The Yin and Yang of Interleukin-21 in Allergy, Autoimmunity and Cancer (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 1.
Published in final edited form as: Curr Opin Immunol. 2008 Jun 12;20(3):295–301. doi: 10.1016/j.coi.2008.02.004
Summary
IL-21 is a type I cytokine that like IL-2, IL-4, IL-7, IL-9, and IL-15 shares the common cytokine receptor γ chain, γc. IL-21 is produced by activated CD4+ T cells, NKT cells, and Th17 cells, and has pleiotropic actions on a range of lymphoid lineages. IL-21 regulates immunoglobulin production and drives B-cell terminal differentiation into plasma cells, cooperatively expands CD8+ T cells and drives Th17 differentiation, has inhibitory effects on antigen-presentation by dendritic cells, and can be pro-apoptotic for B and NK cells. Moreover, IL-21 has potent anti-tumor effects and is implicated in the development of autoimmune diseases. Regulating IL-21 actions in vivo therefore has clinical potential for a range of diseases and is an area of active investigation.
Introduction
IL-21 is the most recently discovered member of the family of cytokines whose receptors share the common cytokine receptor γ chain, γc [1**]. γc is also a component of the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15, and when mutated results in X-linked severe combined immunodeficiency (XSCID) in humans [1**,2]. The simultaneous inactivation of IL-4 and IL-21 signaling in this disease is responsible for the B cell defect in XSCID [3]. IL-21 production was originally thought to be restricted to CD4+ T cells, but it is now clear that IL-21 also is produced by Th17 cells and by NKT cells [1**], indicating roles for IL-21 in innate as well as adaptive immune responses. Although IL-21 production is restricted to lymphoid populations, it acts on a range of both lymphoid and non-lymphoid cells, indicating broad functions [1**]. In the past few years, it has become clear that not only does IL-21 regulate normal lymphoid development and function, but it also serves critical roles in inflammatory responses, allergy, and autoimmunity, and it has anti-tumor actions. We herein review the basic biology and signaling by IL-21 and discuss new insights regarding the function of IL-21 in pathogenic processes and therapeutic approaches for manipulating in vivo levels of IL-21 in allergy, autoimmunity, and cancer.
Regulation of expression of IL-21 and IL-21R and mechanisms of signal transduction
The IL21 gene is adjacent to the IL2 gene, and both genes are potently induced in T cells following TCR stimulation in a fashion that is dependent on a calcium signal and NFAT sites [4]. The IL-21 receptor is expressed on CD4+ and CD8+ T cells, B cells, NK cells, dendritic cells, macrophages, and keratinocytes [1**]. IL-21R is more highly expressed on B than T cells, but its expression within both lineages increases following activation via the antigen receptor [1**,5]. In T cells, this process involves the induction and dephosphorylation of Sp1 [5]. The expression of IL-21R on multiple lineages corresponds to the broad actions mediated by the cytokine [1**]. IL-21 acts through a receptor comprising IL-21R and γc, with Jak1 and Jak3 being rapidly activated and in turn activating Stat3 and to a lesser extent Stat1, Stat5a, and Stat5b [1**,6*]. Tyrosine 510 in the cytoplasmic domain of IL-21R mediates activation of Stat3 and Stat5, and IL-21 also activates PI 3-kinase/Akt and MAP kinase pathways, which together with Jak-STAT pathways contribute to IL-21 signaling [6*].
IL-21 regulation of B cell function
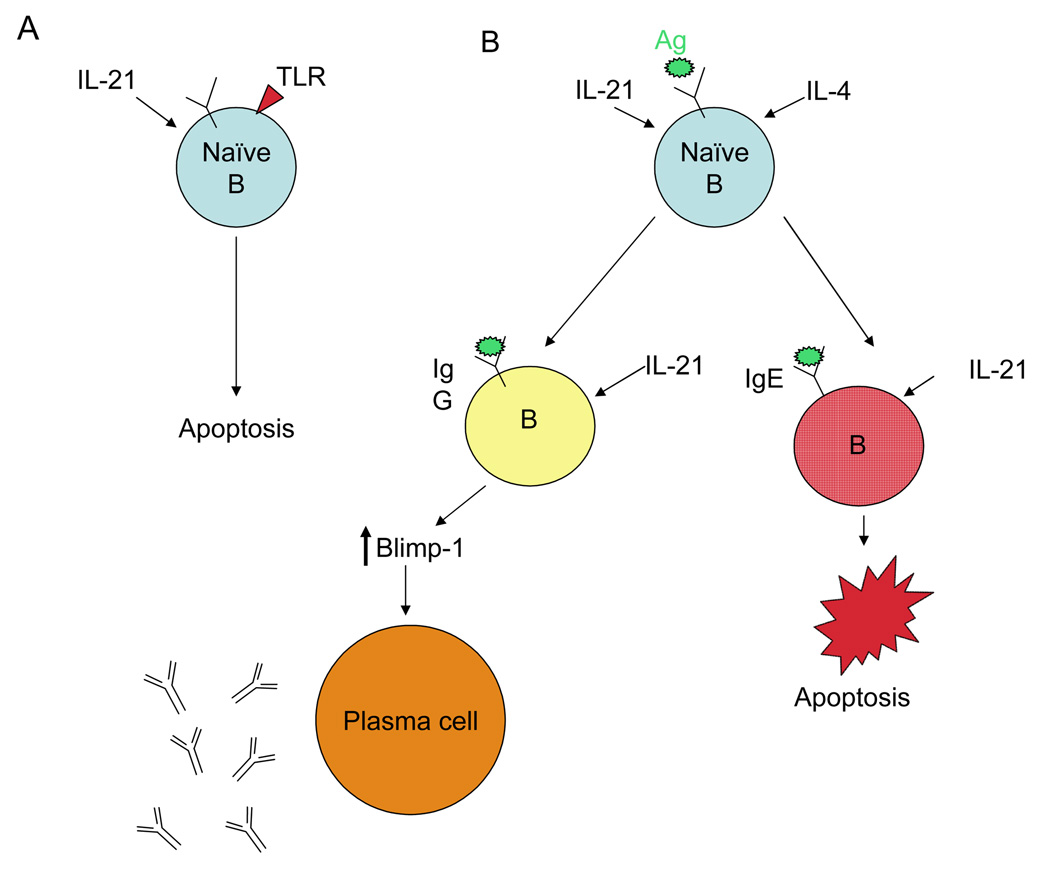
IL-21 plays a central role in the proliferation and survival of B cells and in their differentiation to immunoglobulin (Ig) producing cells [1**,3]. Although IL-21R knockout (KO) and wild type (WT) mice have indistinguishable immature and mature B cell populations [3], IL-21 affects survival and proliferation of mature B cells in vitro, depending on co-stimulatory signals [7–9]. Specifically, although IL-21 augments B-cell proliferation induced by BCR signals or signaling via CD40, IL-21 is pro-apoptotic, alone or when combined with activators of toll-like receptor signaling. These in vitro data suggest that in an immune response, if IL-21 signals without a specific antigen or in the presence of a non-specific polyclonal signal, potentially autoreactive B cells are deleted, whereas IL-21 acting in the context of a specific antigen and T-cell interaction instead induces expansion of responding cells (Figure 1).
Figure 1. Effects of IL-21 on B cell survival and function.
The outcome of IL-21 signaling to the naive B cell is context dependent. (A) In the absence of a signal to the B cell antigen receptor but in the presence of a pathogen-derived signal to toll-like receptors (TLR), IL-21 induces apoptosis of naive B cells. (B) In the presence of a B cell antigen receptor signal as well as a T cell-derived signal such as IL-4, IL-21 enhances naive B cell proliferation, leading to the cooperative regulation of immunoglobulin production mediated by the IL-21 induction of the master switch transcription factor Blimp-1 that leads to plasma cell differentiation. Although IL-21 signals to IgG-expressing B cells are positive, IL-21 induces selective apoptosis of IgE-committed B cells, leading to reduced production of IgE in the presence of IL-21.
The abilities of IL-21 to promote the differentiation of Ig-producing plasma cells [7,10] and regulate specific levels of IgE [3,11] have important implications for B-cell mediated autoimmunity and allergy. Naive IL-21R KO mice have diminished serum IgG1 and elevated serum IgE, and immunization of these mice with T-dependent antigens leads to reduced antigen-specific IgG1 and elevated antigen-specific IgE [3]. The IL-21-mediated downregulation of IgE may result from IL-21-induced expression of the pro-apoptotic Bcl-2-modifying factor, Bmf, in IgE-expressing B cells [12] or the induction of inhibitor of differentiation-2 (Id2) in B cells, leading to suppression of class-switch recombination (CSR) to IgE [13*]. The downregulation of IgE by IL-21 has implications for controlling IgE-mediated allergic responses.
The ability of IL-21 to regulate B-cell function and Blimp-1-dependent plasma cell differentiation (Figure 1) also has therapeutic implications [7,10]. Corresponding to reduced Ig production in IL-21R KO mice and elevated Ig in IL-21 transgenic mice [7], mice constitutively expressing IL-21 have increased plasma cell numbers. In vitro stimulation of naive or memory B cells with IL-21 in conjunction with either BCR or CD40 ligand signals results in the accumulation of Ig-secreting plasma B cells [7,10]. Although IL-21 and IL-4 cooperatively augment Ig-producing cells in vivo [3], these two cytokines have opposing effects on Blimp-1 induction in vitro [10,14] as well as on CSR leading to augmented production of IgE [13*]. IL-4-mediated suppression of IL-21-induced Blimp-1 expression is more potent in naive than memory B cells [14], and memory B cells show augmented response to antigen when IL-21 is present. A distinctive role of IL-21 in B cell biology was underscored in an in vitro CD4+ T cell: B cell collaboration model wherein inhibiting IL-21 but not other cytokines greatly diminished plasma cell differentiation and Ig production [15].
IL-21 effects in allergy and asthma
The ability of IL-21 to downregulate IgE production implied that IL-21 might diminish the severity of allergy and asthma. Indeed, in an ovalbumin-induced mouse model of allergic rhinitis, administering IL-21 during the initial antigen challenge significantly reduced allergic symptoms, with diminished antigen-specific serum IgE and reduced Th2 cytokines (IL-4, IL-5, and IL-13) in the nasal tissue, and decreased IL-4-induced levels of eotaxin-1 and eotaxin-2 in nasal fibroblasts, leading to suppressed eosinophil migration into the nasal tissue [16]. Analogous to the local effects of IL-21 in this allergic rhinitis model, systemic administration of IL-21 blocked antigen-induced anaphylaxis in a mouse food allergy model [13*]. This inhibition was accompanied by the induction of Id2 and corresponding diminished IgE CSR and antigen-specific IgE. The relationship between IL-21-induced prevention of CSR and anaphylaxis was demonstrated by showing that IL-21 could not block anaphylaxis in Id2-deficient mice [13*]. Despite these inhibitory effects of IL-21, in a model of airway inflammation, IL-21R KO mice unexpectedly had less antigen-induced airway hyper-responsiveness than WT littermates, even though they had elevated antigen-specific serum IgE levels [17], suggesting that IgE-mediated mast cell activation is just one component of cytokine involvement in airway inflammation.
IL-21 effects on CD4+ T cells
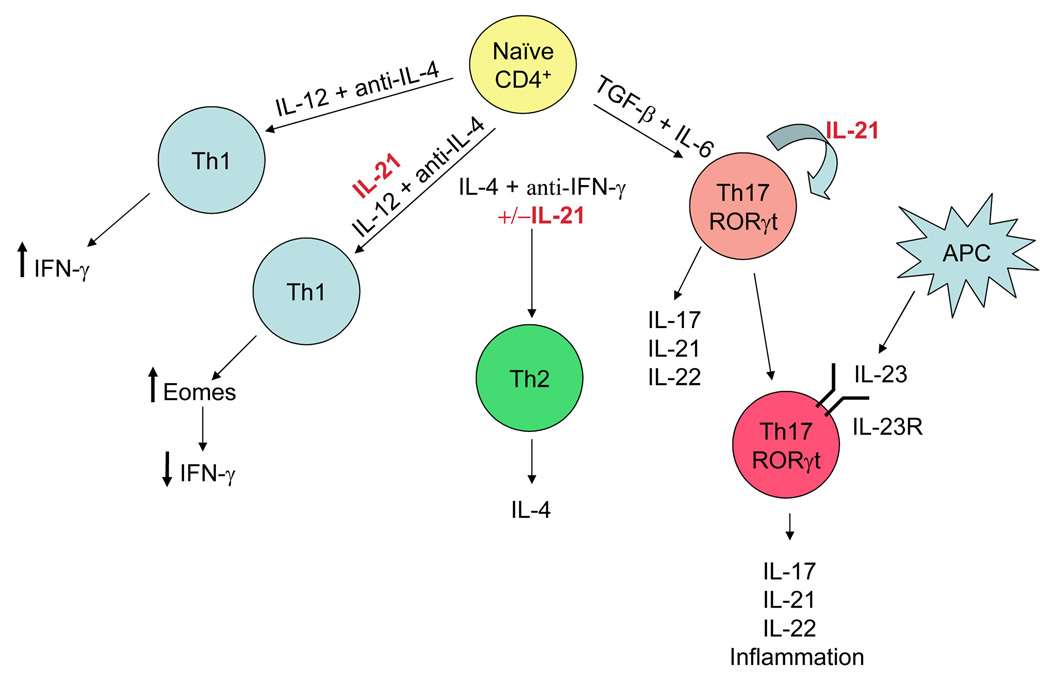
IL-21 is produced in vitro by Th1 [18], Th2 [19], and Th17 [20**,21**,22**] populations of CD4+ T cells as well as by NKT cells [23]. Although in vitro differentiation experiments revealed no difference in Th1 vs. Th2 polarization of WT vs. IL-21R KO CD4+ T cells [3], IL-21R KO CD4+ T cells have a reduced ability to differentiate into Th17 cells [20**,21**]. Infection of IL-21R KO mice with S. mansoni [24] or H. polygyrus [17] parasites resulted in reduced Th2 responses in vivo, even though the levels of Th1 or Th2 cytokines measured ex vivo were similar. Thus, priming in the absence of IL-21 may alter Th2 cytokine production through unknown mechanisms. Interestingly, when IL-21 is added during naive CD4+ T cell priming, it downregulates IFN-γ production without affecting other Th1 cytokines [25] (Figure 2).
Figure 2. Pleiotropic effects of IL-21 on CD4+ T cell subsets.
When IL-21 is present during priming of naive CD4+ T cells under conditions leading to Th1 commitment (IL-12 plus anti-IL-4), levels of the transcription factor Eomes are down-regulated, leading to reduced production of IFN-γ. In contrast, the presence of IL-21 during priming under conditions leading to Th2 differentiation (IL-4 plus anti-IFN-γ) has no apparent effect on the production of Th2 cytokines such as IL-4. Priming of naive CD4+ T cells with TGF-β plus IL-6 leads to expression of RORγt, which initiates Th17 lineage commitment. This leads to the production of high levels of IL-21 which has the further effect of inducing the IL-23R on T cells, thus allowing Th17 cells to respond to IL-23 with further expansion and production of IL-17, IL-21, and IL-22, all of which are involved in the propagation of inflammatory responses.
Role of IL-21 in Th17 differentiation
Th17 cells play a major role in promoting inflammatory responses in T cell-mediated autoimmune diseases [26]. Although there are some unresolved issues regarding factors influencing human vs murine Th17 differentiation, TGF-β and IL-6 are critical in murine Th17 differentiation, with IL-6 shifting the balance from TGF-β-mediated induction of regulatory T cells (Treg cells) to Th17 cells [27]. IL-21 is induced by IL-6 or by transduction of RORγt [20**], a nuclear hormone receptor essential for Th17 differentiation [28]. Interestingly, IL-21 is more highly expressed in Th17 cells than in Th1 or Th2 cells [21**,22**] (Figure 2). Induction of IL-21 by IL-6 and RORγt leads to augmented induction of IL-21 itself and amplification of this pathway. Th17 commitment is stabilized by IL-21-induced upregulation of IL-23R [20**]. IL-23 plays a key role in inflammatory disease [29] but its receptor is not present on naive T cells. The up-regulation of IL-23R by IL-21 promotes responsiveness to IL-23, Th17 commitment and expansion in vivo of these cells.
Role of IL-21 in Treg differentiation and function
Because IL-21 in combination with TGF-β can initiate an IL-6-independent pathway to Th17 development, it is not surprising that IL-21 also down-regulates TGF-β-regulated induction of FoxP3+ Treg cells [21**,22**]. Enhanced FoxP3 expression was observed in CD4+ T cells lacking IL-21, IL-6, or Stat3 following in vitro Th17 differentiation [21**,22**], revealing the negative regulation of Treg development by IL-21 and IL-6. Naive Treg cells express low levels of IL-21R, but TCR stimulation upregulates these levels. In contrast to the ability of IL-2, IL-7, and IL-15 to induce proliferation of Treg cells, IL-21 cannot and inhibits the suppressive function of Treg cells on either CD4+ [30] or CD8+ [31] T cells, but it is unclear whether this results from actions of IL-21 on Treg or responder cells. These studies suggest that IL-21 can overcome Treg-mediated immunosuppression, either by inhibiting their development or their function. These findings have implications for the clinical manipulation of IL-21 levels in autoimmune and inflammatory diseases.
IL-21 regulates proliferation and function of CD8+ T cells
Although IL-21 is produced by CD4+ T cells, most of its proliferative effects are on CD8+ T cells. Naïve CD8+ T cells express low IL-21R levels [8], and IL-21 does not by itself induce proliferation of this subset [32]. However, IL-21 acts synergistically with IL-15 or IL-7 to induce proliferation of both naive and memory phenotype CD8+ T cells [32]. The proliferative response of these cells to IL-21 in the absence of TCR signals implies a role for IL-21 in innate immune responses. In the presence of self-antigen signals, IL-21 can induce CD8+ T-cell expansion, with a cytolytic phenotype characterized by higher avidity TCR interactions and increased surface CD28 [33]. Interestingly, IL-21 can increase perforin expression in CD8+ T cells from HIV patients in the absence of either activation or proliferation, suggesting that IL-21 can upregulate CD8+ cytolytic activity without affecting endogenous HIV gene expression or virus production [34]. The ability of IL-21 to expand antigen-specific cytotoxic CD8+ T cells is limited by Treg cells, as depleting Treg cells augmented IL-21-mediated expansion of tumor-specific CTL [31].
Anti-tumor strategies using IL-21-induced CTL activity
The effects of IL-21 on CD8+ T and NK cell differentiation and cytolytic activity suggested its potential utility in the treatment of tumors and indeed IL-21 appears to possess greater anti-tumor activity than other γc family cytokines. In a study using syngeneic thymomas, IL-21 treatment resulted in longer survival (> 4 months) than seen with IL-2 or IL-15, accompanied by persistent CD8+ memory T cells that responded to secondary tumor challenge [35]. In a study of melanoma and renal tumors, when treatment was administered early after tumor initiation, subcutaneous administration of IL-21 augmented tumor regression and tumor infiltration by CD8+ T cells [36], suggesting that IL-21 might influence the homing of CD8+ T cells to the tumor.
Importantly, IL-21 can synergize with other agents that alone have suboptimal anti-tumor efficacy. For example, when mice with large pre-established poorly antigenic melanomas received in vitro activated tumor-specific Pmel-1 TCR transgenic CD8+ T cells plus peptide vaccine, better tumor regression was seen with the combination of IL-15 plus IL-21 than with individual cytokines [32], consistent with the synergistic effect that IL-15 plus IL-21 have on CD8+ T cell proliferation.
In another study, following adoptive transfer of naive tumor-specific CD8+ T cells, IL-21 and suboptimal IL-2 synergized in treating pre-established melanomas [37], yielding long-term survival of approximately half of mice and persistence of memory phenotype tumor-specific CD8+ T cells. Although IL-2 and IL-21 had synergistic anti-tumor activity when administered in vivo after the delivery of naive CD8+ T cells, in vitro activation with IL-21 enhanced the ability of cells to mediate tumor regression upon adoptive transfer, whereas IL-2 impaired the anti-tumor efficacy of transferred cells [38*].
Monoclonal antibody therapy directed against the TRAIL/DR5 ligand/receptor that controls apoptosis of some tumors was much more effective in suppressing metastases when subsequent IL-21 treatment was added. This suggests that tumor cell apoptosis can prime CTLs to tumor-specific antigens and that IL-21 enhances this priming and CTL effector function [39].
The successful treatment of tumors by IL-21 in animal models led to human clinical trials (reviewed in [1**]). In Phase I clinical trials in patients with advanced metastatic melanoma [40], IL-21 was well-tolerated, with minimal adverse side effects and efficacy in tumor control or regression. Future clinical studies with IL-21 will likely incorporate combination therapies that have been effective in animal models.
IL-21 in autoimmune diseases
Early studies suggested that IL-21 might play a role in autoimmunity, with elevated IL-21 being found in two mouse models of systemic lupus erythematosus. In BXSB.B6-Yaa+ mice, levels of IL-21 correlated with increased serum Ig and development of disease, consistent with the role of IL-21 in plasma cell differentiation [7]. In the Sanroque mutant mouse, there is a defect in Roquin, a protein that negatively regulates follicular T helper cells, a population that produces high levels of IL-21 and regulates the differentiation of follicular B cells [41]. These mice also have high serum levels of IL-21 and develop lupus-like symptoms. The mechanisms leading to elevated IL-21 are unknown but this is believed to lead to increased production of auto-antibodies. One of the genetic loci associated with autoimmune diabetes is the Idd3 locus that contains the genes encoding both IL-21 and IL-2. Although a study of the NOD diabetic mouse model found elevated levels of IL-21 mRNA in T cells [42], a recent study concluded that the critical Idd3-encoded gene that leads to the development of diabetes corresponds to the Il2 rather than the Il21 gene [43].
Therapeutic efforts for the treatment of autoimmune responses with agents that block IL-21 have shown partial success. In the lupus-prone MRL-lpr mouse strain, treatment with soluble IL-21R-Fc fusion protein partially reduced disease [44]. Rheumatoid arthritis is an autoimmune disease in which synovial fluid and tissue have enhanced inflammatory responses to IL-21 and elevated IL-21R expression [45]. In two animal models, collagen-induced arthritis and adjuvant-induced arthritis, treatment with an IL-21-blocking agent ameliorated disease and/or reversed established disease and also lowered levels of IL-6 and IL-17 [46].
In experimental allergic encephalitis (EAE), a model for multiple sclerosis, it was shown that IL-21 is integral in the development of Th17 cells. IL-21 and IL-21R KO mice have dramatically reduced Th17 cells [20**,21**,22**] and greatly reduced progression of the EAE disease [21**,22**]. Although IL-21 treatment at the time of disease initiation can exacerbate EAE symptoms [47], in one study, IL-21-blocking agents have surprisingly exacerbated EAE symptoms, augmenting IL-17 and reducing Tregs numbers and activity [48]. The mechanisms underlying these conflicting results are unknown. A future focus is to understand how/when IL-21 is involved in the development of these autoimmune diseases.
Conclusions
The existence of IL-21 and its receptor were first reported in 2000, with the receptor having been identified as an orphan receptor for which the ligand was then cloned [49,50]. In just over seven years, an enormous amount has been learned about the biology of this sixth γc family cytokine. Not only does its inactivation explain part of the XSCID phenotype, but a range of studies reveal its critical actions for plasma cell differentiation, Th17 differentiation, and other effects on B, T, NK, and dendritic cells. These studies also underscore that there may be a dangerously fine line between levels of IL-21 that function as an effective anti-tumor agent and those levels that lead to pathogenic effects in autoimmunity. The challenge in the field is to understand how this balance is maintained within the normal immune system as well as properly manipulating it in the treatment of pathogenic states.
Acknowledgments
We thank Dr. Jian-Xin Lin, NHLBI for critical comments. This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- **1.Spolski R, Leonard WJ. Interleukin-21: Basic Biology and Implications for Cancer and Autoimmunity. Annu Rev Immunol. 2007 doi: 10.1146/annurev.immunol.26.021607.090316. This review offers the most comprehensive review of the regulation, signaling, and biology of IL-21, including actions within both lymphoid and myeloid cell lineages.
- 2.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 4.Kim HP, Korn LL, Gamero AM, Leonard WJ. Calcium-dependent activation of interleukin-21 gene expression in T cells. J Biol Chem. 2005;280:25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Kim HP, Xue HH, Liu H, Zhao K, Leonard WJ. Interleukin-21 receptor gene induction in human T cells is mediated by T-cell receptor-induced Sp1 activity. Mol Cell Biol. 2005;25:9741–9752. doi: 10.1128/MCB.25.22.9741-9752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. This article provides the most detailed analysis of signaling via IL-21.
- 7.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 8.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 9.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 11.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 12.Harada M, Magara-Koyanagi K, Watarai H, Nagata Y, Ishii Y, Kojo S, Horiguchi S, Okamoto Y, Nakayama T, Suzuki N, et al. IL-21-induced Bepsilon cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med. 2006;203:2929–2937. doi: 10.1084/jem.20062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Kishida T, Hiromura Y, Shin-Ya M, Asada H, Kuriyama H, Sugai M, Shimizu A, Yokota Y, Hama T, Imanishi J, et al. IL-21 induces inhibitor of differentiation 2 and leads to complete abrogation of anaphylaxis in mice. J Immunol. 2007;179:8554–8561. doi: 10.4049/jimmunol.179.12.8554. This article illustrates the strong therapeutic potential of IL-21 as a cytokine that can suppress IgE-mediated anaphylaxis and describes this effect as being mediated by the IL-21 induction of Id2, a repressor of IgE class switch recombination.
- 14.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 15.Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, Ettinger R. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 16.Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, Mazda O. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. 2007;179:7157–7165. doi: 10.4049/jimmunol.179.10.7157. [DOI] [PubMed] [Google Scholar]
- 17.Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 18.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. Articles 20–22 presented the first evidence for the essential role played by IL-21 in the development and function of the recently described Th17 lineage, including its role in the development of autoimmune pathology in experimental allergic encephalitis.
- **21.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 23.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 24.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 34.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, Pahwa RN, Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 36.Sondergaard H, Frederiksen KS, Thygesen P, Galsgaard ED, Skak K, Kristjansen PE, Odum N, Kragh M. Interleukin 21 therapy increases the density of tumor infiltrating CD8(+ )T cells and inhibits the growth of syngeneic tumors. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He H, Wisner P, Yang G, Hu HM, Haley D, Miller W, O'Hara A, Alvord WG, Clegg CH, Fox BA, et al. Combined IL-21 and Low-Dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J Transl Med. 2006;4:24. doi: 10.1186/1479-5876-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008 doi: 10.1182/blood-2007-09-113050. This article presents evidence for a novel programming of tumor-specific CD8+ T cells by IL-21 during in vitro priming which leads to enhanced anti-tumor activity upon secondary encounter with tumor cells in vivo. This IL-21 programming was found to be opposed by the presence of IL-2 during the priming event.
- 39.Smyth MJ, Hayakawa Y, Cretney E, Zerafa N, Sivakumar P, Yagita H, Takeda K. IL-21 enhances tumor-specific CTL induction by anti-DR5 antibody therapy. J Immunol. 2006;176:6347–6355. doi: 10.4049/jimmunol.176.10.6347. [DOI] [PubMed] [Google Scholar]
- 40.Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, Skak K, Lundsgaard D, Frederiksen KS, Thygesen P, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 41.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 42.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 43.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 Has a Pathogenic Role in a Lupus-Prone Mouse Model and Its Blockade with IL-21R.Fc Reduces Disease Progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Shen W, Kong K, Liu Z. Interleukin-21 induces T-cell activation and proinflammatory cytokine secretion in rheumatoid arthritis. Scand J Immunol. 2006;64:515–522. doi: 10.1111/j.1365-3083.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 46.Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007;56:1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]
- 47.Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 2005;174:2696–2701. doi: 10.4049/jimmunol.174.5.2696. [DOI] [PubMed] [Google Scholar]
- 48.Piao WH, Jee YH, Liu RL, Coons SW, Kala M, Collins M, Young DA, Campagnolo DI, Vollmer TL, Bai XF, et al. IL-21 modulates CD4+ CD25+ regulatory T-cell homeostasis in experimental autoimmune encephalomyelitis. Scand J Immunol. 2008;67:37–46. doi: 10.1111/j.1365-3083.2007.02035.x. [DOI] [PubMed] [Google Scholar]
- 49.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]