PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability (original) (raw)
Abstract
Mutations in the phosphatase and tensin homolog (PTEN) gene leading to PTEN protein deletion and subsequent activation of the PI3K/Akt signaling pathway are common in cancer. Here we show that PTEN inactivation in human T cell acute lymphoblastic leukemia (T-ALL) cells is not always synonymous with PTEN gene lesions and diminished protein expression. Samples taken from patients with T-ALL at the time of diagnosis very frequently showed constitutive hyperactivation of the PI3K/Akt pathway. In contrast to immortalized cell lines, most primary T-ALL cells did not harbor PTEN gene alterations, displayed normal PTEN mRNA levels, and expressed higher PTEN protein levels than normal T cell precursors. However, PTEN overexpression was associated with decreased PTEN lipid phosphatase activity, resulting from casein kinase 2 (CK2) overexpression and hyperactivation. In addition, T-ALL cells had constitutively high levels of ROS, which can also downmodulate PTEN activity. Accordingly, both CK2 inhibitors and ROS scavengers restored PTEN activity and impaired PI3K/Akt signaling in T-ALL cells. Strikingly, inhibition of PI3K and/or CK2 promoted T-ALL cell death without affecting normal T cell precursors. Overall, our data indicate that T-ALL cells inactivate PTEN mostly in a nondeletional, posttranslational manner. Pharmacological manipulation of these mechanisms may open new avenues for T-ALL treatment.
Introduction
PI3K catalyzes the production of the second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3), thereby recruiting and activating several downstream kinases. PI3K and its most prominent effector, Akt (also known as PKB), regulate cell viability, metabolism, motility, and proliferation and are extensively implicated in tumorigenesis (1–3). Constitutive activation of the PI3K/Akt signaling pathway in hematological malignancies, including myeloid leukemia, multiple myeloma, and T cell large granular lymphocytic leukemia, has been shown to support tumor cell proliferation and viability in vitro (4–6).
The main negative regulator of the PI3K/Akt pathway, the lipid phosphatase and tensin homolog (PTEN), is frequently inactivated in human cancer as result of various genetic lesions (7, 8), which ultimately result in decreased or absent PTEN protein expression and activity. PTEN deficiency in mice replicates the tumor spectrum observed in humans, including T cell malignancies (9, 10), and T cell–specific deletion of PTEN results in lymphoma-induced death (11). Importantly, PTEN is critically involved in maintaining hematopoietic stem cells and preventing leukemogenesis (12, 13). Several human T cell acute lymphoblastic leukemia (T-ALL) cell lines lack PTEN as a result of deletions or mutations in the gene, which consequently effect constitutive hyperactivation of the PI3K/Akt pathway (14, 15). Enforced expression of PTEN in these cell lines induces apoptosis by inhibiting PI3K/Akt (16), which suggests that this pathway may be important in T-ALL. However, most T-ALL cell lines were established from relapsed patients, have long been in culture, and likely accumulated genomic alterations not associated with the primary disease. Hence, it is unclear whether PTEN mutations and PI3K/Akt hyperactivation are common events in cells of T-ALL patients and whether these putative alterations originate relevant functional consequences.
PTEN inactivation and consequent PI3K/Akt pathway aberrant activation may arise from mechanisms other than those targeting PTEN gene integrity (17). Although not directly implicated in cancer, downregulation of PTEN activity by mechanisms such as phosphorylation and oxidation has been recognized for several years (18–21). PTEN C-terminal phosphorylation appears to stabilize the protein by preventing its ubiquitination and proteasome degradation while decreasing PTEN phosphatase activity (20–23). The serine/threonine protein kinase casein kinase 2 (CK2) has been linked to PTEN phosphorylation (21, 22). Interestingly, CK2 overexpression is observed in human solid tumors (24–26) and is essential for multiple myeloma cell survival (27). Moreover, transgenic mice with targeted expression of CK2 in T cells develop lymphomas (28). In addition, ROS, which are commonly upregulated in cancer cells and proposed to contribute to transformation (29–31), were shown to oxidize PTEN Cys124 in the active site to form a disulfide bond with Cys71, thereby inactivating PTEN (18, 19, 32). However, there is no direct evidence linking CK2, ROS, PTEN phosphorylation, or PTEN oxidation to downregulation of PTEN function in patient tumor cells, and the actual implications of these mechanisms to cancer cell function remain to be determined.
Here we show that constitutive activation of the PI3K/Akt pathway is a common event in primary T-ALL and is critical for leukemia cell viability. PI3K/Akt pathway hyperactivation appeared to result not only from canonical mechanisms involving PTEN gene alterations and consequent protein deletion, but also, in most cases, from PTEN protein stabilization and inactivation due to high CK2 activity and elevated intracellular ROS. Constitutive hyperactivation of the PI3K/Akt pathway occurred not only in PTEN-null, but also in most PTEN-expressing, T-ALL cells, and dependence on PI3K/Akt-mediated signaling was used to selectively target T-ALL cells. In addition, our data suggest that PI3K/Akt activation status, which integrates cues arising from both genetic and posttranslational inactivation of PTEN, could serve as a biomarker for the identification of candidate patients for treatment with inhibitors of PI3K and/or of its downstream targets.
Results
The PI3K/Akt pathway is constitutively hyperactivated in primary T-ALL cells.
Based on evidence from T-ALL cell lines, we hypothesized that PI3K/Akt signaling is hyperactivated in primary disease. To establish how frequently PI3K/Akt constitutive activation actually occurs, we evaluated the integrity of the PTEN/PI3K/Akt axis in T-ALL patient samples collected at diagnosis. Constitutive hyperactivation of the PI3K/Akt pathway was detected in most T-ALL specimens (87.5%; 21 of 24), as evaluated by increased phosphorylation of Akt and/or at least one of its downstream targets GSK-3β and FOXO3a in comparison to normal T cell precursors (Figure 1, A and B, Table 1, and data not shown). Hyperactivation of the PI3K/Akt pathway was not associated with overexpression of total Akt (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI34616DS1) and was consistently observed independently of the stage of developmental arrest of the T-ALL samples (Table 1). Moreover, primary T-ALL cells presented higher PIP3 levels in the plasma membrane, as determined by both confocal microscopy and flow cytometry analysis (Figure 1, C–E). Consistent with previous reports (15, 33) we also observed PI3K/Akt hyperactivation in leukemia T cell lines (Supplemental Figure 2).
Figure 1. The PI3K/Akt pathway is constitutively hyperactivated in primary T-ALL cells.
(A) Cell lysates of normal human thymocytes or primary T-ALL cells collected at diagnosis and immunoblotted with the indicated phosphospecific antibodies or actin as loading control. (B) Levels of phosphorylated Akt (S473) in thymocyte (n = 8) and T-ALL (n = 15) samples were quantified by densitometry analysis. Points represent individual samples, horizontal bars denote mean, and mean ± SEM is shown in parentheses. (C) Surface expression of PIP3 was determined by confocal microscopy after staining with anti-PIP3 antibody. Scale bar: 50 μm. Insets (20 μm square) of 1 representative cell of each sample are shown. (D) Same samples were analyzed by flow cytometry. Values in each histogram indicate PIP3 mean fluorescence intensity; gray histogram represents negative isotypic control; vertical lines indicate peak value in T-ALL samples. (E) Mean fluorescence intensity (MFI) was quantified by flow cytometry and compared in thymocyte and T-ALL samples (n = 4 per group). Values are mean ± SEM.
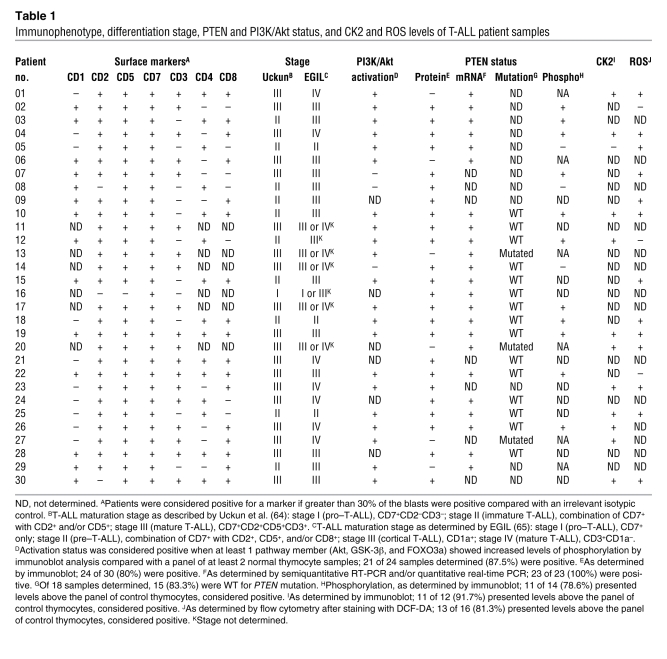
Table 1 .
Immunophenotype, differentiation stage, PTEN and PI3K/Akt status, and CK2 and ROS levels of T-ALL patient samples
Each T-ALL sample essentially consists of a clonal population of leukemia blasts arrested at a particular stage of thymic T cell development (34, 35), whereas normal thymocytes are immunophenotypically and functionally heterogeneous, covering the spectrum of distinct developmental subpopulations. To ensure that our data were not biased by the prevalence of a particular subpopulation in the normal control samples, we next analyzed patient specimens arrested at 2 particular stages of T cell development and compared them with their purified normal equivalents. We selected patients with CD3+CD4+CD8+ (triple positive; TP) and CD3+CD4–CD8+ (CD8 single positive; SP8) immunophenotypes because they were the most prevalent among primary T-ALL samples (Table 1 and Supplemental Figure 3A). Additionally, normal TP and SP8 thymocytes represent 2 distinct subpopulations: TP cells are mostly quiescent, whereas SP8 cells are actively proliferating or have recently done so (36). Both TP and SP8 T-ALL cells revealed clearly higher levels of phosphorylated Akt than their normal immunophenotypic counterparts (Supplemental Figure 3B). Likewise, both CD3– and CD3+ T-ALLs displayed hyperphosphorylation of Akt compared with immunophenotype-matched normal thymocytes (data not shown). Also, PIP3 levels were similar among the different normal thymic subsets and significantly and consistently lower than those of primary T-ALL cells (Supplemental Figure 4). Our data indicate that constitutive hyperactivation of the PI3K/Akt pathway is a common event in T-ALL, not only in immortalized cell lines, but more importantly in primary tumors.
PTEN gene alterations are infrequent, whereas protein expression is commonly deregulated, in primary T-ALL.
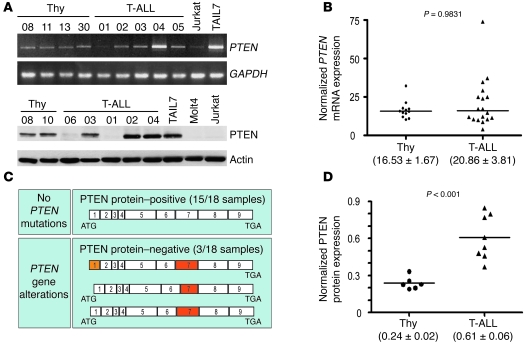
PTEN dephosphorylates PIP3 and is the main negative regulator of the PI3K/Akt pathway. Thus, we next analyzed the expression of PTEN mRNA and protein in primary T-ALL samples. Whereas all 23 samples analyzed expressed PTEN mRNA, a minority of the cases (20%; 6 of 30) lacked PTEN protein (Figure 2A and Table 1). Notably, not only the PTEN protein–negative samples (n = 5), but also most of the PTEN protein–positive samples (84.2%; 16 of 19), showed PI3K/Akt pathway hyperactivation (Table 1). Because point mutations may render PTEN protein inactive with unnoticeable effects on protein expression, we performed mutation analysis of all PTEN exons and flanking intron sequences in 3 PTEN protein–negative and 15 PTEN protein–positive T-ALL cases. Interestingly, all 3 PTEN protein–null samples analyzed showed PTEN gene alterations involving exon 7 (Figure 2C and Table 2), which is mutated in Jurkat cells and is essential for PTEN protein stability (15, 37). One of the samples was also affected in exon 1, which appears to be involved in PTEN membrane binding and activation (38). None of the 15 PTEN protein–positive T-ALLs analyzed presented PTEN mutations in exons 1–9 or in the flanking intronic splice site sequences. Our data indicate that relatively few primary T-ALL samples harbor PTEN gene alterations, in accordance with recent reports that PTEN mutations are rare in primary T cell leukemia specimens (33, 39). In addition, despite some heterogeneity, average PTEN mRNA expression in primary T-ALL cells was comparable to that in normal T cell precursors, as assessed by quantitative RT-PCR (Figure 2B). This observation suggests that PTEN transcription is largely normal in T-ALL, arguing against the possibility that PTEN promoter hypermethylation (40) or Notch-dependent PTEN transcriptional repression (39) plays a broad role in primary disease. Finally, we assessed whether hyperactivation of the PI3K/Akt pathway in PTEN-positive T-ALL cells is caused by decreased PTEN protein abundance. Unexpectedly, we found that PTEN-positive T-ALL samples expressed even higher levels of PTEN protein than did normal controls (Figure 2D), despite displaying constitutive activation of the PI3K/Akt pathway. Similar results were found for PTEN-positive T-ALL cell lines (Supplemental Figure 2). Analysis of PTEN protein expression by flow cytometry revealed no significant differences among the 4 major normal thymic subpopulations (Supplemental Figure 5, A–C). Furthermore, each subpopulation had lower PTEN levels than those of the T-ALL cell line TAIL7 (41), a good representative of PTEN-positive primary T-ALL T cells (Supplemental Figure 5, D–F, and Figure 2A). In addition, immunoblot analysis of TP and SP8 T-ALL cells and normal thymocytes isolated by fluorescence-activated cell sorting (FACS) showed once again that the T-ALL cells expressed higher PTEN protein levels than did their normal counterparts (Supplemental Figure 5G).
Figure 2. PTEN gene alterations and protein expression deregulation in primary T-ALL cells.
(A) Expression of PTEN mRNA (top) and protein (bottom) in normal thymocytes, T-ALL primary samples, and cell lines was assessed by RT-PCR and immunoblotting, respectively. (B) PTEN mRNA levels in thymocyte (n = 12) and T-ALL (n = 18) samples were evaluated by quantitative RT-PCR. (C) None of the 15 PTEN-expressing T-ALL patients presented PTEN gene alterations, whereas all 3 PTEN-negative leukemia samples analyzed harbored mutations in exons 1 and/or 7. (D) PTEN protein levels in thymocyte (n = 6) and T-ALL (n = 8) samples were evaluated by densitometry analysis after immunoblotting. Data are representative of 4 independent analyses involving a total of 9 thymocyte samples and 15 PTEN-expressing T-ALL specimens. In B and D, points represent individual samples, horizontal bars denote mean, and mean ± SEM is shown in parentheses.
Table 2 .
Alterations in PTEN gene coding sequence of PTEN protein–negative T-ALL patients
CK2 regulates PTEN expression and activity in PTEN protein–positive T-ALL cells.
The apparent contradiction between PTEN protein abundance and PI3K/Akt pathway activation in T-ALL samples prompted us to analyze PTEN phosphatase activity. We found that high PTEN expression was not associated with increased activity, but rather with PTEN inactivation, since T-ALL samples showed diminished PTEN in vitro lipid phosphatase activity compared with normal thymocytes (Figure 3A). Furthermore, most malignant specimens showed higher PTEN phosphorylation at different residues in the C-terminal tail (S380, S370, and the cluster including T382/T383/S385) than did normal controls (Table 1, Figure 3B, and data not shown). Thus, most T-ALL cells expressed higher levels of both PTEN (Figure 2A) and phosphorylated PTEN (Figure 3B). Importantly, the ratio of phosphorylated PTEN to total PTEN was significantly higher in phosphorylated PTEN–high T-ALL cases than in normal thymocytes (Supplemental Figure 6). This finding indicates that the higher amounts of phosphorylated PTEN in T-ALL cells are not simply the result of increased PTEN protein availability. Because CK2-mediated phosphorylation of PTEN at the C-terminal tail was previously proposed to downmodulate PTEN activity while increasing its stability (20, 21), we next examined expression and activation of CK2. T-ALL cells expressed higher levels of both the catalytic CK2α and regulatory CK2β subunits compared with normal thymocytes (Table 1, Figure 3, C–E, and Supplemental Figure 7) and showed clearly increased constitutive CK2 kinase activity (Figure 3F). Treatment of TAIL7, HPB-ALL, or primary T-ALL cells with the CK2-specific inhibitor tetrabromobenzotriazole (TBB) resulted in abrogation of C-terminal phosphorylation of PTEN, downregulation of PTEN expression, and simultaneous inhibition of Akt and GSK-3β phosphorylation (Figure 3G). These results suggest that PTEN phosphorylation — leading to PTEN protein stabilization and inactivation — and consequent PI3K/Akt pathway hyperactivation might be secondary to constitutive increase of CK2 activity in T-ALL cells. Because it was previously shown that CK2 may stimulate Akt activity directly (42), we next analyzed the effect of TBB on phosphorylation of Akt in PTEN-null Jurkat T-ALL cells stably expressing PTEN in a Tet-inducible manner (16). CK2 inhibition clearly diminished the levels of Akt S473 phosphorylation in Jurkat cells that expressed PTEN upon doxycycline treatment, whereas it induced minor downregulation of Akt phosphorylation in Jurkat cells that did not express PTEN (Supplemental Figure 8). These data suggest that the effect of CK2 on PI3K/Akt pathway activity in T-ALL cells is largely dependent on the ability of CK2 to regulate PTEN activity. To confirm this, we analyzed the effect of TBB directly on PTEN phosphatase activity. CK2 inhibition dramatically upregulated PTEN activity in TAIL7 (Figure 3H) and HPB-ALL cells (data not shown), suggesting that constitutive aberrant CK2 activity contributed to PI3K/Akt pathway activation, at least in part, by nondeletional posttranslational inactivation of PTEN. Consistent with this hypothesis, patient samples without PI3K/Akt pathway hyperactivation displayed levels of PTEN phosphorylation similar to those of normal thymocytes (e.g., T-ALL patient 14, Figure 3B). Moreover, high CK2 expression in PTEN-positive T-ALL samples was associated with increased phosphorylation of PTEN (Supplemental Figure 9 and Table 1).
Figure 3. PTEN expression and activity are regulated by CK2 in PTEN-positive T-ALL.
(A) PTEN in vitro lipid phosphatase activity was determined after immunoprecipitation of endogenous PTEN from normal thymocytes (n = 5) and PTEN-positive primary leukemia cells (n = 4). PTEN activity was normalized to the levels of immunoprecipitated PTEN in each sample. (B) PTEN phosphorylation at S380 was determined by immunoblotting. Results shown are from 2 independent analyses representative of a total of 8 thymocyte samples and 14 PTEN-positive T-ALL specimens. T-ALL patient 14 showed no PI3K/Akt basal hyperactivation (see Table 1). (C) Immunoblot analysis of CK2α and CK2β expression. (D and E) CK2 levels in normal thymocytes (n = 3) and T-ALL primary cells (n = 4) were quantified by densitometry analysis regarding CK2α (D) and CK2β (E). See Table 1 for expression of CK2 in 12 total cases analyzed. (F) CK2 kinase activity in thymocyte (n = 6) and T-ALL (n = 5) sample lysates was measured in vitro. (G) TAIL7, HPB-ALL, or primary T-ALL cells were treated for 2.5 h with DMSO vehicle control or 25 μM TBB, and levels of expression and phosphorylation of indicated proteins were analyzed by immunoblotting. p-PTEN (sev) indicates PTEN phosphorylation at the cluster S380/T382/T383/S385. (H) TAIL7 cells treated for 2.5 h with DMSO vehicle control (untreated) or 25 μM TBB were lysed, and in vitro lipid phosphatase activity of immunoprecipitated PTEN was assessed in triplicate. Values in A, D–F, and H are mean ± SEM.
High ROS levels downregulate PTEN activity in PTEN protein–positive T-ALL cells.
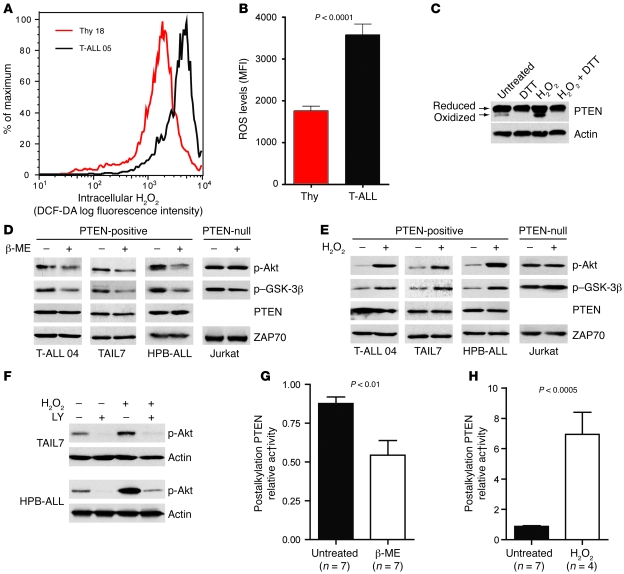
Cancer cells frequently express high levels of ROS, including superoxide anion (30) and H2O2 (29). By using the redox-sensitive dye DCF-DA, we found that the majority of primary T-ALL cells had higher intracellular ROS levels than did normal thymocytes (Table 1, Figure 4, A and B, and Supplemental Figure 10). Because H2O2 was previously shown to oxidize and thereby inactivate PTEN (18, 19, 32), we sought to determine whether diminished PTEN activity in T-ALL cells is also associated with increased ROS. Although most PTEN present in TAIL7 cells was in the reduced form, there were constitutively detectable levels of oxidized PTEN, which were further upregulated by addition of exogenous H2O2 and abrogated by in vitro treatment with the reducing agent dithiothreitol (DTT; Figure 4C). Treatment of PTEN-positive T-ALL cells with another antioxidant, β-mercaptoethanol (β-ME), downregulated constitutive phosphorylation of Akt and GSK-3β (Figure 4D and Supplemental Figure 11A), whereas H2O2 induced the opposite effect (Figure 4E and Supplemental Figure 11B). In contrast, Akt and GSK-3β phosphorylation levels were not significantly affected by modulation of ROS in PTEN-null T-ALL Jurkat cells (Figure 4, D and E), which suggests that oxidation-dependent activation of the PI3K/Akt pathway largely relied on regulation of PTEN. In accordance, the PI3K-specific chemical inhibitor LY294002 abrogated not only constitutive, but also H2O2-promoted, Akt phosphorylation, which indicates that ROS upregulated phosphorylated Akt in a PTEN/PI3K/PIP3-dependent manner and not via alternative mechanisms acting directly on Akt (Figure 4F). To further support the evidence that PTEN activity contributes to ROS-mediated activation of the PI3K/Akt pathway, we performed an indirect PTEN redox assay (19), in which lysis buffers alkylate reduced cysteine residues, thereby irreversibly inactivating PTEN. Oxidized cysteine residues are protected from alkylation, and, because oxidation is reversible, they can be recovered afterward by treatment with a reducing agent for assessment of PTEN activity. The resultant in vitro phosphatase activity reflects the proportion of PTEN that was oxidized and inactivated at the time of lysis. Using this approach, we determined that β-ME (Figure 4G) and H2O2 (Figure 4H) modulated endogenous PTEN redox state and activity in opposite directions. Importantly, addition of β-ME to T-ALL cells reverted constitutive oxidation–induced inactivation of PTEN. These data suggest that high levels of intracellular ROS contributed to PTEN inactivation by oxidation with subsequent PI3K/Akt pathway hyperactivation. Furthermore, combination of low doses of the antioxidant N-acetyl-cysteine (NAC) and the CK2 inhibitor TBB acted synergistically in inhibiting the PI3K/Akt pathway (Supplemental Figure 12). This effect did not appear to result from possible upregulation of CK2 by increased ROS levels in T-ALL cells, since treatment of TAIL7 and HPB-ALL cell lines with β-ME did not significantly affect the expression of CK2α/β (Supplemental Figure 13).
Figure 4. PTEN activity is downregulated by ROS in T-ALL.
(A) Intracellular H2O2 levels were assessed by flow cytometry after incubation with the redox-sensitive fluorescent dye DCF-DA. Shown is 1 representative patient sample and 1 representative normal control. (B) Mean intensity of DCF-DA fluorescence was compared between normal thymocytes (n = 7) and T-ALL (n = 5). Data are representative of most T-ALL samples (see Table 1) analyzed in 4 independent experiments. (C) TAIL7 cells previously treated with or without 1 mM H2O2 for 30 min were lysed in nonreducing conditions and analyzed for expression of PTEN oxidized and reduced bands. DTT was added where indicated to demonstrate the specificity of the lower, oxidized band. (D and E) PTEN-positive TAIL7, HPB-ALL, or primary T-ALL cells and PTEN-null Jurkat cells were treated for 2 h with 0.5 mM β-ME (D) or for 30 min with 1 mM H2O2 (E), and levels of Akt and GSK-3β phosphorylation were determined by immunoblot. (F) TAIL7 and HPB-ALL cells were pretreated for 1 h with 25 μm LY294002 (LY) or with DMSO vehicle control, stimulated with 1 mM H2O2 for 30 min, and analyzed for Akt phosphorylation by immunoblot. (G and H) Effects of β-ME (G) or H2O2 (H) on PTEN activity were measured by using the indirect PTEN redox assay, as described previously (19). More relative PTEN activity in the assay reflects less PTEN activity in the cell. Data from 2 independent experiments were normalized to the highest value in the control conditions. Values in B, G, and H are mean ± SEM.
Targeting PI3K/Akt pathway hyperactivation in T-ALL.
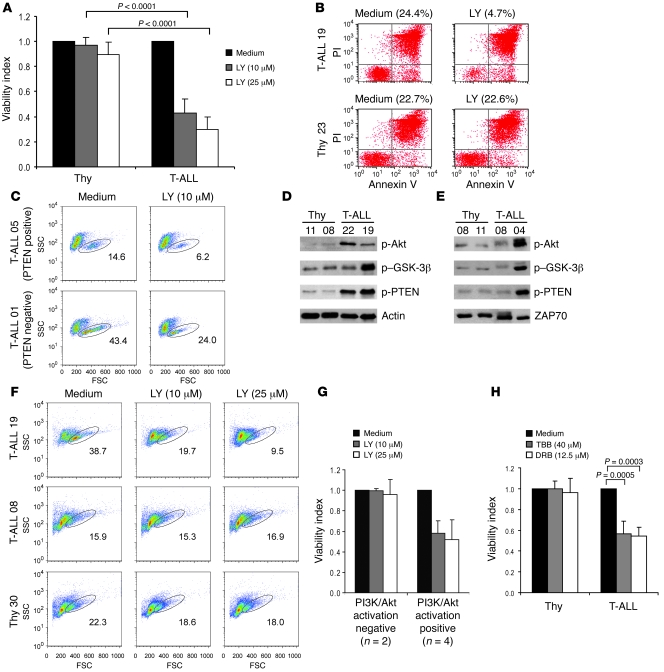
Malignant cell lines lacking PTEN activity as a result of PTEN gene and protein deletion are particularly sensitive to PI3K/Akt pathway inhibition (43). Our present data demonstrated that although they generally expressed PTEN, primary T-ALL cells frequently showed functional inactivation of PTEN phosphatase activity and constitutive activation of the PI3K/Akt pathway. Hence, we next analyzed the effect of the PI3K inhibitor LY294002 on the viability of T-ALL cells. In contrast to normal thymocytes, primary T-ALL cells underwent significant death upon LY294002 treatment in vitro (Figure 5, A and B, and Supplemental Figure 14). The effect of LY294002 on T-ALL cells did not appear to be solely dependent on the expression of PTEN, because it affected not only PTEN-negative but also PTEN-positive patient samples (Figure 5C) and cell lines (Supplemental Figure 15). Moreover, PTEN-positive patient samples with hyperactivation of the PI3K/Akt pathway (e.g., T-ALL patient 19; Figure 5D) were clearly sensitive to PI3K inhibition (Figure 5, F and G). In contrast, PTEN-positive samples without constitutive activation of the PI3K/Akt pathway (e.g., T-ALL patient 08; Figure 5E) were unresponsive to LY294002 treatment (Figure 5, F and G). As expected, these samples showed low levels of PTEN phosphorylation (e.g., T-ALL patient 08, Figure 5E; and T-ALL patient 14, Figure 3B), indicative of normal PTEN activity, in contrast to the samples with hyperactivation of the PI3K/Akt pathway (e.g., T-ALL patients 4, 19, and 22, Figure 5, D and E) that displayed hyperphosphorylation of PTEN. These results suggest that the sensitivity of T-ALL cells to PI3K inhibition is determined by PTEN functional integrity and consequent activation status of the PI3K/Akt pathway, rather than the level of PTEN expression per se.
Figure 5. Inhibition of PI3K induces selective cell death of T-ALL cells displaying hyperactivation of the PI3K/Akt pathway and does not affect normal T cell precursors.
(A) Normal thymocytes (n = 10) and primary leukemia cells (n = 15) were treated with 10 or 25 μM LY294002 for 48 h. Viability index to control untreated samples is shown. (B) Annexin V–FITC versus propidium iodide (PI) dot plots of representative cases. Percent viable cells are shown. (C) PTEN-negative (T-ALL patient 01) and PTEN-positive (T-ALL patient 05) patient samples were analyzed for viability by forward scatter–side scatter distribution. Numbers within plots indicate percent viable cells. FSC, forward scatter; SSC, side scatter. (D and E) T-ALL patients were reevaluated for PI3K/Akt pathway activation by Western blot analysis of phosphorylated Akt, GSK-3β, and PTEN. (F) T-ALL patient 19, with basal PI3K/Akt hyperactivation, and T-ALL patient 08, without such activation, were cultured with 2 different doses of LY294002 and analyzed for viability after 24 h. Also shown is 1 thymocyte sample as a control for unresponsiveness to LY294002 treatment. Numbers within plots indicate percent viable cells for each condition. (G) Responsiveness to LY294002 treatment at 24 h of culture of T-ALL samples without PI3K/Akt constitutive activation (negative) and with hyperactivation of the pathway (positive). Results are representative of 2 independent experiments. (H) Primary leukemia cells were cultured for 48 h in the presence of TBB (n = 12) or DRB (n = 9). TBB and DRB showed no significant effect on viability of normal thymocytes (n = 7). Data in A, G, and H are mean ± SEM. “Medium” denotes culture medium with DMSO vehicle control.
Because abrogation of CK2 activity leads to PTEN activation and PI3K/Akt pathway inhibition, we next treated T-ALL cells with 2 distinct CK2 inhibitors to assess their impact on cell viability. Both TBB and dichlororibofuranosylbenzimidazole (DRB) induced significant cell death in primary T-ALL cells but not in normal thymocytes (Figure 5H). Because we showed that CK2-mediated activation of the PI3K/Akt pathway was largely dependent on regulation of PTEN activity, we next compared the effect of CK2 inhibition on the viability of PTEN-positive versus PTEN-negative Jurkat T-ALL cells. Although TBB and DRB promoted death in PTEN-null T-ALL cells, the effect of both CK2 inhibitors was significantly higher in the cells that expressed PTEN (Supplemental Figure 16). This suggests that PTEN-positive T-ALL cells with hyperactivation of the PI3K/Akt pathway may especially benefit from therapies that include pharmacological inhibitors of CK2. Similarly, the viability of PTEN-expressing Jurkat cells was strikingly diminished upon treatment with β-ME, whereas PTEN-null Jurkat cells were insensitive to the reducing agent (Supplemental Figure 16).
Finally, to evaluate potential cooperative antileukemic effects, we performed experiments combining CK2 inhibition with ROS scavenging. CK2 blockade by TBB cooperated with the antioxidants β-ME and NAC to markedly decrease T-ALL T cell viability, with more than 95% cell death (Figure 6, A and B). We then used each of these agents together with the PI3K antagonist LY294002. Addition of LY294002 markedly increased the antileukemic activity of CK2 inhibitors TBB and DRB (Figure 6, C and D). Finally, PI3K blockade also potentiated the inhibitory effects of ROS scavenging by either β-ME or NAC (Figure 6, E and F). Similar cooperative effects were observed using HPB-ALL and primary T-ALL cells (Supplemental Figures 17 and 18). Together, these data suggest that combinatorial strategies targeting multiple elements related to PTEN regulation and PI3K/Akt activation are promising therapeutic approaches in T-ALL.
Figure 6. Cooperative effects of combinatorial treatment of T-ALL cells with pharmacological antagonists of PI3K, CK2, and ROS.
(A and B) Inhibition of CK2 and ROS scavenging cooperate in inducing T-ALL cell death. TAIL7 cells were cultured with the indicated concentrations of TBB and β-ME (A), TBB and NAC (B), or their combination, and viability was assessed at 72 h. (C–F) Inhibition of PI3K signaling cooperates with inhibition of CK2 and ROS scavenging in inducing T-ALL cell death. TAIL7 cells were cultured with the indicated concentrations of LY294002 and TBB (C), LY294002 and DRB (D), LY294002 and β-ME (E), LY294002 and NAC (F), or their combination, and viability was assessed at 72 h. Percent viability relative to untreated control (vehicle alone) is indicated for each condition. Similar results were obtained using HPB-ALL and primary T-ALL cells (Supplemental Figures 17 and 18). Data are mean ± SEM.
Discussion
PTEN gene alterations occur frequently in human cancers and mainly associate with PTEN protein deletion. However, many so-called “PTEN-null” malignant cells actually express low levels of PTEN protein (44). One possible explanation for this observation is that complete PTEN depletion may be detrimental for tumor function. Indeed, complete loss of PTEN can lead to p53-dependent cellular senescence that antagonizes tumorigenesis (45). Moreover, PTEN was shown to be haploinsufficient for tumor suppression (46), and many human cancers retain a WT copy of PTEN (8). Hence, PTEN does not appear to conform necessarily to the canonical definition of a tumor suppressor that requires inactivation of both alleles. Given this context, it is tempting to speculate that posttranslational inactivation of PTEN may result in levels of activity similar to those seen in cells carrying PTEN heterozygous mutations. Accordingly, aberrant PTEN polyubiquitination and proteasome-dependent degradation were recently shown to potentiate transformation (47). Hence, diminished but detectable PTEN activity likely provides an optimum intracellular context for cancer development. Our data further indicate that decreased PTEN activity, and subsequent activation of the PI3K/Akt pathway, can be uncoupled from PTEN gene lesions and from PTEN protein downregulation and/or deletion in primary T-ALL cells.
Previous studies conducted in cell lines or in a very small number of patient samples suggested that the PI3K/Akt pathway is constitutively activated in T-ALL (33, 48), but did not assess how frequently primary leukemia specimens display PI3K/Akt pathway hyperactivation. Here, we demonstrated that 87.5% of T-ALL primary samples showed hyperactivation of the PI3K/Akt pathway, which suggests that PI3K-mediated signaling is critical for the biology of this malignancy in patients. PTEN expression inversely correlates with phosphorylation of Akt in some T-ALL cells (14, 33) and in many leukemia/lymphoma cell lines (49). Similarly, we found that some T-ALL patient samples present alterations in PTEN gene coding sequence, lack PTEN protein expression, and show constitutive activation of the PI3K/Akt pathway. PTEN inactivating mutations predicted to cause protein truncation were recently reported in 5.2% (33) and 8% (39) of primary T-ALL samples, a low frequency event confirmed in our present study. However, the vast majority of primary T-ALL cells show simultaneously high expression of unmutated PTEN protein and hyperactivation of the PI3K/Akt pathway. We propose 2 posttranslational mechanisms to solve this apparent paradox: CK2-mediated phosphorylation and ROS-dependent oxidation of PTEN in T-ALL cells downregulate PTEN activity and consequently promote PI3K/Akt constitutive hyperactivation without decreasing PTEN expression (Figure 7).
Figure 7. Model for CK2- and ROS-mediated activation of the PI3K/Akt pathway in primary T-ALL.
By as-yet unknown mechanisms, CK2 is overexpressed and hyperactivated in T-ALL cells. Likewise, ROS intracellular levels are clearly elevated. These phenomena lead to posttranslational, nondeletional inactivation of PTEN, thereby contributing to constitutive hyperactivation of the PI3K/Akt pathway in leukemia cells. It is possible that CK2 and ROS may also mediate PTEN-independent activation of the PI3K/Akt pathway in some T-ALL cells.
It was previously shown that CK2 and PTEN can physically interact (22), and CK2-mediated phosphorylation of PTEN affects its activity and stabilizes the protein by preventing proteasome-mediated degradation (20, 21). We demonstrate here — for the first time to our knowledge — that CK2 is overexpressed and hyperactivated in primary T-ALL cells and suggest that CK2-mediated phosphorylation of PTEN stabilizes PTEN expression and inhibits its activity, ultimately leading to PI3K/Akt pathway constitutive activation. The biological impact of these observations was addressed by using 2 distinct pharmacological inhibitors of CK2, both of which promoted T-ALL cell death without affecting the viability of normal T cell precursors, similar to the PI3K-specific inhibitor LY294002. Our present work indicates that increased CK2 expression in T-ALL cells is likely not a consequence of PI3K/Akt hyperactivation itself. Future studies to identify why CK2 is overexpressed and activated in T-ALL are warranted. Interestingly, PTEN phosphorylation on the CK2 target residues S380/T382/T383 was observed in some AML patients and shown to correlate with increased phosphorylation of Akt and decreased overall patient survival (50). These observations suggest that CK2-mediated posttranslational inactivation of PTEN could have important implications in hematological malignancies other than T-ALL. In addition, PTEN-expressing breast cancer cell lines with hyperactivation of PI3K/Akt have concomitant phosphorylation of PTEN, and both can be inhibited by the antitumor agent tetracarcin A (51). Finally, a recent report demonstrated the coexistence of high PTEN protein levels and increased Akt activation in some cases of renal cell carcinoma (52). These studies raise the question of whether the mechanism described herein may extend to solid tumors (17).
We further reported that the intracellular levels of H2O2 are significantly upregulated in primary T-ALL cells and presented evidence indicating that high ROS levels can lead to constitutive oxidation and inactivation of a PTEN pool in T-ALL cells. Although PTEN oxidation is known to be reversible, it appears to significantly contribute to T-ALL cell viability, since the use of β-ME that reverted endogenous oxidation/inactivation of PTEN also promoted T-ALL cell death. This is in accord with previous observations that minor alterations in the pool of oxidized PTEN can significantly impact cellular function (53). Future studies should identify the origin of high ROS in T-ALL. Genes involved in redox regulation, including thioredoxin, peroredoxin, and manganese superoxide dismutase, affect ROS levels and may thereby regulate PTEN (18, 32), but their status in T-ALL is unknown. The Akt substrates FOXO transcription factors were shown to downregulate ROS levels (54, 55), and FOXO1/3/4 full knockout mice develop thymic lymphomas (56). These findings may suggest the existence of a positive feedback loop involving ROS and PI3K/Akt activation in T-ALL. However, we did not find evidence that inhibition of PI3K-mediated signaling affected ROS levels in T-ALL cells (Supplemental Figure 19). Hence, the genetic source of high ROS affecting PTEN function in T-ALL remains to be identified. Our data suggest that ROS-dependent oxidation and CK2-mediated phosphorylation of PTEN contribute to PI3K/Akt pathway hyperactivation and T-ALL cell viability. Although we provided clear evidence that high ROS levels and CK2 expression and activity act upon the PI3K/Akt pathway largely by regulating PTEN, we cannot exclude that in some T-ALL cases they may also act via PTEN-independent mechanisms (Figure 7). Moreover, although PI3K/Akt pathway hyperactivation in the majority of the PTEN-positive specimens analyzed can be explained by at least one of the mechanisms we reported (Supplemental Figure 20), it is conceivable that other posttranslational PTEN modifications, orchestrated by different regulators, may contribute to inactivation of PTEN in T-ALL.
Genetic lesions could be implicated in hyperactivation of PI3K/Akt without PTEN deletion in T-ALL. For instance, PTEN gene alterations could occur, leading to functional inactivation with no effect on protein stability. This possibility is highly improbable, since no PTEN mutations were found in any of the PTEN-expressing samples analyzed. PI3K amplifications and mutations in hematopoietic/lymphoid cancers are very rare (33, 57), and thus unlikely to justify the very high frequency of PI3K/Akt pathway activation that we found in T-ALL. In contrast, Notch1-activating mutations are common, occurring in roughly half of T-ALL cases (58). Notch1 ectopic expression in Jurkat cells upregulates PI3K and Akt activity (59), suggesting a possible link between the pathways in T-ALL. In agreement with this notion, a recent study using T-ALL cell lines showed that mutational deletion of PTEN induces resistance to Notch1 inhibition and that Notch signaling via Hes1 mediates repression of PTEN transcription (39). However, most primary T-ALL samples express abundant, unmutated PTEN. One possible explanation for this discrepancy is that the Notch target gene MYC, which was previously shown to transcriptionally upregulate PTEN (39), may counterbalance the effect of Hes1 in primary leukemia cells but lack this ability in cell lines. Alternatively, Notch-mediated transcriptional downregulation of PTEN may be complemented at the protein level by CK2-mediated PTEN stabilization. In any case, a higher level of complexity appears to exist in the integration of signals regulating PTEN in primary leukemia cells compared with T-ALL cell lines.
Regardless of the underlying mechanism, we showed that dependence on PI3K/Akt pathway hyperactivation could be used to selectively target the malignant cells without affecting their normal counterparts (Supplemental Figure 21). Strikingly, T-ALL samples with normal levels of PTEN expression, PTEN phosphorylation, and PI3K/Akt activation were insensitive to LY294002 treatment, whereas all the remaining PTEN-expressing samples tested that showed PI3K/Akt pathway hyperactivation were clearly affected. We propose that evaluation of the activation status of the PI3K/Akt pathway at diagnosis could be used to determine the endpoint of different mechanisms that affect PTEN function, which are not restricted to gene mutations and deletions affecting PTEN expression. This could be easily performed by flow cytometry analysis of Akt and GSK-3 phosphorylation in patient samples against a panel of known PI3K/Akt activation–negative cell lines. Such simple evaluation should allow for a more accurate assessment of the clinical impact of constitutive PI3K/Akt activation on disease prognosis, treatment response, and patient survival.
In conclusion, we showed that PTEN inactivation was not necessarily synonymous with PTEN deletion in tumor cells. Primary T cell leukemias frequently presented high levels of PI3K/Akt activation as a result of posttranslational, nondeletional inactivation of PTEN by CK2-mediated phosphorylation and ROS-dependent oxidation. Our results indicate that combinations of PI3K/Akt pathway inhibitors with CK2 antagonists or ROS scavengers may be therapeutically beneficial in T-ALL.
Methods
FACS of thymic subpopulations, PTEN intracellular staining, and levels of PIP3 and intracellular ROS in surface-stained cells.
See Supplemental Methods.
Primary samples and T-ALL cell lines.
Institutional review board approval was obtained for all primary leukemia and thymic sample collections from Comitê de ωtica em Pesquisa, Centro Infantil Boldrini (Campinas, São Paulo, Brazil), Gabinete de Investigação Clínica, Instituto Português de Oncologia (Lisbon, Portugal), Comissão de ωtica, Centro Hospitalar de Lisboa Occidental (Carnaxide, Portugal), and Comissão de ωtica, Faculdade de Medicina da Universidade de Lisboa (Lisbon, Portugal). All subjects provided informed consent. T-ALL cells were obtained from the peripheral blood and/or the bone marrow of patients with high leukemia involvement of 85%–100%. Samples were enriched by density centrifugation over Ficoll-Paque (GE Healthcare), washed twice in culture medium (RPMI-1640 supplemented with 10% FBS), subjected to immunophenotypic analysis by flow cytometry, and classified according to their maturation stage (Table 1). Normal thymocytes were isolated from thymic tissue obtained from children undergoing cardiac surgery. The tissue was gently minced in medium, the resulting cell suspension was filtered through a cell strainer, and thymocytes were enriched by density centrifugation to greater than 95% purity. The PTEN-positive T-ALL cell line TAIL7 shares significant similarities with primary leukemia samples (41). The cell lines Jurkat, Molt4, CEM (PTEN-negative), and HPB-ALL (PTEN-positive) have been extensively described (60–63). Jurkat PTEN Tet-inducible clones were provided by A. Weiss (UCSF, San Francisco, California, USA).
Immunoblotting.
Cell lysates were resolved by 10% SDS-PAGE, transferred onto nitrocellulose membranes, and immunoblotted with the following antibodies: ZAP70, p-PTEN (S370), p-PTEN (S380/T382/T383/S385) (Upstate), actin, CK2α, CKβ (Santa Cruz Biotechnology Inc.), Akt, p-Akt (S473), p–GSK-3β (S9), p-FOXO3a (T32), PTEN, and p-PTEN (S380) (Cell Signaling Technology). Where indicated, densitometry analysis was performed using Image Quant software (version 5.2). Each band was analyzed with a constant frame and normalized to the respective loading control.
Detection of PIP3 levels.
Cells were washed in PBS and fixed in 2% paraformaldehyde, blocked with 5% normal goat serum in PBS, and incubated overnight at 4°C with mouse anti-PIP3 monoclonal antibody (Echelon) at 1:10 dilution. Primary antibody was detected with Alexa Fluor 488–conjugated goat anti-mouse antibody (Invitrogen). Samples were split and (a) imaged by confocal microscopy using a Zeiss LSM 510 Meta microscope, using identical acquisition parameters in 1 imaging session, or (b) analyzed by flow cytometry to quantify mean fluorescence intensity using FACSCanto flow cytometer (BD Biosciences) and FlowJo software (Tree Star).
RT-PCR and quantitative RT-PCR.
Total RNA (1 μg) was reverse transcribed using the ImProm II Reverse Transcriptase enzyme (Promega) and random hexamers. PTEN expression was evaluated by RT-PCR using the following primers: forward, CCAAGTCCAGAGCCATTTC; reverse, GTGGGTCCTGAATTGGAGG. The quantitative assessment of PTEN transcripts was made by quantitative RT-PCR on an ABI PRISM 7500 (Applied Biosystems) with the following primers: ABL, TGGAGATAACACTCTAAGCATAACTAAAGGT and GATGTAGTTGCTTGGGACCCA; PTEN, GCTACCTGTTAAAGAATCATCTGG and CATGAACTTGTCTTCCCGT. PTEN primers were designed to provide the most possible difference to the PTEN pseudogene transcript. PCR products were cloned into the pGEM-T Easy vector (Promega), and standard curves were obtained by serial dilutions of uncut plasmid. PTEN transcript values were normalized with respect to the number of ABL transcripts.
PTEN sequencing and mutational analysis.
The entire PTEN coding and flanking intronic sequences were amplified by PCR. Primers (provided by A. Vettore, Ludwig Institute for Cancer Research, São Paulo, Brazil) are listed in Supplemental Table 1. PCR reactions were done using 100 ng DNA and 0.5 U TripleMaster Polymerase (Eppendorf). The PCR products were sequenced directly, on both strands, using the BigDye kit (Applied Biosystems). Sequences were evaluated using Chromas Lite (version 2.0; Technelysium) and Mutation Explorer (version 2.6.1; SoftGenetics LLC) software. All mutations were confirmed by sequencing a newly amplified product. When necessary, PCR amplicons were cloned into pGEM-T vetor (Promega), and several individual clones were sequenced.
Endogenous PTEN in vitro lipid phosphatase assay.
Immunoprecipitations were carried out with an anti-PTEN antibody (Santa Cruz Biotechnology Inc.) overnight and a secondary agarose-conjugate antibody for 3 hours at 4°C. Immunoprecipitated protein was washed, resuspended in enzyme reaction buffer (50 mM Tris, pH 8; 50 mM NaCl; 10 mM DTT; and 10 mM MgCl2), and incubated with 10 μM PIP3 (Echelon) for 30 minutes at 37°C, after which phosphatase reaction was stopped with 100 μl malachite green reagent (Echelon). Free phosphate levels were measured in an ELISA plate reader at 620 nm. Absorbance was converted into pmol phosphate using a phosphate standard curve.
Endogenous CK2 kinase activity assay.
CK2 activity in cell lysates was measured using an appropriate kit from Upstate Biotechnology following the manufacturer’s instructions.
Intracellular ROS levels.
Cell samples were washed in PBS, incubated with 10 μM DCF-DA (Sigma-Aldrich) for 30 minutes in PBS at 37°C, washed, acquired on a FACSCalibur flow cytometer (BD Biosciences), and analyzed with FlowJo software.
Detection of PTEN oxidized form.
Cells were treated with or without 1mM H2O2 in PBS for 30 minutes at 37°C with 5% CO2. Whole cell lysates were prepared in nonreducing lysis buffer (100 mM Tris-Hcl, pH 6.8; 2% SDS; and 50 mM iodoacetamide) and ultracentrifuged at 125,000 g for 10 minutes at 4°C. Lysates were split and incubated with or without 100 mM DTT (Sigma-Aldrich) for 5 minutes at room temperature. Protein was analyzed in denaturing, nonreducing conditions by SDS-PAGE, transferred onto nitrocellulose membranes, and immunoblotted.
Assay of PTEN redox state and activity.
To measure PTEN activity modulation by oxidation the indirect method relying upon the oxidative protection from alkylation was performed as described previously (19).
Assessment of cell viability.
Cells were cultured in 24-well plates as 2 × 106 cells/ml at 37°C with 5% CO2 in control medium or with the indicated concentrations of LY294002 (Calbiochem), TBB, DRB, β-ME, NAC (Sigma-Aldrich), or combinations thereof. At 24, 48, and/or 72 hours, cells were harvested and quantitative determination of viability was performed using an Annexin V–based apoptosis detection kit (R&D Systems), as previously described (3). Alternatively, viability was assessed by forward scatter–side scatter flow cytometry analysis. Viability index was calculated as the ratio of experimental to control conditions.
Statistics.
Differences between populations were calculated using unpaired 2-tailed Student’s t test or Mann-Whitney test, as appropriate. A P value less than 0.05 was considered significant.
Supplementary Material
Supplemental data
Acknowledgments
This work was supported by grants from Fundação para a Ciência e a Tecnologia (FCT; POCI/SAU-OBS/58913), Fundação Calouste Gulbenkian, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 05/02390-4), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 401122/2005-0). A. Silva and B.A. Cardoso have FCT SFRH PhD fellowships. L.R. Martins and P.Y. Jotta have FCT BI and FAPESP scholarships, respectively. We thank Vassiliki Boussiotis for valuable suggestions and critical reading of the manuscript; Ana Luisa Caetano for FACS isolation of thymic subpopulations; Cristina Santos and Inês Cebola for technical support; Francisco Enguita for assistance with CK2 activity assays; André Vettore for providing PTEN primers; and José Rino for help with confocal microscopy and preparation of figures. We also thank Arthur Weiss for kindly providing the Jurkat PTEN Tet-inducible clones. We especially acknowledge the contribution of patients and their families, clinical directors, physicians, and nurses in providing primary samples.
Footnotes
Nonstandard abbreviations used: CK2, casein kinase 2; DRB, dichlororibofuranosylbenzimidazole; DTT, dithiothreitol; β-ME, β-mercaptoethanol; NAC, N-acetyl-cysteine; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PTEN, phosphatase and tensin homolog; SP8, CD8 single positive; T-ALL, T cell acute lymphoblastic leukemia; TBB, tetrabromobenzotriazole; TP, triple positive.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3762–3774 (2008). doi:10.1172/JCI34616
References
- 1.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Luo J., Manning B.D., Cantley L.C. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 3.Barata J.T., et al. Activation of PI3K Is Indispensable for Interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Q., Simpson S.E., Scialla T.J., Bagg A., Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 5.Hsu J., et al. The AKT kinase is activated in multiple myeloma tumor cells. Blood. 2001;98:2853–2855. doi: 10.1182/blood.V98.9.2853. [DOI] [PubMed] [Google Scholar]
- 6.Schade A.E., Powers J.J., Wlodarski M.W., Maciejewski J.P. Phosphatidylinositol-3-phosphate kinase pathway activation protects leukemic large granular lymphocytes from undergoing homeostatic apoptosis. Blood. 2006;107:4834–4840. doi: 10.1182/blood-2005-08-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali I.U., Schriml L.M., Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J. Natl. Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 8.Sansal I., Sellers W.R. The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 9.Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P.P. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 10.Podsypanina K., et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki A., et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/S1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz O.H., et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 14.Sakai A., Thieblemont C., Wellmann A., Jaffe E.S., Raffeld M. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–3415. [PubMed] [Google Scholar]
- 15.Shan X., et al. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol. Cell. Biol. 2000;20:6945–6957. doi: 10.1128/MCB.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z., Stokoe D., Kane L.P., Weiss A. The inducible expression of the tumor suppressor gene PTEN promotes apoptosis and decreases cell size by inhibiting the PI3K/Akt pathway in Jurkat T cells. Cell Growth Differ. 2002;13:285–296. [PubMed] [Google Scholar]
- 17.Cully M., You H., Levine A.J., Mak T.W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.R., et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 19.Leslie N.R., et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez F., Ramaswamy S., Nakamura N., Sellers W.R. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell. Biol. 2000;20:5010–5018. doi: 10.1128/MCB.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres J., Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 22.Miller S.J., Lou D.Y., Seldin D.C., Lane W.S., Neel B.G. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:145–153. doi: 10.1016/S0014-5793(02)03274-X. [DOI] [PubMed] [Google Scholar]
- 23.Tolkacheva T., et al. Regulation of PTEN binding to MAGI-2 by two putative phosphorylation sites at threonine 382 and 383. Cancer Res. 2001;61:4985–4989. [PubMed] [Google Scholar]
- 24.Daya-Makin M., et al. Activation of a tumor-associated protein kinase (p40TAK) and casein kinase 2 in human squamous cell carcinomas and adenocarcinomas of the lung. Cancer Res. 1994;54:2262–2268. [PubMed] [Google Scholar]
- 25.Landesman-Bollag E., et al. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 26.Scaglioni P.P., et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126:269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Piazza F.A., et al. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108:1698–1707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- 28.Seldin D.C., Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267:894–897. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 29.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 30.Irani K., et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 31.Suh Y.A., et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 32.Connor K.M., et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J. Biol. Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 33.Maser R.S., et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrando A.A., et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/S1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 35.Graux C., Cools J., Michaux L., Vandenberghe P., Hagemeijer A. Cytogenetics and molecular genetics of T-cell acute lymphoblastic leukemia: from thymocyte to lymphoblast. Leukemia. 2006;20:1496–1510. doi: 10.1038/sj.leu.2404302. [DOI] [PubMed] [Google Scholar]
- 36.Hare K.J., Jenkinson E.J., Anderson G. An essential role for the IL-7 receptor during intrathymic expansion of the positively selected neonatal T cell repertoire. J. Immunol. 2000;165:2410–2414. doi: 10.4049/jimmunol.165.5.2410. [DOI] [PubMed] [Google Scholar]
- 37.Georgescu M.M., Kirsch K.H., Akagi T., Shishido T., Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vazquez F., et al. Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3633–3638. doi: 10.1073/pnas.0510570103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palomero T., et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat. Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roman-Gomez J., et al. Lack of CpG island methylator phenotype defines a clinical subtype of T-cell acute lymphoblastic leukemia associated with good prognosis. J. Clin. Oncol. 2005;23:7043–7049. doi: 10.1200/JCO.2005.01.4944. [DOI] [PubMed] [Google Scholar]
- 41.Barata J.T., et al. IL-7-dependent human leukemia T-cell line as a valuable tool for drug discovery in T-ALL. Blood. 2004;103:1891–1900. doi: 10.1182/blood-2002-12-3861. [DOI] [PubMed] [Google Scholar]
- 42.Di Maira G., et al. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 43.Uddin S., et al. Inhibition of phosphatidylinositol 3ι-kinase induces preferentially killing of PTEN-null T leukemias through AKT pathway. Biochem. Biophys. Res. Commun. 2004;320:932–938. doi: 10.1016/j.bbrc.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 44.Abbott R.T., et al. Analysis of the PI-3-Kinase-PTEN-AKT pathway in human lymphoma and leukemia using a cell line microarray. Mod. Pathol. 2003;16:607–612. doi: 10.1097/01.MP.0000067423.83712.74. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z., et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Cristofano A., et al. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat. Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 47.Wang X., et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avellino R., et al. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400–1406. doi: 10.1182/blood-2005-03-0929. [DOI] [PubMed] [Google Scholar]
- 49.Dahia P.L., et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum. Mol. Genet. 1999;8:185–193. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- 50.Cheong J.W., et al. Phosphatase and tensin homologue phosphorylation in the C-terminal regulatory domain is frequently observed in acute myeloid leukaemia and associated with poor clinical outcome. Br. J. Haematol. 2003;122:454–456. doi: 10.1046/j.1365-2141.2003.04452.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima H., et al. Apoptosis and inactivation of the PI3-kinase pathway by tetrocarcin A in breast cancers. Biochem. Biophys. Res. Commun. 2007;356:260–265. doi: 10.1016/j.bbrc.2007.02.136. [DOI] [PubMed] [Google Scholar]
- 52.He L., et al. Co-existence of high levels of the PTEN protein with enhanced Akt activation in renal cell carcinoma. Biochim. Biophys. Acta. 2007;1772:1134–1142. doi: 10.1016/j.bbadis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Kwon J., et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kops G.J., et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 55.Tothova Z., et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Paik J.H., et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt P.K., Kang S., Elsliger M.A., Gymnopoulos M. Cancer-specific mutations in phosphatidylinositol 3-kinase. Trends Biochem. Sci. 2007;32:342–349. doi: 10.1016/j.tibs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Weng A.P., et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 59.Sade H., Krishna S., Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J. Biol. Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- 60.Schneider U., Schwenk H.U., Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int. J. Cancer. 1977;19:621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 61.Minowada J., Onuma T., Moore G.E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J. Natl. Cancer Inst. 1972;49:891–895. [PubMed] [Google Scholar]
- 62.Foley G.E., et al. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::AID-CNCR2820180418>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 63.Morikawa S., Tatsumi E., Baba M., Harada T., Yasuhira K. Two E-rosette-forming lymphoid cell lines. Int. J. Cancer. 1978;21:166–170. doi: 10.1002/ijc.2910210207. [DOI] [PubMed] [Google Scholar]
- 64.Uckun F.M., et al. Clinical features and treatment outcome of childhood T-lineage acute lymphoblastic leukemia according to the apparent maturational stage of T-lineage leukemic blasts: a Children’s Cancer Group study. J. Clin. Oncol. 1997;15:2214–2221. doi: 10.1200/JCO.1997.15.6.2214. [DOI] [PubMed] [Google Scholar]
- 65.Bene M.C., et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995;9:1783–1786. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data