fronto-parietal system, whereas bottom-up attention is mediated by a ventral fronto-parietal system. Whereas the dorsal system is involved in “preparing and applying goal-directed selection for stimuli and responses”, the ventral system is involved in the "detection of behaviorally relevant stimuli, particularly when they are salient and unexpected” (p. 202). Within parietal cortex, Corbetta and Shulman's (C&S) attention model assumes that top-down attention depends on DPC, whereas bottom-up attention depends on VPC and superior temporal regions. A similar idea was also proposed by Marois et al (2000).
C&S reviewed evidence of dissociations between the attention functions of DPC and VPC, including functional neuroimaging and patient evidence. For example, one fMRI study found that DPC activity starts with the instruction to the search for a target and is greater during the search period than VPC activity, whereas VPC activity is greater than DPC activity when the target is finally presented and detected (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000). Thus, DPC activity mediates preparatory top-down attention, whereas VPC activity reflects the capture of bottom-up attention by the target. Bottom-up attention is also captured by unexpected stimuli, and VPC has been associated with the detection of unexpected spatial and nonspatial stimuli, particularly in the right hemisphere (e.g., Braver, Barch, Gray, Molfese, & Snyder, 2001; Clark, Fannon, Lai, Benson, & Bauer, 2000; Downar, Crawley, Mikulis, & Davis, 2000, 2001; Kiehl, Laurens, Duty, Forster, & Liddle, 2001; Marois, Leung, & Gore, 2000). According to C&S, right VPC is the most frequent location of lesions causing neglect, which is an attentional deficit in detecting information contralateral to the lesion (Driver & Mattingley, 1998). C&S argue that neglect patients can voluntarily direct attention to the contralesional side and can use cognitive cues to anchor attention to the left visual space. However, neglect patients have a deficit in detecting stimuli that are unattended and outside the focus of processing, which is more consistent with a bottom-up than a top-down attention deficit.
C&S do not describe the difference between the attentional functions of DPC and VPC as a sharp dichotomy but as a more graded difference. First, they note that DPC shows some sensitivity to the presentation of infrequent events, which suggests it is also affected by bottom-up attentional processes. Second, although VPC activity is driven by incoming information, activity in this region is also modulated by task relevancy. For instance, this region will show greater activity to salient, infrequent stimuli in the modality being attended (e.g., visual) but not in a different modality (Downar et al., 2000). Also, an fMRI study found that during search for a red target, right VPC was activated by red distractors, which apparently captured attention bottom-up, but not by distractors in other colors, which did not capture attention (Serences et al., 2005). Thus, to elicit VPC activity, it is not enough that a stimulus is salient; it should be also relevant to current goals. In sum, top-down attentional processes within DPC and bottom-up attentional processes within VPC interact with each other. The detection of unexpected events in VPC can enhance or attenuate sustained goal-driven processes in DPC. Conversely, the detection of targets in VPC is modulated by the definition of what constitutes a target, which depends on top-down behavioral goals supported by DPC.
The dual attentional processes (DAP) hypothesis
Like most attention researchers, C&S define bottom-up attention as attention driven by salient or unexpected events in the environment. The assumption that bottom-up attention is primarily driven by environmental stimuli originates in the experimental paradigms typically employed to investigate bottom-up attention, which generally employ sensory stimuli (Yantis, 2000). Because of these typical paradigms, bottom-up attention is often described as attention driven by sensory stimuli, or "exogenous attention", and opposed to attention driven by goals stored in memory, or "endogenous attention". As a result, the standard view about the relation between memory and attention is that memory modulates top-down attention, via intentions and expectations, whereas bottom-up attention is related to perception but not to memory. Thus, it is not surprising that when describing the attention hypothesis of parietal function in episodic retrieval, Wagner et al. (2005) emphasized the top-down side of attention and cited evidence supporting the role of task demands (recollective-orienting: Dobbins, Foley, Schacter, & Wagner, 2002; Dobbins, Rice, Wagner, & Schacter, 2003; Dobbins & Wagner, 2005).
Yet, memory can modulate attention not only top-down but also bottom-up. The most obvious example of the latter is involuntary remembering, as in the case of the Proustian character who experienced an outpour of autobiographical memories following the taste of a tea-soaked Madeleine. However, the phenomenon of memory-guided bottom-up attention is not limited to involuntary personal memories; it occurs whenever an interesting memory enters consciousness and overtakes attentional resources. Even when the memory search process is voluntary, the outcome of the search can never be completely predicted, and hence, the recovery of relevant memories can always modulate attention in a bottom-up direction. For instance, while trying to remember if you paid the electricity bill, you may recall the wedding invitation that was next to the bill in your mailbox. Although the memory search about the bill was voluntary and driven by top-down attention, the attention shift towards the memory of the wedding invitation is not voluntary and it reflects bottom-up attention.
If memory can interact with attention in both top-down and bottom-up directions, then it is reasonable to expand the attention account of parietal activations during episodic retrieval so it includes both forms of attention. Thus, extending the parietal component of C&S’s attention model to the episodic memory retrieval domain, it is proposed here that DPC is associated with the allocation of attentional resources to memory retrieval according to the goals of the rememberer (top-down attention), whereas VPC is associated with the capture of attentional resources by relevant memory cues and/or recovered memories (bottom-up attention). To distinguish this hypothesis from the attention to internal representation hypothesis discussed by Wagner et al. and from C&S attention model, I call it the dual attentional processes (DAP) hypothesis.
As in C&S’s attention model, the DAP hypothesis assumes that top-down and bottom-up attentional processes interact very closely (see Figure 1). Even if the role of DPC in episodic retrieval reflects mainly attention guided by retrieval goals, these goals may change depending on the nature of information retrieved, and hence DPC is also sensitive to relevant memories. Conversely, even if the role of VPC in episodic retrieval primarily reflects attention guided by the relevancy of the retrieval output, the pertinence of this output depend on retrieval goals, and hence VPC is indirectly sensitive to these goals. Because of these close interactions, one is more likely to find graded differences than sharp dissociations between the contributions of DPC and VPC to episodic retrieval.
Figure 1.
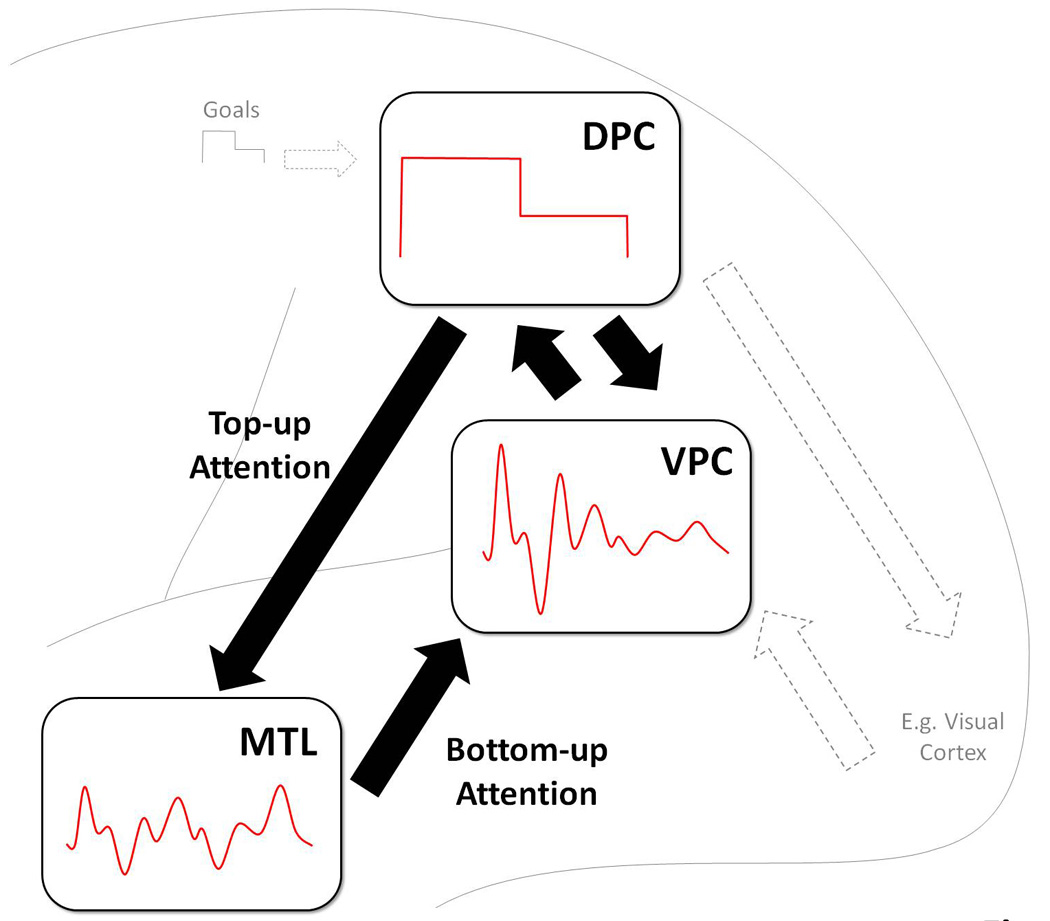
Simple graphical description of the DAP hypothesis. VPC fluctuates, continuously, tracking changes in MTL activity, which in turn reflects the recovery of episodic memories. In contrast, activity in DPC reflects top-down attentional processes guided by retrieval goals. DPC and VPC interact very closely: the goals maintained by DPC define what targets are relevant, and the targets detected by VPC can alter or change behavioral goals. The attentional processes VPC and DPC contribute to episodic retrieval are the same attentional processes these regions contribute to perception.
The DAP hypothesis presumes that VPC monitors the retrieval output continuously without disrupting ongoing processing, unless behavioral goals must be updated. As illustrated by Figure 1, the assumption is that VPC activity fluctuates, constantly, tracking changes in medial temporal lobe (MTL) activity, which in turn reflects the recovery of episodic memories. The signal generated by VPC is not identical to the signal generated by MTL activity because VPC modulates the MTL signal on the basis of behavioral goals maintained by DPC. In Figure 1, this is illustrated by the enhancement of the one part of the signal segment generated by MTL. According to the DAP hypothesis, the roles of DPC and VPC in episodic retrieval are largely the same as their respective roles in perception (see dashed arrows in Figure 1); regardless of whether incoming information arrives from the senses or from memory, DPC mediates top-down attention and VPC, bottom-up attention. It is also worth noting that top-down attention and bottom-up attention are two separate dimensions, rather than the endpoints of a single continuum, and hence, they can be both high or both low at the same time, even if in some situations they tend to vary in opposite directions.
Similarities and differences with related ideas
The C&S attention model and the DAP hypothesis share the basic assumption that DPC and VPC are respectively involved in top-down vs. bottom-up attentional processes. At the same time, there are at least two assumptions that differ between the C&S model and the DAP hypothesis. First, the C&S model assumes that bottom-up attention is attention guided by sensory stimuli (i.e., exogenous attention), whereas the DAP hypothesis defines bottom-up attention as attention guided by incoming information, which may arrive not only from the senses but also from memory. Second, the C&S model assumes that the bottom-up attentional functions of VPC are strongly right lateralized, whereas the DAP hypothesis presumes that these functions are distributed bilaterally and that lateralization differences are related to the verbal/meaningful vs. sensory/meaningless nature of the information (Milner, 1971).
The DAP hypothesis also has similarities and differences with the three hypotheses of parietal contributions to episodic retrieval considered by Wagner et al. (2005). Like the output buffer and mnemonic accumulator hypothesis, the DAP hypothesis assumes that activity in some parietal regions increases as a function of episodic retrieval output. However, the DAP hypothesis differs from these views in at least two ways. First, the DAP hypothesis specifically assumes that as episodic retrieval output increases, VPC and DPC are likely to show different activation functions. This point is elaborated later in the section about functional neuroimaging data. Second, according to the DAP hypothesis, parietal regions show fluctuations in attention as a function of the retrieval output but they do not hold retrieved information or an accruing memory signal. As discussed later, the difference between "holding" retrieved information vs. being modulated by the retrieval output is important as these two assumptions lead to different predictions regarding the effects of VPC damage.
With the attention to internal representation hypothesis, the DAP hypothesis shares the global idea that the role of parietal regions in episodic retrieval is related to attention. However, these two hypotheses also differ in at least two ways. First, the DAP hypothesis does not assume the existence of parietal regions specialized in attention to internal as opposed to external representations; it assumes that the parietal regions that mediate attention to memory representations are the same ones that mediate attention to sensory stimuli. Second, if the attention to internal representations hypothesis is interpreted in terms of top-down attention processes (e.g., Wagner et al., 2005), then this hypothesis overlaps only with one of the two components DAP hypothesis, namely the one focused on the function of DPC.
In summary, this paper proposes a new hypothesis regarding the contributions of parietal regions to episodic retrieval. This DAP hypothesis states that DPC contributes top-down attentional processes guided by retrieval goals, whereas VPC contributes bottom-up attentional processes captured by the retrieval output. The latter idea depends on the assumption that bottom-up attention may be driven not only by sensory stimuli but also by incoming memory information. Like the C&S model, the DAP hypothesis assumes that top-down and bottom-up attentional processes interact; goals determine the relevancy of incoming information and incoming information can alter behavioral goals. Unlike the output buffer and mnemonic accumulator hypotheses, the DAP hypothesis assumes VPC is modulated by the retrieval output but it does not "hold" this output. Finally, unlike the attention to internal representations hypothesis, the DAP hypothesis assume that the same parietal regions mediate attention to both mnemonic and sensory information and it limits the top-down contributions of parietal cortex to DPC. Thus, although closely related to preexistent ideas, the DAP hypothesis is novel in several ways. As a result, the DAP hypothesis yields new predictions regarding functional neuroimaging and lesions findings, which are considered in the next two sections.
Functional neuroimaging evidence
As noted above, activations in lateral parietal cortex are among the most frequent findings in PET and fMRI studies of episodic retrieval (Cabeza & Nyberg, 2000; Rugg & Henson, 2002). Although summarizing the results of many of these studies can be very useful to identify basic patterns of parietal activity during episodic retrieval, as illustrated by the metaanalysis of Vilberg and Rugg (2008), these basic patterns can be accommodated by several competing hypotheses. For example, Vilberg and Rugg's conclusion that VPC shows greater activity during conditions involving recollection, may be interpreted as evidence for the output buffer hypothesis but also as evidence for the DAP hypothesis. A complementary approach to distinguish among competing hypothesis is to identify specific findings that support one hypothesis more than the alternatives. This is the approach used here. The three sections below focus on the results of sets of data that fit better with the DAP hypothesis than with competing hypotheses: (1) memory performance, (2) cross-function comparisons, and (3) encoding-retrieval differences. Each section evaluates how well the data fits with the DAP hypothesis and with the output buffer and mnemonic accumulator hypotheses. The attention to internal representations hypothesis is not discussed because if one assumes that this hypothesis applies mainly to DPC, then this hypothesis is similar to the top-down attention component of the DAP hypothesis.
Memory performance
Let us consider first the application of the DAP hypothesis to a typical old/new recognition test. According to this hypothesis, the instructions of the test set the behavioral goal of retrieving episodic information, which drives top-down attentional processes mediated by DPC. As each recognition cue is presented, DPC supports the attempt to retrieve information. The greater the difficulty of memory search and/or the decision process, the greater the need for top-down attentional control mediated by DPC. Thus, the DAP hypothesis predicts that DPC activity should increase as a function of retrieval effort. In contrast, this hypothesis assumes that VPC activity mediates bottom-up attentional processes, which are captured by relevant memory cues and/or information recovered from memory. As noted above, what is relevant is determined by the goals of the task, which in the case of a recognition task involves distinguishing between old and new items. Thus, the most relevant stimuli in an old/new recognition task are items at the two extremes of the old-new continuum, that is, items that appear as either "extremely old" or "extremely new." Given that factors that enhance memory performance tend to increase both the correct acceptance of old items and the correct rejection of new items (i.e., the phenomenon known as "mirror effect"), the DAP hypothesis predicts that in general VPC activity will tend to be greater when recognition performance is high than when it is low. In sum, the DAP hypothesis predicts a double dissociation between DPC and VPC activity as a function memory performance: DPC should show greater activity when memory performance is low (greater demands of top-down attention), whereas VPC should show greater activity when memory performance is high (stronger capture of bottom-up by relevant old and new stimuli). As noted before, top-down and bottom-up attention are separate dimensions and hence it is possible to have conditions in which they are both high or both low at the same time. In typical recognition tests, however, they tend to vary in opposite directions as memory performance increases.
There are different methods for varying memory performance in functional neuroimaging experiments, including manipulations of study task, number of encoding presentations, and study-test delay. Another method is to let information recovery vary naturally across items, and measure it using confidence ratings. For example, a recent fMRI study (Kim & Cabeza, 2007) compared brain activity for high- vs. low-confidence "old" responses to studied words (hits). As illustrated by Figure 2-A, the study yielded a clear dissociation between DPC and VPC: whereas DPC showed greater activity for low- than high-confidence hits (blue activation), VPC showed greater activity for high- than low-confidence hits (yellow activation) (see also Moritz, Glascher, Sommer, Buchel, & Braus, 2006). This result fits very well with the DAP hypothesis: low-confidence hits require greater top-down attentional resources mediated by DPC, whereas high-confidence hits reflect the retrieval of relevant targets that capture bottom-up attentional resources mediated by VPC.
Figure 2.
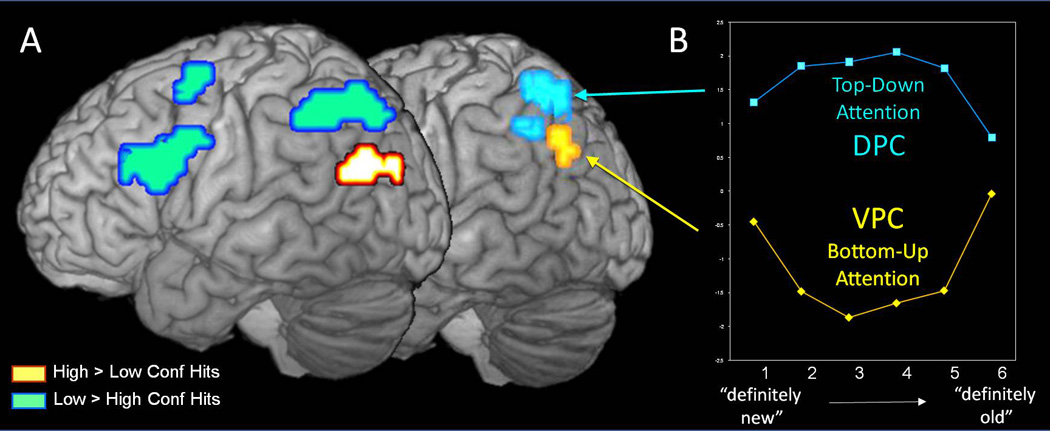
Dissociations between DPC and VPC activations during episodic retrieval that support the DAP hypothesis. A. DPC shows greater activity for low- than high-confidence hits, consistent with its involvement in top-down attention, whereas VPC shows greater activity for high- than low-confidence hits, consistent with its involvement in bottom-up attention (From Kim & Cabeza, 2007). B. The data are from an fMRI study on word recognition reported by Daselaar et al. (2006) and Fleck et al. (2006). Each trial consisted of an old/new decision and followed by confidence rating. The activations in the figure were identified by high > low (yellow activation) and low > high (blue activation) confidence contrasts at a threshold of p < 0.001, uncorrected. For display purposes, activations were masked with a parietal cortex region-of-interest (Pick Atlas ROI). The yellow activation overlaps with the activation labeled “D” in Figure 2 of Daselaar et al. (2006). The blue activation is the lateral extension of a left parietal activation reported in Table 1 of Fleck et al. (2006). The graph displays effect sizes extracted from the two activations and plotted along an oldness scale.
However, the finding of greater VPC activity for high- than low-confidence hits also fits very well with the output buffer hypothesis. This view predicts that, compared to low-confidence, high-confidence hits will elicit greater VPC activity because they involve greater memory recovery and output buffer demands. Thus, both the DAP hypothesis and the output buffer hypothesis predict greater VPC activity for high- than low-confidence hits. However, these two views make different predictions regarding high confidence "new" responses. The DAP hypothesis predicts that "definitely new" items should elicit high VPC activity because these items are highly relevant for the behavioral goals of recognition memory tasks. In contrast, the output buffer hypothesis predicts that "definitely new" items should elicit minimal VPC activity because they involve the least memory recovery and associated working memory load.
To test these different predictions, we examined the results of an fMRI study in which participants made old/new recognition with confidence ratings (Daselaar, Fleck, & Cabeza, 2006; Fleck, Daselaar, Dobbins, & Cabeza, 2006). Whereas reported analyses focused on brain regions where activity increases or decreases with oldness (from "definitely new" to "definitely old"), we focus here on parietal regions where activity shows inverted- or upright-U shapes as a function of oldness. The inverted-U shape reflects greater activity for effortful low-confidence responses (i.e., top-down attention), whereas the upright- U shape reflects greater activity for relevant "definitely new" and "definitely old" responses (i.e., bottom-up attention). Thus, the DAP hypothesis predicts that, as oldness increases, DPC activity should display an inverted-U function whereas VPC activity should display an upright-U function. As illustrated by Figure 2-B, this is exactly what the results show.
The output buffer hypothesis cannot easily account for the results in Figure 2-B, and in particular for the strong VPC response to "definitely new" responses. Why is VPC activity greater for high- than low-confidence "new" responses, if the amount of information retrieved from episodic memory and maintained in working memory is less for the former than for the latter? One possible explanation is that VPC activity for "definitely new" responses reflects the recovery of disconfirmatory evidence, that is, the strategy known as "recall-to-reject". This explanation was suggested by Yonelinas and collaborators (2005), who also found an upright-U activation function in VPC (Fig. 1-C, p. 3005). However, although recall-to-reject may occur in associative recognition (Rotello & Heit, 2000), it is an unlikely strategy in item recognition (Rotello & Heit, 1999), which was the retrieval test investigated by Daselaar et al (2006) and Yonelinas et al (2005).
The mnemonic accumulator hypothesis cannot easily account for either the upright- or the inverted-U responses. As noted before, this hypothesis accounts for evidence that greater parietal activity is associated with "old" decisions regardless of whether they are correct or incorrect (Kahn, Davachi, & Wagner, 2004; Wheeler & Buckner, 2003). In other words, according to this hypothesis parietal activity reflects the accumulation of an "oldness" signal until a response is finally made. Thus, this hypothesis predicts that parietal regions should show a gradual increase from "definitely new" to "definitely old" as the oldness signal accumulates. As shown by Figure 2-B, however, this is not the activation function displayed by either VPC or DPC. However, it is important to point out that some occipito-parietal (Daselaar, Fleck, & Cabeza, 2006) and mid-parietal (Montaldi, Spencer, Roberts, & Mayes, 2006; Yonelinas, Otten, Shaw, & Rugg, 2005) regions have been found to show linear increases in activity as a function of oldness. Thus, it is a challenge for future research to distinguish among parietal regions showing upright-U, inverted-U, and linear response profiles.
In sum, DPC shows greater activity for low- than high-confidence recognition responses whereas VPC shows greater activity for high- than low-confidence recognition responses. These results fit very well with the DAP hypothesis, which associates DPC with top-down attentional control and VPC with bottom-up attention captured by relevant memory targets, whereas neither the output buffer nor the mnemonic accumulator hypotheses can easily account for these data.
Cross-function comparisons
The standard approach in functional neuroimaging has been to focus on a single cognitive function (e.g., episodic retrieval) and attribute the activations found to different aspects of that particular function (postretrieval monitoring, trace recovery, visual imagery, etc.). In contrast with this within-function approach, the cross-function approach asks why the same brain region is activated by very different cognitive functions (Braver et al., 2001; Cabeza, Dolcos, Graham, & Nyberg, 2002; Cabeza et al., 2003; Cabeza & Nyberg, 2002; LaBar, Gitelman, Parrish, & Mesulam, 1999; Nyberg, Forkstam, Petersson, Cabeza, & Ingvar, 2002; Ranganath, Johnson, & D'Esposito, 2003). The cross-function approach is very useful to investigate the role of parietal regions in episodic retrieval because these regions are in fact activated by variety of cognitive tasks, and accounting for these overlaps in activation is likely to constrain theories about their role in episodic retrieval. The DAP hypothesis predicts that (1) DPC activations during episodic retrieval should overlap with DPC activations during cognitive tasks that involve top-down attention, whereas (2) VPC activations during episodic retrieval should overlap with VPC activations during tasks that involve bottom-up attention. Although functional neuroimaging studies relevant to the second prediction do not seem to be available, studies relevant to the first prediction are available and tend to support it.
In a cross-function fMRI study, an episodic retrieval task was compared to a cued attention task (Cabeza et al., 2003) and to a working memory task (Cabeza, Dolcos, Graham, & Nyberg, 2002). In all three tasks, each trial consisted of two 15-sec phases. During the first phase of the episodic retrieval task, participants prepared for a cue-specific recognition decision, and during the second phase, a word was presented and participants made the recognition decision. During the first phase of cued attention task, participants continuously stared at fixation symbol in order to determine if it blipped twice, once, or never during a 12-sec interval, and during the second phase, they indicated the number of blips they detected. There were only a few "catch trials" with blips, and they were excluded from fMRI analyses. Therefore, activity measured during the first phase of the cued attention task reflected mainly the voluntary attempt to detect targets with no targets being detected (i.e., top-down attention). This task is similar to tasks used to investigate top-down attention in functional neuroimaging studies, which typically measure activity while participants are waiting for a target to be presented (for a review, see Corbetta and Shulman, 2002). Finally, during the first phase of the working memory task, participants encoded and maintained four words presented in two columns, and during the second phase, a probe word was presented and participants indicated if the probe words was included in the left column, in the right column, or was a new word. Thus, this task involved the maintenance of integrated verbal-spatial representations in working memory.
As illustrated by Figure 3, DPC was similarly activated by episodic retrieval and cued attention tasks (Figure 3-A), whereas VPC was activated by episodic retrieval but not by cued attention (Figure 3-B). This activation pattern fits well with the DAP hypothesis because both tasks recruited top-down attention but only the episodic retrieval task recruited bottom-up attention. The DAP hypothesis can also explain why VPC was not activated during the working memory task (Figure 3-C). Information maintained in working memory is constant, and hence, there is now new incoming information capturing attentional resources in a bottom-up direction.
Figure 3.
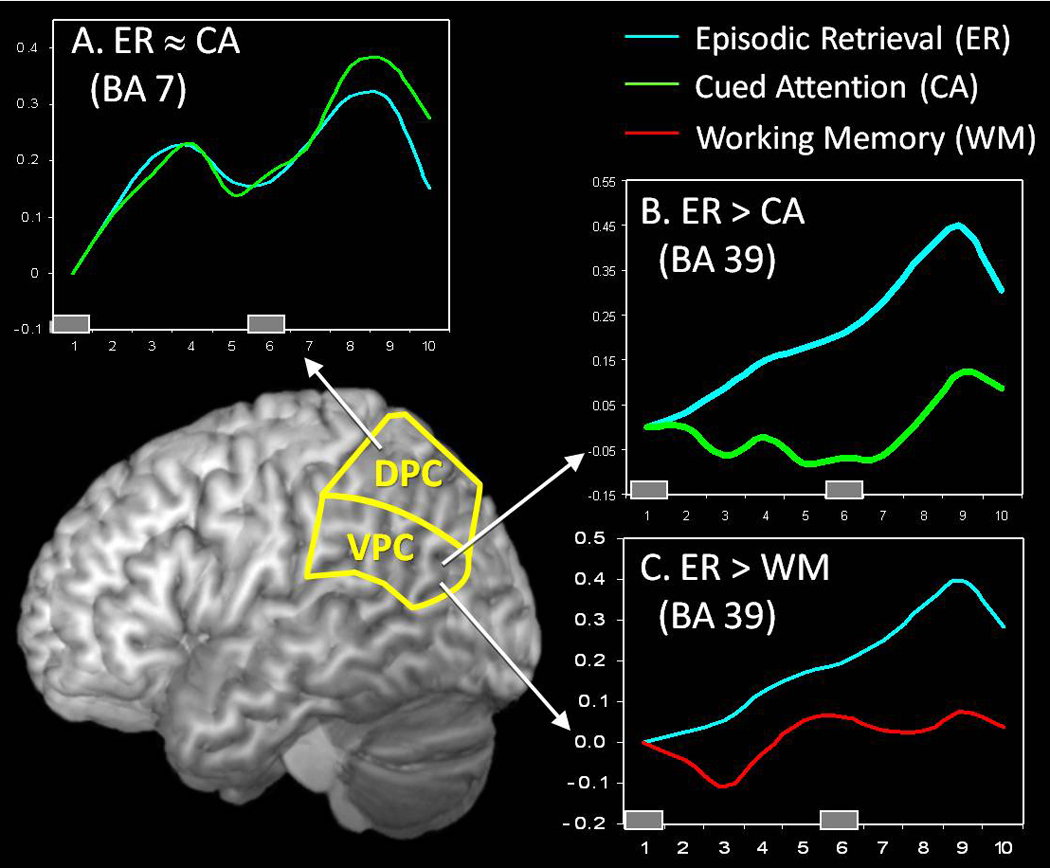
Data from a cross-function fMRI studies comparing episodic retrieval (ER), cued attention (CA), and working memory (WM) (Cabeza, Dolcos, Graham, & Nyberg, 2002; Cabeza et al., 2003) A. ER and CA tasks elicited similar DPC activations, consistent the assumption that this region mediates top-down attentional processes in both tasks. B. In contrast, the ER task but not the CA task activated VPC, consistent with the idea that this region mediates bottom-up attentional processes that are tapped by the ER task but not by the CA task. C. VPC was not activated by a multimodal verbal-spatial working memory (WM) task, inconsistent with the output buffer hypothesis.
In contrast with the DAP hypothesis, the output buffer hypothesis cannot easily accommodate the results in Figure 3-C. This hypothesis predicts that parietal activations during episodic retrieval should overlap with parietal activations during working memory. According to Vilberg and Rugg's (2008) version of the output buffer hypothesis, VPC activations during episodic retrieval reflects the maintenance of information within the "episodic buffer", which is a module specialized in holding integrated multi-domain (e.g., verbal/spatial) representations (Baddeley, 2000). As an example of a cognitive task that engages the episodic buffer, Baddeley (2000) cited the task used by Prabhakaran et al. (2000), in which participants maintained integrated representations of letters and spatial locations. This task is similar to the one that yielded the data in Figure 3 (Cabeza, Dolcos, Graham, & Nyberg, 2002), which also involved maintaining integrated verbal-spatial representations. Yet, this task did not activate VPC at all (Figure 3-C). Also, VPC was not activated in the Prabhakaran et al.'s study, which instead associated the maintenance of integrated verbal-spatial representations with activity in right anterior prefrontal cortex (PFC). Based on this finding, Baddeley (2000) suggested the right anterior PFC could be the site of the episodic buffer. One fMRI study found a right VPC activation while participants encoded a mixed sequence of auditorily-presented numbers and spatial locations (Zhang et al., 2004), but this activation was not found during retrieval phase suggesting the activation during the encoding phase reflected bottom-up attention to modality shifts rather than multimodal working memory per se. At any rate, given the scarcity of fMRI studies on multimodal working memory, it is difficult at present to reach definite conclusions regarding potential overlaps with parietal activations during episodic retrieval.
In sum, regarding cross-function comparisons, the DAP hypothesis predicts that DPC activations during episodic retrieval should overlap with activations during tasks that recruit top-down attention, whereas VPC activations during episodic retrieval should overlap with activations during tasks that recruit bottom-up attention. Supporting the first part of this prediction, a study comparing episodic retrieval to a cued attention task heavily dependent on top-down attention yielded overlapping DPC activations (Figure 3-A). In contrast, VPC was activated by episodic retrieval but not by the cued attention task that did not involve bottom-up attention (Figure 3-B). Finally, inconsistent with the output buffer hypothesis, a verbal/spatial working memory task did not activate VPC (Figure 3-C). Although the mnemonic accumulator hypothesis could explain the VPC activation during episodic retrieval, this hypothesis cannot account for the overlap between episodic retrieval and cued attention in DPC.
Encoding-retrieval differences
Although VPC activity is consistently associated with successful retrieval of episodic information (Vilberg & Rugg, 2008), several studies using the subsequent memory paradigm have linked this region to encoding failure. These studies have shown that during encoding VPC activity is greater for items that are subsequently forgotten than for items that are subsequently remembered (Daselaar, Prince, & Cabeza, 2004; Kao, Davis, & Gabrieli, 2005; Otten & Rugg, 2001; Turk-Browne, Yi, & Chun, 2006; Wagner & Davachi, 2001). Interestingly, this "inverse subsequent memory effect" is not due to stronger activations for subsequently forgotten items but to stronger deactivations for subsequently remembered items (Daselaar, Prince, & Cabeza, 2004; Kao, Davis, & Gabrieli, 2005; Turk-Browne, Yi, & Chun, 2006). In Daselaar et al. (2004), for example, participants encoded semantic associations between words and perceptual associations between words and fonts, and in both cases, associations that were subsequently remembered elicited greater VPC deactivations than those that were subsequently forgotten (see Figure 4). Thus, encoding success is associated with VPC deactivations rather than activations.
Figure 4.
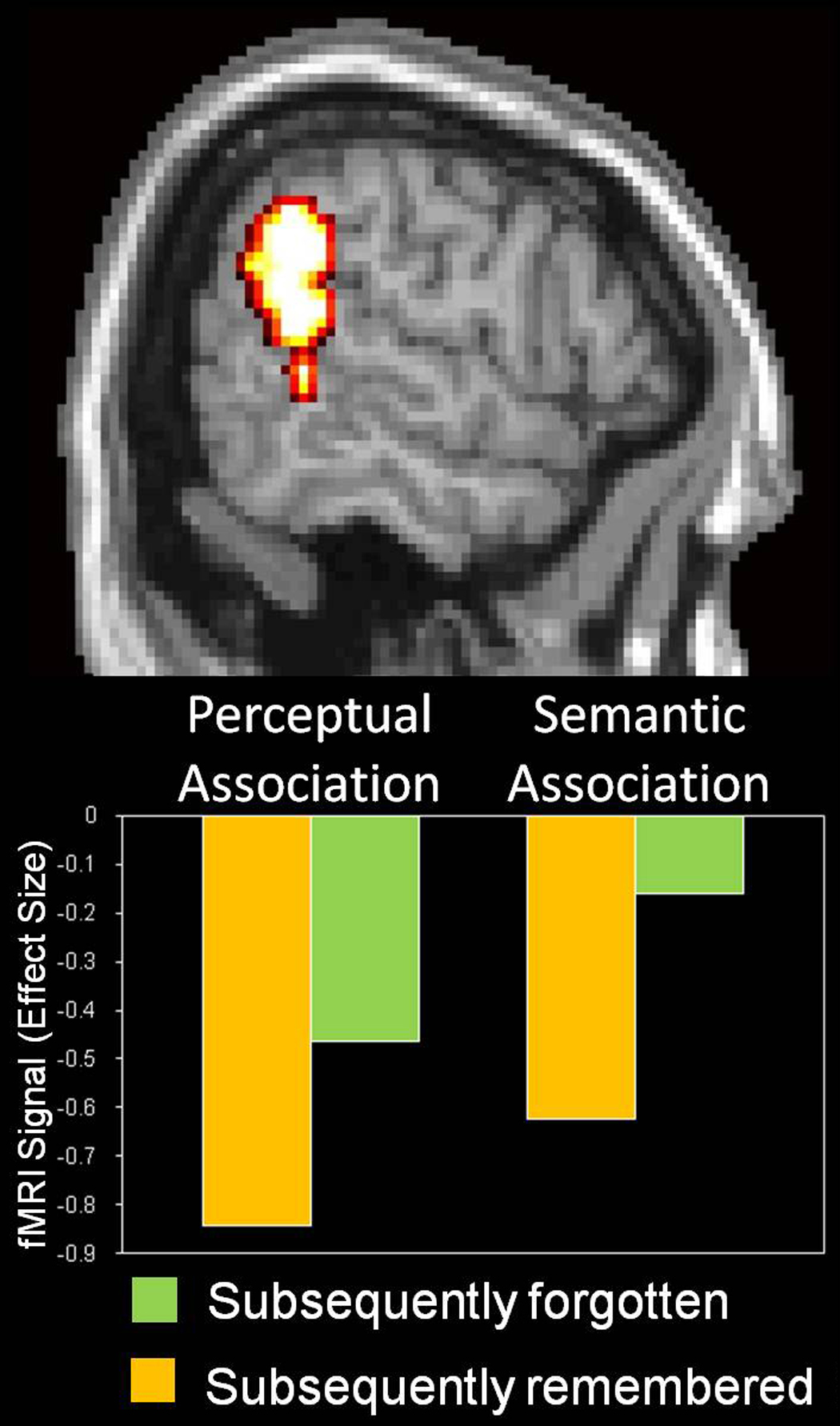
Results from an event-related fMRI study in which participants encoded semantic associations between words and perceptual associations between words and fonts (Daselaar, Prince, & Cabeza, 2004). Bilateral VPC regions (BA 39/40) showed deactivations during encoding, which were greater for items that were later remembered than for those that were later forgotten. These regions overlap with VPC regions that show the opposite pattern during retrieval, namely, greater activity for remembered than forgotten items.
The fact that VPC is deactivated during successful encoding is not surprising given that this region is part of a network of areas (VPC, anterior and posterior midline cortices, etc.) that are typically deactivated during demanding cognitive tasks. According to one view (Gusnard & Raichle, 2001), these default network regions are involved in processes that occur normally during conscious rest and must be "turned off" during demanding cognitive performance. Episodic retrieval seems to be an exception to this general pattern, because it is associated with activations rather than deactivations in these regions. Even if the default network hypothesis has been challenged (e.g., Morcom & Fletcher, 2007), available event-related fMRI data clearly show that greater VPC activity during retrieval is associated with successful retrieval (Vilberg & Rugg, 2008) whereas greater VPC activity during encoding is associated with encoding failure (Daselaar, Prince, & Cabeza, 2004; Kao, Davis, & Gabrieli, 2005; Otten & Rugg, 2001; Turk-Browne, Yi, & Chun, 2006; A. D. Wagner & Davachi, 2001). This dissociation is relevant to the present article because it constraints possible interpretations regarding the cognitive process mediated by VPC, which should be a process that enhance retrieval but disrupts encoding.
The DAP hypothesis can account for the encoding-retrieval dissociation. During typical episodic retrieval tasks, participants search their memory for information relevant to the retrieval decision. Attention is disengaged from a retrieval cue in the present, and turned towards a specific event in the past (Wheeler, Stuss, & Tulving, 1997). When relevant memory targets are found, they capture attentional resources bottom-up, leading to a shift in attention towards these targets. Thus, within the context of typical retrieval tasks, the capture of bottom-up attention often reflects successful recovery of memory targets and is associated with successful retrieval performance. In contrast, during a typical encoding task, participants are asked to make very simple decisions about individual items (e.g., living/nonliving), which are made rapidly without the requirement of a memory search. Relevant information is accessed automatically, without the need to orient attention away from the item being judged, which normally remains unchanged (e.g., on the screen) while the decision is being made. Under these conditions, optimal encoding occurs when attention remains focused on the current item, and shifting attention to other stimuli, such as unrelated thoughts or environmental stimuli, is more likely to weaken than strengthen the encoding of the current item. Thus, within the context of typical encoding tasks, bottom-up attentional processes may become associated with encoding failure rather than success. Thus, the DAP hypothesis can explain why VPC activity tends to be associated with retrieval success but with encoding failure.
However, it is important to note that the association between bottom-up attention and encoding failure applies only to typical encoding tasks in which the items whose memory is later tested are the focus of top-down attention and are processed via simple decision judgments (e.g., living/nonliving). It is perfectly possible that for tasks without these two characteristics, bottom-up attention during encoding could be associated with learning success. For example, if the items whose memory is later tested are not at the center of attention but are items which capture attention bottom-up (e.g., peripheral stimuli), then the DAP hypothesis would predict that VPC activity would be associated with better learning. Consistent with this prediction, an fMRI study showed that deviant stimuli were associated with better encoding (von Restorff effect) as well as with greater interactions between VPC and frontal regions (Strange, Henson, Friston, & Dolan, 2000). Also, if instead of simple decision judgments, the encoding task involves a memory search task whereby retrieval-related bottom-up attention is associated with deeper encoding of retrieval cues, then VPC activity is also likely to be associated with successful learning. This may occur, for example, when episodic retrieval attempts function as additional encoding opportunities (Karpicke & Roediger, 2008).
In contrast with the DAP hypothesis, the output buffer and mnemonic accumulator hypotheses cannot easily account for the dissociation between the role of VPC in encoding vs. retrieval. The output buffer hypothesis cannot explain why VPC activity is associated with enhanced retrieval but with impaired encoding. Working memory processes are generally assumed to contribute to both encoding and retrieval (Moscovitch, 1992). The output buffer hypothesis could accommodate the association of VPC activity with encoding failure if this activity is attributed to the continuous maintenance of irrelevant information in working memory. As for the mnemonic accumulator hypothesis, this idea cannot account for VPC deactivations during encoding because typical encoding tasks require semantic retrieval decisions, which are also likely to involve the accumulation of a memory signal. It could be argued that the VPC activity related to encoding failure reflects the accumulation of irrelevant information, but this possibility is unlikely given the demands of typical encoding tasks.
To summarize, the DAP hypothesis can account for evidence that VPC activity is associated with successful retrieval but with unsuccessful encoding. An explanation based on this hypothesis is that VPC activity during retrieval reflects the capture of bottom-up attention by relevant memory targets, whereas VPC activity during encoding reflects the capture of bottom-up attention by unrelated thoughts or environmental stimuli. In contrast, the output buffer and mnemonic accumulator hypotheses cannot account as well for the encoding vs. retrieval dissociation.
Lesion evidence
Although functional neuroimaging evidence that parietal regions are frequently activated during episodic retrieval suggests that these regions play an important role in episodic retrieval, this idea is challenged by neuropsychological evidence that parietal lesions do not typically yield severe episodic memory deficits. Yet, if the contributions of parietal regions to episodic retrieval are related to their role in attention, then severe episodic memory deficits should not be expected. In general, attention enhances cognitive processes but is not essential for these processes; when attention fails, cognitive operations are weakened but they are not completely obliterated. In the case of vision, for example, attention deficits may produce neglect but they do not normally lead to blindness (Driver & Mattingley, 1998). For the same reason, the effect of attention deficits on episodic memory should not be expected to yield amnesia. At most, attention deficits should produce a memory deficit analogous to the neglect syndrome in vision. This hypothetical syndrome is called here "memory neglect."
The characteristics of memory neglect may be inferred from those of visual neglect. As mentioned before, patients with visual neglect typically have lesions in right VPC, and hence, according to C&S model, they should have a deficit in bottom-up attention rather than a deficit in top-down attention. Consistent with this idea, these patients have a deficit in detecting stimuli that are unattended and outside the focus of processing but they can voluntarily direct attention to the contralesional side (Corbetta & Shulman, 2002). By analogy, the DAP hypothesis predicts that VPC lesions could also yield a memory neglect deficit whereby patients cannot spontaneously report relevant details in retrieved memories (bottom-up attention) but can access these details if guided by specific questions (top-down attention). A recent study by Berryhill et al. (2007) provided direct support for these predictions.
This study investigated autobiographical memory in two patients whose parietal damage was greater for VPC than DPC and compared them to ten age- and education-matched controls. A great advantage of investigating autobiographical memory is that it is possible to assess memory for events that happen before the parietal lesions, thereby controlling for the potential contributions of parietal cortex to encoding processes. The parietal lesions in the two patients were due to infarcts involving the posterior cerebral artery or the watershed between posterior and middle cerebral arteries and were greater for VPC than DPC. Both patients are middle aged, university educated, and suffer from simultanagnosia (the inability to visually recognize two or more things at the same time). However, they both have normal object and color perception, as well as language comprehension and production. Autobiographical memory in patients and controls was assessed using text analyses of subjects’ freely reported memories for different time periods (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002), as well as specific probe questions regarding each event retrieved (Levine, 2004). The main dependent variables were the number of details relating to event, place, time, perceptual, and associated emotions or thoughts. Given that total number of words uttered was similar in patients and controls, differences in autobiographical recall cannot be attributed to language deficits.
The results of the autobiographical memory test were consistent with the aforementioned prediction of the DAP hypothesis: when participants spontaneously reported their autobiographical memories, they produced fewer details than controls across all categories, but when they were asked specific questions about the same memories, the memory deficit disappeared. The deficit in spontaneous report of memory details was ubiquitous, and was not limited to spatial or perceptual aspects of memory. Additional tests showed that the spontaneous memory deficit cannot be explained by a general difficulty with mental imagery. The finding that VPC damage impaired autobiographical memory recall is consistent with the fact that this region is one of areas most frequently activated in functional neuroimaging studies of autobiographical memory (e.g., Addis, Moscovitch, Crawley, & McAndrews, 2004; Conway et al., 1999; Gilboa, Winocur, Grady, Hevenor, & Moscovitch, 2004; Levine et al., 2004; Maguire & Frith, 2003; Nyberg, Forkstam, Petersson, Cabeza, & Ingvar, 2002; for a metaanalysis, see Svoboda, McKinnon, & Levine, 2006).
Thus, VPC lesions impair the ability to spontaneously detect relevant details in representations retrieved from memory (bottom-up attention) but not the ability of identifying these details when guided by external probes (top-down attention). In other words, consistent with the idea of memory neglect, memories are intact but they do not capture bottom-up attention by themselves. An alternative interpretation is that these patients have difficulty in processing multiple memory details simultaneously, which is a deficit that could be called "memory simultanagnosia." Distinguishing between memory neglect and memory simultanagnosia would require specifically-designed studies, but both ideas are consistent with the DAP hypothesis because both assume that the deficit is in attention not memory. The idea that parietal lesions impair attentional rather mnemonic processes could also explain why parietal lesions do not impair episodic retrieval in tasks that focus participants' attention on a single dimension of the stimuli (Simons et al., 2008).
In contrast with the DAP hypothesis, the results of the Berryhill et al.'s study cannot be easily accommodated by the output buffer or mnemonic accumulator hypotheses. Regarding the output buffer hypothesis, if VPC holds retrieved episodic information, then damage to this region should impair the ability to maintain recovered information available while the memory is being reported, and hence, deficits should be observed not only during spontaneous report but also while answering specific memory probes. It could be argued that specific probes reduce the amount of information to be loaded on the buffer, thereby attenuating deficits due to a malfunctioning buffer. However, this idea assumes that memory details are loaded to the buffer before they are verbalized rather than being reported as soon as they are accessed. Further testing of parietal patients would be required to address this question. As for the mnemonic accumulator hypothesis, given that the core assumption of this hypothesis is that parietal cortex holds a summary memory signal rather than memory contents per se, it is unclear why parietal lesions would lead to a deficit in spontaneously retrieving episodic details.
To sum up, patients with VPC lesions showed a deficit in spontaneously reporting details of retrieved autobiographical memories but not in answering specific questions about the same memories. This finding suggests that these patients do not have a deficit in retrieving episodic memories but in the mechanism whereby retrieved memories capture bottom-up attentional resources. The fact that VPC patients can access memory details when guided by specific probes suggests that their ability to deploy bottom-up attentional resources during memory search is largely intact. This pattern of results fits very well with the DAP hypothesis but not with the output buffer or the mnemonic accumulator hypotheses.
Open Questions
Before concluding, it is important to consider several open question regarding the DAP hypothesis. These include questions about (1) the overlap of activations in episodic retrieval and attention studies, (2) the domains of functional neuroimaging evidence accounted by the DAP hypothesis, and (3) potential future extensions of the DAP hypothesis.
Overlap of activations in episodic retrieval and attention studies
An important piece of evidence supporting the DAP hypothesis is that the regions associated with top-down and bottom-up attentional processes in fMRI studies of episodic retrieval match well with the regions associated with these processes in fMRI studies of attention, namely DPC and VPC. However, even if activation patterns overlap at the broad level, differences may still exist regarding the relative strength of left vs. right hemisphere activations or the spatial distribution within specific subregions of parietal cortex.
A difference in lateralization may exist for VPC activations related to bottom-up attention, which tend to stronger in the left hemisphere in fMRI studies of episodic retrieval (Vilberg & Rugg, 2008), but in the right hemisphere in fMRI studies of attention (Corbetta & Shulman, 2002). However, this is not a serious problem for the DAP hypothesis. First, although detection-related VPC activations in fMRI studies of attention may be stronger on the right hemisphere, they are usually significant in both hemispheres (e.g., Clark, Fannon, Lai, Benson, & Bauer, 2000; Downar, Crawley, Mikulis, & Davis, 2000, 2001; Kiehl, Laurens, Duty, Forster, & Liddle, 2001; Marois, Leung, & Gore, 2000). Thus, left VPC contributes to bottom-up attention in both episodic retrieval and attention fMRI studies. Second, most fMRI studies of episodic retrieval studies have employed meaningful verbal materials while most fMRI studies of attention have employed meaningless perceptual materials. Therefore, differences in lateralization can be explained by well-known hemispheric asymmetries in processing verbal vs. sensory stimuli (e.g., Milner, 1971)
A difference in the localization of VPC activations related to bottom-up attention may also exist within hemispheres because in fMRI studies of episodic retrieval (Vilberg & Rugg, 2008), these activations tend to extend posteriorly from the supramarginal gyrus (towards the angular gyrus), whereas in fMRI studies of attention (Corbetta & Shulman, 2002), they tend to extend anteriorly and ventrally from the supramarginal gyrus (towards anterior supramarginal and superior temporal regions). This apparent difference in anterior-posterior localization is not a problem for the DAP hypothesis because the two distributions show substantial overlap in the supramarginal gyrus. Also, there are many exceptions to anterior-posterior differences localization. For example, several episodic retrieval studies have found recollection-related activations in anterior supramarginal and superior temporal regions (e.g., Eldridge, Knowlton, Furmanski, Bookheimer, & Engle, 2000; Yonelinas, Otten, Shaw, & Rugg, 2005), and several attention studies have found detection-related activations in the angular gyrus (e.g., Arrington, Carr, Mayer, & Rao, 2000; Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000; Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005). Thus, within the data available, similarities in localization seem more consistent than the potential differences.
In sum, although there are some differences in the localization of VPC activations related to bottom-up attention in episodic retrieval and attention studies, these differences do not represent a serious problem for the DAP hypothesis. Lateralization differences may be explained by differences in stimuli, and anterior-posterior differences are not consistent. At any rate, further research directly comparing the localization of VPC activations across domains is warranted.
Domains of functional neuroimaging evidence accounted by the DAP hypothesis
The section on functional neuroimaging evidence focused on a few data sets directly relevant to the DAP hypothesis, but did not consider how well this hypothesis accounts for other functional neuroimaging findings, such as retrieval success activations and activations supporting alternative hypotheses.
In the domain of functional neuroimaging of episodic retrieval, the term "retrieval success" is typically used to describe activations identified by comparing hits (correct "old" responses) to correct rejections (correct "new" responses). Retrieval success activations have been often found in parietal regions. A question arises because according to the DAP hypothesis VPC responds to both high-confidence old and new responses, and DPC responds to both low-confidence old and new responses (see Figure 2-B). If parietal regions respond to both old and new items, why would they show greater activity for hits than correct rejections? One possible answer is that contrasts between hits and correct rejections have been often confounded with differences in bottom-up and/or top-down processing. For example, when hits are more confident that CRs, the DAP hypothesis predicts VPC activations. As illustrated by Figure 2-B, if one compares "definitely old" trials (level 6) to "most likely new" trials (level 2), one is likely to find VPC activations. Conversely, when hits involve more demanding search or decision processes than correct rejections, the DAP hypothesis predicts activation in DPC. Importantly, given that the DAP hypothesis assumes that top-down and bottom-up processing are separate dimensions, differences in both dimensions may occur simultaneously, thereby yielding activations in both VPC and DPC. Thus, unless confounding factors are controlled, retrieval success activations are difficult to interpret in relation to the DAP hypothesis.
The section on functional neuroimaging evidence focused on data supporting the DAP hypothesis, but how about evidence supporting alternative hypotheses, such as the mnemonic accumulator and the output buffer hypotheses? The main finding supporting the mnemonic accumulator hypothesis is evidence that certain parietal regions show greater activity for "old" than for "new" responses regardless of whether the "old" responses are correct (hits) or incorrect (false alarms) (Kahn, Davachi, & Wagner, 2004; Wheeler & Buckner, 2003). As noted above, the old-new difference may reflect a confound in confidence levels. A similar response for hit and false alarms fits well with the DAP hypothesis because parietal regions are assumed to mediate attentional processes rather than memory processes per se. An item perceived as old will tend grab attention regardless of the accuracy of this perception. Unlike the mnemonic accumulator hypothesis, however, the DAP hypothesis assumes that a strong feeling of novelty may also drive parietal responses in VPC (see Figure 2-B).
Turning to the output buffer hypothesis, the main functional neuroimaging evidence supporting this account is evidence that VPC shows greater activity for recollection than for familiarity (Vilberg & Rugg, 2008). As noted above, the DAP hypothesis can account for this evidence because recollected trials are also highly relevant, and hence, they grab attentional resources in a bottom-up fashion. In other words, VPC is associated with recollection not because this region holds recollected information but because it mediates bottom-up attentional processes. The DAP hypothesis can also account for the involvement of DPC in familiarity-based recognition decisions (Vilberg & Rugg, 2008). In general, familiarity-based decisions are more demanding than recollection-based decisions because they are closer to the old-new decision criterion. Consistent with this idea, in the Remember/Know paradigm, familiarity-based Know responses are much slower (e.g., 500–800 ms) than recollection-based Remember responses (e.g., Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Wheeler & Buckner, 2004; Woodruff, Johnson, Uncapher, & Rugg, 2005). Thus, according to the DAP hypothesis, DPC is associated with familiarity not because this region is specialized in processing a special quality of the familiarity signal but because familiarity-based decisions place greater demands on top-down attention.
In sum, the DAP hypothesis can accommodate retrieval success activations as well as functional neuroimaging evidence supporting the mnemonic accumulator and the output buffer hypotheses. However, accounting for this evidence requires additional assumptions, such as the notion that retrieval success contrasts (hits vs. correct rejections) are often confounded by differences in confidence, which require independent validation.
Potential future extensions of the hypothesis
Finally, another open question is whether the DAP hypothesis could be expanded to account for the ventral-dorsal distribution of episodic retrieval activations within PFC. As noted before, C&S model assumes that, in the attention domain, dorsal PFC regions are more involved in top-down attention and ventral PFC regions, in bottom-up attention. Could that be also the case for episodic retrieval? It is not difficult to find examples supporting this idea. For example, in the data shown in Figure 2-A, not only dorsal parietal but also dorsolateral PFC activity was greater for low than high confidence recognition hits (Kim & Cabeza, 2007). The involvement of dorsolateral PFC in low confidence recognition has been also found in other fMRI studies (e.g., Fleck, Daselaar, Dobbins, & Cabeza, 2006; Henson, Rugg, Shallice, & Dolan, 2000). For instance, consistent with the idea that these activations reflect a general top-down attentional mechanism rather than memory-specific processes, one study (Fleck, Daselaar, Dobbins, & Cabeza, 2006) found an overlap between episodic retrieval and visual perception (see Figure 5-A). Although the C&S model associates top-down attention with frontal eye fields, it is possible that dorsolateral PFC plays a more important role for nonspatial stimuli. Turning to bottom-up attention, several fMRI studies have found activations in ventrolateral PFC regions as a function of the amount of information recovered from episodic and semantic memory (e.g., Prince, Tsukiura, Daselaar, & Cabeza, 2007; Thompson-Schill, D. Esposito, Aguirre, & Farah, 1997). Moreover, one study (Daselaar, Fleck, & Cabeza, 2006) found that ventrolateral PFC regions showed an upright-U response (see Figure 5-B) very similar to the one displayed by VPC in the same study (see Figure 2-B). At any rate, the literature on PFC activity during episodic retrieval is large and complex, and reviewing it is beyond the scope of this article. A reasonable conclusion at this point is that it is not implausible that the DAP hypothesis could be extended to include also PFC regions.
Figure 5.
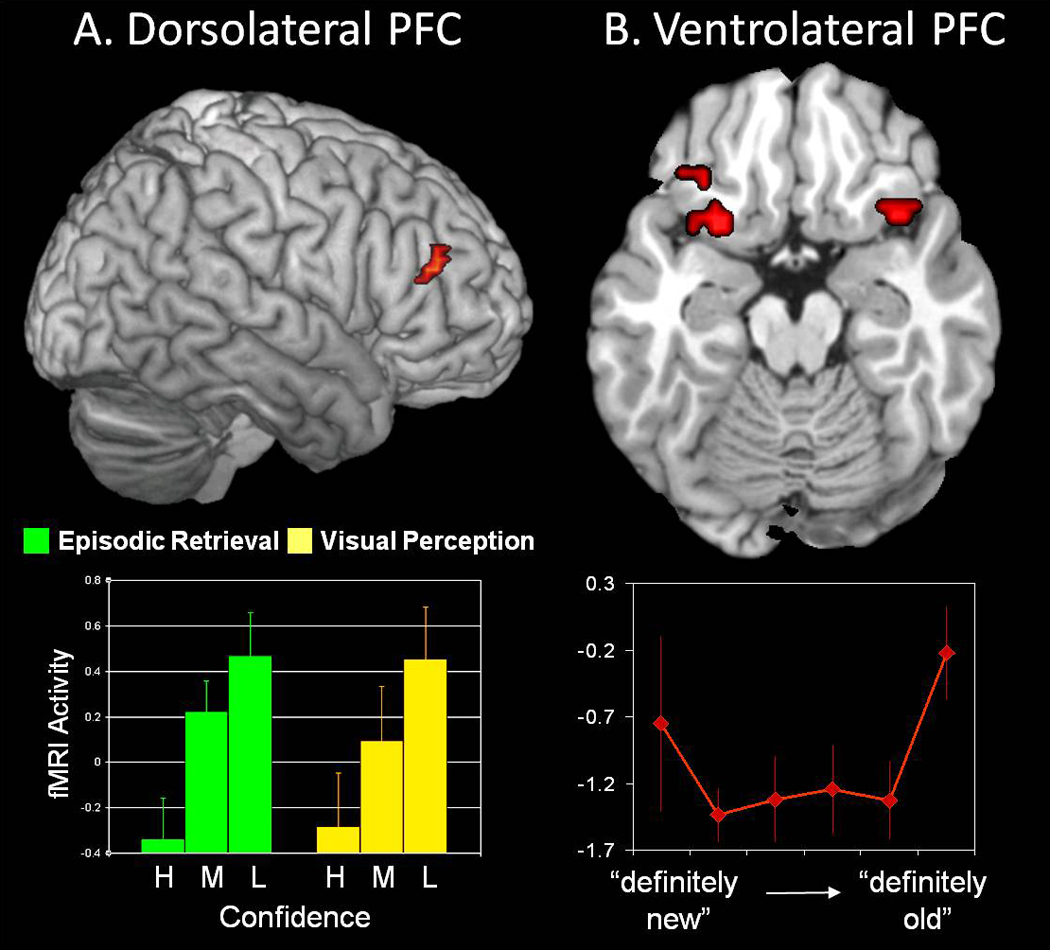
Evidence supporting a possible extension of the DAP hypothesis to the PFC. A. Consistent with the possibility that dorsal PFC regions contribute top-down attentional process to episodic retrieval, activity in a dorsolateral PFC region (BA 46/10) increased parametrically from high (H), to medium (M), to low (L) confidence during a word recognition task (Fleck, Daselaar, Dobbins, & Cabeza, 2006). The idea that this effect is related to top-down attention rather than to memory-specific operations is supported by the fact that the same region showed similar confidence effects during a visual perception task (area size comparison). B. Consistent with the possibility that ventral PFC regions contribute bottom-up attentional process to episodic retrieval, activity in a ventrolateral PFC region (BA 47) showed greater activity for highly relevant "definitely new" and "definitely old" responses in a word recognition test (Daselaar, Fleck, & Cabeza, 2006). The upright-U activation pattern in response to perceived oldness is almost identical to the one displayed by VPC in the same study (see yellow region in Figure 2-B).
Conclusions
In sum, the present article introduced a new hypothesis regarding the contributions of parietal regions to episodic retrieval. According to this DAP hypothesis, DPC contributes to top-down attentional processes guided by retrieval goals, whereas VPC contributes bottom-up attentional processes captured by the retrieval output. Although different, these processes interact very closely: goals determine the relevancy of incoming information and incoming target may alter behavioral goals. The DAP hypothesis is closely related to preexisting ideas, including Corbetta and Shulman's (2002) attention model and other hypotheses regarding parietal cortex and episodic retrieval reviewed by Wagner et al. (2005), but it differ from these previous ideas in several ways, yielding new predictions for functional neuroimaging and neuropsychological research.
Three sets of neuroimaging findings were considered: memory performance, cross-function comparisons, and encoding-retrieval differences. First, regarding memory performance, the DAP hypothesis predicts that DPC should display greater activity when memory performance is low (greater demands of top-down attention), whereas VPC should show greater activation when memory performance is high (stronger capture of bottom-up by relevant old and new stimuli). This prediction is consistent with many published studies, including those distinguishing between recollection and familiarity (Vilberg & Rugg, 2008). Unlike the output buffer account of VPC function, the DAP hypothesis attributes the role of this region to bottom-up attention processes, and hence, it predicts that this region should be also activated for high-confidence new responses. The data in Figure 2-B directly supports this specific prediction. Second, regarding cross-function comparisons the DAP hypothesis predicts that DPC activations during episodic retrieval should overlap with DPC activations during tasks that recruit top-down attention. In keeping with this prediction, a study found that a cued attention task heavily dependent on top-down attention activated the same DPC regions as episodic retrieval but did not activate VPC regions recruited by episodic retrieval (Figure 3). Finally, the DAP hypothesis can account for the finding that VPC activity is associated with successful retrieval but with unsuccessful encoding. During retrieval, VPC activity reflects the capture of bottom-up attention by relevant memory targets successfully retrieved from episodic memory, whereas during encoding, VPC activity reflects the capture of bottom-up attention by unrelated thoughts or environmental stimuli, which diverts resources from encoding.
Turning to lesion evidence, the DAP hypothesis predicts that VPC lesions should produce a deficit that, by analogy with the effects of parietal lesions in vision, could be described as "memory neglect." Patients with memory neglect should have difficulties spontaneously reporting relevant details in retrieved memories (bottom-up attention) but they should be able to access these details when guided by specific questions (top-down attention). Consistent with this prediction, a recent study (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007) found that patients with VPC lesions showed a deficit in spontaneously reporting details of retrieved autobiographical memories but not in answering specific questions about the same memories. This finding suggests that these patients do not have a deficit in retrieving episodic memories but in the mechanism whereby retrieved memories capture bottom-up attentional resources. In contrast, their ability to access memory details when guided by specific probes suggests their top-down attentional processes are intact.
Although the foregoing findings are consistent with the DAP hypothesis, the present article did not provide a comprehensive review of the functional neuroimaging and lesion literature, and hence, it is possible that findings inconsistent with this hypothesis can be found. However, given that no theoretical account of parietal involvement in episodic retrieval can account for all available data, the existence of some inconsistent findings does not necessarily hinder the value of the DAP hypothesis when compared to alternative hypotheses. At any rate, broad distinctions such as the one proposed by the C&S model and extended by the DAP hypothesis should be considered as useful heuristics that help guide research, which eventually should replace these simple distinctions with more complex and accurate models of neurocognitive function.
Acknowledgements
Special thanks to Marian Berryhill, Nancy Dennis, Scott Hayes, Morris Moscovitch, Lars Nyberg, Ingrid Olson, Michael Rugg, Kaia Vilberg, and three anonymous reviewers for insightful comments. This work was funded by NIH grants AG19731 and AG23770.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: Object-based selection of a region in space. Journal of Cognitive Neuroscience. 2000;12:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince S, Rice H, Weissman D, Nyberg L. Attention-related activity during episodic memory retrieval: A cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging Cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberga L. Seeing the forest through the trees: The cross-function approach to functional neuroimaging. In: Zani A, Proverbio AM, editors. The Cognitive Electrophysiology of Mind and Brain. San Diego: Academic Press; 2002. pp. 41–68. [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. Journal of Neurophysiology. 2000;83(5):3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Conway MA, Turk DJ, Miller SL, Logan J, Nebes RD, Meltzer CC, et al. A positron emission tomography (PET) study of autobiographical memory retrieval. Memory. 1999;7(5–6):679–702. doi: 10.1080/096582199387805. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple Dissociation in the Medial Temporal Lobes: Recollection, Familiarity, and Novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiplle prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and Domain-sensitive Prefrontal Mechanisms for Recollecting Events and Detecting Novelty. Cereb Cortex. 2005 doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2005;14(6):1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Driver J, Mattingley JB. Parietal neglect and visual awareness. Nat Neurosci. 1998;1(1):17–22. doi: 10.1038/217. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engle SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3(11):1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;16(11):1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14(11):1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:635–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: Dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12:913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24(17):4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Davis ES, Gabrieli JD. Neural correlates of actual and predicted memory formation. Nat Neurosci. 2005;8(12):1776–1783. doi: 10.1038/nn1595. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Roediger HL. The critical importance of retrieval for learning. Science. 2008;319(5865):966–968. doi: 10.1126/science.1152408. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. Neural sources involved in auditory target detection and novelty processing: An event-related fMRI study. Psychophysiology. 2001;38(1):133–142. [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: Dissociating the neural correlates of confidence in veridical vs. illusory memories. Journal of Neuroscience. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience. 2005;25(18):4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage. 1999;10(6):695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Levine B. Autobiographical memory and the self in time: brain lesion effects, functional neuroanatomy, and lifespan development. Brain and Cognition. 2004;55:54–68. doi: 10.1016/S0278-2626(03)00280-X. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16(9):1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126(Pt 7):1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Marois R, Leung HC, Gore JC. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25(3):717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16(5):504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37(4):1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Moritz S, Glascher J, Sommer T, Buchel C, Braus DF. Neural correlates of memory confidence. Neuroimage. 2006;33(4):1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4(3):257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Forkstam C, Petersson KM, Cabeza R, Ingvar M. Brain imaging of human memory systems: Between-systems similarities and within-system differences. Cognitive Brain Research. 2002;13:281–292. doi: 10.1016/s0926-6410(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Current Biology. 2001;11(19):1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JDE. Intergration of diverse information in working memory within the frontal lobe. Nature Neuroscience. 2000;3:85–89. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Prince SE, Tsukiura T, Daselaar SM, Cabeza R. Distinguishing the neural correlates of episodic memory encoding and semantic memory retrieval. Psychological Science. 2007 doi: 10.1111/j.1467-9280.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41(3):378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Heit E. Two-process models of recognition memory: Evidence for recall-to-reject? Journal of Memory and Language. 1999;40(3):432–453. [Google Scholar]
- Rotello CM, Heit E. Associative recognition: a case of recall-to-reject processing. Mem Cognit. 2000;28(6):907–922. doi: 10.3758/bf03209339. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Hove: Psychology Press; 2002. pp. 3–37. [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16(2):114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the pareital lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12(4):425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49(6):917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from event-related fMRI. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Shannon B, Kahn I, Buckner R. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Davachi L. Cognitive neuroscience: forgetting of things past. Curr Biol. 2001;11(23):R964–R967. doi: 10.1016/s0960-9822(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Buckner RL. Functional dissociation among components of remembering: Control, perceived oldness, and content. Journal of Neuroscience. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21(4):1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43(7):1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yantis S. Goal-directed and stimulus-driven determinants of attentional control. In: Monsell S, Driver J, editors. Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 73–103. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang XC, Sun XW, Li ZH, Wang ZX, He S, et al. Cross-modal temporal order memory for auditory digits and visual locations: an fMRI study. Human Brain Mapping. 2004;22(4):280–289. doi: 10.1002/hbm.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]