Cannabinoid Sensitivity and Synaptic Properties of 2 GABAergic Networks in the Neocortex (original) (raw)
Abstract
Distinct networks of γ-aminobutyric acidergic interneurons connected by electrical synapses can promote different patterns of activity in the neocortex. Cannabinoids affect memory and cognition by powerfully modulating a subset of inhibitory synapses; however, very little is known about the synaptic properties of the cannabinoid receptor–expressing neurons (CB1-positive irregular spiking [CB1-IS]) in the neocortex. Using paired recordings in neocortical slices, we 1st report here that synapses of CB1-IS cells, but not synapses of fast-spiking (FS) cells, are suppressed by release of endocannabinoids from pyramidal neurons. CB1-IS synapses were characterized by a very high failure rate that contrasted with the high reliability of FS synapses. Furthermore, CB1-IS cells received excitatory inputs less frequently compared with FS cells and made significantly less frequent inhibitory contacts onto local pyramids. These distinct synaptic properties together with their characteristic irregular firing suggest that CB1-IS cells play different role in neocortical function than that of FS cells. Thus, whereas the synaptic properties of FS cells can allow them to impose high-frequency rhythmic oscillatory activity, those of CB1-IS cells may lead to disruption of fast rhythmic oscillations. Our results suggest that activity-dependent release of cannabinoids, by blocking CB1-IS synapses, may alter the role of inhibition in neocortical circuits.
Keywords: CB1, DSI, endocannabinoids, fast-spiking cells, gap junctions, microcircuits, neocortex
Introduction
Cannabinoids, including the active component of marijuana and endogenously released cannabinoids, are powerful modulators of inhibition in the cerebral cortex. They activate CB1 receptors in inhibitory axonal terminals and suppress inhibitory postsynaptic potentials (IPSPs) in pyramidal neurons (Freund et al. 2003; Piomelli 2003). Following postsynaptic depolarization, endogenous cannabinoids can be released from pyramidal cells to produce a brief suppression of inhibitory inputs at the postsynaptic cells (Ohno-Shosaku et al. 2001; Wilson et al. 2001; Trettel and Levine 2002, 2003; Chevaleyre and Castillo 2003; Fortin et al. 2004; Trettel et al. 2004; Bodor et al. 2005; Foldy et al. 2006; Glickfeld and Scanziani 2006; Fortin and Levine 2007). This form of postsynaptic activity–induced retrograde signaling via presynaptic CB1 receptors has been termed depolarization-induced suppression of inhibition (DSI) and may underlie cannabinoid actions in vivo (Alger 2002; Wilson and Nicoll 2002; Freund et al. 2003).
Interestingly, physiological studies of nonspecific inhibitory inputs at pyramidal cells suggest that only a subset of inhibitory synapses are suppressed by cannabinoids (Wilson et al. 2001; Trettel and Levine 2003; Bodor et al. 2005; Glickfeld and Scanziani 2006). In agreement, anatomical studies have reported that the expression of CB1 receptors is cell specific and only a subset of inhibitory terminals express functional CB1 receptors (Bodor et al. 2005; Glickfeld and Scanziani 2006). Yet, the synaptic properties and connectivity of the inhibitory neurons expressing CB1 receptors in the neocortex remain mostly unknown.
Distinct networks of γ-aminobutyric acidergic (GABAergic) interneurons interconnected by electrical synapses are responsible for promoting different patterns of coordinated activity within the neocortex (Galarreta and Hestrin 1999; Gibson et al. 1999; Wilson and Nicoll 2002; Freund 2003; Klausberger et al. 2005; Somogyi and Klausberger 2005). Two major types of GABAergic interneurons provide inhibitory terminals at somatic and proximal regions of pyramidal neurons: parvalbumin-positive, CB1-negative fast-spiking (FS) cells and parvalbumin-negative, CB1-positive irregular-spiking (CB1-IS) cells (Galarreta et al. 2004; Bodor et al. 2005). Using paired recordings, we report here that synapses of CB1-IS cells, but not synapses of FS cells, are suppressed by release of endocannabinoids from pyramidal neurons. Unitary CB1-IS synapses showed, in contrast to those of FS cells, a very high failure rate, long latency, and variable short-term plasticity. Furthermore, CB1-IS cells received less efficient excitatory drive from local pyramidal neurons than FS cells. It was previously shown that networks of FS cells can produce rhythmic activity, whereas networks of CB1-IS cells are more likely to exhibit nonrhythmic activity (Gibson et al. 1999; Galarreta et al. 2004; Klausberger et al. 2005). Taken together, these data suggest that CB1-IS and FS cells have different impact on cortical networks and that activity-dependent release of endocannabinoids, by blocking CB1-IS synapses and not those of FS cells, may control the relative impact of these 2 inhibitory networks on cortical activity.
Materials and Methods
Slice Preparation and Cell Identification
CB1-IS cells were identified in a mouse strain expressing an enhanced green fluorescent protein (EGFP) under the control of the promoter for glutamic acid decarboxylase (GAD) 65 (Lopez-Bendito et al. 2004). We have used immunohistochemistry methods (see below) to determine the proportion of EGFP-expressing cells that also express CB1 receptors. We found that 38% of CB1-expressing cells in layer 2/3 also expressed EGFP (45 of 118 cells). In addition, we have found that about 1% of EGFP-expressing neurons were CB1 immunopositive (17 of 1226 cells). Experiments with fast-spiking cells were carried out in a mouse strain expressing EGFP under the control of the promoter for GAD67 (Chattopadhyaya et al. 2004). Juvenile mice of both sexes (14–20 days old) were anesthetized by inhalation of isofluorane and decapitated. Parasagittal cortical slices (300-μm thick, 30° angle) were obtained in an ice-cold extracellular solution containing (in millimoles) 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgSO4, 0.5 CaCl2, 25 NaHCO3, 25 glucose, 75 sucrose, and 1 kynurenic acid (pH 7.4, 315 mOsm). After dissection, the slices were incubated at 32–34 °C for 25 min in the same slicing solution and then transferred into a solution containing (in millimoles) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 26 NaHCO3, 20 glucose, 4 lactic acid, 2 pyruvic acid, 0.4 ascorbic acid, and 1 kynurenic acid (pH 7.4, 315 mOsm). This solution was continuously bubbled with a gas mixture of 95% O2 and 5% CO2 and kept at room temperature (20–22 °C). After at least 30 min, individual slices were transferred to a submersion-type recording chamber in which a solution containing (in millimoles) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 26 NaHCO3, 20 glucose, 4 lactic acid, 2 pyruvic acid, and 0.4 ascorbic acid was perfused at a rate of 2–4 mL/min.
Fluorescent neurons in the somatosensory cortex were visualized using an upright microscope (Axioskop, Zeiss, Thornwood, NY) illuminated with a xenon lamp (150 W, Opti-Quip, Highland Mills, NY) and equipped with a ×40 water immersion objective lens and EGFP filters (XF100, Omega Optical, Brattleboro, VT). Once a fluorescent neuron was selected, it was visualized using infrared differential interference contrast video microscopy (C2400, Hamamatsu, Bridgewater, NJ) and recorded using conventional patch-clamp techniques (Stuart et al. 1993). Whole-cell recording from CB1-IS cells was accomplished as previously described (Galarreta et al. 2004). Briefly, to identify CB1-IS cells, we selected EGFP-expressing cells in layers 2/3 that had large somata (the width and length were 12.8 ± 2.3 μm and 17.2 ± 3.8 μm; N = 42 cells). Of these cells, we further selected cells that in response to current injection generated irregular-spike patterns. We have previously showed that most of the cells selected by these criteria were immunoreactive to CB1 antibody (Galarreta et al. 2004). Multipolar EGFP-positive cells in line G42 transgenic mice (Chattopadhyaya et al. 2004) showing characteristic discharges of high-frequency (>30 Hz) nonaccommodating spikes in response to near-threshold current injection (Kawaguchi and Kubota 1997) were classified as FS cells. Layer 2/3 pyramidal cells were selected on the basis of their characteristic dendrosomatic appearance and regular pattern of action potentials in response to current injection (McCormick et al. 1985).
Immunohistochemistry
To detect the presence of CB1 receptors, a section (about 5 mm) of cortical hemisphere was fixed in 4% paraformaldehyde in 0.01 M phosphate buffer and 0.2% picric acid for 2 h at 4 °C. After washing the hemisphere section with tris-buffered saline (TBS), the tissue was resectioned to produce 50-μm sections. The sections were blocked for 2 h and then were incubated overnight at 4 °C with a rabbit anti-CB1 antibody (1:2000; Ken Mackie, Department of Psychological and Brain Sciences, Indiana University). Next, the sections were washed again in TBS and incubated overnight at 4 °C in the secondary antibody goat anti-rabbit IgG (H + L) Alexa Fluor 555 (1:500; #A21428, Molecular Probes, Eugene, OR). Finally, the sections were washed in TBS and mounted with Vectashield.
Paired Recording and Data Analysis
Simultaneous somatic whole-cell recordings were made with patch electrodes (3–4 MΩ) filled with a solution containing (in millimoles) 130 K-methylsulfate, 6.3 KCl, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 4 MgATP, 20 phosphocreatine(Na), 0.3 NaGTP, 0.2 ethyleneglycol-bis(2-aminoethylether)-N,N,_N_‘,_N_‘-tetra acetic acid (EGTA), and 0.3% biocytin or 106 K-methylsulfate, 40 KCl, 10 HEPES, 4 MgATP, 20 phosphocreatine(Na), 0.3 NaGTP, 0.2 EGTA, and 0.3% biocytin or 130 KCl, 10 HEPES, 4 MgATP, 20 phosphocreatine(Na), 0.3 NaGTP, 0.2 EGTA, and 0.3% biocytin (pH 7.3, 295 mOsm). We recorded from pairs of CB1-IS in layer 2/3 (average distance and standard deviation [SD]: 102 ± 57 μm; range: 48–200 μm), pairs CB1-IS → P in layer 2/3 (average distance and SD: 57 ± 32 μm; range: 32–162 μm), pairs of FS → P in layer 5 (average distance and SD: 30 ± 12 μm; range: 19–67 μm), pairs of FS → P in layer 2/3 (average distance and SD: 46 ± 30 μm; range: 21–112 μm), and pairs of FS → FS in layer 5 (average distance and SD: 50 ± 23 μm; range: 21–87 μm). Recordings were performed at 31–32 °C. The error due to the liquid junction potential was not corrected. We did not compensate for the series resistance that ranged between 10 and 25 MΩ. Cells were recorded using 2 Axopatch 200B amplifiers (Axon Instruments, Union City, CA). The voltage and current output were filtered at 5 kHz and digitized at 16-bit resolution (ITC-18, Instrutech, Port Washington, NY) with a sampling frequency of 10 kHz. IPSPs at resting potential were recorded keeping both the pre- and the postsynaptic cell under the current-clamp configuration. Inhibitory postsynaptic currents (IPSCs) were recorded by keeping the postsynaptic cell under voltage-clamp configuration at different holding potentials.
IPSP/Cs were recorded in the presence of the AMPA and kainate receptor antagonist DNQX (10 μm, Sigma, St Louis, MO). DNQX, WIN55,212-2, and AM251 (Tocris, Ellisville, MO) were applied in the bath. WIN55,212-2 and AM251 were dissolved in dimethyl sulfoxide (10 mM stock solution).
The strength of the electrical coupling in pairs of CB1-IS cells is reported as the mean of the coupling coefficients (CCs) from cell 1 to cell 2 and that measured from cell 2 to cell 1. The CC was calculated as the ratio between the change of membrane voltage produced in the noninjected cell and that in the injected cell. Assuming a simple model of 2 isopotential neurons connected by a single electrical junction, the gap junction conductance (Gc) was calculated according to the equation: Gc = 1/[(Rin2/CC)−Rin2], where Rin2 is the input resistance of the postsynaptic neuron and CC is the step CC.
The latency of individual IPSCs was measured from the peak of the presynaptic spike to the beginning of the IPSC. The beginning of the IPSC was determined as the intersection between a 2-ms baseline and a line fit to the slope of the synaptic current. The jitter is the SD of the latency of individual IPSCs.
In the ‘DSI’ experiments, a train of 8 action potentials at 20 Hz was generated in the presynaptic cell every 6 s. To estimate the baseline postsynaptic response, we averaged the 8 IPSPs amplitudes in response to the 8 action potential train and then averaged this average for all trains at the same time point relative to the postsynaptic depolarization (Fig. 1_B_). DSI was induced by a 2- to 4-s-long depolarizing current pulse (500–1000 pA) in the postsynaptic cell. The depolarizing current produced between 100 and 200 action potentials. Typically, action potentials were generated for about 1–2 s at a frequency of about 100 Hz. The presynaptic train of 8 action potentials was interrupted during the postsynaptic depolarization and resumed 2 s after it.
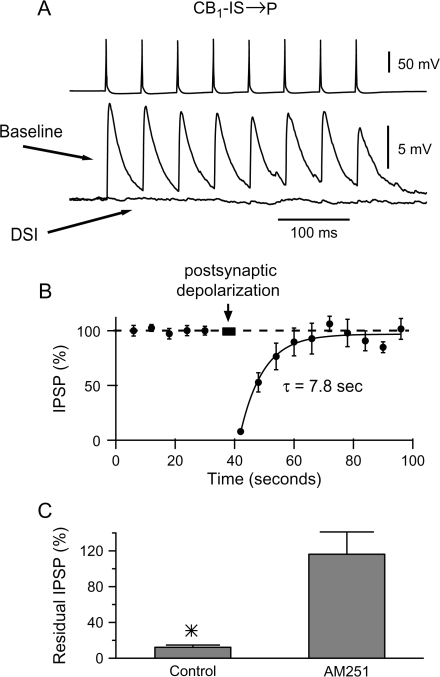
Figure 1.
DSI at unitary layer 2/3 CB1-IS → P connections. (A) Simultaneous recording from synaptically connected pair of neurons composed of a presynaptic CB1-IS cell and a postsynaptic pyramid ([Cl−]in = 130 mM). Both cells were recorded under current clamp. A train of 8 action potentials at 20 Hz was generated in the presynaptic cell every 6 s. To induce DSI, the presynaptic train of 8 action potentials was interrupted and a 4-s-long depolarizing current pulse (500 pA) was injected in the postsynaptic cell (labeled postsynaptic depolarization in (B). The averages of 11 responses to the 8-spike train before (Baseline) and after (DSI) the postsynaptic depolarization are shown. (B) Summary data from 7 CB1-IS → P connections in layer 2/3 are shown. (C) DSI at layer 2/3 CB1-IS → P connections depends on activating CB1 receptors. Summary data from 3 CB1-IS → P connections. Control, residual amplitude of the IPSP 2 s after the postsynaptic depolarization of DSI with a 4-s-long postsynaptic depolarizing pulse under control conditions. AM251, residual amplitude of the IPSP in the same pairs 2 s after the postsynaptic depolarization of DSI in the presence of the CB1 receptor antagonist AM251 (10 μM). Control = 8.8 ± 1.9%; AM251 = 112.7 ± 24.3% (n = 3; P < 0.05).
Data are given as mean ± standard error of the mean. To analyze DSI and WIN effects, we applied a 2-tailed paired Student's t test on the logarithmically transformed data. Testing the logarithmically transformed data is preferred when the ratio (treated/control) is more consistent than the difference (treated − control). We used Fisher's exact test to compare the rate of connectivity of different classes of cells. We used 2-tailed unpaired t test to determine the statistical significance of differences of synaptic properties. Differences were considered statistically significant if P < 0.05.
Results
CB1-IS → P Connections Exhibit DSI
We 1st used paired recordings to study whether CB1-IS → P connections or FS → P connections are sensitive to endogenous cannabinoids. CB1-IS cells were identified using a transgenic line of mice expressing EGFP under the control of a GAD65 promoter (Lopez-Bendito et al. 2004). We recorded simultaneously from a presynaptic CB1-IS cell and its postsynaptic pyramidal target (Galarreta et al. 2004). Because the IPSPs produced by CB1-IS cells are highly variable (see below), we tested the strength of these inhibitory connections using an average response to 20-Hz train of 8 presynaptic spikes (Fig. 1_A_). To induce DSI, we depolarized the postsynaptic pyramidal cells for 2–4 s generating a train of postsynaptic spikes. After recovery of the IPSPs, we repeated the sequence with a cycle time of 100 s (Fig. 1_B_). Comparison of the average 8 IPSPs before (Fig. 1_A_, ‘baseline’) and 2 s after the postsynaptic depolarization (Fig. 1_A_, DSI) indicated that this procedure completely suppressed the postsynaptic response. The time course of recovery from DSI was obtained by a sequence consisting of baseline stimulation (8 spikes at 20 Hz) every 6 s followed by a DSI-inducing depolarization. Figure 1_B_ illustrates the average time course of recovery following DSI of 7 CB1-IS to pyramid connections. The recovery time course of each experiment was fitted with a single-exponential function. Similar results were obtained for 7 CB1-IS to pyramid connections and the time course of the recovery from DSI of the pooled data was 7.8 s (Fig. 1_B_). Postsynaptic depolarization produced an almost complete suppression of the IPSP. We found that the average residual IPSP measured 2 s after postsynaptic depolarization was 8 ± 2% (P = 0.0004, n = 7 experiments). CB1-IS to pyramid connections exhibit high failure rates. We therefore asked whether DSI affects the failure rates at these synapses. We have found that the failure rate increased from an average of 34.9% at the baseline to 88% after DSI (n = 7 DSI experiments, P = 0.0004). Thus, DSI increases the failure rate as would be expected for a presynaptic mechanism.
To confirm that the suppression of the IPSPs was mediated by CB1 receptors, we studied the effect of postsynaptic depolarization in the presence of the CB1 receptor–specific antagonist, AM251 (Freund et al. 2003). In control conditions, postsynaptic depolarization strongly suppressed the IPSPs in CB1-IS → P connections (Fig. 1_C_; 8% of control, P = 0.007, n = 3 experiments). When the same connections were tested for DSI after bath application of 10-μM AM251, we found no depression of the unitary IPSPs (Fig. 1_C_; 112.7% of control, P = 0.8). These experiments indicate that activity-dependent release of endogenous cannabinoids can dramatically depress the inhibitory connections mediated by CB1-IS cells onto pyramidal neurons. Further, these results indicate that CB1-IS cells underlie neocortical DSI.
Specificity of Cannabinoid Sensitivity at Unitary Connections
We next asked whether the sensitivity to cannabinoids was specific to CB1-IS cells. To address this question, we used paired recordings of FS → P connections. Previous anatomical studies showed that terminals of parvalbumin-expressing cells, including FS neurons, are immunonegative to CB1 receptors (Bodor et al. 2005). It should be noted, however, that the sensitivity of immunological approach may not be sufficient to detect low levels of CB1 expression. We thus tested whether unitary connections from FS cell to pyramids are physiologically sensitive to exogenously applied cannabinoids. We found that IPSPs in pyramidal cells induced by action potentials in FS cells were insensitive to the application of the cannabinoid agonist WIN. Twenty minutes in 1-μM WIN had no effect on the amplitude of the IPSPs at unitary connections (P = 0.9, n = 3 pairs; data not shown). Furthermore, we found that 4-s-long depolarization of the postsynaptic pyramidal cells did not depress the unitary IPSPs mediated by FS cells. We found that the residual IPSP was 90 ± 7% at FS → P connections (n = 6 experiments, P = 0.9; Fig. 2_D_). Thus, endocannabinoids released by postsynaptic pyramids suppress the inhibitory synapses mediated by CB1-IS cells, whereas FS contacts are not sensitive to cannabinoids and are unaffected.
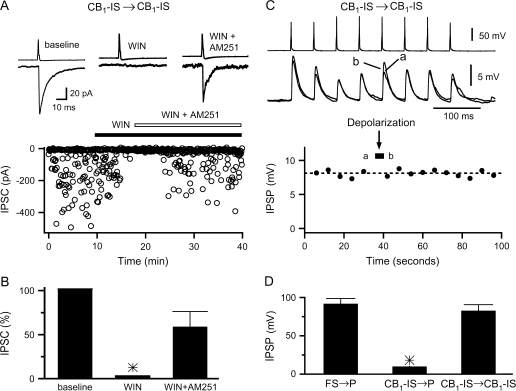
Figure 2.
CB1-IS → CB1-IS synapses are suppressed by CB1 receptor activation but do not exhibit DSI. (A) Inhibitory connections among layer 2/3 CB1-IS neurons ([Cl−]in = 130 mM) are suppressed by the cannabinoid receptor agonist WIN55,212-2 (1 μM). The inhibitory transmission recovered after the addition of the CB1-selective antagonist AM251 (10 μM) indicating that the WIN effect was indeed mediated by the activation of CB1 receptors. The top panel shows the average responses (n = 200 IPSCs) in control (marked ‘baseline’), in the presence of WIN (marked ‘WIN’; n = 50 IPSCs), and in the presence of WIN and AM251 (marked ‘WIN + AM251’; n = 100 IPSCs). The lower panel shows the entire course of this experiment. (B) Summary data from 4 experiments among CB1-IS cells in layer 2/3. (C) Paired recording between 2 CB1-IS cells in layer 2/3. Both cells were recorded under current clamp, and internal chloride was 130 mM. A train of 8 action potentials at 20 Hz were generated in the presynaptic cell every 6 s (top panel). The traces in the top panel are averages of five 8-spike trains before and after postsynaptic depolarization. At the time labeled as postsynaptic depolarization, the 8 action potential presynaptic train was interrupted and a 4-s-long depolarizing current pulse (500 pA) was injected in the postsynaptic cell. Symbols in lower panel represent the average of 5 trials. (D) Summary data on the residual IPSP following postsynaptic depolarization for 3 types of synaptic connections. FS → P: 90 ± 7% (n = 6 pairs; 3 in layer 2/3 and 3 in layer 5); CB1-IS → P: 8 ± 2% (n = 7 in layer 2/3); CB1-IS → CB1-IS: 81 ± 8% (n = 4 pairs in layer 2/3).
Our current and previous (Galarreta et al. 2004) results show that at pyramidal cells the axons of CB1-IS, but not those of FS cells, express functional CB1 receptors that can be activated by postsynaptic depolarization. However, whether synaptic inputs among CB1-IS cells express CB1 receptors and can exhibit DSI is not known. We 1st asked whether inhibitory inputs among CB1-IS cells are suppressed by CB1 receptor activation. We found that bath application of 1-μM WIN completely suppressed IPSCs at a unitary CB1-IS → CB1-IS connection (Fig. 2_A_). Furthermore, this effect was partially reversed by subsequent bath application of the CB1 receptor antagonist, AM251 (10 μM). Similar results were obtained for 4 unitary CB1-IS → CB1-IS connections (Fig. 2_B_). We next tested whether postsynaptic depolarization of CB1-IS cells can release endocannabinoids and suppress these same synapses. We found that prolonged depolarization of postsynaptic CB1-IS cells did not lead to suppression at connections among these cells (Fig. 2_C_,D). Comparison of the impact of postsynaptic depolarization shows that CB1-IS → P connections were completely suppressed. We found that the residual IPSP at CB1-IS → P connections was 8 ± 2% (P = 0.0004, n = 7 experiments; Fig. 2_D_), whereas at both FS → P and CB1-IS → CB1-IS connections, no attenuation was observed (Fig. 2_D_). We found that the residual IPSP was 90 ± 7% at FS → P connections (n = 6 experiments; P = 0.9) and 81 ± 8% at CB1-IS → CB1-IS connections (n = 6 experiments; P = 0.8).
Thus, although functional CB1 receptors are expressed at CB1-IS terminals contacting both pyramidal cells and other CB1-IS cells, under our experimental conditions, only CB1-IS → P connections exhibited DSI (see Discussion).
Connectivity and Synaptic Properties of CB1-IS and FS Cells
It has been proposed that networks of inhibitory neurons that could be defined by selective connections via electrical synapses may underlie different network rhythms (Klausberger et al. 2002; Hajos et al. 2004; Hestrin and Galarreta 2005; Klausberger et al. 2005). We and others have shown evidence suggesting that FS cells and CB1-IS cells form 2 distinct such networks (Freund 2003; Galarreta et al. 2004; Klausberger et al. 2005). The nature and the effectiveness of the interaction between an inhibitory network and cortical activity depend on the connectivity and synaptic properties of the interneurons. Thus, we next asked whether the synaptic properties and synaptic connectivity of these 2 cell types differ.
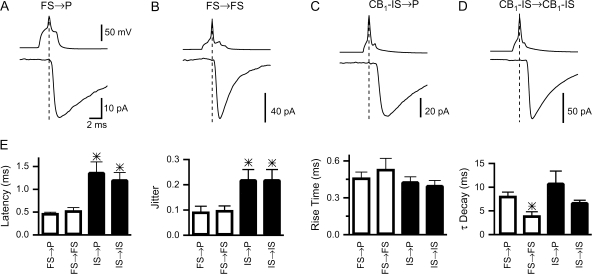
It has been shown that the kinetics of synaptic interactions among interneurons can determine the synchronization of their activity (Jefferys et al. 1996; Bartos et al. 2002). Thus, we 1st compared the synaptic properties of the IPSCs at inhibitory connections of FS and CB1-IS cells (Fig. 3_A_–D). FS → P and FS → FS inhibitory synapses had a remarkably brief synaptic latency (0.5 ± 0.03 ms, n = 8 FS → P pairs and 0.5 ± 0.07 ms, n = 5 FS → FS pairs) and a low degree of trial-to-trial variability in the synaptic latency (coefficient of variation: 0.09 ± 0.02 and 0.1 ± 0.02; Fig. 3_E_). In contrast, CB1-IS → P (n = 6 pairs) and CB1-IS → CB1-IS (n = 5 pairs) had relatively longer synaptic latencies (1.4 ± 0.2 and 1.2 ± 0.2 ms, respectively) and a larger degree of ‘jitter’ (coefficient of variation: 0.2 ± 0.04 and 0.2 ± 0.04, respectively; Fig. 3_E_). The rise time of the IPSCs was similar at the 4 types of connections (see Fig. 3_E_), suggesting that the synaptic contacts of these cells are similarly located on the proximal regions of the postsynaptic neurons. We found that the decay time constants of the IPSCs at FS → FS synapses were significantly faster than those at FS → P and at connections made by CB1-IS cells (3.9 ± 0.8 at FS → FS vs. 8.0 ± 0.9 at FS → P, 10.9 ± 2.5 at CB1-IS → P and 6.8 ± 0.5 at CB1-IS → CB1-IS).
Figure 3.
Kinetics of IPSCs mediated by FS and CB1-IS cells. (A) A pair between a presynaptic FS cell and a postsynaptic pyramid ([Cl−]in = 130 mM, holding Vm = −70 mV) in layer 2/3. (B) A pair between 2 FS cells in layer 5 ([Cl−]in = 6 mM, holding Vm = −90 mV). (C) A pair between a CB1-IS cell and a pyramid ([Cl−]in = 130 mM, holding Vm = −70 mV) in layer 2/3. (D) A pair between 2 CB1-IS cells in layer 2/3 ([Cl‑] = 130 mM, holding Vm = −70 mV). (E) Summary data. Latency (FS → P, 0.47 ± 0.03 ms, n = 8 pairs; FS → FS, 0.53 ± 0.07 ms, n = 5 pairs; IS → P, 1.37 ± 0.2 ms, n = 6 pairs; CB1—IS → CB1-IS, 1.2 ± 0.16 ms, n = 5 pairs). Jitter (FS → P, 0.09 ± 0.02 ms; FS → FS, 0.1 ± 0.02 ms; CB1—IS → P, 0.2 ± 0.04 ms; CB1—IS → CB1-IS, 0.22 ± 0.04 ms). Rise Time (FS → P, 0.46 ± 0.05 ms; FS → FS, 0.5 ± 0.1 ms; CB1—IS → P, 0.43 ± 0.04 ms; CB1—IS → CB1-IS, 0.4 ± 0.04 ms). Decay (FS → P, 8.1 ± 0.1 ms; FS → FS, 3.9 ± 0.8 ms; CB1-IS → P, 10.9 ± 2.5 ms; CB1-IS → CB1-IS, 6.5 ± 0.5 ms).
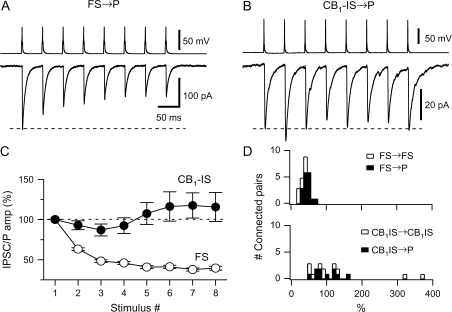
Signaling among specific types of cortical neurons depends on their synaptic dynamics (Thomson and Deuchars 1994; Fuhrmann et al. 2002). To study short-term plasticity of IPSCs, we generated a train of 8 presynaptic action potentials at 20 Hz in FS → P and FS → FS connections and at CB1-IS → P and CB1-IS → CB1-IS connections. The average response of different unitary FS → P and FS → FS connections exhibited similar synaptic depression (Fig. 4_A_,D) (Galarreta and Hestrin 1998; Reyes et al. 1998). The average response of different unitary CB1-IS → P and CB1-IS → CB1-IS connections, however, exhibited variable responses (Fig. 4_B_,D). We found that the average postsynaptic response to 8 presynaptic spikes steadily declined to approximately 40% of the initial IPSC amplitude for the eighth spike at FS → P connections. The average responses for unitary CB1-IS → P and CB1-IS → CB1-IS connections were variable and averages ∼100% (Fig. 4_C_). We found that FS → P synapses and FS → FS connections showed similar synaptic depression. The ratios of the eighth response and the 1st response were 43.6 ± 2.4% (N = 17 connections) and 30.6 ± 4.2% (N = 8 connections) at FS → P and FS → FS synapses, respectively. Unitary CB1-IS → P and CB1-IS → CB1-IS connections exhibited variable short-term plasticity (Fig. 4_D_). We found that the ratio of the eighth response and the 1st response was 97.1 ± 11% (N = 10 connections) and 137 ± 10% (N = 10 connections) at CB1-IS → P and CB1-IS → CB1-IS connections, respectively. Summary data showing the average response for all connections with a presynaptic FS cell (open circles, Fig. 4_C_) and a presynaptic CB1-IS cell (filled circles, Fig. 4_C_) highlight the difference in the averaged responses. A plot of the ratio of the eighth and the 1st inhibitory postsynaptic response for the 2 types of connections. The short-term plasticity of different pairs of CB1-IS → P and CB1-IS → CB1-IS connections ranged from facilitation to depression, whereas FS → P and FS → FS synapses consistently exhibited synaptic depression (Fig. 4_D_).
Figure 4.
The short-term plasticity of the inhibitory connections of FS and of CB1-IS cells differs. (A) Recordings from a pair composed of a presynaptic FS cell and a postsynaptic pyramid ([Cl−] = 6 mM) in layer 5 is shown. A train of 8 action potentials at 20 Hz was generated in the presynaptic cell. The average of 20 responses is shown in the bottom trace. (B) Recordings from a pair composed of a presynaptic CB1-IS cell and a postsynaptic pyramid ([Cl−]in = 130 mM) in layer 2/3 is shown. A train of 8 action potentials at 20 Hz was generated in the presynaptic cell. The average of 20 responses is shown in the lower trace. (C) Summary data from a total of 25 pairs with a presynaptic FS cell (17 FS → P connections and 8 FS → FS connections) and 20 pairs with a presynaptic CB1-IS cell (10 CB1-IS → P and 10 CB1-IS → CB1-IS recorded in current-clamp or voltage-clamp mode. (D) Histograms showing the distribution of the ratio between the eighth and the 1st IPSC or IPSP for both types of inhibitory connections. Open symbols represent data among interneurons. Filled symbols represent data between interneurons and pyramidal cells.
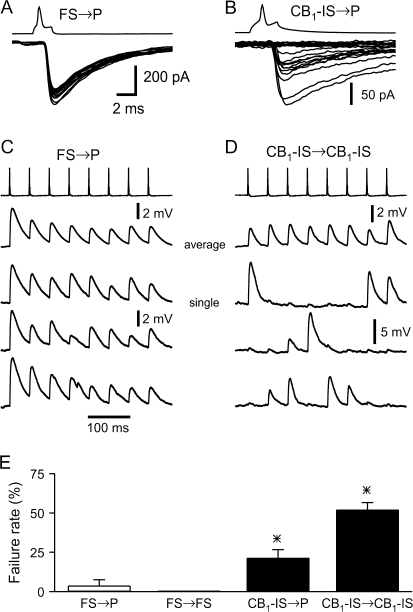
In addition to differences in their short-term plasticity, inhibitory synapses of FS and CB1-IS cells exhibited different trial-to-trial variability. Variability of the postsynaptic response including response failure would impact synaptic efficacy and the ability of diverging inputs to coordinate the activity of postsynaptic targets. The individual postsynaptic responses to a presynaptic action potential in an FS cell appeared to be much more reliable when compared with postsynaptic responses to presynaptic action potentials in a CB1-IS cell (Fig. 5_A_,B). Furthermore, we found that both FS → P and FS → FS synapses had only small number of failures, whereas both CB1-IS → P and CB1-IS → CB1-IS synapses were often dominated by response failures. The averaged responses to 8 presynaptic action potentials at synapses of FS and CB1-IS are generally similar (Fig. 5_C_,D; top traces). However, when the responses to single trials are examined, the difference between the 2 types of synapses is quite striking (Fig. 5_C_,D; bottom 3 traces). Moreover, we found that the failure rates were 3.8% and 0% at FS → P (n = 5 pairs) and FS → FS (n = 6 pairs) connections, respectively, whereas the failure rates were 52% and 21% at CB1-IS → CB1-IS (n = 12 pairs) and CB1-IS → P (n = 9 pairs) connections (Fig. 5_E_). We found that synaptic transmission of FS cells is significantly more reliable than those of CB1-IS cells and that CB1-IS → P connections were significantly more reliable than CB1-IS → CB1-IS connections.
Figure 5.
Synaptic responses mediated by CB1-IS cells show much more variability than those mediated by FS cells. (A) Superimposition of 15 consecutive IPSCs in a pair between a presynaptic FS cell and a postsynaptic pyramid ([Cl−] = 130 mM) in layer 2/3. Note the lack of failures. (B) Superimposition of 20 consecutive IPSCs in a pair between a presynaptic CB1-IS cell and a postsynaptic pyramid ([Cl−] = 130 mM) in layer 2/3. Note that about half of the responses are failures. (C) Connection between a presynaptic FS cells and postsynaptic pyramid in layer 5. Both cells are under current clamp. Trains of 8 action potentials at 20 Hz were generated in the FS cell every 5 s. Depolarizing IPSPs were recorded from the postsynaptic cell ([Cl−] = 40 mM, Vm = -90 mV). The top trace shows the average of 20 trials. The 3 lower traces are individual trials. (D) Connection between 2 CB1-IS cells in layer 2/3 that was also electrically coupled. Both cells are under current clamp. Trains of 8 action potentials at 20 Hz were generated in the presynaptic CB1-IS cells every 6 s. Depolarizing IPSPs were recorded from the postsynaptic cell ([Cl−] = 130 mM) at resting potential. The top trace shows the average of 50 trials. The 3 lower traces are individual trials. Note that many presynaptic action potentials failed to produce a postsynaptic potential. However, the weak electrical coupling produced small postsynaptic depolarization following each presynaptic spike. (E) Summary data of failure rate from the 4 types of connections (FS → P, 3.8% n = 5 pairs; FS → FS, 0% n = 6 pairs; CB1-IS → CB1-IS, 52% n = 12 pairs and CB1-IS → P, 21% n = 9).
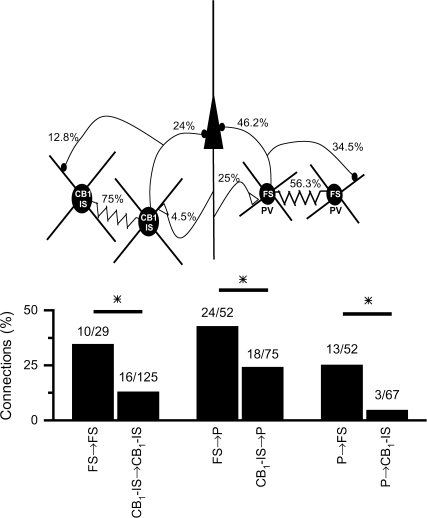
The patterns of connectivity among interneurons and between interneurons and pyramidal cells determine how these networks affect local circuits. We thus compared the local intracortical synaptic connectivity among FS and among CB1-IS and between these 2 types of inhibitory cells and pyramidal neurons (Fig. 6). Both types of inhibitory neurons were highly interconnected by electrical synapses 75% and 56% (P = 0.13; Fisher's exact test) for CB1-IS → CB1-IS and FS → FS, respectively (Fig. 6_A_). However, GABAergic connections among FS cells were significantly more common than GABAergic connections among CB1-IS cells (Fig. 6_B_; 34.5% vs. 12.8%, per connection tested, P = 0.01; Fisher's exact test). FS cells also synapse onto local pyramidal cells with a significantly higher probability than CB1-IS cells (46.2% vs. 24%, P = 0.01; Fisher's exact test). Moreover, excitatory synapses from local pyramids to FS cells were more than 5-fold more common than connections from local pyramids onto CB1-IS cells (25% vs. 4.5%, P = 0.002; Fisher's exact test). Thus, although both cell types are heavily interconnected by electrical synapses, FS cells receive excitatory synapses from neighboring pyramidal cells and project inhibitory synapses onto neighboring pyramids and FS cells with significantly higher probability than CB1-IS cells.
Figure 6.
Synaptic connectivity between FS, CB1-IS, and pyramidal cells. (A) Schematic drawing illustrating the synaptic connectivity among pyramidal, FS, and CB1-IS cells. Numbers represent the percentage of functional connections out of the total connections tested. (B) Summary data on synaptic connectivity. Numbers show the ratio between connected and all the pairs tested for each kind of connection.
Discussion
We have 3 main findings. First, we have shown that depolarization of postsynaptic pyramidal cells can completely suppress unitary IPSPs at CB1-IS → P connections while having no effect at FS → P connections, thus demonstrating that CB1-IS cells contribute to DSI in the neocortex. Second, we have found that inhibitory synapses among CB1-IS cells express CB1 receptors but do not exhibit DSI. Finally, we found that both inhibitory cell types are densely interconnected by electrical synapses. However, whereas FS and pyramidal cells form highly reliable and frequent chemical contacts, CB1-IS cell interactions with pyramids were remarkably unreliable and significantly less frequent. Together, our data suggest that CB1-IS and FS cells are activated under different conditions and likely to have different impact on cortical networks. Thus, their selective modulation by activity-dependent release of endocannabinoids may change both the strength and properties of inhibition in the neocortex.
Unitary Connections of CB1-IS cells, but not FS cells, exhibit DSI
It has been shown previously that depolarization of hippocampal and neocortical pyramidal cells release endocannabinoids suppressing their inhibitory inputs (Alger 2002; Wilson and Nicoll 2002; Freund et al. 2003; Trettel and Levine 2003). However, identification and characterization of the cells that can generate DSI in the cortex has not been obtained. In the neocortex, postsynaptic depolarization did not completely suppress extracellularly generated IPSPs, suggesting that only a subset of inhibitory inputs onto pyramids is endocannabinoid sensitive (Trettel and Levine 2002, 2003; Fortin et al. 2004; Trettel et al. 2004; Bodor et al. 2005; Fortin and Levine 2007). These physiological data can be explained by anatomical findings showing that CB1 receptors are expressed in only a subset of inhibitory axons (Bodor et al. 2005). Specifically, previous anatomical studies have shown that layer 2/3 pyramidal cell somata is surrounded by inhibitory terminals of parvalbumin expressing but CB1-negative terminals and by parvalbumin-negative but CB1-positive terminals (Bodor et al. 2005). However, the identity and properties of the inhibitory cells with endocannabinoid-sensitive terminals have not been studied systematically. We previously identified a class of neurons (CB1-IS cells) expressing CB1 receptors and exhibiting irregular spiking in response to step current injections (Galarreta et al. 2004). Here, we showed for the 1st time that depolarization of a postsynaptic pyramid leads to complete suppression of the IPSPs at unitary CB1-IS → P connections. This DSI was blocked by AM251 indicating that it was mediated by CB1 receptors. These data indicate that CB1-IS cells play a role in generating DSI in the neocortex.
We have found that postsynaptic depolarization of pyramidal cells did not produce DSI at FS → P connections. Thus, activity of pyramidal cells can selectively and completely suppress IPSPs originating from CB1-IS cells without affecting those originating from FS cells. We then showed that WIN did not attenuate FS → P connections providing functional evidence for lack of CB1 receptor expression at FS terminals (Bodor et al. 2005). We have also found that postsynaptic depolarization did not produce DSI at CB1-IS → CB1-IS connections. However, bath application of WIN did suppress IPSPs at these connections. This suppression was blocked by AM251 indicating that functional CB1 receptors are present at CB1-IS inhibitory terminals on neighboring CB1-IS cells. Postsynaptic depolarization, under our experimental conditions, did not produce DSI. This may indicate that CB1-IS cells are not capable of releasing endocannabinoids under our experimental conditions. However, it is possible that trains of action potentials in CB1-IS cells do not result in sufficiently high concentration of calcium to generate release of endocannabinoids. A recent paper has shown that in different types of neocortical interneurons the increase in calcium concentration generated by action potential trains is similar to that producing DSI in pyramidal cells (Lemtiri-Chlieh and Levine 2007). Furthermore, it was reported that those neocortical interneurons do not exhibit DSI of spontaneous IPSCs (Lemtiri-Chlieh and Levine 2007). What are the physiological conditions that could activate CB1 receptors at contacts among CB1-IS cells? It is possible that modulators of endocannabinoid synthesis may allow DSI at these connections (Varma et al. 2001; Kim et al. 2002). Alternatively, it is also possible that endocannabinoids released from neighboring pyramidal cells could ‘spillover’ and activate CB1 receptors at CB1-IS → CB1-IS connections. It has been reported that inhibitory connections among Schaffer collateral–associated (SCA) interneurons generate DSI (Ali 2007). The DSI observed among SCA cells was about 50%, and their inhibitory output displayed strong facilitation suggesting that those cells are different from neocortical CB1-IS cells.
Distinct Connectivity and Synaptic Properties in CB1-IS and FS Cell Networks
In the hippocampus, the class of inhibitory neurons expressing CB1 receptors may be activated under different conditions and are associated with different oscillations than the class of inhibitory cells that include FS cells (Freund 2003; Klausberger et al. 2005; Somogyi and Klausberger 2005). The patterns of connectivity and the properties of synaptic interactions determine the impact of inhibitory networks on local circuits. We thus asked whether neocortical FS and CB1-IS cells have cell-type–specific synaptic properties and connectivity. We found that, although both cell types were highly interconnected by electrical synapses, FS cells made and received inhibitory and excitatory synapses at significantly higher rates than CB1-IS cells. GABAergic connections among FS and CB1-IS and between these cells and pyramidal neurons were 2- to 3-fold more likely for FS cells compared with CB1-IS cells. Excitatory inputs from neighboring pyramidal cells were more than 5-fold more likely onto FS cells compared with CB1-IS cells. These data imply that to generate inhibitory output from CB1-IS cells would require a higher level of converging excitatory inputs compared with FS cells.
We found that the decay time constants of the IPSCs at FS → FS synapses were significantly faster than those at FS → P and at connections made by CB1-IS cells. Fast decay time constants of IPSCs among FS cells have been shown previously (Galarreta and Hestrin 2002). Furthermore, it was shown that IPSCs among FS cells in the dentate gyrus are faster than those between FS cells and granule cells (Bartos et al. 2001; Bartos et al. 2002). These data may be explained by different expression patterns of GABAA subunit found at basket cells synapses (Nyiri et al. 2001) that may underlie the differences in IPSC kinetics. The rise times of both FS and CB1-IS inputs to pyramidal cells were about 0.4 ms. The relatively fast rise time is compatible with proximal input of both cell types on pyramidal neurons. The synaptic latency and synaptic jitter were significantly larger at inhibitory synapses of CB1-IS cells compared with those of FS cells. Similar differences in jitter and latency were also reported in the hippocampus for connections between CB1-positive and CB1-negative inhibitory neurons and pyramidal cells (Glickfeld and Scanziani 2006) and between CCK cells and PV cells and granule cells in the dentate gyrus (Hefft and Jonas 2005). Inhibitory synapses of FS cells exhibited short-term depression, as been shown previously (Galarreta and Hestrin 1998; Reyes et al. 1998; Maffei et al. 2006), whereas the population average of CB1-IS synapses did not show depression. Furthermore, whereas the amplitude of IPSPs of FS cells was relatively invariable, that originating from CB1-IS cells was highly variable. In addition, whereas the failure rate at FS → FS and FS → P synapses was less than 5%, the failure rate at CB1-IS → CB1-IS was 52% and that at CB1-IS → P was 21%. These properties are generally similar to hippocampal CB1/CCK expressing inhibitory neurons (Losonczy et al. 2004; Hefft and Jonas 2005). It should be noted that 2 types of CCK-expressing cells are found in the neocortex (Kubota and Kawaguchi 1997; Bodor et al. 2005). However, only the large CCK cells express CB1 receptors and provide basket-like input to pyramidal cells and are thus similar to the CB1-IS cells (Bodor et al. 2005). Together, these studies show that FS and CB1-IS cells are different not only in their intrinsic properties but also in their synaptic properties and synaptic connectivity.
Functional Implications
We have demonstrated here that CB1-IS cells are sensitive to endocannabinoids and that, following pyramidal cell activity, unitary IPSPs at CB1-IS → P are completely suppressed. These data suggest that CB1-IS cells underlie DSI in the neocortex (Trettel and Levine 2003; Trettel et al. 2004; Bodor et al. 2005; Fortin and Levine 2007). Previous studies of cortical DSI either examined responses to extracellular stimulation or spontaneous IPSPs (Trettel and Levine 2003; Trettel et al. 2004; Fortin and Levine 2007). In either of these approaches, DSI did not completely suppress IPSPs. The differences between our results and previous data can be simply explained by the heterogeneous populations of terminals involved in extracellular stimulation or spontaneous activity. However, we cannot rule out the possibility that neurons other than CB1-IS cells may also exhibit DSI in the neocortex. Recent work has suggested that in the neocortex CB1 receptors may be expressed in multiple GABAergic cell types (Bacci et al. 2004; Bodor et al. 2005; Hill et al. 2007). We have provided direct evidence that CB1-IS → P, but not FS → P, exhibit DSI. It would be interesting to test whether IPSPs between other classes of inhibitory cells and pyramidal cells express functional CB1 receptors and are sensitive to endocannabinoids. It should be noted that EGFP expression in the G42 line is limited to about 50% of parvalbumin cells (Chattopadhyaya et al. 2004); thus, the FS cells studied here represent a subset of all FS cells.
It has been shown that in the hippocampus, only a subset of GABAergic neurons are sensitive to DSI (Martin et al. 2001; Wilson et al. 2001; Foldy et al. 2006; Glickfeld and Scanziani 2006). The endocannabinoid-sensitive GABAergic neurons in the hippocampus generally exhibit functional similarities to neocortical CB1-IS cells (but see Ali 2007). In the hippocampus, excitatory input at CB1 receptor–positive cells was weak compared with that at CB1-negative cells (Glickfeld and Scanziani 2006). We have shown here that the probability of P → CB1-IS connection is 5-fold lower than that of P → FS synapses. Furthermore, we have also shown that inhibitory connections of CB1-IS cells were also sparser than those of FS cells. Anatomical studies have shown that parvalbumin-expressing basket cells in the hippocampus receive significantly more inputs than CCK-expressing basket cells thought to express CB1 receptors (Freund 2003; Matyas et al. 2004). Together these data suggest that endocannabinoid-sensitive interneurons may both receive and project sparse excitatory and inhibitory synaptic connections compared with FS cells.
What may be the functional consequences of the different connectivity and synaptic properties of CB1-IS and FS cells? We have found that electrical coupling among CB1-IS cells was very high (>75%) suggesting that gap junctions may be particularly important in determining the activity of these cells. Furthermore, we have previously shown that electrical coupling among CB1-IS cells can produce coherent activity in coupled cells (Galarreta et al. 2004). Electrical coupling among FS cells also produce coherent activity among these cells (Galarreta and Hestrin 1999; Gibson et al. 1999). However, whereas crosscorrelograms of pairs of FS cells showed a narrow peak centered about 0 ms, crosscorrelograms of pairs of CB1-IS cells showed 2 broader peaks at ±1-2 ms (Gibson et al. 1999; Galarreta et al. 2004). Moreover, pairs of FS cells fired rhythmically at relatively high frequencies, whereas pairs of CB1-IS cells fired nonrhythmically. We have found that synaptic connections of CB1-IS cells are highly unreliable compared with those of FS cells. Thus, together these data suggest that the connectivity and synaptic properties allow FS cells to impose fast rhythmic oscillatory activity in neocortical circuits, whereas those of CB1-IS cells may disrupt fast rhythmic oscillations. It has been shown that DSI induced by brief trains of action potentials in layer 2/3 pyramidal cells increases their excitation (Fortin et al. 2004). We have shown that FS → P in layer 2/3 and layer 5 do not exhibit DSI. Thus, following brief trains of action potentials, by suppressing inputs from CB1-IS cells, pyramidal cells are more likely to be influenced by FS cells allowing fast rhythmic oscillations. Moreover, the same mechanism could allow a group of pyramidal cells to be paced by FS cells and synchronize their activity.
Funding
National Eye Institute, National Institutes of Health (EY12114 and EY09120).
Acknowledgments
We thank Z.J. Huang for providing EGFP mice, Sally Pak for histology, Ken Mackie for CB1 antibodies, and Solange Brown for useful comments. Conflict of Interest: None declared.
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Ali AB. Presynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J Neurophysiol. 2007;98:861–869. doi: 10.1152/jn.00156.2007. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Geiger JR, Jonas P. Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci. 2001;21:2687–2698. doi: 10.1523/JNEUROSCI.21-08-02687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol. 2004;92:2105–2112. doi: 10.1152/jn.00351.2004. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G, Segev I, Markram H, Tsodyks M. Coding of temporal information by activity-dependent synapses. J Neurophysiol. 2002;87:140–148. doi: 10.1152/jn.00258.2001. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Traub RD, Whittington MA. Neuronal networks for induced ‘40 Hz’ rhythms. Trends Neurosci. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Two distinct subgroups of cholecystokinin-immunoreactive cortical interneurons. Brain Res. 1997;752:175–183. doi: 10.1016/s0006-8993(96)01446-1. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. Lack of depolarization-induced suppression of inhibition (DSI) in layer 2/3 interneurons that receive cannabinoid-sensitive inhibitory inputs. J Neurophysiol. 2007;98:2517–2524. doi: 10.1152/jn.00817.2007. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Sturgess K, Erdelyi F, Szabo G, Molnar Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–1133. doi: 10.1093/cercor/bhh072. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Martin LA, Wei DS, Alger BE. Heterogeneous susceptibility of GABA(A) receptor-mediated IPSCs to depolarization-induced suppression of inhibition in rat hippocampus. J Physiol. 2001;532:685–700. doi: 10.1111/j.1469-7793.2001.0685e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Freund TF, Gulyas AI. Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci. 2004;19:1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneuron structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends Neurosci. 1994;17:119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurons in juvenile mouse neocortex. J Physiol. 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Levine ES. Cannabinoids depress inhibitory synaptic inputs received by layer 2/3 pyramidal neurons of the neocortex. J Neurophysiol. 2002;88:534–539. doi: 10.1152/jn.2002.88.1.534. [DOI] [PubMed] [Google Scholar]
- Trettel J, Levine ES. Endocannabinoids mediate rapid retrograde signaling at interneuron right-arrow pyramidal neuron synapses of the neocortex. J Neurophysiol. 2003;89:2334–2338. doi: 10.1152/jn.01037.2002. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]