Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation (original) (raw)
Abstract
The conserved Hippo tumor suppressor pathway is a key signaling pathway that controls organ size in Drosophila. To date a signal transduction cascade from the Cadherin Fat at the plasma membrane into the nucleus has been discovered. However, how the Hippo pathway is regulated by extracellular signals is poorly understood. Fat not only regulates growth but also planar cell polarity, for which it interacts with the Dachsous (Ds) Cadherin, and Four-jointed (Fj), a transmembrane kinase that modulates the interaction between Ds and Fat. Ds and Fj are expressed in gradients and manipulation of their expression causes abnormal growth. However, how Ds and Fj regulate growth and whether they act through the Hippo pathway is not known. Here, we report that Ds and Fj regulate Hippo signaling to control growth. Interestingly, we found that Ds/Fj regulate the Hippo pathway through a remarkable logic. Induction of Hippo target genes is not proportional to the amount of Ds or Fj presented to a cell, as would be expected if Ds and Fj acted as traditional ligands. Rather, Hippo target genes are up-regulated when neighboring cells express different amounts of Ds or Fj. Consistent with a model that differences in Ds/Fj levels between cells regulate the Hippo pathway, we found that artificial Ds/Fj boundaries induce extra cell proliferation, whereas flattening the endogenous Ds and Fj gradients results in growth defects. The Ds/Fj signaling system thus defines a cell-to-cell signaling mechanism that regulates the Hippo pathway, thereby contributing to the control of organ size.
Keywords: Drosophila, organ growth, imaginal discs, Fat
The regulation of growth is fundamental to animal development and organs grow until they have reached their proper size. The number of cell divisions is often not programmed into precursor cells and the regulation of cell proliferation must thus involve cell-to-cell communication. However, the signals that regulate cell proliferation to control organ size are not understood (1).
The Hippo tumor suppressor pathway has emerged as a key signaling pathway that controls tissue size in Drosophila (2). Hippo signaling suppresses cell proliferation and animals that lack Hippo pathway activity have severely overgrown imaginal discs and corresponding adult structures. The Hippo pathway is therefore a prime candidate to transduce cell-to-cell signals that control imaginal disc size. However, how the Hippo pathway is regulated by extracellular signals has yet to be elucidated.
Several components of the Hippo pathway have been discovered and a signal transduction pathway from the plasma membrane into the nucleus has emerged (2). Known components include the adaptor proteins Merlin (Mer) and Expanded (Ex) that act in parallel and activate a kinase cascade involving the Hippo (Hpo) and Warts (Wts) kinases. Wts then inhibits the transcriptional coactivator Yorkie (Yki) that normally induces the expression of target genes that drive cell proliferation and cell survival. Recently, the atypical Cadherin Fat was identified as an upstream component of the Hippo pathway and it was proposed that Fat acts as a receptor that activates the Hippo pathway (3–6).
Notably, Fat not only regulates growth, but it also regulates planar cell polarity (PCP) (7, 8). PCP describes the polarity of cells within an epithelial tissue and manifests itself in the orientation of cellular structures such as hairs on insect cuticles. Fat acts in a cell-to-cell signaling process that aligns polarity between neighboring cells, for which it interacts with Dachsous (Ds), an atypical cadherin related to Fat, and Four-jointed (Fj), a Golgi-associated kinase that phosphorylates the extracellular domains of Fat and Ds (7–9). Fat and Ds bind each other and they may act as receptor and ligand, whereas Fj modulates the interaction between Fat and Ds (7, 8, 10). Ds and Fj are expressed in complementary gradients in many tissues which may provide directionality to the signaling, thereby contributing to establish the direction of PCP (7, 8).
Interestingly, Ds and Fj are also required for normal growth. ds and fj mutants have reduced growth in the proximal-distal axis resulting in shorter legs and wings (11–16). These growth phenotypes suggest that Ds and Fj also modulate the growth control function of Fat, in addition to their effect on Fat in PCP. In addition, Ds and Fj, like Fat, regulate the expression of wingless (wg) in the hinge region of developing wing discs (17), where wg expression is sensitive to Hippo signaling (6, 17). Ds and Fj may thus act as extracellular signals that modulate the Hippo pathway. However, how Ds and Fj modulate the Hippo pathway and their signaling logic is not understood. Here, we describe results that suggest a model in which Ds and Fj modulate the Hippo pathway but through an unusual mechanism.
Results
Boundaries of Ds and Fj Activity Up-regulate Hippo Target Genes.
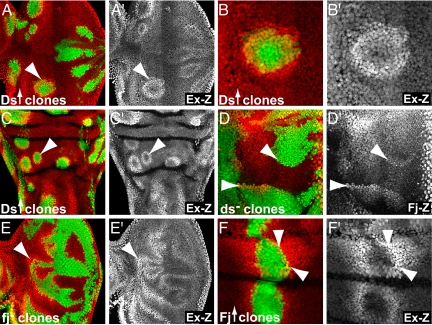
To investigate the role of Ds in regulating the Hippo pathway, we first tested the effects of Ds overexpression on Hippo pathway activity. We monitored the activity of the Hippo pathway by assaying the expression of lacZ reporter insertions into the ex, diap1, and fj genes, known transcriptional targets of the Hippo pathway (2, 5, 6, 18). We found that Ds overexpressing clones caused an up-regulation of the expression of these reporters [Fig. 1 A–C, supporting information (SI) Fig. S1 A and B (6)]. The expression of ex, diap1, and fj is negatively regulated by the Hippo pathway and their up-regulation thus indicated that Ds overexpressing clones suppressed the activity of the Hippo pathway, thereby derepressing the expression of target genes. Similar to the effects of loss of Hippo activity, the up-regulation of diap1 and fj was strongest in eye discs, whereas ex was induced in various imaginal discs including eye, wing, and leg discs (Fig. 1 A–C, Fig. S1 A and B, and data not shown). Consistent with a signaling function of Ds, the up-regulation of target gene expression occurred not only in Ds expressing cells but also in neighboring cells that did not overexpress Ds (Fig. 1 and Fig. S1). Remarkably, however, Hippo target genes were not up-regulated in all cells within Ds expressing clones, but were up-regulated only along the borders of Ds expressing clones (Fig. 1 and Fig. S1) (6). Thus, even though cells in the center of Ds expressing clones are exposed to high levels of Ds, these cells did not up-regulate Hippo target genes. It could be that high levels of Ds expression renders a cell refractory to receiving the Ds signal, similar to the _cis_-inhibition effects observed for Notch ligands (19). However, we found that cells on both sides of Ds expressing clone borders up-regulated Hippo target genes, arguing against _cis_-inhibition (Fig. S1_A_). Together, the observed effects therefore suggested a model in which it is the juxtaposition of cells expressing different levels of Ds (discontinuities or boundaries of Ds activity), rather than a certain amount of Ds, that triggers the up-regulation of target genes. Ds may therefore not simply act as a ligand, which would induce target genes proportional to its concentration.
Fig. 1.
Boundaries of Ds and Fj activity induce Hippo target genes. Third instar imaginal discs, which contain clones of cells either overexpressing or mutant for ds or fj. Clones induced the expression of β-Gal expression from lacZ enhancer trap insertions into the Hippo target genes ex (A–C, E, and F) and fj (D). β-Gal induction was observed specifically at clone borders and induction was seen on both sides of clone borders except for dsUA071 mutant clones, which induced fj-lacZ expression only in cells outside mutant clones. Cells overexpressing Ds (A–C) or Fj (F) were positively marked by coexpression of GFP. Clones mutant for dsUAO71 (D) and fjd1 (E) were marked by the absence of GFP. A, B, D, and E show eye imaginal discs and C and F show wing discs. B is a close-up of a clone shown in A. (F) Wing disc with clones overexpressing Fj in the hinge region marked by GFP expression. For all stainings, GFP is shown in green and β-Gal antibody stainings are shown in red. A′_–_F′ show β-Gal antibody stainings in gray. Anterior is to the left in all discs and arrowheads point to clone borders.
The boundary model can be distinguished from a simple ligand model by comparing the effects of loss and gain of Ds function. The ligand model predicts that loss of Ds causes effects opposite to those caused by Ds overexpression. In contrast, the boundary model predicts that loss and gain of Ds function may cause similar effects, because loss- and gain-of-function clones create borders where cells with different amounts of Ds are confronted. To distinguish between the two models, we thus produced ds loss-of-function clones and compared their effects of with those of Ds overexpression clones. Strikingly, we found that ds loss-of-function clones also caused an up-regulation of fj-lacZ expression outside of clone borders (Fig. 1D). These effects were most conspicuous toward the poles of the eye discs, in regions that express high levels of Ds where ds clone borders produce maximal disparity in Ds levels. Interestingly, fj-lacZ was only up-regulated in wild-type cells but not in ds mutant cells, indicating that Ds is required for cells to respond to Ds boundaries (further discussed below). Nevertheless, the up-regulation of fj-lacZ around ds mutant clones is similar to that caused by Ds overexpression and thus supports the boundary model.
Next, we tested whether manipulating Fj also caused boundary effects. In particular, we wanted to test whether loss and gain of Fj caused similar or opposite effects. We found that fj mutant clones and clones overexpressing Fj resulted in the up-regulation of Hippo target genes around clone borders in eye and wing discs (Fig. 1 E and F and Fig. S1 C and D). In the case of Fj, Hippo target genes were up-regulated on both sides of loss- as well as gain-of-function clones, although the effects were more pronounced on the side that expressed less Fj. Loss and gain of Fj thus caused similar phenotypes further supporting the boundary model while contradicting a simple ligand model.
In summary, we conclude that Ds does not simply act as an antagonistic ligand for Fat. Instead, it appears that disparities of Ds and Fj activity between cells rather than the absolute amounts of Ds and Fj presented to a cell modulate the Hippo pathway. Hippo target genes are thus up-regulated along boundaries where cells with different levels of Ds/Fj activity are confronted and cells on both sides of Ds/Fj boundaries induce Hippo target genes in gradients up to several cells away from Ds/Fj boundaries (Fig. 1 and Fig. S1).
Because Ds is known to interact with Fat on neighboring cells (7), Ds boundaries may require Fat signaling to regulate Hippo targets. To test this, we first generated Ds expressing clones in fat mutant discs. Although ex-lacZ and diap1-lacZ are already elevated in fat mutant cells (3–6), Ds expressing clones did not cause further up-regulation of ex-lacZ and diap1-lacZ in fat mutant discs (Fig. S2 A and B, data not shown). This indicates that the Ds boundary effect requires Fat. Second, we tested whether components acting downstream of Fat are required for Ds boundary action. We turned to Dachs, an unconventional Myosin, which acts downstream of Fat and is required for the overgrowth phenotypes and target gene induction seen in fat mutants (6, 17, 20). We found that loss of Dachs suppressed the up-regulation of Hippo target genes at Ds boundaries (Fig. S2_C_). These results are consistent with a model in which Ds boundaries suppress the activity of Fat and the Hippo pathway to regulate gene expression.
Ds and Fj Regulate the Level of Ex at the Plasma Membrane.
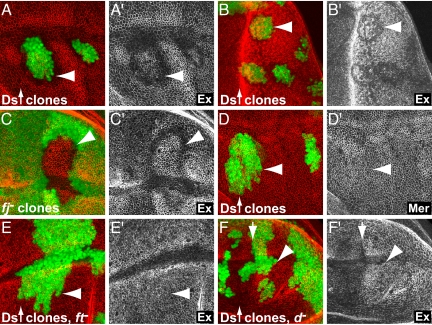
Fat is required for proper stability and localization of Ex to the plasma membrane (3–5); however, whether Fat acts through Ex is controversial (6, 21). We thus tested whether Ds/Fj boundaries affect the amount of Ex protein localized to the plasma membrane. Strikingly, we found that boundaries of Ds activity, generated at the borders of Ds overexpression or fj mutant clones, resulted in a decrease of Ex protein at the plasma membrane of cells on both sides of such clone borders (Fig. 2 A–C). In contrast to Ex, levels of Mer at the plasma membrane were not affected along Ds boundaries (Fig. 2D). The reduction of Ex was not due to reduced transcription, because ex transcription is increased in these cells as shown above (Fig. 1). The effect of Ds boundaries on Ex levels required Fat and Dachs because Ds boundaries did not cause a reduction of Ex at the plasma membrane in fat or dachs mutant discs (Fig. 2 E and F). These results indicate that Ds boundaries act through Fat and Dachs to regulate the localization and stability of Ex. Ds boundaries may thus suppress the activity of the Hippo pathway, at least in part, through a Fat- and Dachs-dependent mechanism that regulates the localization and stability of Ex. Because Fat and Dachs, but not Ex regulates the stability of Wts (6), Fat and Dachs may regulate the activity of Wts through Ex dependent and independent mechanisms.
Fig. 2.
Boundaries of Ds and Fj activity regulate Ex stability and localization. Ex protein was reduced at the plasma membrane along the borders of clones of cells overexpressing Ds in a wing (A) and an eye disc (B) and along the borders of fjd1 mutant clones (C). (D) Ds overexpressing clones did not affect Mer levels (wing disc). Ds overexpressing clones did not affect Ex in a fatfd/422 (E) or a dGC13 homozygous mutant wing disc (F). (Left) GFP in green and antibody staining in red. (Right) Antibody staining in gray. Arrowheads point to clone boundaries. Anterior is to the left in all discs.
Ds Is Required to Sense the Boundary Signal.
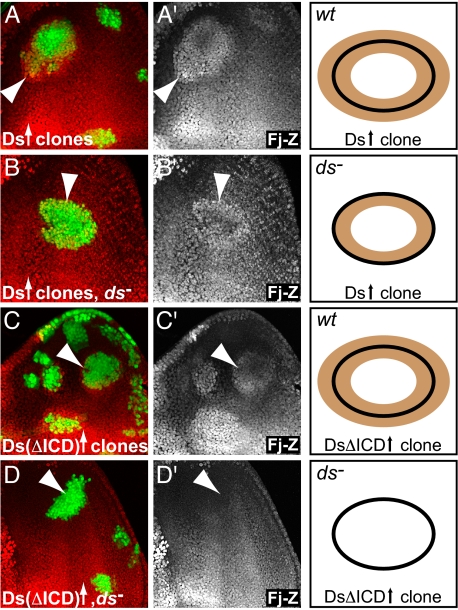
Our data above show that clones overexpressing Ds trigger an up-regulation of Hippo target genes in neighboring cells. This effect is consistent with a signaling function of Ds. Surprisingly, Ds is also required to sense the boundary signal. Thus, clones overexpressing Ds in ds homozygous mutant discs caused Hippo target gene induction, but only in Ds expressing cells (Fig. 3 A and B). Similarly, although ds mutant clones generate a Ds activity boundary and a boundary signal that causes the up-regulation of Hippo target genes, target genes were up-regulated only in wild-type cells but not ds mutant cells (Fig. 1D). We conclude that Ds is required cell-autonomously for cells to respond to the boundary signal, indicating that Ds has receptor-like functions in addition to its signaling function.
Fig. 3.
Ds is required for sensing of the boundary signal. (A) Overexpression of Ds in a wild-type background induced fj-lacZ expression inside and outside of clone borders. (B) Overexpression of Ds in dsUA071 homozygous mutant eye discs also induced fj-lacZ expression, but only in cells within the clone. (C and D) Overexpression of a version of Ds that lacks the intracellular domain, Ds(ΔICD), was sufficient to induce fj-lacZ expression in wild-type (C), but not dsUA071 homozygous mutant discs (D). For all stainings: β-Gal expression is shown in red and overexpression clones are marked by coexpression of GFP (green) (Left); anti-β-Gal staining is shown in gray (Center); schematic representations of the four experiments illustrating the requirement of the Ds intracellular domain for the sensing of the boundary signal (Right). These schematics depict the pattern of Hippo target gene induction (brown) as a result of clones overexpressing different versions of Ds.
Ds is a transmembrane protein with extensive extra- and intracellular domains. Similar to its activity in PCP, we found that overexpressing a version of Ds that lacks the intracellular domain (DsΔICD; ref. 10) was as potent as overexpression of full-length Ds in causing an up-regulation of Hippo target genes (Fig. 3C). The intracellular domain of Ds is thus not required for its signaling function. In contrast, however, the intracellular domain of Ds was required for the receptor-like function of Ds, because clones of cells expressing wild-type Ds, but not DsΔICD, up-regulated Hippo target genes when expressed in ds mutant discs (Fig. 3D). Therefore, although overexpression of DsΔICD is sufficient to generate a boundary signal, it is not sufficient to render a cell capable of responding to the boundary signal (schematically represented in Fig. 3 Right). The observation that the intracellular domain of Ds is required specifically for the sensing of the boundary further emphasizes that Ds does not simply act as a ligand for Fat, but that Ds has signaling and receptor-like functions that can be genetically distinguished by removing the intracellular domain.
Ds and Fj Boundaries Induce Cell Proliferation.
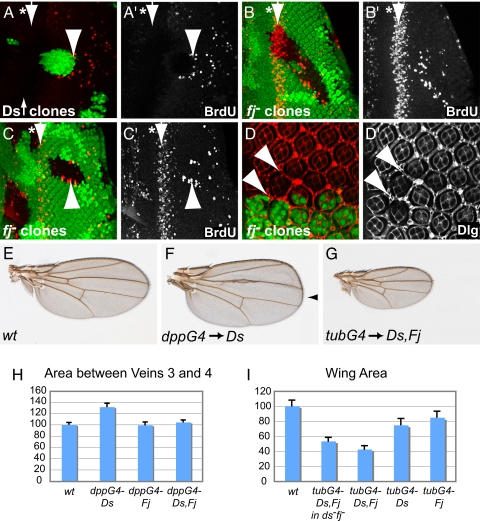
Because Ds/Fj boundaries induce Hippo target genes, we tested whether Ds and Fj boundaries mimic the overproliferation phenotypes of hpo mutants. Ectopic cell proliferation in hpo mutants is most conspicuously observed in developing eye discs posterior to the second mitotic wave where cells normally exit the cell cycle and start to differentiate. We found that Ds expressing or fj mutant clones caused extra cell proliferation posterior to the second mitotic wave, as evidenced by ectopic BrdU incorporation (Fig. 4 A–C). Strikingly, ectopic cell proliferation was observed specifically along clone borders and in mutant (32%) and wild-type cells (68%). We also observed extra interommatidial cells at clone borders in pupal retinae, another phenotype characteristic for hpo mutants (Fig. 4D). Ds/Fj activity boundaries thus regulate cell proliferation and mimic hpo mutant phenotypes.
Fig. 4.
Discontinuities of Ds/Fj activities drive cell proliferation and tissue growth. Eye imaginal discs containing a Ds overexpressing clone marked by GFP expression (A) and fjd1 mutant clones marked by the absence of GFP expression (B and C). These discs were labeled for BrdU incorporation (red in A–C and gray in A′_–_C′). Wild-type cells arrest in G1 in the morphogenetic furrow (asterisks) and nondifferentiating cells go through one synchronous S phase in the second mitotic wave (arrows). Ds expressing and fjd1 mutant clones posterior to the second mitotic wave show ectopic cell proliferation along clone borders in mutant and wild-type cells (arrowheads). (The second mitotic wave in A is out of focus.) (D) Midpupal retina carrying fjd1 mutant clones marked by the absence of GFP expression stained with Discs large (Dlg) antibodies to mark cell outlines. Extra interommatidial cells were produced at clone borders (arrowheads). (E–G) Images of wings from adults of the indicated genotypes. (F) _dpp-Gal4_-driven overexpression of Ds caused an enlargement of the distance between veins 3 and 4 (arrowhead). (G) _tub-Gal4_-driven overexpression of Ds and Fj caused small wings. (H) Quantification of the area between veins 3 and 4 of flies shown in E and F, as well as additional genotypes including overexpression of Fj. The areas were normalized to sibling wild-type control flies. (I) Quantification of the wing area of flies that overexpressed Ds and Fj under the tub-Gal4 driver shown in G as well as additional genotypes that expressed Ds and Fj in ds, fj double mutants, and Ds and Fj by themselves. The wing areas were normalized to sibling wild-type control flies.
Ds and Fj Boundaries Regulate Growth.
To test whether ectopic Ds/Fj boundaries are sufficient to induce extra growth, we ectopically expressed Ds and Fj in a stripe along the anterior–posterior compartment boundary in the developing wing by using the dpp-Gal4 driver. Overexpression of Ds by dpp-Gal4 caused an enlargement of the region between veins 3 and 4 where dpp-Gal4 is expressed (Fig. 4 E, F, and H). Artificial discontinuities of Ds expression are thus sufficient to drive extra wing growth.
In contrast to Ds, overexpression of Fj had no significant overgrowth phenotype (Fig. 4H). This difference may be explained by the endogenous expression patterns of Ds and Fj. Ds and Fj are expressed in complementary expression patterns in many different tissues, including the eye and wing imaginal discs (7). In wing discs, for example, the highest levels of Ds are found in proximal parts that will develop into the hinge, whereas the highest levels of Fj are expressed in the wing pouch (7). Overexpression of Fj in the pouch may thus not cause extra growth because it is already expressed there. In addition, coexpression of Fj with Ds suppressed the growth induced by Ds expression, consistent with a model in which Fj negatively regulates the interaction between Ds and Fat (7, 8).
Are the endogenous patterns of Ds and Fj expression important for growth? Flies homozygous mutant for ds or fj have growth defects and show shortened legs and wings, indicating that Ds and Fj are required for normal growth (11–16). However, ds homozygotes have slightly overgrown wing discs, which may at least in part be due to partial loss of Fat function, because Ds is required for proper Fat localization (22, 23). To avoid effects on Fat localization, we flattened the gradients of Fj and Ds without completely removing their expression by uniformly expressing Ds and Fj in ds, fj double mutant, or otherwise wild-type animals by using the ubiquitous tub-Gal driver. Animals with such uniform Ds/Fj expression had significantly smaller bodies, and comparatively smaller wings and legs (Fig. 4 G and I and Fig. S3). Similarly, overexpression of Ds and Fj throughout the posterior compartment by hh-Gal4 resulted in wings with significantly smaller posterior compartments (Fig. S4). Notably, uniform expression of Ds and Fj together had stronger phenotypes than overexpression of Ds and Fj individually, indicating that the gradients of both, Ds and Fj are important for proper growth (Fig. 4I). Taken together, our data show that ectopic Ds/Fj boundaries drive growth, whereas flattening the endogenous gradients suppresses growth, indicating that the endogenous expression patterns of Ds and Fj are required for proper growth control.
Discussion
Our data show that Ds and Fj regulate the Hippo pathway in an unusual manner. Most interestingly, we found that discontinuities or boundaries of Ds and Fj activity, rather than their absolute amounts, modulate the Hippo pathway. The effects of Ds and Fj on wg expression in the hinge region are also consistent with the proposed boundary model (6, 17). Importantly, artificial Ds/Fj boundaries cause an up-regulation (de-repression) of Hippo pathway target genes and drive extra cell proliferation, whereas flattening of the endogenous Ds and Fj gradients reduced normal growth (this study and refs. 15 and 24). Together, these data are consistent with a model in which Ds/Fj discontinuities suppress the activity of the Hippo pathway, thereby driving imaginal disc growth and thus contributing to the regulation of organ size.
How much growth is controlled by Ds/Fj signaling? Flies with uniform Ds/Fj expression have significantly reduced wings, legs, and other body parts, but growth is not abolished. The Ds boundary effect thus accounts for some but not all growth control. Given that fat mutants have severely overgrown imaginal discs, how do these growth defects caused by flat Ds/Fj expression fit with a model that Ds and Fj act through Fat to regulate growth? The dachs mutant phenotype gives us insights into that question. Dachs acts downstream of Fat and is required for the growth control function of Fat (17, 20). Unlike Fat, however, Dachs is a positive regulator of growth. Fat thus suppresses growth by inhibiting Dachs (17, 20), and the dachs mutant phenotype thus reflects the situation where Fat is fully (hyper) active. because Fat functions through the inactivation of Dachs, the growth defects caused by Fat hyperactivation cannot be stronger than the dachs mutant phenotype. The boundary model proposes that flattening the Ds and Fj gradients results in hyperactivation of Fat, thereby causing reduced growth. Remarkably, dachs mutants have small wings and short legs, and the strength of these growth defects are similar to those caused by uniform Ds and Fj expression (this study and refs. 15, 20, and 24). The phenotypes caused by uniform Ds and Fj expression are thus consistent with our model that discontinuities of Ds and Fj inactivate Fat signaling to promote growth.
The observation that flies with uniform Ds/Fj expression as well as dachs mutants retain some growth indicates that other signaling mechanisms act in addition to the Ds boundary effect to control imaginal disc size. The Ds boundary effect is thus one of possibly several separate mechanisms that contribute to control the final size of imaginal discs. These other, currently unknown signals may act in parallel to the Hippo pathway to regulate tissue growth. In addition, other signals may regulate the Hippo pathway independently of the Ds boundary effect. For example, Mer acts in parallel to Fat, thus identifying another input into the Hippo pathway (4, 5). It will be interesting to elucidate these additional signaling systems and to understand how they cooperate with Hippo signaling to control imaginal disc growth.
Ds, Fj, and Fat regulate growth and planar cell polarity (PCP). Interestingly, discontinuities in Ds/Fj activity rather than their absolute amounts also regulate PCP. Ommatidial polarity reversals are associated with ds and fj mutant clone borders and ommatidia inside and outside of clones are affected (7). Boundary effects of Ds and Fj are also observed on hair polarity in the wing and abdomen (7). Thus, Ds/Fj discontinuities modulate Hippo signaling and PCP. However, the effects on the Hippo pathway are different from those on PCP. In contrast to the effects on Hippo target genes, which are induced all around clone borders, PCP effects are observed only on one side of clones (7). This difference can be explained because the Hippo readout is scalar (levels of target gene expression), whereas the PCP readout is vectorial (direction of polarity) (Fig. S5). Thus, the direction of the Ds/Fj gradients determines the direction of cell polarity, whereas the disparity in Ds/Fj activity (steepness of the gradients) modulates Hippo signaling.
The effect of Ds/Fj boundaries appears to spread over several cells. Although β-Gal perdurance may contribute to this effect when assaying reporter gene expression, we found that Ex degradation as well as up-regulation of DIAP1 protein, which has a short (30-min) half-life, is also observed over several cell diameters (Fig. S1_C_ and Fig. 2), indicating that the boundary signal is propagated over several cells. A similar propagation is also observed for the effects of Ds/Fj boundaries on PCP (7, 8), and it has been suggested that Ds/Fj boundaries cause an asymmetric localization of Ds and Fat which may then propagate between cells (8, 26).
The boundary model proposes that cells respond to disparities in the levels of Ds/Fj between cells. How do cells sense Ds/Fj disparities to modulate downstream effectors? Ds forms heterodimers with Fat on neighboring cells (22, 23, 25) and Fat cell autonomously regulates the activity of the Hippo pathway (3–6). This suggested that Fat and Ds may act as receptor and ligand, respectively. Surprisingly, however, Ds and Fat do not behave like a classical ligand–receptor pair. First, Ds does not regulate Fat in a dose-dependent manner, but rather acts through a boundary effect. Second, Ds is required in signal-sending cells as well as in responding cells, indicating that Ds has ligand- and receptor-like functions. This is true for the regulation of Hippo signaling as well as for PCP signaling in the abdomen (this study and ref. 26). The finding that the intracellular domain of Ds is not required for the generation but for the sensing of the boundary signal further exposes this dual function of Ds. Two alternative models could explain how cells sense Ds discontinuities. In a first model, cells may compare the amount of Ds presented by neighboring cells on opposite sides (26). Cells may then sense a differential in the number of bound Fat molecules from one side of the cell to the other. In an alternative model, cells may compute the difference between the amount of Ds presented by neighboring cells (sensed by the amount of bound Fat molecules) with the amount of Ds expressed by a cell itself. This model may explain why Ds and its intracellular domain are required cell-autonomously to respond to the boundary signal. In both models, a differential in Ds activity between cells may regulate the activity of Fat, which then transduces the signal to downstream components. Fat is cell-autonomously required to regulate the Hippo pathway, and the intracellular domain of Fat is sufficient to promote the growth control and at least some PCP functions of Fat (10). The intracellular domain of Fat may thus transduce the boundary signal to downstream components regulating PCP and Hippo. Because Ex, Dachs, Hpo, Wts, and Yki do not or only slightly affect PCP (2, 20, 21), Fat may engage different downstream effectors to regulate PCP and the Hippo pathway. It will be fascinating to decipher the molecular mechanisms of how boundaries of Ds/Fj activity regulate the activity of Fat and how they are translated into a vector to control PCP and a scalar to modulate the Hippo pathway.
Materials and Methods
Mutant clones were induced by using the FLP/FRT system with fjd1 (11) and dsUAO71 (13) alleles and the corresponding _ubiGFP_-marked FRT chromosomes. Overexpression was done with the UAS-Gal4 system by using dpp-Gal4, hh-Gal4, tub-Gal4, UAS-ds (25), UAS-ds_Δ_ICD (10), UAS-fj (15), and hsFLP; _act_>y+>Gal4, UAS-GFPS65T, and hsFLP; _act_>_CD2_>Gal4, UAS-GFPS65T. Other stocks were: ex697-lacZ, diap1-lacZ, fj-lacZ (fjP1), fjN7, ds38k, ftfd, ft422, and dGC13. Antibody stainings of imaginal discs and BrdU incorporations were done as described in ref. 5. The following antibodies were used (source and dilutions in parentheses): guinea-pig α-Mer (R. Fehon, 1:4,000), guinea-pig α-Ex (R. Fehon, 1:2,000), mouse α-BrdU (Becton-Dickinson, 1:50), mouse α-DIAP-1 (B. Hay, 1:200), mouse α-Dlg (DSHB, 1:300), and mouse α-βGal (Promega, 1:2000).
Supplementary Material
Supporting Information
Acknowledgments.
We thank Seth Blair, Peter Bryant, Richard G. Fehon, Allen Laughon, Bruce Hay, Kenneth D. Irvine, Michael A. Simon, Gary Struhl, David Strutt, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank (University of Iowa) for fly stocks and antibodies, Leisa McCord for her help with artwork, Marlese Pisegna and Kathleen Gajewski for technical help, and members of the Halder Lab for discussions. This work was supported by a National Institutes of Health grant (to G.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stanger BZ. Organ size determination and the limits of regulation. Cell Cycle. 2008;7(3):318–324. doi: 10.4161/cc.7.3.5348. [DOI] [PubMed] [Google Scholar]
- 2.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21(8):886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 3.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16(21):2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16(21):2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Willecke M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16(21):2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38(10):1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 7.Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. BioEssays. 2005;27(12):1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence PA, Struhl G, Casal J. Planar cell polarity: One or two pathways? Nat Rev Genet. 2007;8(7):555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321(5887):401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development (Cambridge, U.K.) 2006;133(12):2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky MH, Steller H. Positional information along the dorsal-ventral axis of the Drosophila eye: Graded expression of the four-jointed gene. Dev Biol. 1996;173(2):428–446. doi: 10.1006/dbio.1996.0038. [DOI] [PubMed] [Google Scholar]
- 12.Clark HF, et al. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9(12):1530–1542. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- 13.Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development (Cambridge, U.K.) 1998;125(5):959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- 14.Villano JL, Katz FN. Four-jointed is required for intermediate growth in the proximal-distal axis in Drosophila. Development (Cambridge, U.K.) 1995;121(9):2767–2777. doi: 10.1242/dev.121.9.2767. [DOI] [PubMed] [Google Scholar]
- 15.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228(2):181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 16.Waddington CH. The development of some “leg genes” in Drosophila. Drosophila J Genet. 1943;45:29–43. [Google Scholar]
- 17.Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development (Cambridge, U.K.) 2004;131(18):4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- 18.Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8(1):27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 19.Schweisguth F. Regulation of notch signaling activity. Curr Biol. 2004;14(3):R129–R138. [PubMed] [Google Scholar]
- 20.Mao Y, et al. Dachs: An unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development (Cambridge, U.K.) 2006;133(13):2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Irvine KD. Fat and expanded act in parallel to regulate growth through warts. Proc Natl Acad Sci USA. 2007;104(51):20362–20367. doi: 10.1073/pnas.0706722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421(6922):543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 23.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: Dishevelled-independent functions of frizzled. Dev Cell. 2002;3(6):851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 24.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development (Cambridge, U.K.) 2004;131(24):6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 25.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development (Cambridge, U.K.) 2004;131(15):3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 26.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development (Cambridge, U.K.) 2006;133(22):4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information