Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression (original) (raw)
. Author manuscript; available in PMC: 2008 Oct 10.
Published in final edited form as: J Allergy Clin Immunol. 2007 Oct 1;121(1):148–157.e3. doi: 10.1016/j.jaci.2007.08.014
Abstract
Background
Cutaneous lymphocyte-associated antigen (CLA) is a surface glycoprotein expressed by skin-homing T cells. This carbohydrate moiety expressed on mucin-like surface glycoproteins, including P-selectin glycoprotein ligand 1 and CD43, confers binding activity to dermal endothelial E-selectin and is critical for T-cell recruitment to the skin. Vitamin A (retinoic acid [RA]) and the active form of vitamin D3 (1,25 dihydroxyvitamin D3 [1,25D(3)]) have been used to treat certain T cell-mediated inflammatory skin diseases, as well as cutaneous T-cell lymphomas; however, their effect on CLA expression has not been studied.
Objective
We analyzed the effects of RA and 1,25D(3) on expression of CLA and other lymphocyte-homing receptors on human T cells.
Methods
We cultured human T cells with 1,25D(3) and RA and analyzed the expression of CLA and other homing receptors. We also pretreated mice with either vitamin and then induced an antigen-dependent contact hypersensitivity response.
Results
Both RA and 1,25D(3) downregulated expression of the CLA and, in parallel, functional E-selectin ligand. Whereas RA increased expression of the gut-homing receptor α4β7 and reduced L-selectin expression, 1,25D(3) had no effect on other homing receptors. In an in vivo assay treatment with RA or 1,25D(3) downregulated the skin infiltration of effector CD4+ T cells.
Conclusion
These findings suggest that 1,25D(3) can selectively downregulate CLA expression without influencing lymphocyte migration patterns to other tissues.
Keywords: 1, 25-Dihydroxyvitamin D(3), retinoic acid, cutaneous lymphocyte-associated antigen, chemokine receptor
Lymphocyte trafficking to the skin is dependent on the expression of cutaneous lymphocyte-associated antigen (CLA), a sialyl Lewisx-related epitope expressed on P-selectin glycoprotein ligand 1 (PSGL-1) and CD43.1-6 CLA is highly expressed on skin-infiltrating T cells in inflammatory skin diseases, including psoriasis, allergic contact dermatitis, and atopic dermatitis.2-5,7-9 Malignant clonal T cells in cutaneous T-cell lymphoma (CTCL) also express CLA.10 Because CLA is a major E-selectin ligand for dermal vascular E-selectin and promotes T-cell entry into the skin, this molecule might be a useful target for the treatment of T cell-mediated skin diseases.3,4,9 Recent studies suggest that reduction of CLA or E-selectin ligand expression on effector T cells can ameliorate T-cell trafficking to inflamed skin and development of the T cell-dependent cutaneous inflammatory response.4 CLA function is dependent on α1,3 fucosylation of PSGL-1 O-glycans by α1,3-fucosyltransferase (FucT) VII1-4 and appears to be controlled in part by IL-12 and TGF-β expression5,11,12; however, other factors regulating CLA expression or fucosyltransferase expression have not been determined.
Vitamin D(3) (cholecalciferol) is produced in the skin after exposure to sunlight and is converted to its biologically active metabolite, 1, 25-dihydroxyvitamin D(3) (1,25D[3]). In the liver and kidney 1,25D(3) has been shown to influence the development of autoimmune diseases, including ulcerative colitis and Crohn’s disease,13,14 as well as skin diseases, such as psoriasis.5 1,25D(3) has been reported to decrease the production of IFN-γ and IL-2 in T cells, while increasing their production of IL-4.14,15 These effects on cytokine production suggest that 1,25D(3) can skew T-cell development from a TH1 to a TH2 phenotype. 1,25D(3) is also known to inhibit the differentiation of human dendritic cells from monocytes, as well as their maturation into antigen-presenting cells.16 Specific alterations of T cell-homing receptor expression by 1,25D(3) in the treatment of psoriasis, atopic dermatitis, or CTCL have not been reported. On the other hand, retinoic acid (RA) synthesis is accomplished by 2 sequential oxidation steps in which retinol is oxidized to retinal and retinal is oxidized to RA. Interestingly, the induction of lymphocyte-homing receptor expression can be triggered by vitamin A analogues, as evidenced by a recent report that showed RA induces murine T cells to express gut-homing molecular features.17
In the present study we analyzed the effect of 1,25D(3) and RA on the expression of CLA, as well as other gut- and lymph node (LN)-homing receptors and chemokine receptors (CCRs), on human CD3+ T cells. Using flow cytometry and Western blotting/immunoprecipitation approaches, we found that 1,25D(3) at concentrations as low as 1 nM decreased the decoration of PSGL-1 with the CLA epitope, whereas RA at similar pharmacologically attainable concentrations also ablated CLA expression. RA also decreased the expression of the skin-homing receptor CCR10 and the LN-homing receptor L-selectin (CD62L) and increased the expression of the gut-homing receptors α4β7 integrin and CCR9. 1,25D(3), on the other hand, did not alter the expression of gut- or LN-homing receptors or of CCRs. Quantitative PCR of glycosyltransferase mRNAs showed that reduction of CLA expression mediated by both 1,25D(3) and RA was associated with reduced FucT-VII mRNA levels. Finally, using a murine model of contact hypersensitivity, we found that in vivo treatment with 1,25D(3) or RA resulted in the inhibition of skin infiltration of CD4+ T cells. These findings suggest that 1,25D(3) might selectively downregulate CLA expression without influencing lymphocyte migration patterns to other tissues.
METHODS
Cell purification and culture
Fresh whole blood was collected from healthy donors in compliance with institutional review board policies, and PBMCs were prepared by means of density gradient centrifugation over Ficoll-Histopaque (Sigma Chemical Co, St Louis, Mo). CD3+ T cells were purified with the pan-T cell isolation kit II (Miltenyi Biotec, Auburn, Calif). For analysis of CLA and PSGL-1 expression, CD3+ T cells were cultured in XVIVO15 medium (BioWhittaker, Walkersville, Md) supplemented with 2 mM l-glutamine, 0.5 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 80 U/mL recombinant human IL-2.11 For analysis of other tissue-homing receptor and CCR expression, CD3+ T cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, Calif) supplemented with 2 mM l-glutamine, 0.5 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FCS. Cells were plated initially at 2 × 106 cells/mL in 24-well, plastic, tissue-culture plates (Costar, Cambridge, Mass) coated with azide-free murine anti-human CD3 mAb on goat anti-mouse IgG. To these cultures, 1,25D(3) (active vitamin D3), cholecalciferol (the inactive precursor of vitamin D3), RA (all-trans-RA, the active form of vitamin A), retinol (an inactive precursor of vitamin A), and retinal (all-trans-retinal, an active intermediate form of vitamin A) were added at final concentrations of 1 pM to 1 μM. After 2 days, cells were harvested, suspended in the same medium, and added to non-antibody-coated plates. Half of the medium was replaced with fresh medium every other day, and cells were collected on day 7. Cells were washed twice with PBS and then analyzed by means of flow cytometry, or cell lysates were prepared for Western blotting/immunoprecipitation experiments.
Flow cytometric studies
Flow cytometric analysis was performed by using directly conjugated mAbs to human CD3, CLA, CD162, CCR4, CCR6, CCR9, CD62L, α4 integrin, β7 integrin, CD25, and CD69, and isotype controls for these antibodies were purchased from BD Biosciences (San Diego, Calif). Fluorescein isothiocyanate (FITC)-annexin-V and 7-amino-actinomycin (7-AAD) were also purchased from BD Biosciences. Monoclonal antibodies to human CCR7 and CCR10 were purchased from R&D Systems (Minneapolis, Minn). Immunophenotypic analysis of cells was performed on a Becton Dickinson FACScan instrument with CellQuest software (Becton Dickinson, Mansfield, Mass).
Detection of E- and P-selectin ligands
Cultured T cells were first stained with FITC-conjugated anti-CLA antibody. The cells were washed and subsequently incubated with 10 μg/mL E- or P-selectin human IgG Fc chimera (R&D systems) in HBSS supplemented with 2 mM calcium, 5% FCS, and 1 mM HEPES. After incubation for 30 minutes at 4°C, the cells were gently washed and incubated with phycoerythrin-conjugated goat F(ab’)2 anti-human IgG (Beckman Coulter) for 30 minutes at 4°C.
Quantification of mRNA expression levels
We analyzed mRNA levels of 7 principal glycosyltransferases involved in the synthesis of CLA18 in CD3+ T cells cultured in the presence or absence of 1,25D(3), RA, or diluent control (ethanol). For quantitative RT-PCR analysis, total RNA was extracted with the Clontech RNA purification kit (Clontech, Palo Alto, Calif), according to the manufacturer’s instructions. Total RNA (2.5 μg; A260/A280 = 1.7-2.0) was reverse transcribed with oligo-dT primers and Powerscript Reverse Transcriptase (Clontech, Palo Alto, Calif) in a final volume of 20 μL. One microliter of the cDNA was amplified by means of PCR to a final volume in 50 μL with SYBR Green PCR Core reagents (Biosystems, Warrington, United Kingdom) and 200 nM of glycosyltransferase primers. cDNAs were also probed for the expression of β-actin to control for fidelity and efficiency of cDNA synthesis from each cell preparation. The following glycosyltransferase mRNAs were probed: core 2 β1, 6 N-acetylglucosaminyltransferase; N-acetylglucosamine-6-O-sulfotransferase 2; β1,4 galactosyl-transferase I and III (β4GalT-I and β4GalT-III), α2,3-sialyltransferase III and IV (ST3Gal-III and -IV), and FucT-VII. The primer sequences were as follows (5′-3′): core 2 β1, 6 N-acetylglucosaminyltransferase, ATCCGAAACACCTCTCTTTTCTGGC and GGTCAGTGTTTTAATGTCTCCAAAG18; N-acetylglucosamine-6-O-sulfotransferase 2, GCACACTAGTCATAAAGGGTGTGC and TTGCGTGCAGATACCACGAAAGGC18; β4GalT-I, AAG CAGAACCCAAATGTGAAGATG and GGGCGAGATATAGACATGCCTC18; β4GalT-III, TCTACCACCTGCACCCCTTCTTGC and GCTGTGATGTTGGTATAAAGAGGC18; ST3Gal-III, ATGGAGGCGTTCTTGCCAACAAG and ATGCGAACGGTCTCATAGTAGTG18; ST3Gal-IV, TTGAACAATGCCCCAGTGGCTGG and TCTTGGGAGACATTATGGCCTGAC18; FucT-VII, CCC ACC GTC GCC CAG TAC CGC TTC and CTG ACC TCT GTG CCC AGC CTC CCG T19; and β-actin, GTGGGGCGCCCCAGGCACCA and CTCCTTAATGTCACGCACGATTTC. A series of standard dilutions of a plasmid were used to quantify these messages. Specific signals for all transcripts were readily detected in human CLA+ T cells. Standard dilutions were amplified with the pGEM-T Easy Vector System (Promega, Madison, Wis) from the PCR amplifiers above. Analysis was performed at 40 cycles, which was within the linear amplification range. PCR analyses were conducted twice, and the specificity of PCR products was confirmed by means of sequence analysis. The mRNA level of each glycosyltransferase was normalized to mRNA levels of β-actin.
Cell lysate preparation and immunoprecipitations
For lysate preparation, CD3+ T cells cultured with 1,25D(3), RA, or diluent control were washed 3 times in ice-cold PBS and lysed in buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 0.02% NaAzide, 20 μg/mL phenylmethylsulfonyl fluoride, complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, Ind), and 2% Nonidet P-40 (NP-40; 250 μL/100 × 106 cells). After a 2-hour incubation on ice, insoluble cellular debris was pelleted by means of centrifugation for 30 minutes at 10,000_g_ at 4°C, and solubilized protein lysate was collected and quantified by using the Bradford protein assay (Sigma).
For immunoprecipitation of PSGL-1, 2 μg of the anti-PSGL-1 mAb KPL-1 or mouse IgG isotype was added to cell lysates, which had been precleared with recombinant protein G-agarose (Invitrogen, Carlsbad, Calif). The antibody-lysate mixture was added to protein G-agarose and incubated overnight at 4°C on a rotator. Immunoprecipitates were washed 5 times with lysis buffer/2% NP-40/1% SDS/1% BSA, 3 times with lysis buffer/2% NP-40, and then boiled in reducing sample buffer for SDS-PAGE analysis.
Western blotting
For Western blot analysis, cell lysates (25 mg of total protein per lane) or immunoprecipitates were boiled in reducing sample buffer and separated on 4% to 20% SDS-PAGE gradient gels (Bio-Rad, Hercules, Calif). Resolved protein was transferred to immunoblot membrane (Bio-Rad) and blocked with FBS for 1 hour at room temperature. Blots were incubated with the rat IgM anti-human CLA mAb HECA-452 (1 μg/mL), the mouse IgG anti-human PSGL-1 mAb KPL-1 (2 μg/mL), or recombinant mouse E-selectin/human immunoglobulin chimera (R&D Systems, Inc; 1 μg/mL) for 1 hour at room temperature. Isotype control immunoblots using either rat IgM, mouse IgG, or human IgG were performed in parallel to evaluate nonspecific staining. After 3 washes with PBS/0.1% Tween-20, blots were incubated with respective secondary antibodies, alkaline phosphatase (AP)-conjugated goat anti-rat IgM (1:400), goat anti-mouse IgG (1:5000), or AP-goat anti-human IgG. AP substrate, Western Blue (Promega, Madison, Wis), was then added to develop blots.
Model of allergic contact hypersensitivity
Seven-week-old C57BL/6 male mice were purchased from Japan SLC Co (Shizuoka, Japan) and were used at the age of 8 weeks. All animals were cared for according to ethical guidelines approved by the Institutional Animal Care and Use Committee of Mie University.
Mice were pretreated with subcutaneous injection of 10 mmol of 1,25D(3), RA, or diluted ethanol (as a control) around both ears every 2 days from day -20 to day -2. Mice were also pretreated with 10 mmol of 1,25D(3), RA, or diluted ethanol (as a control) by means intraperitoneal injection every 2 days from day -20 to day -2. Mice were initially sensitized by applying 100 μL of 5% oxazolone solution to their shaved abdominal areas 7 days before the first challenge (day -7), and then 20 μL of 1% oxazolone solution was applied topically to both ears on days 0 and 1.
Immunohistochemistry
Skin samples were obtained on day 2 of the contact hypersensitivity protocol. For the detection of mouse CD4 on skin-infiltrating cells, tissues were snap-frozen, and sections prepared at 7-μm thickness were subjected to a blocking procedure with 5% normal goat serum (Vector Laboratories, Burlingame, Calif). Sections were incubated with FITC-conjugated rat anti-mouse CD4 antibody (Beckman Coulter, Fullerton, Calif) and were examined under Fluoview FV1000 laser scanning confocal microscopy (Olympus, Tokyo, Japan). Cell counts were performed at ×100 magnification, and 10 randomly chosen fields of 5 samples were evaluated.
Statistical analysis
The Friedman test was used for statistical comparison of CLA expression levels, mRNA expression levels, CCR expression levels, and the number of skin-infiltrating CD4+ T cells.
RESULTS
Effect of 1,25D(3) and vitamin A on T-cell CLA expression
To determine whether CLA expression is altered by vitamins D or A, we cultured human CLA+ T cells ex vivo with concentrations of active and inactive forms of vitamin D or vitamin A, ranging from 1 pM to 1 μM. These treatments did not affect growth, as measured by means of thymidine incorporation (data not shown). Flow cytometric analysis of treated cells revealed that CLA expression was completely abolished at 1 nM RA and 10 nM retinal. Reduction of CLA expression was seen at concentrations of RA as low as 10 pM. The active form of vitamin D, 1,25D(3), completely blocked CLA expression at 100 nM, and levels as low as 100 pM significantly inhibited CLA expression (Fig 1 and see Fig E1 in the Online Repository at www.jacionline.org). Inactive precursors of vitamin A (retinol) and vitamin D (cholecalciferol) had relatively little inhibitory action (Fig 1, A). Of note, there were additive, but not synergistic, effects on CLA levels when cells were cultured with both 1,25D(3) and RA (data not shown). Thus at levels of RA and 1,25D(3) that are readily attainable through diet supplementation or sun exposure, CLA expression is significantly inhibited.
FIG 1.
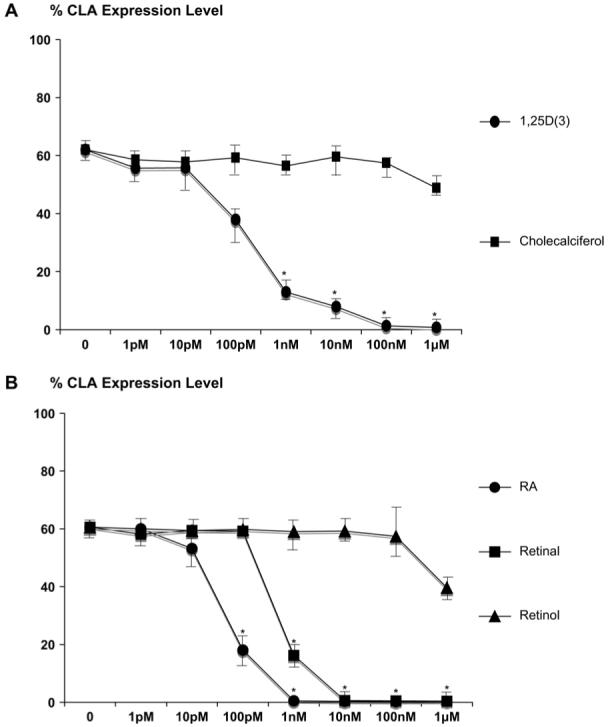
Normal T cells were cultured with vitamin D or vitamin A. A, CLA expression levels were reduced with 1 nM 1,25D(3), whereas cholecalciferol, an inactive precursor of vitamin D, had no effect. B, CLA expression levels were also decreased with 100 pM RA, 1 nM retinal, and 1 mM retinol (n = 6 per group). *Statistical difference: P < .05.
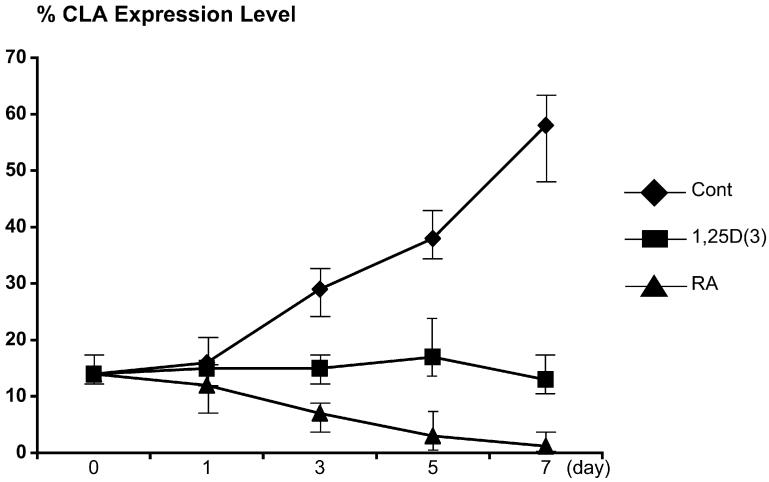
FIG E1.
CLA expression levels were decreased slightly on 1,25D(3)- or RA-treated cells compared with those seen in control-treated cells on day 3 and significantly after day 5.
Effect of 1,25D(3) and RA on other lymphocyte trafficking receptors
Different combinations of homing receptors and CCRs help guide T cells into distinct lymphoid and peripheral tissues.5 To determine whether expression of other tissue-specific lymphocyte-homing molecules was also affected by 1,25D(3) or RA treatment, we measured the skin-homing markers (CLA, CCR4, CCR6, and CCR10) in comparison with homing receptors for peripheral LNs (L-selectin/CD62L and CCR7) and for intestinal lamina propria (α4β7 integrin and CCR9), using specific antibodies and flow cytometry. CLA expression was significantly reduced or eliminated by 1,25D(3) and RA at 1 nM. In contrast, RA, but not 1,25D(3), increased the expression of the gut-homing receptors α4β7 integrin and CCR9 and reduced the expression of the LN-homing receptor L-selectin (Fig 2). Expression of the skin-homing receptors CCR4 and CCR6 was not affected by either 1,25D(3) or RA treatments, which is in agreement with a prior published report.20 On the other hand, the expression level of the skin-homing receptor CCR10 was decreased with the addition of RA. 1,25D(3) neither increased nor decreased the expression of CCR10 in these experiments. The LN-homing receptor CCR7 was also unaffected by either 1,25D(3) or RA. We also examined both the activation and growth status of these cells by assaying for CD69 and CD25 and for the apoptotic markers annexin-V and 7-AAD, respectively. Expression of CD69 was increased with RA, but not with 1,25D(3), whereas CD25 levels were unaffected by either compound. Expression of annexin-V and 7-AAD was not appreciably affected by treatments, suggesting that vitamin action on homing receptor expression was not due to induction of apoptosis (Fig 2).
FIG 2.
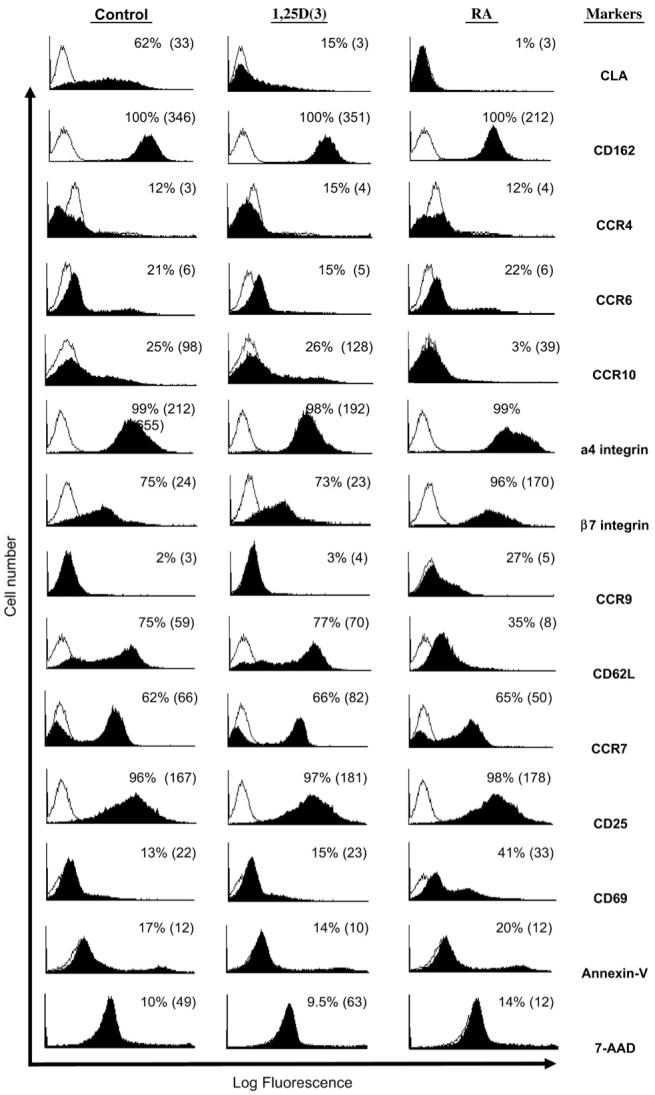
Effect of 1,25D(3) and RA on other lymphocyte trafficking receptors. By using flow cytometry, CLA expression (percent positive and mean fluorescence intensity) was decreased with both vitamins at 1 nM, whereas RA also increased the expression of gut-homing receptors, α4 and β7 integrins, and CCR9 and decreased expression of the LN-homing receptor L-selectin (CD62L). Data are representative of at least 5 independent experiments.
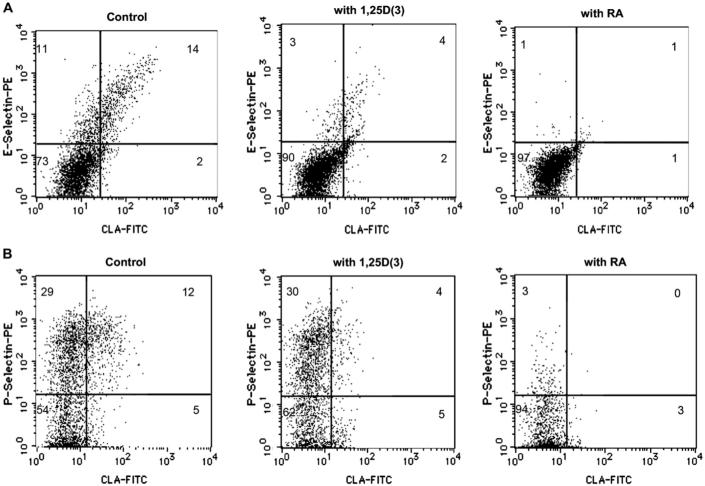
Detection of E- and P-selectin ligands on T cells
Almost all untreated CLA+ T cells bound E-selectin, and this activity was markedly reduced in cells treated with either RA or 1,25D(3) (Fig 3, A). Furthermore, the number of P-selectin ligand-positive T cells was significantly less after culturing in RA, whereas T-cell P-selectin ligand activity was only slightly decreased by treatment with 1,25D(3) (Fig 3, B). Values shown are the percentage of positive cells in each quadrant.
FIG 3.
Detection of E- and P-selectin ligands. A, Most CLA+ T cells bound E-selectin/immunoglobulin, and this activity was reduced by treatment with either vitamin A or vitamin D. B, The number of T cells staining positive with P-selectin/immunoglobulin was significantly less after culturing cells in RA, although it was only slightly decreased by treatment with 1,25D(3). Values shown are the percentage of positive cells in each quadrant.
Quantification of glycosyltransferase mRNA expression levels
To determine whether 1,25D(3) and RA treatments resulted in altered expression of glycosyltransferases involved in CLA biosynthesis, we analyzed glycosyltransferase mRNA levels using real-time RT-PCR in CD3+ T cells cultured with 1,25D(3), RA, or diluent control. The level of FucT-VII mRNA was significantly decreased by both 1,25D(3) and RA treatment (P < .05 compared with diluent control, Fig 4). ST3Gal-III mRNA was also significantly decreased with RA, although not by 1,25D(3) (Fig 4). These results indicated that 1,25D(3) and RA elicited overlapping inhibitory activity on FucT-VII expression and that reduced CLA expression in treated cells might reflect reduction of FucT-VII activity.
FIG 4.
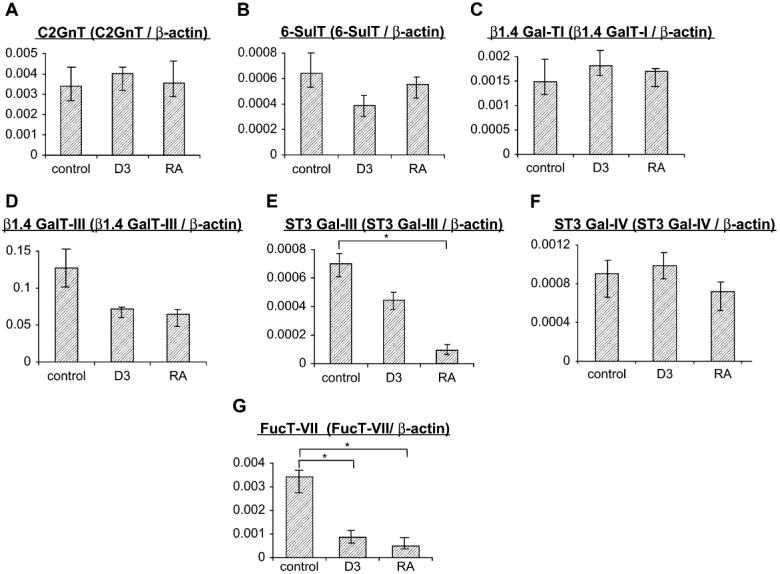
mRNA levels of glycosyltransferases involved in CLA biosynthesis (A-G) were analyzed on CD3+ T cells cultured with non-growth-inhibitory concentrations of 1,25D(3), RA, or diluent control. At 100 nM, mRNA expression of FucT-VII was significantly decreased by 1,25D(3) and RA treatment. ST3Gal-III mRNA was also significantly decreased by RA (n = 8 per group). *Statistical difference: P < .05. C2GnT, Core 2 β1,6 N-acetylglucosaminyltransferase; 6-SulT, N-acetylglucosamine-6-O-sulfotransferase 2.
Effects of 1,25D(3) and RA on CLA and P-selectin glycoprotein ligand expression on human CLA+ T cells
To determine whether 1,25D(3) or RA treatments inhibited the abundance of the protein scaffolds bearing CLA epitopes, we performed SDS-PAGE on lysates from CLA+ T cells treated with 1,25D(3) or RA at low (100 pM), intermediate (10 nM), and high (1 μM) concentrations. These lysates were Western blotted with mAb HECA-452, the E-selectin/immunoglobulin chimera, and PSGL-1, the major protein scaffold of the CLA/HECA-452 antigen on T cells.1 Although PSGL-1 expression (120-kd monomeric and 250-kd dimeric forms) did not appear to be significantly changed across all 1,25D(3) and RA concentrations, binding of HECA-452 antibody and the E-selectin/immunoglobulin chimera were eliminated in cells grown in intermediate or high concentrations of 1,25D(3), as well as with all concentrations tested of RA (Fig 5, A). To directly examine HECA-452 antigen expression on the PSGL-1 protein scaffold, we immunoprecipitated PSGL-1 from lysates of treated cells with mAb KPL-1 and immunoblotted with either mAb KPL-1 and mAb HECA-452. In these blots PSGL-1 from cells treated with intermediate to high levels of 1,25D(3) or with low to high levels of RA did not show staining with HECA-452 antibody (Fig 5, B). These data demonstrate that 1,25D(3) and RA can specifically disrupt the expression of the CLA carbohydrate epitope on the PSGL-1 protein scaffold and eliminate its function as an E-selectin ligand.
FIG 5.
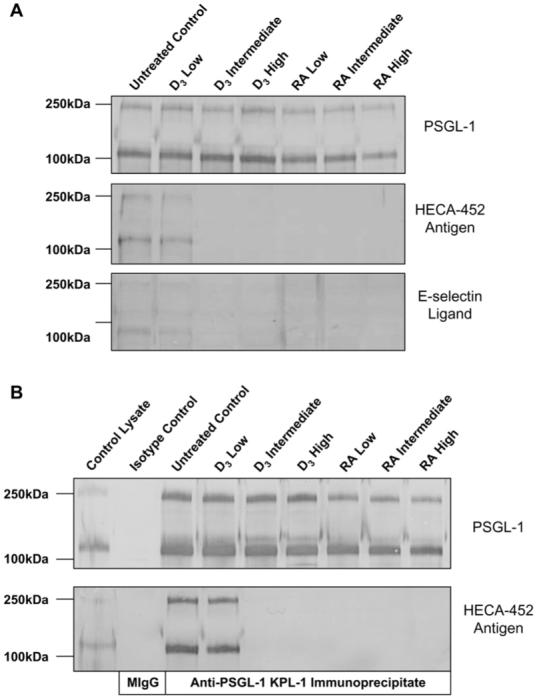
A, Western blotting showed that although PSGL-1 expression was unchanged, HECA-452 antigen and E-selectin glycoprotein ligands were eliminated in cells grown with 1,25D(3) or RA. B, Western blotting of anti-PSGL-1 immunoprecipitates showed that although PSGL-1 was expressed, HECA-452 antigen on PSGL-1 from cells treated with 1,25D(3) or RA was eliminated.
Effector TH cell trafficking in a model of contact hypersensitivity
To explore the in vivo efficacy of both 1,25D(3) and RA on the skin-infiltrating activity of effector T cells, we used an antigen-dependent contact hypersensitivity mouse model. We pretreated mice with either vitamin by means of subcutaneous or intraperitoneal injection and then induced an oxazolone-induced contact hypersensitivity response. We found that the number of CD4+ T cells in the inflamed skin was significantly decreased in mice treated with 1,25D(3) or RA compared with that seen in the control-treated mice (Fig 6 and see Figs E2 and E3 in the Online Repository at www.jacionline.org).
FIG 6.
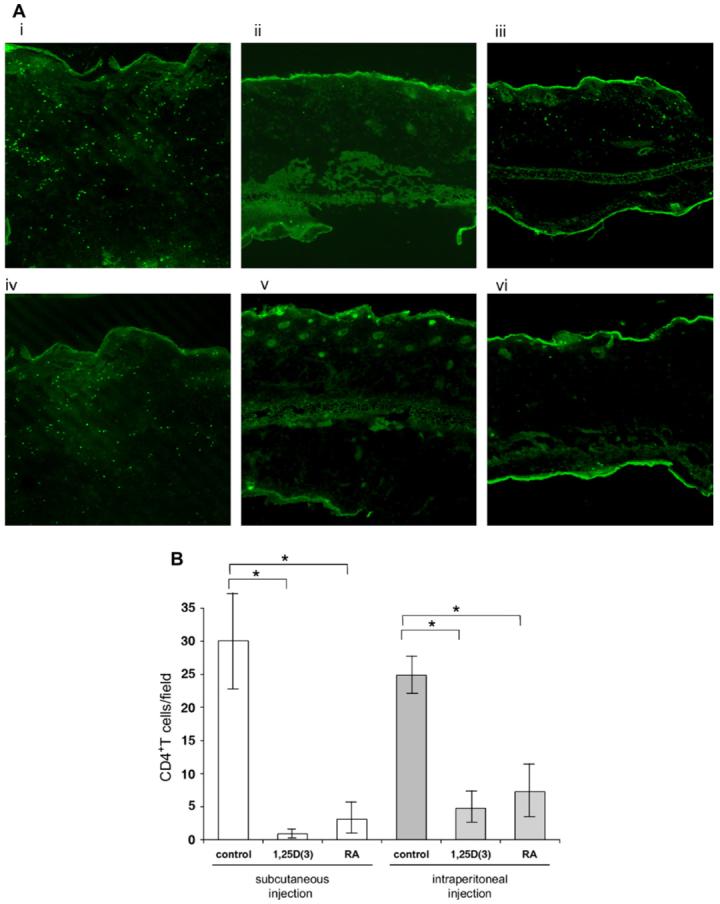
In vivo efficacy of 1,25D(3) and RA in contact hypersensitivity. A, CD4+ T cells were visualized in sections from subcutaneously injected mice (control-treated [i], 1,25D[3]-treated [ii], and RA-treated [iii] mice) and intraperitoneally injected mice (control-treated [iv], 1,25D[3]-treated [v], and RA-treated [vi] mice). CD4+ T cells were routinely detected in control-treated mice (i and iv). Fewer CD4+ cells were detected in 1,25D(3)-treated mice (ii and v) and RA-treated mice (iii and vi) than in control-treated mice. B, The number of skin-infiltrating CD4+ T cells was significantly less in 1,25D(3)- and RA-treated mice. *Statistical significance: P < .01.
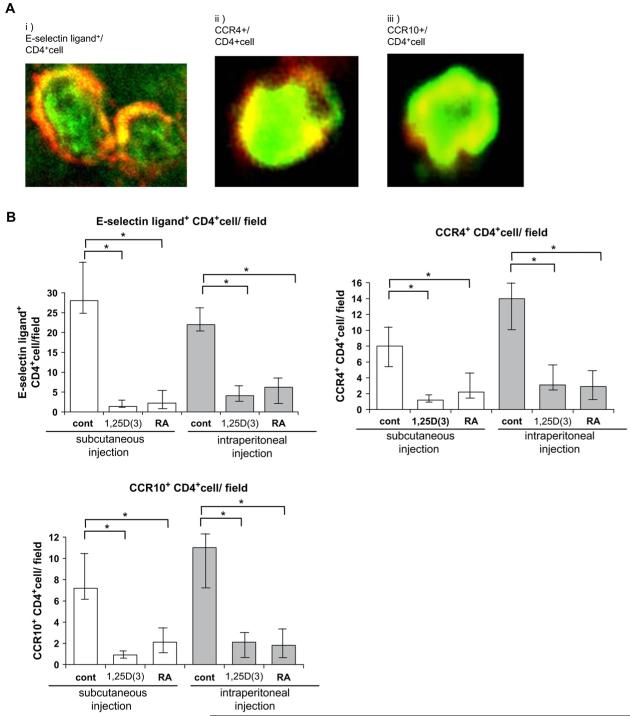
FIG E2.
Phenotypic analysis on skin-infiltrating CD4+ T cells. Mouse functional E-selectin ligand-positive CD4+ T cells, CCR4+CD4+ T cells, and CCR10+CD4+ T cells were detected in control-treated mice (A); however, the number of these cells was decreased in both groups of vitamin-treated mice (B).
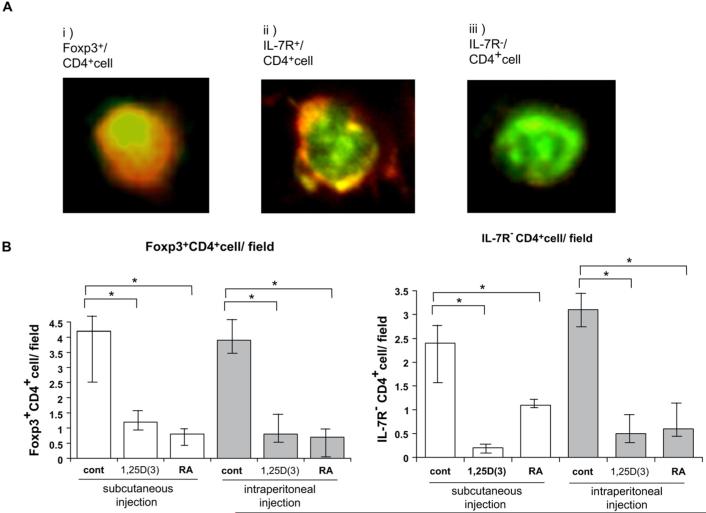
FIG E3.
Foxp3 or IL-7 receptor (IL-7R) expression on skin-infiltrating CD4+ T cells. Foxp3+CD4+ T cells were detected in control-treated mice (A); however, the number of these T cells was reduced by injection of both vitamins (B).
DISCUSSION
In this study we analyzed the effect of RA and 1,25D(3) on the expression of lymphocyte-homing receptors on cultured human T cells. We found that both RA and 1,25D(3) completely eliminated the human T-cell skin-homing marker CLA at physiologically attainable levels, whereas inactive/precursor forms of vitamin A and vitamin D had little inhibitory action. In parallel, functional E-selectin ligand activity was also disabled by RA and 1,25D(3). Additionally, RA decreased the expression of the skin-homing receptor CCR10 and increased the expression of the gut-homing receptors α4β7 and CCR9, as reported previously in a murine model,17 and reduced L-selectin expression, whereas 1,25D(3) had no effect on these other homing receptors. Thus in activated normal human T cells, RA not only blocked skin-homing and LN-homing receptor expression but also enhanced gut-homing receptor expression. In contrast, the effects of 1,25D(3) were limited to blocking CLA expression. To explore the possible indirect mechanisms of action of RA and 1,25D(3), we examined both the activation and growth status in treated cells. Only CD69 expression was increased after RA treatment. Likewise, T-cell proliferation, as determined by means of thymidine incorporation, in the presence of either vitamin treatment was unchanged (data not shown), suggesting that the observed effects on homing receptor expression were not due to cell growth inhibition.
A recent article shows the induction of epidermotropism of lymphocytes by the upregulation of CCR10 with 1,25D(3),20 and the authors speculated that this might occur during the interaction between dermal dendritic cells and lymphocytes in normal physiologic circumstances. In our studies we did not observe either a positive or negative effect of 1,25D(3) on the expression of CCR10 (Fig 2). We cannot explain this discrepancy with the article cited above but suggest that such a putative upregulation might require the presence of dendritic cells. Whether CCR10 is always upregulated by 1,25D(3), the fact that CLA is so readily downregulated suggests that on balance, 1,25D(3) in skin is inhibitory to the skin-homing T-cell function. This is also consistent with the observed clinical efficacy of 1,25D(3) compounds for inflammatory skin diseases.
To further explore the mechanism of CLA downregulation, we analyzed mRNA levels of 7 principal glycosyltransferases associated with sialyl Lewisx synthesis and CLA expression in human T cells. Interestingly, mRNA levels of FucT-VII were significantly decreased in T cells cultured with RA or 1,25D(3), whereas ST3Gal-III mRNA was significantly decreased by RA, although not by 1,25D(3). These results suggest that vitamins A and D have overlapping effects on FucT-VII gene expression. The fact that nuclear receptors for RA and 1,25D(3) act as transcriptional regulators and trigger common signaling pathways might explain the parallel inhibitory effects of these 2 agents on FucT-VII expression.21-23 Additional regulation of the glycosylation machinery responsible for CLA biosynthesis could also be controlled by the induction of endogenous glycosidases (eg, sialidase), the inhibition of glycosyltransferase activity, and/or enhanced glycosyltransferase degradation. These potential mechanisms are currently under investigation.
An immunostaining approach with anti-CLA mAb (HECA-452) and the E-selectin/immunoglobulin chimera revealed a distinct loss of CLA epitopes on cells treated with RA and 1,25D(3). Furthermore, E-selectin ligand function was also significantly suppressed by both compounds. PSGL-1 protein, a major carrier of CLA antigen and E-selectin ligand, was minimally downregulated by high levels of RA but was unaffected by 1,25D(3). The downregulation of E-selectin ligand activity by RA and 1,25D(3) was also demonstrated directly on the immunoprecipitates with anti-PSGL-1. Together, these results suggest that RA and 1,25D(3) do not affect PSGL-1 expression but rather inhibit CLA synthesis, E-selectin ligand expression, or both on glycoprotein scaffolds in T cells.
To determine the inhibitory effects of RA and 1,25D(3) on the skin infiltration of T cells in vivo, we performed antigen-dependent contact hypersensitivity experiments and analyzed infiltrating T cells by means of immunostaining. In this model epicutaneous application of oxazolone results in a site-restricted cytokine profile shift into the TH1 milieu in the acute phase,24 which allowed us to study the effects of these vitamins on CD4+ T cells. The number of skin-infiltrating CD4+ T cells was decreased by both RA and 1,25D(3) compared with that seen in control-treated mice.
Both vitamin D analogs and retinoids have been used successfully for the treatment of T cell-mediated skin diseases, including CTCL, vitiligo, and psoriasis.2 Based on the evidence provided above, it seems plausible that their mechanism of action might be related in part to inhibition of CLA expression on activated T cells. In our preliminary data the similar decrease in CLA level caused by RA and 1,25D(3) was observed on T cells from 3 patients with atopic dermatitis (58% ± 7%, 4% ± 2%, 11% ± 2% for control, RA, and 1,25D(3), respectively). Although prior studies from our laboratory reporting on the efficacy of an inhibitor of CLA synthesis, peracetylated-4-fluorinated glucosamine, show that CLA downregulation can be achieved in the low micromolar range,4,9 the present studies show that CLA expression can be eliminated with concentrations of vitamins A and D in the low nanomolar to picomolar ranges, which are well within physiologically attainable limits. Moreover, because 1,25D(3) treatment does not appear to influence non-skin-homing receptor expression, 1,25D(3) might be an efficacious agent for controlling T cell-mediated cutaneous inflammation without disrupting T-cell trafficking to other tissues.
In summary, these studies reveal a potent effect of vitamins A and D on CLA inhibition and suggest that T cell-mediated skin diseases dependent on E-selectin ligand expression could be dampened by treatment with 1,25D(3) without affecting T-cell growth and differentiation or other non-skin tissue-homing patterns.
Acknowledgments
We thank Jaylyn Olivo for critical reading of this manuscript and Dr Kazuki Hirahara for helpful discussion.
Supported by a SPORE in Skin Cancer from the National Institutes of Health (NIH)/National Cancer Institute (NCI) (to T.S.K.) and by NIH/NCI grant CA118124 (to C.J.D.) and American Cancer Society grant RSG-06-024-01-CSM.
Abbreviations used
7-ADD
7-Amino-actinomycin
AP
Alkaline phosphatase
CCR
Chemokine receptor
CLA
Cutaneous lymphocyte-associated antigen
CTCL
Cutaneous T-cell lymphoma
1,25D(3)
1, 25-Dihydroxyvitamin D(3)
FITC
Fluorescein isothiocyanate
FucT-VII
α1,3-Fucosyltransferase
β4GalT
β1,4-galactosyltransferase
LN
Lymph node
NP-40
Nonidet P-40
PSGL-1
P-selectin glycoprotein ligand 1
RA
Retinoic acid
ST3Gal
α2,3-sialyltransferase
Footnotes
Disclosure of potential conflict of interest: C. J. Dimitroff has received grant support from the American Cancer Society. The rest of the authors have declared that they have no conflict of interest.
Clinical implications: Both RA and 1,25D(3) can improve malignant and benign diseases of T-cell infiltration into the skin by interfering with CLA expression.
REFERENCES
- 1.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–28. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 3.Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J Immunol. 2002;168:5645–51. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- 4.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J Clin Invest. 2003;112:1008–18. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–6. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santamaria LF, Perez Soler MT, Hauser C, Blaser K. Allergen specificity and endothelial transmigration of T cells in allergic contact dermatitis and atopic dermatitis are associated with the cutaneous lymphocyte antigen. Int Arch Allergy Immunol. 1995;107:359–62. doi: 10.1159/000237032. [DOI] [PubMed] [Google Scholar]
- 8.Sigmundsdottir H, Gudjonsson JE, Jonsdottir I, Ludviksson BR, Valdimarsson H. The frequency of CLA+ CD8+ T cells in the blood of psoriasis patients correlates closely with the severity of their disease. Clin Exp Immunol. 2001;126:365–9. doi: 10.1046/j.1365-2249.2001.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitroff CJ, Bernacki RJ, Sackstein R. Glycosylation-dependent inhibition of cutaneous lymphocyte-associated antigen expression: implications in modulating lymphocyte migration to skin. Blood. 2003;101:602–10. doi: 10.1182/blood-2002-06-1736. [DOI] [PubMed] [Google Scholar]
- 10.Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119:1405–10. doi: 10.1046/j.1523-1747.2002.19610.x. [DOI] [PubMed] [Google Scholar]
- 11.Armerding D, Kupper TS. Functional cutaneous lymphocyte antigen can be induced in essentially all peripheral blood T lymphocytes. Int Arch Allergy Immunol. 1999;119:212–22. doi: 10.1159/000024197. [DOI] [PubMed] [Google Scholar]
- 12.Akdis M, Klunker S, Schliz M, Blaser K, Akdis CA. Expression of cutaneous lymphocyte-associated antigen on human CD4(+) and CD8(+) Th2 cells. Eur J Immunol. 2000;30:3533–41. doi: 10.1002/1521-4141(2000012)30:12<3533::AID-IMMU3533>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 14.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(suppl):1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 15.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 16.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 17.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama F, Teraki Y, Kudo T, Togayachi A, Iwasaki H, Tamatani T, et al. Expression of cutaneous lymphocyte-associated antigen regulated by a set of glycosyl-transferases in human T cells: involvement of alpha1, 3-fucosyltransferase VII and beta1,4-galactosyltransferase I. J Invest Dermatol. 2000;115:299–306. doi: 10.1046/j.1523-1747.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 19.Wagers AJ, Lowe JB, Kansas GS. An important role for the alpha 1,3 fucosyl-transferase, FucT-VII, in leukocyte adhesion to E-selectin. Blood. 1996;88:2125–32. [PubMed] [Google Scholar]
- 20.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 21.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–54. [PubMed] [Google Scholar]
- 23.Li M, Indra AK, Warot X, Brocard J, Messaddeq N, Kato S, et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–6. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 24.Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484–91. [PubMed] [Google Scholar]