A Precursor-specific Role for Hsp40/Hsc70 during Tail-anchored Protein Integration at the Endoplasmic Reticulum (original) (raw)
Abstract
Tail-anchored (TA) protein synthesis at the endoplasmic reticulum (ER) represents a distinct and novel process that provides a paradigm for understanding post-translational membrane insertion in eukaryotes. The major route for delivering TA proteins to the ER requires both ATP and one or more cytosolic factors that facilitate efficient membrane insertion. Until recently, the identity of these cytosolic components was elusive, but two candidates have now been suggested to promote ATP-dependent TA protein integration. The first is the cytosolic chaperone complex of Hsp40/Hsc70, and the second is a novel ATPase denoted Asna-1 or TRC40. In this study we focus on the role of the Hsp40/Hsc70 complex in promoting TA protein biogenesis at the ER. We show that the membrane integration of most TA proteins is stimulated by Hsp40/Hsc70 when using purified components and a reconstituted system. In contrast, when both Hsp40/Hsc70 and Asna-1/TRC40 are provided as a complete system, small molecule inhibition of Hsp40/Hsc70 indicates that only a subset of TA proteins are obligatory clients for this chaperone-mediated delivery route. We show that the hydrophobicity of the TA region dictates whether a precursor is delivered to the ER via the Hsp40/Hsc70 or Asna-1/TRC40-dependent route, and we conclude that these distinct cytosolic ATPases are responsible for two different ATP-dependent pathways of TA protein biogenesis.
Tail-anchored (TA)2 proteins are a distinct class of integral membrane proteins defined by the C-terminal location of their subcellular targeting signal (1). A recent study estimated there are 400 TA proteins in humans (2), and their primary sites of biosynthesis are the endoplasmic reticulum (ER) and the mitochondrial outer membrane (3,4). In the case of the ER, once membrane-integrated, TA proteins can also be sorted to most other locations within the secretory pathway (5–7). TA proteins play many diverse roles within the cell (7) and share only the common feature of displaying the bulk of their polypeptide chain in the cytoplasm with the C-terminal region acting as a membrane anchor. It has been apparent for some time that TA protein biosynthesis at the ER is quite distinct from the classical signal recognition particle (SRP)-dependent route used by the majority of membrane proteins (see Refs.6,8). In particular, the location of the TA sequence that acts as an ER-targeting signal dictates that targeting to, and insertion into, the ER membrane is post-translational. Furthermore, both the nucleotide triphosphate requirements and the cytosolic factors that mediate TA protein biosynthesis at the ER are different from the classical SRP-dependent pathway (9). This distinction has led to a number of studies aimed at identifying the cytosolic factors that deliver TA proteins to the ER and defining the membrane components that facilitate their subsequent integration.
Over the recent years there has been significant progress in the identification of the cytosolic factors that promote TA protein integration at the ER (9,10). First, a novel SRP-mediated, GTP-dependent route for the post-translational delivery of TA proteins to the ER was observed (11) and was found to exhibit substrate specificity (12). This pathway most likely overlaps/competes with one or more alternative routes for TA delivery (13,14). Second, the membrane integration of most if not all TA proteins is strongly dependent on ATP and one or more cytosolic proteins (13,15,16). Notably, both the Hsp40/Hsc70 chaperone pair (12) and the ATPase Asna-1/TRC40 (14,17) have been proposed to mediate the ATP-dependent delivery of TA proteins into the ER. Recent studies of the Asna-1/TRC40 component have compared multiple TA protein substrates and found clear evidence of substrate specificity for both Asna-1/TRC40 binding and its ability to mediate membrane integration (14,17). We recently used a reconstituted system to demonstrate that purified Hsp40/Hsc70 chaperones catalyze the integration of Sec61β (12). However, a detailed analysis of substrate specificity during the chaperone-mediated integration of TA proteins has been lacking.
In this study we have addressed this issue by studying the behavior of eight different TA proteins (Ramp4, Sec61β, Synaptobrevin2-Syb2-, Cytochromeb5-Cytb5-, Ubc6 J1, Syntaxin1A-Syn1A-, PTP1B, and Bcl2) with distinct properties (2,18). We have also investigated whether the chaperones are necessary or sufficient to promote this process. To achieve this feat, we both employed our established reconstituted system (12) and examined the effect of specifically inhibiting Hsc/Hsp70 activity in a “complete” system that also contains functional Asna-1/TRC40.
This comparative approach enabled us to distinguish the TA proteins that must use the Hsp40/Hsc70-mediated route and TA proteins that can use this route in the absence of an alternative pathway. We find that TA proteins with an obligatory requirement for the Hsp40/Hsc70-mediated route have TA regions with comparatively low hydrophobic index. In contrast, those proteins with more hydrophobic TA regions favor an alternative pathway for membrane integration, almost certainly driven by Asna-1/TRC40. Most strikingly, chimeric substrates were then used to show that the Hsp40/Hsc70 dependence is dictated by the TA core. We therefore conclude that the Hsp40/Hsc70-mediated delivery of TA proteins to the ER membrane is a vital and physiologically relevant pathway for a specific subset of precursor proteins. The identification of two distinct ATP-dependent targeting routes for TA protein biosynthesis is in agreement with the recent discovery of two different mechanisms for their membrane insertion (18).
EXPERIMENTAL PROCEDURES
_Chemicals and Compounds_—Small molecule Hsc/Hsp70 chaperone modulators used in this study were synthesized, as described previously (19,20), and were solubilized in DMSO to a final concentration of 30 mm. Intermediate dilutions were made in DMSO, so that the final volume in the reaction was 5%.
Unless otherwise stated, all other chemicals were purchased from Sigma. Recombinant Hsc70 and recombinant Hsp40 and antibodies to Hsp70/Hsc70 (clone N27F3-4, to Hsp40 (rabbit polyclonal) and to Hsp90 (rabbit polyclonal) were from Stress-Gen. Monoclonal anti-Asna-1/TRC40 antibody was from Abnova. Anti-SRP antibody was a gift from B. Dobberstein (ZMBH, Heidelberg, Germany).
_cDNA and Transcription_—cDNAs encoding human Sec61β, Cytb5, Bcl2, Ubc6 J1, mouse Ramp4, and rat Syb2 and Syn1A were subcloned into pCDNA5 (Invitrogen) in-frame with a 13-amino acid glycosylation sequence from bovine opsin (OPG) (GPNFYVPFSNKTG). cDNAs for Sec61β, Cytb5, Syb2, and Syn1A were as described previously (11,21). Ubc6 J1 (IMAGE ID 4137664) and Ramp4 (IMAGE ID 3489738) were from Geneservice. Human PTP1B, also bearing an opsin glycosylation sequence, OPG2 (SGMRGTEGPNFYVPFSNKTVDMMM), was cloned in pGEM4 and was kindly provided by N. Borgese (CNR Institute for Neuroscience, Milan, Italy). cDNAs encoding the chimeras of Ramp4 and Cytb5 (Ramp4(Cb5TM)OPG and Cb5(Ramp4TM)OPG) were generated by sequential PCR from the relevant parental vectors.
In all cases, the mRNAs were translated lacking the stop codon causing the resulting polypeptides to remain associated with the ribosome after synthesis. Transcripts were synthesized using SP6 or T7 RNA polymerase, according to the manufacturer's instructions (Promega).
_Preparation of Semi-intact HeLa Cells (sp-HeLa)_—HeLa cells were permeabilized using a mild digitonin treatment (40 μg/μl; Calbiochem) for 10 min on ice in KHM buffer (110 mm KOAc, 2 mm MgOAc, 20 mm HEPES, pH 7.2). The cells were then washed with HEPES buffer (90 mm HEPES, pH 7.2, 50 mm KOAc) and treated with micrococcal nuclease and DNase to remove any contaminating endogenous messenger. The cells were then washed and resuspended in KHM buffer.
Reconstitution of ER Integration_—Ribosome-nascent chain complexes (RNCs) were generated by translating transcripts lacking a stop codon for 15 min in rabbit reticulocyte lysate (RRL) in the presence of [35S]methionine, according to the manufacturer's instructions (Promega). In this case, the lysate was pre-spun for 3 min at 100,000 ×_g to remove trace amounts of ER membrane. Reactions of 200 μl were supplemented with 2.5 mm cycloheximide and 500 mm KOAc, and the sample was layered over 500 μl of HSCC (500 mm sucrose, 500 mm KOAc, 5 mm MgOAc; 50 mm HEPES, pH 7.9, supplemented with 2.5 mm cycloheximide and 1 mm dithiothreitol), followed by centrifugation at 213,000 × g for 20 min. The pellet was resuspended in 10 μl of low sucrose cushion. Membrane-insertion reactions included 2 μl of isolated RNCs made up to a final volume of 10 μl by low sucrose cushion and various additions. Hsp40 was added at 2.5 μm, and Hsc70 was added at 1.5 μm (final concentrations), and pre-spun reticulocyte lysate was added at 20% v/v. ATP was added at a final concentration of 1 mm. Following the addition of all cytosolic targeting factors and treatments, puromycin was added at 1 mm, and the sample was incubated for 10 min at 30 °C. Membrane insertion was achieved by incubation with sp-HeLa cells (eq to ∼ 2 × 105 cells per reaction) at 30 °C for 20 min. Membrane were recovered by a short spin on a benchtop centrifuge, and TA protein insertion was evaluated on the basis of _N_-glycosylation efficiency.
_Inhibition Assays_—Proteins trapped on the ribosome were synthesized using RRL in the presence of [35S]methionine at 30 °C for 15 min. The translation reaction was then treated with puromycin (1 mm) and immediately split, and the small molecule inhibitors solubilized in DMSO or DMSO alone were added at the desired concentration (5% of final volume) with subsequent incubation at 30 °C for 20 min. When indicated, apyrase (1 IU/20 μl) was added concomitantly to the small molecule inhibitors to hydrolyze ATP and ADP into AMP. sp-HeLa cells (∼2 × 105 per reaction) were added as a source of membrane and were analyzed as mentioned above. Where indicated, deglycosylation was performed with endoglycosidase H (EndoH) according to the manufacturer's instructions (New England Biolabs).
_Analysis of Chaperone Oligomerization_—A total of 50 μl of RRL was incubated with the small molecule inhibitors (100 μm, 5% volume) for 20 min at 30 °C. The reaction was then centrifuged for 1 h at 4°C at 100,000 × g (55,000 rpm) in a TLA-100 rotor. Supernatant (diluted 1:4 in SDS sample buffer) and pellet fractions (resuspended in 200 μl of SDS sample buffer) were analyzed by SDS-PAGE followed by Western blotting.
_Gel Electrophoresis_—Samples were heated at 70 °C for 10 min in SDS-PAGE sample buffer and then resolved on 10–18% polyacrylamide Tris-glycine gels under denaturing conditions. Gels were fixed, dried, and then exposed to phosphorimager plates, which were read using a Fuji BAS-3000 PhosphorImager. Radiolabeled products separated by SDS-PAGE were quantified using Aida software. Alternatively, the resolved proteins on gels were transferred to polyvinylidene difluoride membranes, and the membranes were blotted with the indicated primary antibodies in Tris-buffered saline with 0.05% (v/v) Tween 20, 5% milk, followed by relevant secondary horseradish peroxidase-conjugated antibodies (1/2000, Sigma). Secondary antibody location was detected using ECL (Roche Applied Science).
_Statistical Analysis_—Results are expressed as means ± S.E. Results were analyzed by analysis of variance followed by Dunnett's post-tests. Post-tests were only performed when the analysis of a variance test showed a significant difference (p < 0.05) between groups. * indicates p < 0.05, and ** indicates p < 0.01 throughout.
RESULTS
_Post-translational Integration of TA Proteins in Semi-permeabilized HeLa Cells_—Our primary assay for the unambiguous integration of TA proteins at the ER is the _N_-glycosylation of a short C-terminal extension, as previously exploited in several studies (12,13,18). Hence, each TA protein is fused in-frame at its C terminus with a 13-amino acid fragment of bovine opsin containing a consensus site for _N_-glycosylation (denoted by the suffix OPG throughout). This extension is short enough so as not to perturb post-translational integration, and _N_-glycosylation can only occur if this region is fully translocated into the ER lumen (12,13,18).
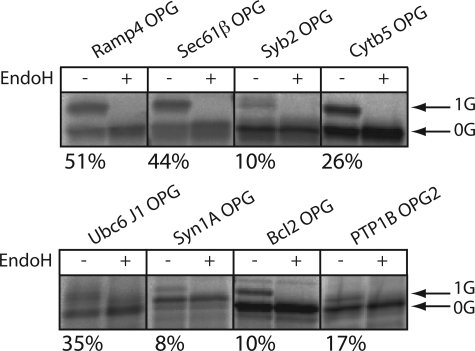
In contrast to our previous studies (cf. Refs.11,12,21), we chose to use semi-permeabilized mammalian tissue culture cells as a source of ER membrane for this work (see 22). First, we obtained efficient and reproducible levels of _N_-glycosylation in this system, and second, this approach will ultimately enable the use of mammalian cells that have been depleted of specific ER components using RNA interference (23). Ribosome-bound nascent chains (RNCs) were prepared for each of the selected model TA proteins using in vitro translation of mRNAs encoding the entire polypeptide but lacking a translation termination stop codon (11,12). These translation products can then be released from the ribosome by treatment with puromycin in the presence of sp-HeLa, such that membrane integration only occurs via a post-translational route. Under these conditions, we observed an endoglycosidase H-sensitive higher molecular weight species for each of the eight proteins studied (Fig. 1_A_), consistent with membrane integration and_N_-glycosylation in each case. The fraction of each precursor that was glycosylated varied from 8% with Syntaxin 1A to 51% with RAMP4 (Fig. 1_A_). This variability most likely reflects subtle differences in the access of the respective _N_-glycosylation sites to the oligosaccharyltransferase complex (12,24). Furthermore, the proportion of each precursor that is _N_-glycosylated is almost certainly an underestimate of the total percentage of correctly membrane-inserted polypeptides (12). Nevertheless, authentic integration of the _N_-glycosylated proteins is ensured, and hence this technique provides a robust quantitative method for comparative assays.
FIGURE 1.
_N_-Glycosylation reports the TA protein integration in the ER membrane. 35S-Metabolically labeled TA precursors fused in-frame with the N-terminal glycosylation sequence of opsin and lacking a stop codon were synthesized in RRL. The nascent chain was puromycin-released, and sp-HeLa were added. Membranes were isolated after another 20 min of incubation and RNase-treated, and half of the reaction was EndoH-treated, as indicated. The samples were then solubilized in Laemmli buffer before being analyzed by SDS-PAGE. The gels were then subjected to autoradiography. Values_below_ each panel represent the fraction of _N_-glycosylated material.
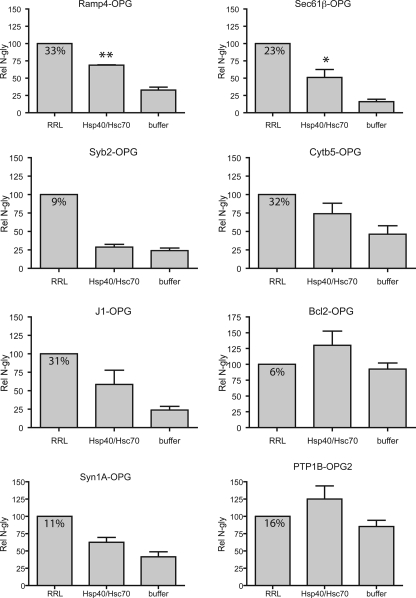
_Purified Chaperones Can Promote the Integration of Many Tail-anchored Proteins in a Reconstituted System_—The majority of TA proteins studied to date show a clear ATP dependence for efficient integration at the ER membrane, and we have previously shown that a combination of Hsp40 and Hsc70 can significantly promote the ATP-dependent insertion of Sec61β in a minimal reconstituted system (12). To investigate the ability of Hsp40/Hsc70 to promote the integration of a diverse range of TA proteins, RNCs for each precursors were isolated, and the nascent chains were released from the ribosome by puromycin treatment in the presence of RRL, recombinant Hsp40/Hsc70, or buffer. The capacity of these chains to be integrated into the ER of sp-HeLa cells was then assayed by comparing the relative efficiency with which the various TA proteins were_N_-glycosylated (cf. Fig. 1; see also Ref.12).
We found that a minimal combination of Hsc70 and Hsp40 stimulated the membrane integration of seven of the eight precursors analyzed to a level that was greater than that obtained in buffer (Fig. 2). Thus, for most of the TA proteins studied, these results support our previous analysis of Sec61β in which we found that Hsp40/Hsc70 could promote membrane integration in this purified system (12). Only in the case of Syb2 did we not find evidence of any stimulation of membrane integration by this chaperone combination (Fig. 2,Syb2-OPG panel, cf. Hsp40/Hsc70 and buffer samples), supporting the proposal that this precursor is unable to exploit the chaperone-mediated route for membrane integration (11,12).
FIGURE 2.
Hsp40/Hsc70 stimulate TA protein integration in vitro. 35S-Metabolically labeled TA precursors fused in-frame with the N-terminal glycosylation sequence of opsin and lacking a stop codon were translated in RRL and trapped on the ribosome by cycloheximide treatment. The labeled proteins were then released from the isolated RNCs by puromycin treatment and incubated with RRL, chaperones/ATP, or with buffer, as shown. Samples were incubated for 10 min at 30 °C before sp-HeLa was added as a membrane source. The membranes were isolated after another 20 min of incubation and processed as described inFig. 1. The fraction of_N_-glycosylated material recovered is indicated in the DMSO_box_. The relative amount of _N_-glycosylation (Rel N-gly) was normalized to the _N_-glycosylated material recovered in the DMSO control that was set to 100. Results are expressed as the means ± S.E. (n = 4) and were analyzed by ANOVA. If the ANOVA gave significant results (p < 0.05), statistical analyses were performed using Dunnett's post-test using the buffer lane as a control. **,p < 0.01; *, p < 0.05.
In most cases, the Hsp40/Hsc70 combination was less efficient at promoting membrane integration of the purified TA proteins than the addition of complete RRL (Fig. 2, see Ramp4-OPG, Sec61_β_-OPG, Cytb5-OPG, J1-OPG, and Syn1A-OPG) indicating either that this minimally reconstituted system is suboptimal for the chaperone-mediated process or that additional factors in RRL stimulate membrane integration. In two cases (Bcl2-OPG and PTP1B-OPG2) the Hsp40/Hsc70 combination stimulated membrane integration more effectively than RRL. Notably, these two TA proteins displayed an unusually high level of basal integration in the presence of buffer (Fig. 2). This basal integration most likely reflects the capacity of a precursor to use any residual chaperones derived from the RNC preparation (12) and/or the sp-HeLa cells (supplemental Fig. S1), combined with its potential for unassisted insertion into ER-derived membranes (15,18). We therefore conclude that the majority of TA proteins can exploit the Hsp40/Hsc70-mediated route for membrane insertion when this is the only pathway available.
_Selective Inhibition of Hsc/Hsp70s and Hsp40s in RRL_—Small molecule modulators of Hsc/Hsp70s have been shown to effectively inhibit the post-translational translocation of the yeast secretory protein, prepro-α-factor, across the ER membrane in vitro (19,25), consistent with a role for these chaperones in promoting this specialized translocation pathway (26). These inhibitors have also been used to show essential roles for Hsc/Hsp70 in maintaining breast cancer and multiple myeloma cell survival. However, these compounds are not generally cytotoxic and do not appear to target other essential factors (20,27).3
To assess the contribution of Hsp70 family members on the RRL-dependent stimulation of TA protein integration, we included three small molecules in the RRL-containing reactions. MAL3-101 (see supplemental Fig. S2) was selected because it is a potent inhibitor of both prepro-α-factor translocation and Hsp40-stimulated Hsp70 ATPase activity (19). MAL3-051 is a related compound that has little effect in these assays (19). We also included a newer analog, DMT002220 (denoted 2220 hereafter), on the basis that it affects both the endogenous and Hsp40-stimulated ATPase activities of Hsp70 (20).
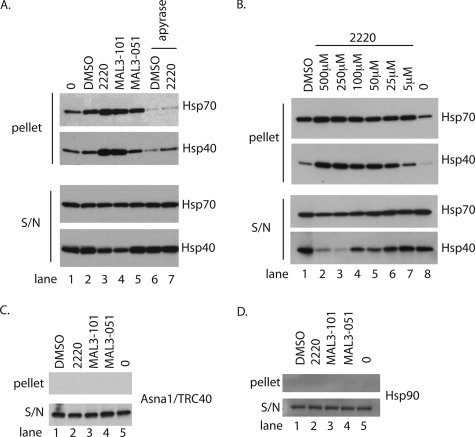
Because some of these dihydropyrimidinone-peptoid derivatives can induce the oligomerization of purified chaperones (25), we first investigated the effects of these compounds on selected factors present in RRL by using an established centrifugation assay (cf. Ref.25). When RRL was incubated with the solvent (DMSO), we observed a modest increase in the amount of Hsp/Hsc70s recovered in the pellet after centrifugation (Fig. 3_A_, pellet, cf. lanes 1 and 2). When 100 μm MAL3-051, the negative control, was included, the level of Hsp/Hsc70 in the pellet showed no obvious difference from the DMSO-treated sample (Fig. 3_A_, Hsp70 pellet, cf. lanes 2 and 5). However, the addition of 100 μm MAL3-101 or 2220 clearly increased the amount of Hsp/Hsc70 recovered in the pellet (Fig. 3_A_, pellet, cf. lanes 3 and_4_). Because Hsp/Hsc70s are relatively abundant in reticulocyte lysate (estimated to be 2 μm) (28), a substantial proportion of these chaperones remains soluble under the conditions of this assay (Fig. 3_A_, Hsp70 S/N, cf. lanes 1–5). When the Hsp70 co-chaperone Hsp40 was analyzed, the effect of the drug treatment was even more striking, and again the amount of material recovered in the pellet increased relative to the control reactions (Fig. 3_A_, Hsp40 pellet), and in this case the soluble pool was clearly depleted (Fig. 3_A_, S/N, cf. lanes 3–5). Interestingly, if nucleotide di- and triphosphates, including ADP and ATP, are removed with apyrase before drug treatment, the selective loss of Hsp/Hsc70 and Hsp40 was no longer observed (Fig. 3_A_, all panel,s see lanes 6 and_7_). We conclude that the small molecules MAL3-101 and 2220 affect Hsp/Hsc70 and Hsp40 function in RRL via a mechanism that requires ADP/ATP binding and/or hydrolysis. This results in an increase in the proportion of these chaperones present in higher order complexes (see “Discussion”).
FIGURE 3.
Hsp40/Hsc70 oligomerize in RRL in the presence of small molecule chaperone inhibitors. A, 100 μl of RRL was incubated with the indicated compound at 100 μm for 20 min at 30 °C before being subjected to centrifugation for 1 h at 100,000 × g. The supernatant and the pelletted fractions were recovered and the resolved proteins were immunoblotted for Hsp70/Hsc70 and Hsp40 (equivalent of 5 μl of RRL). Where indicated, the RRL was concomitantly treated with apyrase (1 IU/20 μl). B, same as A, but the RRL was treated with the indicated concentration of compound. C, same as A, except the fractions were immunoblotted for Hsp90. D, same as A, except the fractions were immunoblotted for Asna-1/TRC40.
Next, we titrated compound 2220 into these reactions to establish a dose dependence for any increase in the levels of Hsp/Hsc70 and Hsp40 recovered in the pellet (Fig. 3_B_, pellet). At concentrations of 250 and 500 μm 2220, the soluble levels of Hsp/Hsc70 were visibly diminished, and Hsp40 was now barely detectable (Fig. 3_B_, S/N). To confirm the specificity of the small molecule inhibitors, we studied their effects on other cytosolic components, specifically the TA protein targeting factor Asna-1/TRC40, an ATPase present in RRL at >20 nm (14), and the molecular chaperone Hsp90, another ATPase found in RRL at ∼2 μm (28). Neither Asna-1/TRC40 nor Hsp90 was found to pellet following incubation with the various compounds (Fig. 3, C and_D_), indicating selective targeting of Hsp70 family members as reported previously (19,25). We previously defined a role for SRP in the post-translational targeting of selected TA proteins (11), and we found that compound 2220 also had no effect on the behavior of cytosolic SRP (supplemental Fig. S3). We conclude that compounds MAL3-101 and 2220 provide the basis for selectively inhibiting the Hsp70-dependent route for TA protein integration at the ER, and that MAL3-051 offers a control for compound specificity.
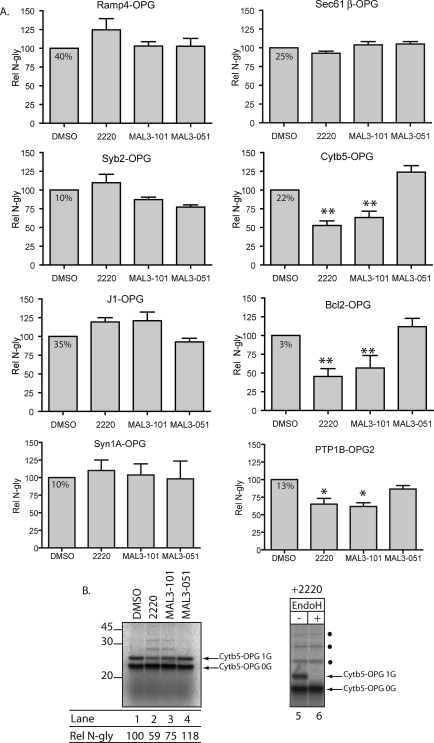
_Hsp70 Modulators Inhibit the ER Integration of Specific TA Proteins_—Based on the above data, we then investigated the effects of these compounds upon the RRL-dependent stimulation of TA protein integration at the ER (Fig. 2) (12). The eight model precursors were again synthesized as RNCs. In this case, the precursors were not further purified but rather were maintained in the RRL. Nascent chains were then released from the ribosome by puromycin treatment, and samples were incubated with DMSO, 2220, MAL3-101, or MAL3-051 before sp-HeLa cells were added as a source of ER membrane. Using _N_-glycosylation as a measure of membrane insertion, we found that the integration of three TA proteins (Cytb5-OPG, Bcl2-OPG, and PTP1B-OPG2) was significantly inhibited by 2220 and MAL3-101 (Fig. 4_A_). In contrast, MAL3-051 was without effect (Fig. 4_A_). These data are consistent with the ability of purified Hsp40/Hsc70 to stimulate the insertion of these proteins (Fig. 2). In contrast, the insertion of Ramp4-OPG, Sec61β-OPG, J1-OPG and Syn1A-OPG was not inhibited, and in some cases was even somewhat stimulated (Fig. 4_A_). Hence, the ability of purified Hsp40/Hsc70 to stimulate the insertion of a precursor in a minimal reconstituted system (Fig. 2) does not necessarily correlate with the use of this pathway in RRL, which likely contains alternative factors (i.e. Asna1/TRC40).
FIGURE 4.
Small molecule chaperone modulators inhibit the membrane integration of a subset of TA proteins. A, metabolically 35S-labeled TA precursors were synthesized in RRL with no further purification, released from ribosomes with puromycin. The reactions were then split four ways, and each fraction was incubated with either DMSO, 2220, MAL3-101, or MAL3-051 at 100 μm each for 20 min at 30 °C. sp-HeLa were then added for another 20 min at 30 °C, isolated, RNase-treated, and processed for SDS-PAGE and autoradiography. The fraction of _N_-glycosylated material recovered is indicated in the DMSO box. The relative amount of_N_-glycosylation (Rel N-gly) was normalized to the_N_-glycosylated material recovered in the DMSO control that was set to 100. Results are expressed as the means ± S.E. (n = 4). Statistical analyses were performed using Dunnett's post-test using MAL3-051 as a control, only if the ANOVA yielded significant results (p < 0.05): **, p < 0.01; *, p < 0.05, relative to the MAL3–51 control. B, left panel, gel sample for Cytb5-OPG. Molecular weight markers (×10–3) are indicated.Right panel, the EndoH treatment was performed on a fraction of the isolated membranes for 2 h and processed as described above. Filled circles denote higher molecular weight species that correspond to putative ubiquitinated material (see text).
The integration of Syb2-OPG was not promoted by Hsp40/Hsc70 alone (Fig. 2), and we likewise found that the small molecule modulators of Hsp/Hsc70 activity did not inhibit its membrane insertion. These data suggest that Syb2 does not utilize the chaperone-dependent route for TA insertion. The fact that the integration of five of the eight precursors studied was unaffected by compounds MAL3-101 and 2220 also allowed us to exclude the trivial possibility that these drugs simply interfere with N_-glycosylation or another general process. We did, however, notice a faint ladder of higher molecular mass products following 2220 and MAL3-101 treatment when Cytb5-OPG, Bcl2-OPG, and PTP1B-OPG2 were examined (Fig. 4_B_, cf. lanes 1–4,_ and data not shown). Although the singly N_-glycosylated species of Cytb5-OPG was EndoH-sensitive (Fig. 4_B_, lanes 5_ and 6; see alsoFig. 1_A_), the other products were not (Fig. 4_B_, lanes 5 and 6, filled circles), and most likely correspond to ubiquitinated species that accumulate when membrane integration is inefficient (data not shown and cf. Ref.11).
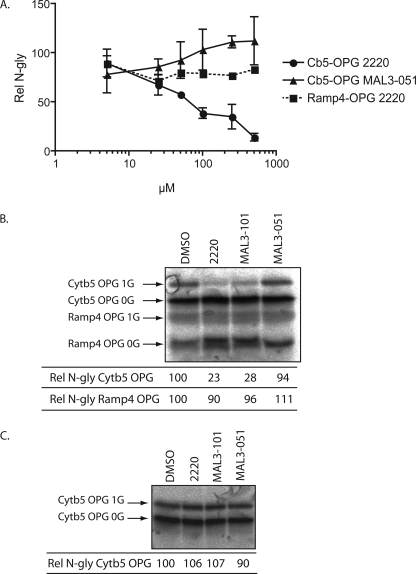
To understand why only certain TA proteins show a strong dependence on Hsc/Hsp70 activity for efficient integration at the ER, we first considered the possibility that different precursors may simply require different levels of chaperones; hence, a 100 μm concentration of the compounds may be suboptimal in some cases. To test this hypothesis we performed a dose-response analysis for the inhibition of Ramp4-OPG and Cytb5-OPG integration with compound 2220. We observed a stepwise increase in the inhibition of Cytb5-OPG integration as the concentration of 2220 increased from 5 to 500 μm with the highest concentration of the compound resulting in 90% inhibition relative to the control (Fig. 5_A_). MAL3-051 showed no such effect, and if anything may slightly stimulate integration at the very highest concentrations. In contrast, Ramp4-OPG integration was largely unaffected even by the highest concentration of drug tested (Fig. 5_A_), a level that appears to effectively inactivate the bulk of the Hsp40 present in RRL (see Fig. 3). We conclude that Ramp4 integration occurs independently of the Hsp40/Hsc70 activity rather than being facilitated by lower concentration of active chaperone complex. This analysis also suggests that the ∼50% level of Cytb5 integration observed in the presence of 100 μm 2220 (Fig. 4_A_) is because of incomplete inhibition of the Hsp40/Hsc70 complex, and that the majority of Cytb5 integration is dependent on an ATP-dependent, chaperone-mediated pathway (Fig. 5_A_) (16,17).
FIGURE 5.
Small molecule inhibition is dose-dependent and substrate-specific. A, dose-response curve. Cytb5-OPG and Ramp4-OPG were translated in RRL as described in Fig. 4 with the indicated concentrations of 2220 or MAL3-051. For each value, the relative amount of _N_-glycosylation was normalized to the_N_-glycosylation obtained in the same experiment in the presence of DMSO, which was set to 100. Results are expressed as the means ± S.E. (n = 2). B, competition experiment to assess chaperone-dependent TA integration. The reactions were assembled as inFig. 4, except that Cytb5-OPG and Ramp4-OPG were translated in the same RRL mix. C, sp-HeLa were treated for 20 min at 30 °C with DMSO, 2220, MAL3-101, and MAL3-051 at 100 μm, washed, and used as a source of membrane to assess the integration of 35S-labeled Cytb5-OPG. The relative amounts of_N_-glycosylation in B and C were calculated as described in Fig. 4.
A difference in the requirement for distinct cytosolic factors between different classes of TA proteins is further supported by competition experiments. Ramp4-OPG and Cytb5-OPG were synthesized in the same reaction, and the effect of small molecule inhibitors upon membrane integration was analyzed in concert. We again observe a marked inhibition of Cytb5-OPG insertion by 2220 and MAL3-101, but no substantial effect was noted on Ramp4-OPG integration (Fig. 5_B_, cf. 1st 4 lanes). This result strongly suggests that the membrane-targeting pathways utilized by the two precursors act independently and do not share common cytosolic components.
As a final test for the specificity and site of action of the small molecule inhibitors, we analyzed the effect of preincubating sp-HeLa cells with the compounds before assaying for TA protein integration. This treatment had no effect upon Cytb5-OPG membrane insertion, showing that ER function and bilayer integrity were unaffected by the compounds (Fig. 5_C_) (25).
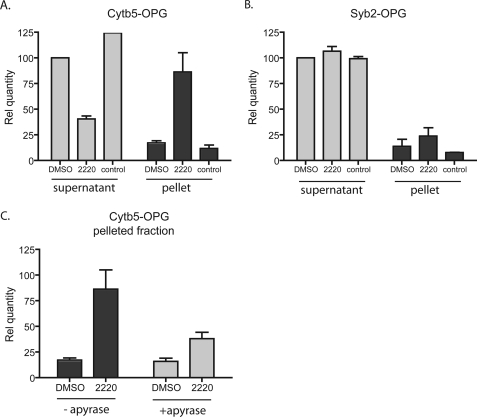
_Hsc70 Associates Selectively with TA Protein Substrates_—Both cross-linking and biochemical studies indicate that cytosolic factors, including Hsp40/Hsc70, SRP, and Asna-1/TRC40, bind directly to TA proteins (11,12,14,17). Given the effect of compound 2220 on Hsc/Hsp70 and Hsp40 solubility (Fig. 3), we postulated that if the chaperones were in a complex with particular TA proteins, then the associated polypeptides may also be selectively recovered in the pellet fraction after treatment with the compound. Therefore, an RNC complex containing radiolabeled Cytb5-OPG was synthesized as described inFig. 4_A_, and the translation reaction was treated with puromycin to release the nascent chains. These species were then incubated in the presence or absence of DMSO or 2220 and in the absence of ER-derived membranes. A pelleting assay was then carried out as described previously (Fig. 3), and the relative proportion of the Cytb5-OPG protein recovered in the supernatant and pellet fractions was determined. Treatment with compound 2220 resulted in a substantial increase in the amount of Cytb5-OPG recovered in the pellet fraction (>3-fold that of control reactions, seeFig. 6_A_) and a corresponding decrease in amount of precursor in the supernatant (Fig. 6_A_). This result strongly suggests that compound 2220 influences Hsp/Hsc70 function while bound to Cytb5-OPG. However, to address the possibility that the drug had a direct effect upon TA protein solubility, we carried out an identical analysis using Syb2-OPG, a TA protein for which Hsp40/Hsc70 plays no role during membrane integration (Fig. 2 andFig. 4) (11,12). In this case we found that 2220 has no effect on Syb2-OPG solubility (Fig. 6_B_). Furthermore, the effect of the drug on Cytb5-OPG solubility was dependent upon the presence of nucleotide di- and triphosphates, because prior apyrase treatment largely negated its effect (Fig. 6_C_), as anticipated (Fig. 3). On the basis of these data, we surmise that Cytb5-OPG binds Hsp40/Hsc70 to promote ER integration (cf. 12). Compounds 2220 and MAL3-101 do not prevent chaperone binding to Cytb5-OPG, but instead inhibit delivery of the precursor to the ER membrane.
FIGURE 6.
Small molecule chaperone inhibitors compromise Hsc/Hsp70 function after substrate binding. The small molecule 2220 induces an increased fraction of the Cytb5 nascent chain recovered in the pellet without significantly affecting Syb2. A, 35S-metabolically labeled Cytb5-OPG lacking a stop codon was translated in RRL. The nascent chain was puromycin-released and incubated with either DMSO or 2220 for 20 min at 30 °C. The mix was then centrifuged for 1 h at 100,000 × g. The supernatant (light gray histogram) and the pellet fraction (dark gray histogram) were recovered separately and resolved by SDS-PAGE. The radioactive material recovered under each condition was assessed after autoradiography and was calculated relative to the quantity recovered in the supernatant treated with DMSO, which was set to 100 (n = 3).B, same as in A for Syb2-OPG. C, effect of apyrase treatment of the RRL on the same set of experiment. The RRL was apyrase-treated (1 IU/20 μl) simultaneously with compound.
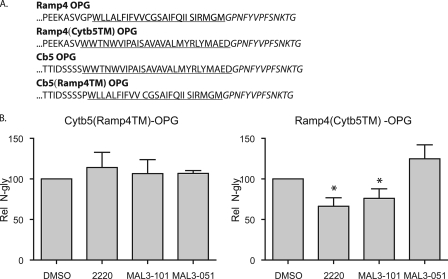
_Hsp70 Requirement during TA Insertion Is Defined by the Hydrophobic Core_—Having established that the membrane integration of a subset of TA proteins depends upon members of the Hsp40/Hsc70 family, we next consider what feature(s) of the precursors dictate this chaperone requirement. A number of previous studies have indicated that the hydrophobic core of the sequence plays a key role in recruiting specific cytosolic factors, namely SRP (11), Hsc70 (12), and Asna-1/TRC40 (14,17). We therefore hypothesized that if the specific properties of the TA regions of Cytb5-OPG and Ramp4-OPG are responsible for Hsp40/Hsc70 dependence, then the TA region would be necessary and sufficient to recapitulate the appropriate chaperone requirements. To test this model, we made chimeras in which the TA regions of Cytb5-OPG and Ramp4-OPG were exchanged to generate Cytb5(Ramp4TM)-OPG and Ramp4(Cytb5TM)-OPG (Fig. 7_A_). We then tested the effect of the small molecule inhibitors on their ER integration (cf. Fig. 4_A_). In contrast to the substantial effect observed with Cytb5-OPG (Figs.4_A_ and5), the membrane integration of the Cytb5(Ramp4TM)-OPG chimera bearing the TA region from Ramp4 was not inhibited by MAL3-101 or 2220 (Fig. 7_B_). Even more compelling, we observed that the ER integration of the complementary chimera, Ramp4(Cytb5TM)-OPG, with the TA region from Cytb5 was significantly inhibited by MAL3-101 and 2220 (Fig. 6_B_), despite the fact that the parental Ramp4-OPG protein was unaffected by these compounds (Figs. 4_A_ and5). Thus, a requirement for the Hsp40/Hsc70 chaperone system during the integration of a TA protein at the ER reflects the physicochemical properties of the hydrophobic core of its TA sequence.
FIGURE 7.
The TA core region dictates Hsp40/Hsc70 dependence. A, C-terminal sequences of the native TA and the chimera proteins studied. The transmembrane domains are underlined, and the opsin glycosylation site is in italics. Ramp4(Cytb5TM)-OPG indicates the core structure of Ramp4 fused with the C-terminal sequence of Cytb5, and Cb5(Ramp4TM)-OPG indicates the core structure of Cytb5 fused with the Ramp4 C-terminal sequence. B, inhibition of the integration of TA by small molecule chaperone modulators is mediated by the C-terminal sequence. Experiments were performed as described in Fig. 4 with the indicated constructs. Results are expressed as the means ± S.E. (n = 3). Statistical analyses were performed using Dunnett's post-test in comparison with MAL3-101, only if the ANOVA gave significant results (p < 0.05).
DISCUSSION
In comparison with the SRP-dependent co-translational integration of membrane proteins via the classical route (29), the insertion of TA proteins into the ER requires a distinct and novel biosynthetic pathway (6). Hence, the majority of TA proteins are integrated post-translationally via an ATP-dependent pathway(s) that exploits distinct cytosolic factors (but see also Ref.11). To date, two candidates that mediate the ATP-dependent integration of TA proteins have been identified, namely Hsp40/Hsc70 and Asna-1/TRC40 (12,14,17). To clarify the specific contribution made by the Hsp40/Hsc70 chaperone combination during TA protein biosynthesis, we have taken two complementary approaches. First, we used our previously established reconstitution assay (12) to show that seven of the eight TA protein substrates tested can utilize the Hsp40/Hsc70 system to stimulate their membrane integration. Second, we analyzed Hsp40/Hsc70 usage under more physiological conditions, i.e. by providing all of the factors implicated in the ATP-dependent delivery of TA proteins to the ER membrane via the addition of RRL. We then compared the effectiveness of this complete system to stimulate TA protein integration in the presence or absence of small molecule inhibitors of Hsc/Hsp70. The compounds employed (MAL3-101 and 2220) selectively inhibited the Hsp40/Hsc70 system in a dose-dependent fashion, primarily by inducing the ADP/ATP-dependent self-association of the chaperones (cf. Refs.30,31). This effect was previously noted for a related compound and most likely arises from the extended peptide-like motif embedded within these small molecule inhibitors (25). A control compound, MAL3-051, which has no effect on purified Hsc/Hsp70 activity (19), similarly had no effect on TA insertion or on Hsp40/Hsc70 oligomerization. Furthermore, two other ATPases, Asna-1/TRC40 and Hsp90, were unaffected by the compounds employed in this study.
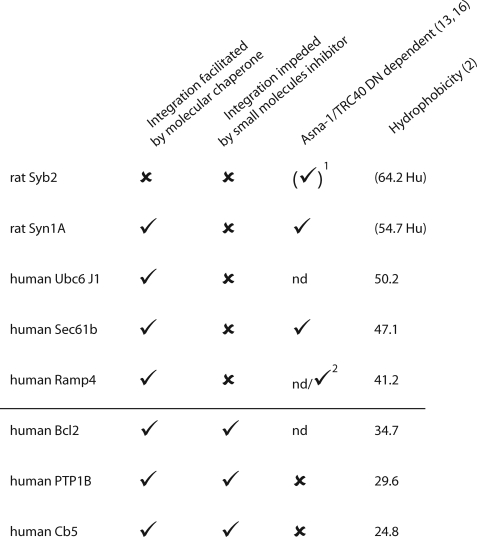
_A Subset of TA Proteins Are Obligatory Clients for Hsp40/Hsc70_—Our studies indicate that a subset of TA proteins display an obligatory requirement for the chaperone-mediated pathway when multiple factors are available to promote TA protein targeting (Fig. 8). In contrast, other TA proteins are unaffected by the small molecule inhibitors, although they can exploit the Hsp40/Hsc70 combination when no alternative routes are available (Fig. 8). We therefore conclude that the Hsp40/Hsc70 route for TA protein biosynthesis plays a physiologically relevant and major role during the biosynthesis of specific TA proteins. Most strikingly, the TA proteins that we identify as “obligatory” substrates for the chaperone-mediated route correlate with those previously shown to be independent of the Asna-1/TRC40 route (Fig. 8) (14,17). Hence, precursors that others have suggested employ the Asna-1/TRC40 route (i.e. Sec61β, Ramp4, see Refs.14,17) are unaffected by the small molecule Hsc/Hsp70 inhibitors. Evidence for these distinct pathways was perhaps best demonstrated by examining the insertion of both an Hsp40/Hsc70-dependent and an Hsp40/Hsc70-independent precursor together (Cytb5 and Ramp4, see Fig. 6_B_).
FIGURE 8.
TA hydrophobicity correlates with Hsp40/Hsc70 dependence for membrane integration. Summary of results obtained in the reconstitution experiment and with the small molecule inhibitors. TAs are classified with respect to the hydrophobicity values estimated as described (2). The values given for rat Syb2 and rat Syn1A are the human counterparts. The dependence toward Asna-1/TRC40 has been evaluated by Stefanovic and Hegde (14) and Spasic and co-workers (17). 1 indicates a chimera of rat Syb2 (Sec61β fused with the hydrophobic core of rat Syb2) was used (14). 2 indicates cross-linking evidence (17).
What then is the relevance of the Hsp40/Hsc70-mediated stimulation of the majority of proteins observed in our reconstituted system? Our data suggest that for many TA proteins there is sufficient redundancy between the two pathways that the Hsp40/Hsc70-dependent route may partly compensate for a loss of Asna-1/TRC40 function. Such redundancy has already been suggested in the case of Saccharomyces cerevisiae (14,32) and may also explain the apparently modest phenotype observed in other organisms following perturbation of Asna-1/TRC40 function (32–34). This is in striking contrast to the substantial effect on cell growth observed after perturbing the SRP-dependent targeting pathway in S. cerevisiae (35). Nevertheless, disruption of the gene encoding the Asna-1/TRC40 protein in mice results in embryonic lethality (36,37). This suggests that the biogenesis of at least one essential TA protein requires Asna-1/TRC40 function and/or that Asna-1/TRC40 plays some other crucial role(s) (33,37).
When the chaperone inhibitors were present at 100 μm, all three of the affected TA proteins were still inserted with at least 50% efficiency (Fig. 4_A_). To establish whether the remaining integration is because of a partial inhibition of the Hsp40/Hsc70 pathway or reflects the use of alternative factors or pathways, we carried out a dose-response analysis for Cytb5 integration. By increasing the concentration of compound 2220 to 500 μm, we could achieve 90% inhibition of Cytb5 membrane insertion (Fig. 6_A_). In contrast, Ramp4 integration was unaffected under these conditions. We therefore conclude that for Cytb5, and most likely other Hsp40/Hsc70-dependent TA proteins, membrane insertion primarily depends upon the presence of active chaperone complexes. Hence, whereas the levels of ATP required for efficient Cytb5 membrane integration are extremely low, being in the nanomolar range (16,18), we propose that this nucleotide requirement reflects a role for Hsc70/Hsp40. It also seems likely that this complex is equivalent to a previously undefined cytosolic protein required for maintaining Cytb5 in a translocation-competent form that is capable of efficient integration at the ER (16).
_Hydrophobicity of the TA Region Dictates the Delivery Route_—Finally, we considered what feature(s) of a TA protein dictate the primary route of its delivery to the ER membrane. Previous studies identified the importance of signal sequence composition in driving both the selective recruitment of cytosolic factors (38) and the mechanisms that underlie subsequent membrane translocation/integration (18). Cross-linking studies suggested that both Hsc70 (12) and Asna-1/TRC40 (14,17) bind to TA sequences. We therefore generated chimeras between an Hsp40/Hsc70-dependent precursor (Ramp4) and an Hsp40/Hsc70-independent precursor (Cytb5) by swapping the hydrophobic cores of their respective TA regions (Fig. 7_A_) and then investigated whether the behavior of the resulting chimeras reflected the origin of this hydrophobic core. We found that the TA insertion pathway reflects the origin of their TA core. Thus, when the TA core of Cytb5 is replaced with that from Ramp4, the resulting protein no longer depends upon Hsp40/Hsc70 for membrane integration. Even more compellingly, the reciprocal Ramp4 chimera with a Cytb5 TA core now depends upon Hsp70/Hsp40 for membrane integration (Fig. 7_B_).
When our results for Hsp70/Hsc40 dependence are combined with the physicochemical properties of TA proteins (2,18) and the ability of Asna-1/TRC40 to promote their membrane integration (14,17), we find a clear correlation between the predicted net hydrophobicity of the TA core and the preferred targeting route taken by the precursor (Fig. 8). Specifically, all of the proteins tested with a net hydrophobicity of ≤35 display an obligatory requirement for Hsp40/Hsc70 and, where known, do not utilize the Asna-1/TRC40-dependent route (Fig. 8). Conversely, none of the TA proteins with a net hydrophobicity of ≥40 are solely dependent upon the Hsp40/Hsc70 route, and three of the five precursors are known to bind to and/or utilize Asna-1/TRC40 (Fig. 8). We conclude that the hydrophobicity of the TA core primarily dictates which of two distinct ATP-dependent routes promotes TA protein integration. Intriguingly, the hydrophobicity of the TA core also defines the nature of TA protein translocation after delivery to the ER membrane. Hence, the membrane insertion of TA proteins with low net hydrophobicity is facilitated by very low ATP levels in vitro. Furthermore, such proteins are capable of unassisted integration into the ER bilayer via a process that requires no receptor or translocase (16–18). In contrast, proteins with more hydrophobic TA cores need higher levels of ATP and are incapable of unassisted translocation, requiring one or more unidentified membrane components to facilitate their insertion (1,11,17,18). Taking all currently available knowledge together, we propose that the low [ATP] route is mediated by Hsc70/Hsp40, and the high [ATP] route is mediated by Asna-1/TRC40 (see17). This would strongly suggest that the mode of ATP-dependent delivery utilized by a TA protein determines whether its subsequent membrane insertion is facilitated or unassisted (10).
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank Nica Borgese for kindly supplying the plasmid containing PTP1B. We also thank Martin Pool and Blanche Schwappach for their help during manuscript preparation.
_Note Added in Proof_—M. Schuldiner et al. (2008)Cell 134, 634–645, have recently shown that the yeast Asna-1 homologue, Get 3, promotes TA protein membrane insertion at the ER via interaction with the Get1/Get2 membrane receptor complex.
*
This work was supported, in whole or in part, by National Institutes of Health Grant P50-GM067082 from the NIGMS for support of the P50 Chemical Methodologies and Library Development Program at the University of Pittsburgh (to P. W.). This work was also supported by a Biotechnology and Biological Sciences Research Council project grant (to S. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
S⃞
The on-line version of this article (available athttp://www.jbc.org) contains supplemental Figs. S1, S2, and S3.
Footnotes
2
The abbreviations used are: TA, tail-anchored; ER, endoplasmic reticulum; EndoH, endoglycosidase H; OPG, opsin _N_-glycosylation motif; RNC, ribosome-nascent chain complex; DMSO, dimethyl sulfoxide; RRL, rabbit reticulocyte lysate; sp-, semi-permeabilized; ANOVA, analysis of variance; SRP, signal recognition particle.
3
M. J. Braunstein, C. M. Scott, S. Behrman, P. Walter, P. Wipf, E. L. P. Smith, S. S. Scott, J. L. Brodsky, and O. Batuman, manuscript submitted for publication.
References
- 1.Kutay, U., Hartmann, E., and Rapoport, T. A. (1993) Trends Cell Biol. 3 72–75 [DOI] [PubMed] [Google Scholar]
- 2.Kalbfleisch, T., Cambon, A., and Wattenberg, B. W. (2007) Traffic 8 1687–1694 [DOI] [PubMed] [Google Scholar]
- 3.Borgese, N., Brambillasca, S., Soffientini, P., Yabal, M., and Makarow, M. (2003) Biochem. Soc. Trans. 31 1238–1242 [DOI] [PubMed] [Google Scholar]
- 4.Wattenberg, B. W., Clark, D., and Brock, S. (2007) Biosci. Rep. 27 385–401 [DOI] [PubMed] [Google Scholar]
- 5.Borgese, N., Brambillasca, S., and Colombo, S. (2007) Curr. Opin. Cell Biol. 19 368–375 [DOI] [PubMed] [Google Scholar]
- 6.High, S., and Abell, B. M. (2004) Biochem. Soc. Trans. 32 659–662 [DOI] [PubMed] [Google Scholar]
- 7.Borgese, N., Colombo, S., and Pedrazzini, E. (2003) J. Cell Biol. 161 1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson, D. J., Mostov, K. E., and Blobel, G. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 7249–7253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore, R. (1993) Cell 75 589–592 [DOI] [PubMed] [Google Scholar]
- 10.Rabu, C., and High, S. (2007) Curr. Biol. 17 R472–R474 [DOI] [PubMed] [Google Scholar]
- 11.Abell, B. M., Pool, M. R., Schlenker, O., Sinning, I., and High, S. (2004) EMBO J. 23 2755–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abell, B. M., Rabu, C., Leznicki, P., Young, J. C., and High, S. (2007) J. Cell Sci. 120 1743–1751 [DOI] [PubMed] [Google Scholar]
- 13.Kutay, U., Ahnert-Hilger, G., Hartmann, E., Wiedenmann, B., and Rapoport, T. A. (1995) EMBO J. 14 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanovic, S., and Hegde, R. S. (2007) Cell 128 1147–1159 [DOI] [PubMed] [Google Scholar]
- 15.Kim, P. K., Janiak-Spens, F., Trimble, W. S., Leber, B., and Andrews, D. W. (1997) Biochemistry 36 8873–8882 [DOI] [PubMed] [Google Scholar]
- 16.Yabal, M., Brambillasca, S., Soffientini, P., Pedrazzini, E., Borgese, N., and Makarow, M. (2003) J. Biol. Chem. 278 3489–3496 [DOI] [PubMed] [Google Scholar]
- 17.Favaloro, V., Spasic, M., Schwappach, B., and Dobberstein, B. (2008) J. Cell Sci. 121 1832–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brambillasca, S., Yabal, M., Makarow, M., and Borgese, N. (2006) J. Cell Biol. 175 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fewell, S. W., Smith, C. M., Lyon, M. A., Dumitrescu, T. P., Wipf, P., Day, B. W., and Brodsky, J. L. (2004) J. Biol. Chem. 279 51131–51140 [DOI] [PubMed] [Google Scholar]
- 20.Wright, C. M., Chovatiya, R. J., Jameson, N. E., Turner, D. M., Zhu, G., Werner, S., Huryn, D. M., Pipas, J. M., Day, B. W., Wipf, P., and Brodsky, J. L. (2008) Bioorg. Med. Chem. 16 3291–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abell, B. M., Jung, M., Oliver, J. D., Knight, B. C., Tyedmers, J., Zimmermann, R., and High, S. (2003) J. Biol. Chem. 278 5669–5678 [DOI] [PubMed] [Google Scholar]
- 22.Setoguchi, K., Otera, H., and Mihara, K. (2006) EMBO J. 25 5635–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson, C. M., and High, S. (2007) J. Cell Sci. 120 648–657 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson, I. M., and von Heijne, G. (1993) J. Biol. Chem. 268 5798–5801 [PubMed] [Google Scholar]
- 25.Fewell, S. W., Day, B. W., and Brodsky, J. L. (2001) J. Biol. Chem. 276 910–914 [DOI] [PubMed] [Google Scholar]
- 26.Ngosuwan, J., Wang, N. M., Fung, K. L., and Chirico, W. J. (2003) J. Biol. Chem. 278 7034–7042 [DOI] [PubMed] [Google Scholar]
- 27.Rodina, A., Vilenchik, M., Moulick, K., Aguirre, J., Kim, J., Chiang, A., Litz, J., Clement, C. C., Kang, Y., She, Y., Wu, N., Felts, S., Wipf, P., Massague, J., Jiang, X., Brodsky, J. L., Krystal, G. W., and Chiosis, G. (2007) Nat. Chem. Biol. 3 498–507 [DOI] [PubMed] [Google Scholar]
- 28.Frydman, J., Nimmesgern, E., Ohtsuka, K., and Hartl, F. U. (1994) Nature 370 111–117 [DOI] [PubMed] [Google Scholar]
- 29.Egea, P. F., Stroud, R. M., and Walter, P. (2005) Curr. Opin. Struct. Biol. 15 213–220 [DOI] [PubMed] [Google Scholar]
- 30.King, C., Eisenberg, E., and Greene, L. E. (1999) Biochemistry 38 12452–12459 [DOI] [PubMed] [Google Scholar]
- 31.Chang, Y. W., Sun, Y. J., Wang, C., and Hsiao, C. D. (2008) J. Biol. Chem. 283 15502–15511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuldiner, M., Collins, S. R., Thompson, N. J., Denic, V., Bhamidipati, A., Punna, T., Ihmels, J., Andrews, B., Boone, C., Greenblatt, J. F., Weissman, J. S., and Krogan, N. J. (2005) Cell 123 507–519 [DOI] [PubMed] [Google Scholar]
- 33.Kao, G., Nordenson, C., Still, M., Ronnlund, A., Tuck, S., and Naredi, P. (2007) Cell 128 577–587 [DOI] [PubMed] [Google Scholar]
- 34.Auld, K. L., Hitchcock, A. L., Doherty, H. K., Frietze, S., Huang, L. S., and Silver, P. A. (2006) Genetics 174 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutka, S. C., and Walter, P. (2001) Mol. Biol. Cell 12 577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhopadhyay, R., Ho, Y. S., Swiatek, P. J., Rosen, B. P., and Bhattacharjee, H. (2006) FEBS Lett. 580 3889–3894 [DOI] [PubMed] [Google Scholar]
- 37.Lee, M. J., and Dohlman, H. G. (2008) Curr. Biol. 18 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng, D. T., Brown, J. D., and Walter, P. (1996) J. Cell Biol. 134 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]