Columnar Architecture Sculpted by GABA Circuits in Developing Cat Visual Cortex (original) (raw)
. Author manuscript; available in PMC: 2008 Oct 7.
Published in final edited form as: Science. 2004 Mar 12;303(5664):1678–1681. doi: 10.1126/science.1091031
Abstract
The mammalian visual cortex is organized into columns. Here, we examine cortical influences upon developing visual afferents in the cat by altering intrinsic γ-aminobutyric acid (GABA)–mediated inhibition with benzodiazepines. Local enhancement by agonist (diazepam) infusion did not perturb visual responsiveness, but did widen column spacing. An inverse agonist (DMCM) produced the opposite effect. Thus, intracortical inhibitory circuits shape the geometry of incoming thalamic arbors, suggesting that cortical columnar architecture depends on neuronal activity.
Columnar architecture is the hallmark of the mammalian neocortex. What determines the final dimensions of individual columns, however, remains largely unknown. Manipulations of early visual experience indicate that the segregation of eye-specific columns is a competitive process between axons serving the two eyes in primary visual cortex (1, 2). Cortical target neurons detect patterns of input activity to strengthen those connections correlated with their own firing, while weakening un-correlated afferent input. Gross disruption of postsynaptic activity supports such a correlation-based mechanism of refinement (3, 4 ). Yet, recent reports have shown that initial clustering of individual thalamocortical arbors occurs at least a week before the critical period (5), leading to a suggestion that it may be largely genetically predetermined (6 ) rather than emerging from an initially overlapping configuration.
Computational models based on traditional, self-organizing principles offer specific predictions about column spacing (7,8). Local excitatory connections within cortex may spread incoming afferent activity over a certain radius, which is ultimately limited by farther-reaching inhibition. Modulating the relative balance of excitation to inhibition alters the shape of this interaction function. Activity-dependent processes acting upon narrowed or broadened central excitatory regions would ultimately yield slender or wide columns, respectively (7, 8). Here, we tested this hypothesis in developing kitten visual cortex.
We measured the final size of ocular dominance columns after locally altering the inhibition intrinsic to visual cortex in vivo for 1 month starting 2 weeks after birth. At this age, physiological and anatomical data show that columns are beginning to segregate (5). Yet, individual thalamocortical arbors in the cat extend sparse, broadly distributed processes that have yet to focus their branching (9). Because direct activation of γ-aminobutyric acid (GABA) receptors with muscimol disrupts column formation (3), we used a local enhancement of intracortical inhibition during development that does not silence cortex (Fig. 1A) (10).
Fig. 1.
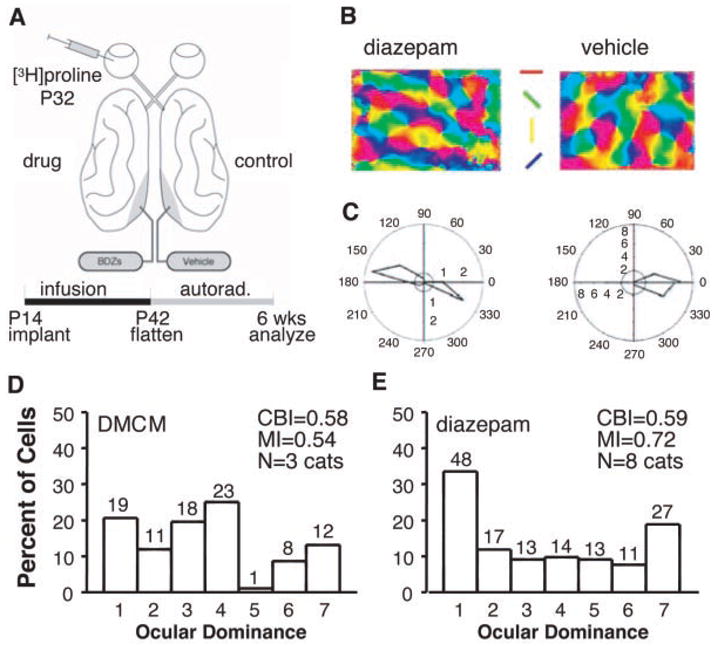
(A) Protocol for local modulation of intrinsic inhibition in vivo. Local benzodiazepine agonist (diazepam), inverse agonist (DMCM), or vehicle (propylene glycol) was infused from osmotic minipumps (0.2 or 2.5 μl/hour) into kitten striate cortex for 4 weeks starting at 14 to 17 days after birth (10). Monocular [3H]proline (2 mCi) was injected 10 days before electrophysiological recording. Labeled columns were measured in tangential sections through layer 4 of unfolded and flattened visual cortex (10). (B and C) Physiological properties of diazepam-treated cortical neurons. Robust orientation tuning in optical imaging angle maps (B) and single-unit responses (C). Spontaneous activity and response strength are indicated as respective concentric-circle radii. The absence of directionality in diazepam-treated cortex is apparent in this example (fig. S2). (D and E) Ocular dominance distribution after DMCM infusion was binocular, as in controls (D) (1, 2), but diazepam-treated hemispheres were significantly less binocular (E) (P < 0.001, χ2 test, DMCM versus diazepam). CBI, contralateral bias index; MI, monocularity index. The number of cells per ocular dominance group is as indicated.
Visual stimulation releases the endogenous inhibitory neurotransmitter GABA (11). Benzodiazepines are bidirectional, al-losteric modulators at GABAA receptors of particular subunit stoichiometry (12). Agonists, such as diazepam, reversibly potentiate chloride flux through the GABAA receptor ionopore (13), whereas inverse agonists, such as β-carboline derivatives (methyl-6,7-dimethoxy-4-ethyl-β-carbo-line, DMCM), decrease GABAA currents (14 ). Benzodiazepines are inert in the absence of released GABA (12), making them an ideal tool to examine the endogenous role of intracortical interactions during column formation. Binding sites are highest in input layer 4 of cats at all ages but are not altered by surgical undercutting of afferents (15), indicating that benzodiazepine receptors are associated with intrinsic cortical elements rather than thalamocortical axons or other subcortical input.
Chronic treatment with a benzodiazepine agonist (diazepam) had little effect on the functional properties of mature cortical neurons in vivo (10, 16, 17 ), although inhibitory postsynaptic currents were enhanced by the drug in vitro even after 4 weeks at body temperature (37°C) (fig. S1). Optical imaging of intrinsic signals revealed robust orientation maps, and single units remained well tuned for orientation (Fig. 1, B and C). Although response strength, habituation, and spontaneous activity were not significantly affected (fig. S2), direction selectivity was consistently reduced (Fig. 1C and fig. S2). These results are typical of enhanced inhibition in computational models of cortical processing normally operating in a regime of balanced excitation and inhibition (18), supporting the notion that the visual cortex can recalibrate itself following alterations in neuronal activity.
However, unlike the representation in normal (1, 2) or inverse agonist-treated hemispheres (Fig. 1D), a reduced binocularity appeared in the ocular dominance distribution of single units near a chronic diazepam infusion site (Fig. 1E). We then examined the overall layout of [3H]proline-labeled ocular dominance columns in flattened visual cortex (10). Columns near the diazepam infusion site (3.5 mM; 2.5 μl/hour) were wider than columns in regions farther away or behind the cannula (Fig. 2, A and B). Such a pattern would be expected from a point source of drug flowing unidirectionally anterior to the cannula bevel (~100-fold dilution within brain tissue) (10, 16). Pumping at a slower rate (0.2 μl/hour) and higher dose (35 mM) to create a larger radius of drug diffusion produced a region of unsegregated columns in front of the cannula and wide columns behind (Fig. 3B). These results reflect a continuum of GABA action approaching saturation, because potent, direct activation of GABAA receptors with muscimol desegregates columns (3).
Fig. 2.
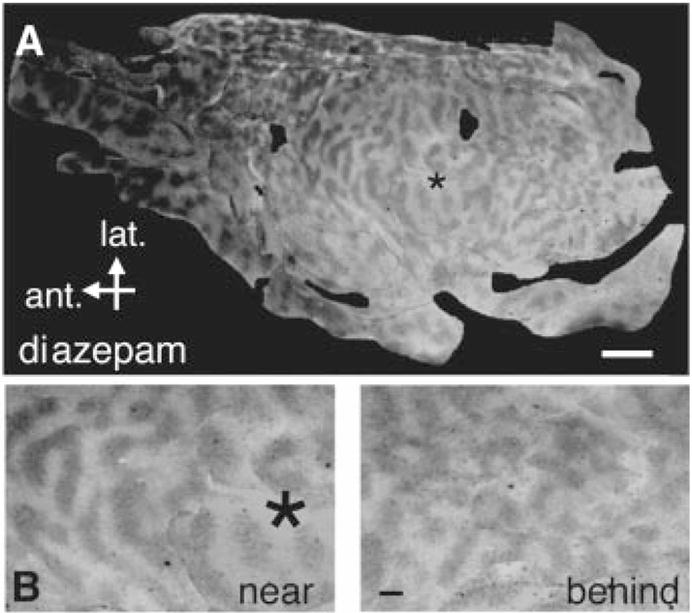
(A) Chronic diazepam infusion widens ocular dominance columns locally. Flatmount of overall layout of labeled (dark) and unlabeled (white) ocular dominance bands in layer 4 of diazepam-infused (*) (3.5 mM, 2.5 μl/hour) kitten visual cortex. (B) Wide, crisp columns just anterior to the cannula site compared to an area behind and away from the infusion. Scale bars: 5 mm (A); 1 mm (B).
Fig. 3.
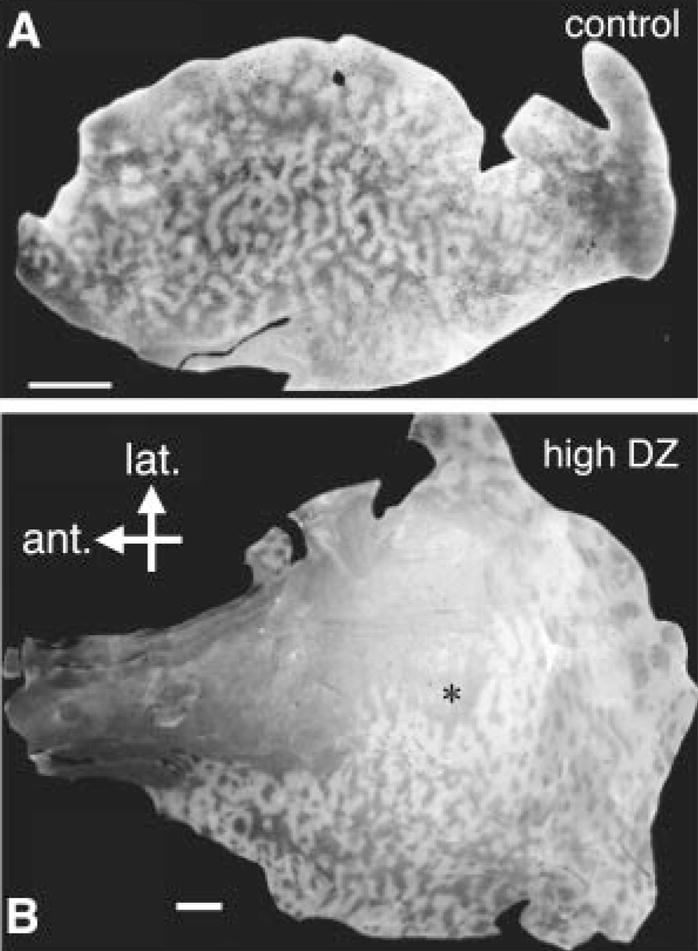
(A) Control hemisphere exhibits relatively homogeneous column spacing across a homologous extent of area 17. (B) Graded effect of diazepam treatment. A high dose (35 mM, 0.2 μl/hour) yields an area of column desegregation (anterolateral to cannula site) (*) reminiscent of direct GABAA receptor activation with muscimol (3). Scale bar: 5 mm.
In contrast to the local, graded changes in column width seen with diazepam, control hemispheres exhibited a homogeneous spacing across a similar extent of area 17 (Fig. 3A). To further verify the specificity of diazepam action, we successfully infused the benzodiazepine inverse agonist DMCM in three animals, but at a necessarily less than saturating dose (50 μM) to avoid the toxicity of excessive intracortical excitation. Regardless of the dose, final column spacing exhibited a trend that was the reverse from that of diazepam: Columns just in front of the cannula site were less discrete and narrower than columns farther away, behind, or in control hemispheres (Fig. 4).
Fig. 4.
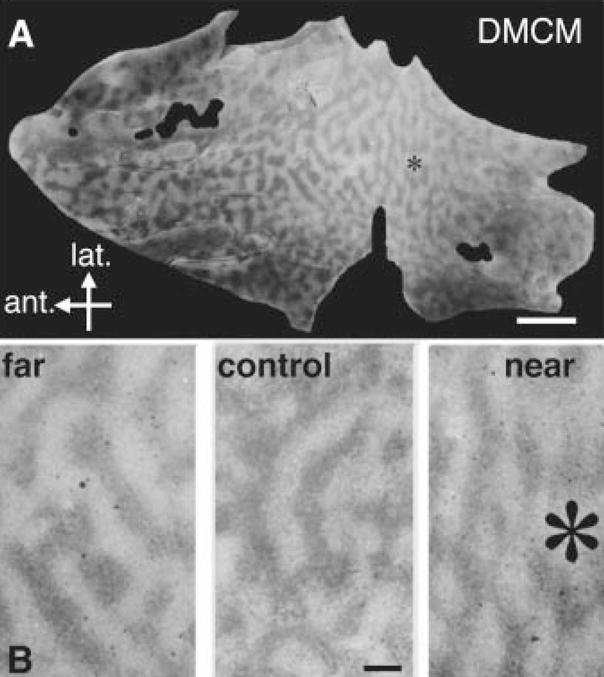
(A) Chronic inverse agonist infusion locally reduces column size. Overall layout of labeled ocular dominance bands (dark) surrounding a DMCM infusion site (*) (50 μM, 2.5 μl/hour). (B) Columns near a DMCM source were blurred and narrow (right) compared to distal (left) or homologous regions in control hemispheres (middle). Scale bars, 5 mm (A); 1 mm (B).
We used a computer-based wavelet analysis algorithm to quantify the changes in periodicity (19, 20). Interindividual variability in column spacing would obscure changes in a group comparison (20, 21). We therefore expressed column widths of experimental regions as a percentage change from the mean observed in regions far from the cannula site. Column spacing across the similar extent of control hemispheres was also calculated as a fraction of the mean. We further analyzed a large control sample from the literature of flattened kitten hemispheres labeled with either 2-deoxyglucose or [3H]proline (3, 22, 23). Intrinsic variability in column spacing spanned ±10 to 20% distal to a cannula site or when no drug or vehicle was infused (Fig. 5) (20). In contrast, the percentage increase for five of six diazepam-treated regions was greater than that in any of the controls. The remaining experimental area was near the upper extreme of the control range, whereas reducing intrinsic inhibition with subsaturating doses of inverse agonist (DMCM) consistently yielded column shrinkage toward the opposite extreme (Fig. 5).
Fig. 5.
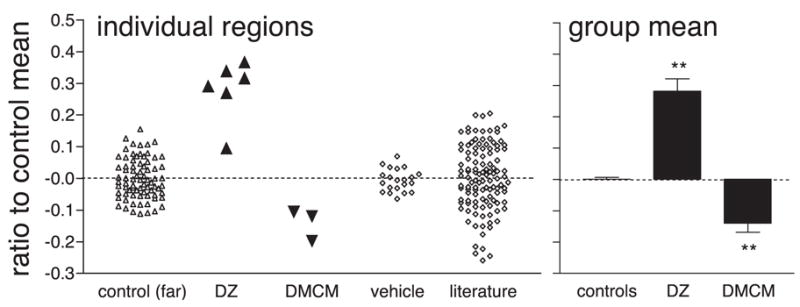
Bidirectional control of developing column size by cortical inhibition. Wavelet measurements (10, 20) of spacing in individual regions were normalized to control mean far from the cannula, or across vehicle and untreated control hemispheres from literature (3, 22, 23). Statistical comparison is by group. **P < 0.001, t test versus control.
A straightforward interpretation of our findings is that GABA-mediated inhibition controls the local competition between right and left eye inputs in visual cortex (16, 17 ). Enhanced competition by diaze-pam would explain reduced binocularity of single-unit responses (Fig. 1E), as well as wider and sharper anatomical segregation near the infusion site. All three aspects are reminiscent of strabismic cats (23) and monkeys (24 ) in which the two eyes’ inputs are maximally decorrelated (1, 2). Intrinsic differences in the range of intracortical inhibition may underlie the large variability of anatomical column spacing normally found across individuals (20, 21, 25).
Not all GABA circuits may be relevant. Lateral inhibition is thought to enhance the contrast between neighboring columns in normal animals (26 ). Indeed, the model of Miller et al. (7 ) predicts the bidirectional changes observed here when benzodiaz-epines act preferentially through long range rather than local inhibition (fig. S4). Large basket cells in cat visual cortex extend a wide, horizontal axonal plexus that can span ocular dominance columns (27 ), which is useful for discriminating input coming from the two eyes. Among the great diversity of GABAA receptors (12), only those containing the α1 subunit drive the functional shift of ocular dominance following monocular deprivation in mice (28). These receptors are enriched at perisomatic synapses innervated by parvalbumin-positive large basket cells (29), which form coupled networks that are exquisitely sensitive for the detection and propagation of synchronized signals on a columnar scale (30).
Thus, our results demonstrate that inhibitory circuits intrinsic to the neocortex instruct both functional (16) and anatomical refinement of incoming presynaptic input (9) and support a self-organizing segregation process based on correlated neuronal activity (7, 31) rather than a prespecified patterning of molecular cues (6).
Supplementary Material
References and Notes
- 1.Wiesel TN. Nature. 1982;299:583. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- 2.Daw N. Visual Development. Plenum; New York: 1995. [Google Scholar]
- 3.Hata Y, Stryker MP. Science. 1994;265:1732. doi: 10.1126/science.8085163. [DOI] [PubMed] [Google Scholar]
- 4.Hata Y, Tsumoto T, Stryker MP. Neuron. 1999;22:375. doi: 10.1016/s0896-6273(00)81097-1. [DOI] [PubMed] [Google Scholar]
- 5.Crair MC, Horton JC, Antonini A, Stryker MP. J Comp Neurol. 2001;430:235. doi: 10.1002/1096-9861(20010205)430:2<235::aid-cne1028>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley JC, Katz LC. Curr Opin Neurobiol. 2002;12:104. doi: 10.1016/s0959-4388(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 7.Miller KD, Keller JB, Stryker MP. Science. 1989;245:605. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- 8.Willshaw DJ, von der Malsburg C. Proc R Soc London B. 1976;194:431. doi: 10.1098/rspb.1976.0087. [DOI] [PubMed] [Google Scholar]
- 9.Antonini A, Stryker MP. J Neurosci. 1993;13:3549. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Materials and methods are available as supporting material on Science Online.
- 11.Shirokawa T, Ogawa T. Brain Res. 1992;589:157. doi: 10.1016/0006-8993(92)91177-g. [DOI] [PubMed] [Google Scholar]
- 12.Sieghart W. Pharmacol Rev. 1995;47:181. [PubMed] [Google Scholar]
- 13.Eghbali M, Curmi JP, Birnir B, Gage PW. Nature. 1997;388:71. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- 14.Capogna M, et al. Neuropharmacology. 1994;33:875. doi: 10.1016/0028-3908(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 15.Shaw C, Aoki C, Wilkinson M, Prusky G, Cynader M. Brain Res. 1987;465:67. doi: 10.1016/0165-3806(87)90229-x. [DOI] [PubMed] [Google Scholar]
- 16.Hensch TK, et al. Science. 1998;282:1504. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagiolini M, Hensch TK. Nature. 2000;404:183. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 18.Somers DC, Nelson SB, Sur M. J Neurosci. 1995;15:5448. doi: 10.1523/JNEUROSCI.15-08-05448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaschube M, Wolf F, Geisel T, Löwel S. J Neurosci. 2002;22:7206. doi: 10.1523/JNEUROSCI.22-16-07206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaschube M, et al. Eur J Neurosci. 2003;18:3251. doi: 10.1111/j.1460-9568.2003.02979.x. [DOI] [PubMed] [Google Scholar]
- 21.Horton JC, Hocking DR. J Neurosci. 1996;16:7228. doi: 10.1523/JNEUROSCI.16-22-07228.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löwel S, Singer W. Exp Brain Res. 1987;68:661. doi: 10.1007/BF00249809. [DOI] [PubMed] [Google Scholar]
- 23.Löwel S. J Neurosci. 1994;14:7451. doi: 10.1523/JNEUROSCI.14-12-07451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roe AW, Ghose GM, Smith EL, Chino YM, Tso DY. Soc Neurosci Abstr. 1995;121:689.9. [Google Scholar]
- 25.Adams DL, Horton JC. Nature Neurosci. 2003;6:113. doi: 10.1038/nn1004. [DOI] [PubMed] [Google Scholar]
- 26.Sillito AM, Kemp JA, Patel H. Exp Brain Res. 1980;41:1. doi: 10.1007/BF00236673. [DOI] [PubMed] [Google Scholar]
- 27.Buzas P, Eysel UT, Adorjan P, Kisvarday ZF. J Comp Neurol. 2001;437:259. doi: 10.1002/cne.1282. [DOI] [PubMed] [Google Scholar]
- 28.Fagiolini M, et al. Science. 2004;303:1681. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 29.Klausberger T, Roberts JD, Somogyi P. J Neurosci. 2002;22:2513. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galaretta M, Hestrin S. Science. 2001;292:2295. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- 31.Adams DL, Horton JC. Science. 2002;298:572. doi: 10.1126/science.1074887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.We thank S. Harris for assistance during minipump implant surgery; J. C. Horton for advice on flat-mount histology; Y. Hata and S. Löwel for flat-mount image data of labeled ocular dominance columns; M. Kaschube and F. Wolf for the “wavelet” analysis program; and K. D. Miller and M. Fagiolini for critical comments and discussion. Supported in part by a Howard Hughes Medical Institute Predoctoral Fellowship (T.K.H.) and grants from NIH (M.P.S.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.