Muscle fiber size and function in elderly humans: a longitudinal study (original) (raw)
Abstract
Cross-sectional studies are likely to underestimate age-related changes in skeletal muscle strength and mass. The purpose of this longitudinal study was to assess whole muscle and single muscle fiber alterations in the same cohort of 12 older (mean age: start of study 71.1 ± 5.4 yr and end of study 80 ± 5.3 yr) volunteers (5 men) evaluated 8.9 yr apart. No significant changes were noted at follow-up in body weight, body mass index, and physical activity. Muscle strength, evaluated using isokinetic dynamometry, and whole muscle specific force of the knee extensors were significantly lower at follow-up. This was accompanied by a significant reduction (5.7%) in cross-sectional area of the total anterior muscle compartment of the thigh as evaluated by computed tomography. Muscle histochemistry showed no significant changes in fiber type distribution or fiber area. Experiments with chemically skinned single muscle fibers (n = 411) demonstrated no change in type I fiber size but an increase in IIA fiber diameter. A trend toward an increase in maximal force in both fiber types was observed. Maximum unloaded shortening velocity did not change. In conclusion, single muscle fiber contractile function may be preserved in older humans in the presence of significant alterations at the whole muscle level. This suggests that surviving fibers compensate to partially correct muscle size deficits in an attempt to maintain optimal force-generating capacity.
Keywords: aging, compensatory hypertrophy, single muscle fibers
the loss of muscle mass (sarcopenia) and strength in elderly is one of the most significant physiological and clinical problems in gerontology and geriatrics. The incidence of sarcopenia increases with advancing age and is not limited by geographic region or ethnic group (4). In older men and women, muscle weakness and atrophy are causally related to the loss of functional independence, increase in morbidity, higher rate of hospitalization, and mortality after bone fractures due to falls (26). In the elbow and knee extensors and flexors of aging humans, the loss of muscle strength ranges from 1 to 3% per year (13). In the vastus lateralis, this strength loss could partly be explained by a 1% reduction per year in the number of muscle fibers (23).
Several studies have reported age-related decrements in muscle size and function at the whole muscle level (8, 9, 13). However, the loss is neither universal nor homogeneous. It is noteworthy that, in one longitudinal study, approximately one-third of the participating individuals (mean age at start of study = 60.4 yr) showed no loss of muscle strength over 10 yr (15). We have reported significant differences in specific force in single skinned muscle fibers between young and older men (10). In that study, however, there were no significant differences in muscle fiber cross-sectional area, and the reduction in specific force was due to a reduction in maximal single fiber force generation. This lack of muscle atrophy was also noted using histochemical techniques in a longitudinal study of nine older men, suggesting that surviving muscle fibers may compensate by maintaining their size (8).
Sarcopenia has been studied using both cross-sectional and longitudinal research designs. Cross-sectional studies are likely to underestimate the losses and represent a comparison only with those that have survived advanced age. In other words, persons with stronger muscles may have a better chance of being included in cross-sectional studies because they have survived to old age. The true magnitude and nature of the loss of muscle size and function in older men and women can only be determined in longitudinal studies of the same cohort of individuals. The fact that these types of studies are difficult to carry out may explain the limited longitudinal data in the scientific literature. To our knowledge, no study has looked at changes in single muscle fiber contractility and muscle quality (force adjusted by fiber size) in a longitudinal fashion. Further, no longitudinal study has combined whole muscle and single fiber experiments in the same cohort of individuals. The purpose of the present investigation was to examine changes in whole muscle and single fiber size and function in a cohort of aging humans over ∼9 yr.
MATERIALS AND METHODS
Subjects.
Twenty-four subjects (12 men) completed the study protocol in 1997–1998 (T1) including knee extensor strength testing, computed tomography (CT) scans of the thigh, and biopsies of the vastus lateralis muscle. In 2005–2006 (T2), attempts were made to contact all 24 prior participants. Four had died, two moved away, two subjects could not be located to send recruitment materials, and four subjects were contacted by mail and phone but elected not to participate. The remaining 12 subjects (5 men) enrolled and completed the study. All 12 subjects completed strength testing and CT scans of the thigh. For two subjects we did not have the CT data for 1997–1998 and thus the comparison is limited to 10 subjects (Table 1). Nine subjects underwent muscle biopsies, and the other three were excluded because of concurrent coumadin therapy.
Table 1.
General characteristics of subjects (n = 12)
| 1997–1998 | 2005–2006 | P | |
|---|---|---|---|
| Age, yr | 71.1±5.4 (62-81) | 80.0±5.3 (71-90) | <0.001 |
| Body weight, kg | 71.2±7.7 (59-87) | 72.6±10.4 (58-87) | 0.40 |
| Height, m | 1.67±0.1 (1.56-1.80) | 1.65±0.1 (1.56-1.76) | 0.53 |
| Body mass index, kg/m2 | 25.8±2.8 (21-30) | 26.6±3.2 (22-34) | 0.16 |
| Duration of follow-up, yr | 8.9±0.7 | ||
| Physical activity index, kcal/wk | 2,919±1,631 (84-5,232) | 2,261±1,620 (84-5,095) | 0.37 |
The volunteers received a complete explanation of the purposes and procedures and gave their written consent. A comprehensive medical evaluation, including a medical history, physical examination, routine blood and urine tests, and a resting electrocardiogram, was performed before their participation in the study at both time points. The level of recreational activity and sports participation over the last year was estimated using a questionnaire (22). This study was approved by the Human Investigation Review Committees of Tufts University-Tufts Medical Center and Spaulding Rehabilitation Hospital.
Muscle strength measurements.
An isokinetic dynamometer (Cybex II, Medway, MA) was used to measure strength (N·m) of the nondominant knee extensors and flexors at 60°/s and 240°/s, as previously reported (9). The placement of the lever arm with relation to the subject's leg was carefully controlled. The subjects performed five maximal voluntary contractions at each velocity, and the peak torque was recorded and adjusted for leg weight.
CT.
A CT scan of the nondominant thigh was performed at the midpoint of the femur for each subject. The length of the femur was determined from a coronal scout image as the distance between the intercondylar notch and the trochanteric notch. At T1, the scanner was a GE Highspeed Advantage CT scanner (Milwaukee, WI) operating at 100 kV and 170 mA. Scans were obtained at T2 using a Siemens Somotom Scanner (Erlangen, Germany) operating at 120 kV and 100 mA. Technical factors were slice width of 10 mm and a scanning time of 1 s.
All scans were analyzed by a single investigator in a blinded manner using SliceOmatic v4.2 software (Montreal, Canada). Images were reconstructed on a 512 × 512 matrix with a 25-cm field of view. From the images, the cross sectional areas (CSAs) for normal density muscle and low density muscle, subcutaneous adipose tissue (AT), subfascial AT, and intramuscular AT were measured using manual tracing. A one-pixel width line was used to separate anterior from posterior muscle groups, using, when applicable, intermuscular AT as a guide. To ensure proper placement of this line on T2 scans, the T1 analysis was consulted as a guide. Muscle CSA was measured in the range of 0-100 Hounsfield units (HU) and calculated as the sum of low-density muscle and normal-density muscle CSA (areas of AT not included). AT areas were measured in the range of −190 to −30 HU. One tracing was drawn between, on the inside edge of the fascia, to distinguish between subcutaneous AT and subfascial AT. Similarly, another tracing was drawn to separate subfascial AT from intramuscular AT, defining subfascial AT as that lying just below the fascial plane. Intermuscular AT was defined as AT lying between and among muscle groups. These methods have been previously described (12, 16).
Whole muscle specific force.
For each of the muscle compartments, whole muscle specific force was calculated as the ratio of muscle strength and muscle CSA.
Muscle biopsy.
Muscle biopsies were taken from the vastus lateralis muscle at the level of the CT scan using a 5-mm Duchenne biopsy needle (3) and suction (6). The same biopsy site was used on each occasion (T1 and T2) since it was possible to identify the scar of the biopsy at T1 in all subjects.
The specimen was divided in two pieces. One piece was laid down longitudinally in a plastic cryomold, mounted in embedding medium (OCT, Sakura Finetek, Torrance, CA), frozen in isopentane slush, cooled to the temperature of liquid nitrogen, and stored in liquid nitrogen for later sectioning and staining.
For histochemical analysis, serial 10-μm cross sections were cut in a cryostat (Leica CM1850) at −24°C. To obtain values of fiber-type mean area, sections were stained for myofibrillar ATPase at pH 9.40 after preincubation at pH 4.35. The mean number of fibers counted per biopsy was 69 ± 26 (range 32-107) in T1 and 442 ± 229 (range 56–716) in T2. Measurements of type I and II fiber areas were performed using the NIH Image-based software Scion Image for Windows (Scion, version alpha 4.0.3.2). All measurements were done once for calculation of muscle fiber areas. Only areas without artifacts and distinct cell borders were measured. The mean number of fibers measured per biopsy was 33 ± 9 (range 17–40) in T1 and 228 ± 133 (range 12–421) in T2.
Single muscle fiber experiments.
The second piece of the biopsy was placed in relaxing solution (see below) at 4°C within 1-2 min of being obtained. Bundles of ∼30 fiber segments were dissected free from the samples and then tied with surgical silk to glass capillary tubes at slightly stretched lengths. The fiber segments were chemically skinned for 24 h in relaxing solution containing 50% (vol/vol) glycerol at 4°C and were subsequently stored at −20°C for up to 4 wk before use.
A detailed explanation of the general methods used in this study has been published by others (20). Briefly, on the day of an experiment, fiber segments were placed for 30 min in relaxing solution containing 0.5% Brij-58 (polyoxyethylene 20 cetyl ether; Sigma, St. Louis, MO) before mounting in an experimental apparatus, similar to that described previously (25). A fiber segment length of 1-2 mm was left exposed to the solution between connectors leading to a force transducer (model 400A; Aurora Scientific, Aurora, Ontario, Canada) and a DC torque motor (model 308B; Aurora Scientific). The apparatus was mounted on the stage of an inverted microscope (Olympus IX70, Tokyo, Japan). While the fiber segments were in relaxing solution, sarcomere length (SL) was set to 2.75-2.85 μm by adjusting the overall segment length (10).
The sarcomere length, the segment diameter, and the length of segment between the connectors were measured with an image analysis system (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). Fiber depth was measured by recording the vertical displacement of the microscope nosepiece while focusing on the top and bottom surfaces of the fiber. The focusing control of the microscope was used as a micrometer. In our hands the coefficient of variation for three measurements done by the same observer is 0.5% for diameter and 3.7% for depth. Fiber cross-sectional area was calculated from the diameter and depth, assuming an elliptical circumference. Maximum force (Po) was adjusted for fiber cross-sectional area after adjusting fiber area for the 20% swelling that is known to occur during skinning (11, 25).
Relaxing and activating solutions contained (in mM) 4 MgATP, 1 free Mg2+, 20 imidazole, 7 EGTA, 14.5 creatine phosphate, and KCl to adjust the ionic strength to 180 mM. The pH was adjusted to 7.0. The concentrations of free Ca2+ were 10−9 M (relaxing solution) and 10−4.5 M (maximum activating solution) and are expressed as pCa (−log [Ca2+]). Apparent stability constants for Ca2+-EGTA were corrected for temperature (15°C) and ionic strength (180 mM) (7). A computer program (7) was used to calculate the concentrations of each metal, ligand, and metal-ligand complex.
Immediately preceding each activation, the fiber was immersed for 10–20 s in a solution with a reduced Ca2+-EGTA buffering capacity. This solution was identical to the relaxing solution except that EGTA was reduced to 0.5 mM, which resulted in a faster attainment of steady tension during subsequent activation. Maximum active force (Po) was calculated as the difference between the total force in activating solution (pCa 4.5) and the resting tension measured in the same segment while in the relaxing solution. All contractile measurements were carried out at 15°C. Fibers with visible tears and fibers demonstrating a loss of force >10% of the baseline value were not used for the analysis.
Maximum unloaded shortening velocity was measured using the slack test (5).
Myosin heavy chain composition.
After mechanical measurements, each fiber was placed in SDS sample buffer in a plastic microfuge tube and stored at −20°C for up to 1 wk or at −80°C if the gels were to be run later. The myosin heavy chain (MyHC) composition of single fibers was determined by SDS-PAGE (19). The acrylamide concentration was 4% (wt/vol) in the stacking gel and 6% in the running gel, and the gel matrix included 30% glycerol. Sample loads were kept small (equivalent to ∼0.05 mm of fiber segment) to improve the resolution of the MyHC bands (types I, IIA, IIB). The conditions in which the SDS-PAGE were run include constant current (24 mA) for 5.5 h. Proteins were identified using a combination of human myosins from vastus lateralis muscles and from reports in the literature (20).
Statistical analysis.
All variables were examined for normality both graphically and statistically. Means and SDs were calculated. Paired samples _t_-tests and Wilcoxon matched pairs signed-rank tests were used to detect significant differences between T1 and T2. Statistical significance was accepted if the two-tailed P value was <0.05. Data were analyzed using SPSS statistical software (version 15.0 for Windows, Chicago, IL).
RESULTS
Subjects.
The characteristics of the subjects are presented in Table 1. After 8.9 yr of follow up, the subjects were older, but body weight, body mass index, and the physical activity index had not changed significantly.
Muscle strength.
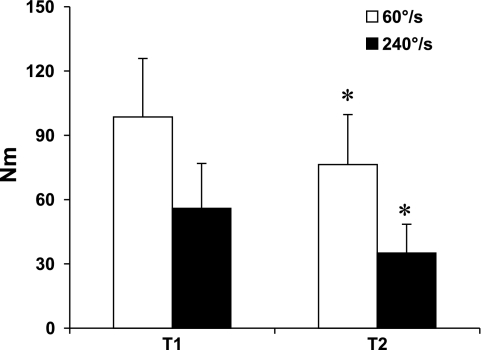
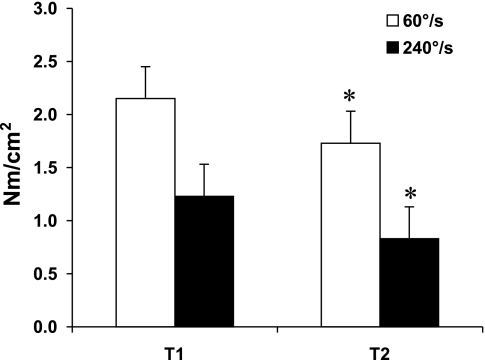
Significant losses in muscle strength of the knee extensors were noted at slow and fast angular velocities (Tables 2 and 3; Figs. 1 and 2). The average percent loss per year was 2.56 at 60 °/s and 4.20 at 240°/s. No significant changes were noted in the knee flexors. Whole muscle specific force was lower in the knee extensors but did not change in the knee flexors.
Table 2.
Longitudinal changes in isokinetic muscle strength (N·m) of the nondominant leg (n = 12)
| 1997–1998 | 2005–2006 | Delta | %Change | %Change/yr | P Value | |
|---|---|---|---|---|---|---|
| Knee extensors | ||||||
| 60°/s | 98.5±27.4 | 76.3±23.4 | −22.2±11.7 | −22.4±8.9 | −2.56±1.1 | <0.001 |
| 240°/s | 55.9±20.9 | 35±13.5 | −20.9±10.4 | −37.1±10.2 | −4.20±1.2 | <0.001 |
| Knee flexors | ||||||
| 60°/s | 56.7±16.9 | 59.5±18.9 | 2.8±10.7 | 5.9±19.4 | 0.68±2.2 | 0.38 |
| 240°/s | 35.1±14.7 | 33.1±15.6 | −2.0±8.1 | −4.2±15 | −0.50±1.6 | 0.39 |
Table 3.
Longitudinal changes in specific peak torque (N·m/cm2) of the nondominant leg (n = 10)
| 1997–1998 | 2005–2006 | Delta | %Change | %Change/yr | P Value | |
|---|---|---|---|---|---|---|
| Knee extensors | ||||||
| 60°/s | 2.15±0.3 | 1.73±0.3 | −0.42±0.2 | −19.3±7.7 | −2.2±1 | <0.001 |
| 240°/s | 1.23±0.3 | 0.83±0.3 | −0.40±0.2 | −32.9±12.2 | −3.7±1.3 | <0.001 |
| Knee flexors | ||||||
| 60°/s | 1.08±0.2 | 1.16±0.3 | 0.08±0.3 | 8.4±24 | 1.03±2.8 | 0.34 |
| 240°/s | 0.7±0.23 | 0.65±0.2 | −0.05±0.2 | −4.9±20 | −0.54±2.3 | 0.29 |
Fig. 1.
Longitudinal changes in isokinetic knee extensor muscle strength. T1: 1997–1998. T2: 2005–2006. Values are means ± SD. *P < 0.001.
Fig. 2.
Longitudinal changes in specific isokinetic knee extensor muscle strength. Values are means ± SD. T1: 1997–1998. T2: 2005–2006. *P < 0.001.
Thigh composition.
A significant reduction in total muscle cross-sectional area (4.5%) and total anterior muscle area (5.7%) was observed (Table 4). The posterior muscle group did not change.
Table 4.
Skeletal muscle and thigh adipose tissue area (n = 10)
| 1997–1998 | 2005–2006 | Delta | %Change | P Value | |
|---|---|---|---|---|---|
| Total area, cm2 | 186.5±45.0 | 173.8±35.2 | −13.2±24 | −6.3±9 | 0.06 |
| Total muscle area, cm2 | 98.3±21.8 | 92.9±15.4 | −5.4±8 | −4.5±6 | 0.03 |
| Total subcutaneous adipose tissue, cm2 | 80.8±46 | 69.9±39 | −9.2±21 | −9.2±16 | 0.17 |
| Anterior muscle groups | |||||
| Total muscle, cm2 | 45.9±8.1 | 43.2±8 | −2.7±2.2 | −5.7±4.5 | 0.005 |
| Subfascial adipose tissue, cm2 | 0.91±0.4 | 0.77±0.3 | −0.14±0.3 | −12.7±23 | 0.11 |
| Intermuscular adipose tissue, cm2 | 0.54±0.3 | 0.73±0.5 | 0.2±0.6 | 58.8±102 | 0.20 |
| Posterior muscle groups | |||||
| Total muscle, cm2 | 52.4±14.7 | 49.7±9.2 | −2.7±7 | −3.2±9 | 0.20 |
| Subfascial adipose tissue, cm2 | 3.1±1.6 | 3.37±2.0 | 0.31±99 | 16.2±46 | 0.34 |
| Intermuscular adipose tissue, cm2 | 2.9±1.3 | 2.7±1.5 | −0.25±1.2 | −6.2±32 | 0.33 |
Histochemical analysis of muscle fiber composition and size.
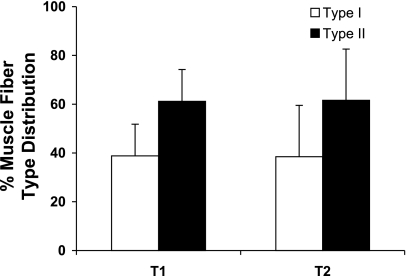
No changes were noted at T2 in fiber type distribution (Table 5 and Fig. 3). No significant changes were seen in average fiber area.
Table 5.
Longitudinal changes in muscle fiber type and area (n = 8)
| 1997–1998 | 2005–2006 | Delta | %Change | P Value | |
|---|---|---|---|---|---|
| Distribution, % | |||||
| Type I | 38.8±13 | 38.5±21 | −0.39±15 | −2.1±35 | 0.94 |
| Type II | 61.2±13 | 61.6±21 | 0.39±15 | −0.3±27 | 0.94 |
| Area, μm2 | |||||
| Type I average area | 4,069±1,080 | 4,524±1,324 | 455±1,275 | 13.98±30 | 0.35 |
| Type II average area | 3,649±1,506 | 3,664±1,213 | 15±1,818 | 9.2±37.6 | 0.67 |
Fig. 3.
Longitudinal analysis of muscle fiber-type distribution. T1: 1997–1998. T2: 2005–2006.
Size and contractile properties of single muscle fibers.
A total of 411 fibers were studied (247 at T1). At T2, type I fibers showed no change in diameter (P = 0.16) or cross-sectional area (P = 0.80) (Table 6). Type IIa fibers had a larger (P = 0.02) diameter at T2 and a nonsignificant increase (P = 0.17) in cross-sectional area. A trend toward an increase (P = 0.07) in maximal force was noted in both type I and IIa fibers. Specific force showed the same trend (P = 0.07) only in type I fibers. Maximum unloaded shortening velocity was unchanged in either fiber type.
Table 6.
Longitudinal changes in single muscle fiber size and contractile properties (n = 9)
| 1997–1998 | 2005–2006 | P Value | |
|---|---|---|---|
| Type I | |||
| No. of fibers | 183 | 105 | |
| Diameter, μm | 92±13 | 100±13 | 0.16 |
| Cross-sectional area, μm2 | 5020±1140 | 4910±1190 | 0.80 |
| Maximal force, μN | 493±108 | 605±191 | 0.07 |
| Specific force, N/cm | 14.8±2.0 | 19±5.7 | 0.07 |
| Shortening velocity, fiber lengths/s | 0.75±0.12 | 0.73±0.17 | 0.72 |
| Type IIA | |||
| No. of fibers | 64 | 59 | |
| Diameter, μm | 83±6 | 101±16 | 0.02 |
| Cross-sectional area, μm2 | 4,230±530 | 4,840±1,260 | 0.17 |
| Maximal force, μN | 482±113 | 541±168 | 0.07 |
| Specific force, N/cm2 | 17±3.5 | 17.2±5.0 | 0.88 |
| Shortening velocity, fiber lengths/s | 1.54±0.25 | 1.26±0.32 | 0.13 |
DISCUSSION
To our knowledge, this is the first study of longitudinal changes in whole muscle and single muscle fiber contractile properties in the same cohort of elderly subjects. The main observations of the present longitudinal study are 1) a significant loss of maximal knee extensor muscle force; 2) a significant reduction in whole muscle area, particularly of the anterior compartment of the thigh; 3) no change in fiber-type distribution or mean type I and II fiber area; 4) a significant increase in type IIa fiber diameter; and 5) no decrease in single fiber maximal force or specific force in both fiber types. Of particular interest is the contrast between the changes at the whole muscle level vs. the microscopic level. While in vivo muscle strength and whole muscle size showed the expected age-associated losses, the analysis of cross sections stained with histochemical techniques and the experiments performed with permeabilized single fibers suggest that individual muscle fibers that survived the effects of aging compensated for the loss of motor units by maintaining size, force production, and single fiber quality.
Whole muscle size and function.
The rate of loss of strength of the knee extensors found in the present study compares favorably with a recent three year follow-up of a large cohort of 1,880 volunteers with a mean age of 73.5 yr (13). These investigators reported annualized losses of knee extensor muscle strength of 2.6 to 4.1%. The losses were higher in men than women. Our present longitudinal findings showed larger reductions in muscle strength of the knee extensors expressed as percent change per year than our previous cross-sectional studies (9): 2.5 and 4.2 vs. 1.8 and 1.1 at slow and fast angular velocities, respectively. In general, these observations confirm our earlier findings of greater rates of muscle strength decline when the same cohort of subjects is measured longitudinally compared with cross-sectional differences among various age groups (15). When similar testing methods are used, this difference between studies of different designs is most likely due to selection bias of healthy older subjects in cross-sectional studies. Persons with stronger muscles may have a better chance of being included in cross-sectional studies because they have survived to old age.
A significant difference between the present study and previous longitudinal investigations (8, 15) is the fact that, in the present study, the flexors of the knee maintained their strength at follow-up at both slow and fast angular velocities. An explanation for this discrepancy is not immediately obvious. Some muscles may be more susceptible to the effects of aging than others. For example, in women, the loss of strength in the elbow extensors and flexors is less than the loss of strength in the knee extensors and flexors (15). In addition to possible intrinsic muscle susceptibility, several factors such as the level of limb-specific physical activity and the presence of diseases with regional effects (i.e., knee osteoarthritis) may also contribute to larger losses in some muscle groups.
It has been argued that the age-related decline in muscle strength is partially due to the loss of muscle mass (8, 13). In the present study, the pattern of muscle atrophy is similar to that of the loss of strength. In other words, the reduction in total muscle cross-sectional area can be explained by losses in the anterior (extensor) compartment of the thigh while no changes were observed in the posterior (flexor) compartment. Thus the absence of muscle loss in the flexor compartment may have contributed to the preservation of the strength of the flexor muscle group. It is interesting that the absolute and relative losses in the anterior compartment in the present study are smaller than in our previous study (8). This may be due to the fact that the subjects in the present study were older and had a smaller muscle size at baseline. Another possibility is that they maintained a similar level of physical activity during the period of observation (Table 1).
Muscle fiber type distribution and size.
As shown in Fig. 3, histochemical analysis of muscle cross sections stained for myofibrillar ATPase demonstrated no changes in muscle fiber-type distribution. This is in contrast with our previous longitudinal report (8) of a reduction in percentage of type I fibers after 12 yr. However, in that study, the volunteers were younger at baseline (mean age 65 yr). Also, the percentage of type I fibers at follow-up (40%) was very similar to the 38% reported at baseline and follow-up in the present study. The differences between studies may also be due to the inherent variability in fiber-type distribution in various areas of the vastus lateralis and the limitations of the muscle biopsy technique. On the other hand, in both the present and our previous study, no statistically significant change in mean cross-sectional area in either fiber type was observed. In fact, in the present study, a nonsignificant trend (P = 0.35) was in the direction of an increase in average type I fiber area. The lack of statistical significance may be related to a relatively small number of subjects and/or fibers and the possibility of a type II error.
Experiments with permeabilized single muscle fibers.
We are reporting for the first time longitudinal changes in permeabilized single muscle fibers obtained with the percutaneous muscle biopsy technique. Of importance, both type I and IIA fibers showed no atrophy after 10 yr using diameter or cross-sectional area as indexes of fiber size. In fact, the diameter of type IIA fibers was significantly larger (P = 0.02) at follow-up although the fiber cross-sectional area was not significantly larger. This mismatch between changes in diameter and cross-sectional area could be explained by flattening of the fibers that has been reported to be more pronounced among type II fibers (2). Maximal force production at the single fiber level tended to be higher (P = 0.07) at follow-up in both type I and IIA fibers. Because type I fiber size did not change, specific force (an index of muscle quality) also tended to be higher (P = 0.07) at follow-up. In the case of type IIA fibers, specific force did not change.
These data differ from a cross-sectional study published by our group (10). A cross-sectional comparison of young and older groups with an average difference in age of 37 yr demonstrated significantly lower maximal force and specific force in type I and IIA fibers in elderly. The inclusion of healthier older survivors in the present study could explain these differences between studies. Another possible explanation is the fact that the subjects in the present study were more physically active and maintained their weekly caloric expenditure over the 10 yr. In addition, a time span of 9 yr may have been too short to detect a significant change in contractile function in the very old, and the relatively small number of subjects increases the possibility of a type II statistical error.
Compensatory muscle hypertrophy.
Compensatory hypertrophy in animal models is a well-known phenomenon that occurs after, for example, selective tenotomy of agonist muscles. The presence of atrophied and hypertrophied fibers has been reported in late middle-aged rat soleus muscle (14). Finally, a mutation of the gene for IIB myosin heavy chain that leads to the loss of muscle fibers results in compensatory hypertrophy of all fiber types in the mouse (1). Therefore, different pathological and age-related conditions can be associated with compensatory hypertrophy. In humans, this phenomenon has been described and documented using magnetic resonance imaging at the whole muscle level after selective neurectomy (21). Selective neurectomy of the nerve to the medial gastrocnemius resulted in atrophy of the medial gastrocnemius and compensatory hypertrophy of the lateral gastrocnemius and part of the soleus muscle. A similar lesion of the nerve to the soleus resulted in hypertrophy of the gastrocnemius muscle. However, in that study, the evaluation of muscle size was limited to whole muscle and muscle fibers were not studied.
It is possible that some of the age-associated changes in skeletal muscle are induced by disuse. Thus it may seem contradictory to find no change in muscle size or strength in single fibers obtained from aged muscle. It could be that the relative maintenance of levels of physical activity in the present study had a preventative effect. However, other human models of prolonged disuse showed similar findings. The absence of atrophy and weakness in single muscle fibers after prolonged disuse has been reported in patients with chronic spinal cord injury (SCI) (24), amyotrophic lateral sclerosis (18), and inclusion body myositis (17). Patients with chronic SCI, both complete and incomplete, demonstrated nonsignificant increases in cross-sectional area and maximal force of type I and IIa single fibers compared with normal control subjects. The authors speculated that the main effect of chronically reduced neuromuscular activity is a loss of muscle cells, leaving a population of surviving fibers that have equal or better contractile performance. Similar to the findings in our study, this preservation of size and contractile performance was not dependent on the fiber type.
Our findings suggest that sarcopenia should not be conceptualized as a linear process that begins after 30 yr of age and progresses at a constant rate beyond age 80 yr. The loss of muscle mass with advancing age may be more like a hyperbola with a slower rate of muscle loss in older age groups partially due to compensatory single muscle fiber hypertrophy or maintenance of muscle fiber size. These findings suggest a dissociation between changes in muscle size and the process that results in the loss of muscle strength.
Conclusion.
We report longitudinal changes in whole muscle size and strength in older adults that is consistent with previous reports. We also show for the first time that despite these changes at the whole muscle level, single muscle fiber contractile function is preserved with advancing age. These data suggest that with loss in whole muscle size, existing fibers that have survived may compensate to partially correct these deficits in an attempt to maintain optimal force-generating capacity even at very old age (80 yr).
GRANTS
This work was supported by National Institute on Aging Grant AG-18844 and by the USDA under Agreement No. 58-1950-7-707.
Acknowledgments
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture (USDA).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA. Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol 280: C637–C645, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JL Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13: 40–47, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J Muscle electrolytes in man.. Scand J Clin Lab Invest 14, _Suppl_1–68, 1962. [Google Scholar]
- 4.Doherty TJ Aging and sarcopenia. J Appl Physiol 95: 1717–1727, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Edman KA The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1982. [PubMed] [Google Scholar]
- 7.Fabiato A Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378–417, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88: 1321–1326, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol 71: 644–650, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Godt RE, Maughan DW. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J 19: 103–116, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 90: 2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Hepple RT, Ross KD, Rempfer AB. Fiber atrophy and hypertrophy in skeletal muscles of late middle-aged Fischer 344 × Brown Norway F1-hybrid rats. J Gerontol A Biol Sci Med Sci 59: 108–117, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56: B209–217, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr 54: 509–515, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Krivickas LS, Amato AA, Krishnan G, Murray AV, Frontera WR. Preservation of in vitro muscle fiber function in dermatomyositis and inclusion body myositis: a single fiber study. Neuromuscul Disord 15: 349–354, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Krivickas LS, Yang JI, Kim SK, Frontera WR. Skeletal muscle fiber function and rate of disease progression in amyotrophic lateral sclerosis. Muscle Nerve 26: 636–643, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 20.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472: 595–614, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CJ, Park JH, Park SW. Compensatory hypertrophy of calf muscles after selective neurectomy. Aesthetic Plast Surg 30: 108–112, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lee IM, Paffenbarger RS Jr, Hsieh CC. Time trends in physical activity among college alumni, 1962-1988. Am J Epidemiol 135: 915–925, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol 102: 340–349, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Moss RL Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol 292: 177–192, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rantanen T, Sakari-Rantala R, Heikkinen E. Muscle strength before and mortality after a bone fracture in older people. Scand J Med Sci Sports 12: 296–300, 2002. [DOI] [PubMed] [Google Scholar]