CD34+ Cord Blood Cell-Transplanted Rag2−/− γc−/− Mice as a Model for Epstein-Barr Virus Infection (original) (raw)
Abstract
Recent studies suggest that Epstein-Barr virus (EBV) can infect naïve B cells, driving them to differentiate into resting memory B cells via the germinal center reaction. This hypothesis has been inferred from parallels with the biology of normal B cells but has never been proven experimentally. Rag2−/− γc−/− mice that were transplanted with human CD34+ cord blood cells as newborns were recently shown to develop human B, T, and dendritic cells, constituting lymphoid organs in situ. Here we used this model to better define the strategy of EBV infection of human B cells in vivo and to compare this model system with different conditions of EBV infection in humans. Our results support the model of EBV persistence in vivo in cases that were characterized by follicular hyperplasia and a relatively normal CD4+ and CD8+ T-cell distribution. Intriguingly, in cases that were characterized by nodular and diffuse proliferation with a preponderance of CD8+ T cells, similar to infectious mononucleosis, EBV still infects naïve B cells but also induces clonal expansion and ongoing somatic mutations without germinal center reactions. Our results reveal different strategies of EBV infection in B cells that possibly result from variations in the host immune response. Future experiments might allow understanding of the mechanisms responsible for persistent EBV infection and provide targets for more highly tailored therapeutic interventions.
In developing countries and in low socioeconomic groups Epstein-Barr virus (EBV) infection is usually acquired in childhood, most often without specific symptoms. When infection is delayed it can cause a benign self-limited lymphoproliferation or infectious mononucleosis characterized by variable case histories. In whatever way the disease first manifests in healthy individuals, primary infection is followed by a symptom-free carrier state.1
Early in the course of primary infection EBV infects B lymphocytes. However, it is not known where B lymphocytes are infected and whether this involves epithelial cells.1 EBV does not usually replicate in B lymphocytes but instead establishes a latent infection, which is characterized by the limited expression of a subset of virus latent genes.2 These patterns of latent infections are classified as type I, type IIa, type IIb, and type III.1 Recent work highlights the role of these EBV genes in the colonization of the B-cell pool and the establishment of persistent infection.3,4 These studies have led to the following scenario of the viral strategy to establish latency in the B-cell compartment: EBV first infects naïve B cells and activates a growth program in these cells (also termed latency III), which is characterized by expression of nine latent viral genes. A fraction of these cells will be driven into germinal center (GC) reactions and will express only three latent viral genes (default program or latency II). In proliferating GC B cells, the process of somatic hypermutation, which modifies the DNA of the variable region of immunoglobulin (Ig) genes, is activated, and GC B cells finally differentiate into memory B cells or plasma cells. The virus thus gains access to the memory B-cell compartment, its main reservoir during persistence, where no latent viral genes are expressed.5 An exception occurs when the latently infected memory cells divide, in which case they express the EBNA-1 protein (latency I), thereby allowing viral DNA to replicate.5
However, the scenario discussed above for persistent infection does not consequently hold true for primary infection associated with infectious mononucleosis. In this condition there is no indication for a preferential infection and clonal expansion of naïve B cells.6,7 Apparently, GC and/or memory B cells can be directly infected and it is these cells that clonally expand preferentially. In addition, EBV is associated with a number of tumors, in particular B-cell lymphomas, but the viral and host factors necessary for the development and control of EBV-associated malignancies are still poorly understood.8,9 EBV infects most humans by adulthood, yet the overwhelming majority of EBV-infected individuals do not suffer from EBV-associated malignancies. The degree of immunosuppression is one risk factor for EBV-infected B-cell lymphomas, as demonstrated by the increased frequency of EBV-associated lymphomas in patients aggressively treated with immunosuppressive drugs after organ transplantation8 and in patients with poorly controlled or more advanced stages of AIDS.10 However, immunosuppression alone is not sufficient for EBV-induced lymphomagenesis, because EBV-induced B-cell lymphomas still develop in only a minority of immunosuppressed transplant patients or patients with AIDS. Furthermore, EBV-associated lymphomas occur in patients without overt immunosuppression (eg, Burkitt’s lymphoma and Hodgkin’s lymphoma).
Existing models for studying EBV infection are limited in their ability to address the multiple factors leading to the development and control of EBV-associated malignancies in vivo. Tissue culture systems are valuable for dissecting molecular pathways usurped by specific viral genes to immortalize B-cell growth,11,12 but tissue culture systems are unable to address why some EBV-infected individuals develop malignancies whereas others do not. Inoculation of severe combined immunodeficient (SCID) mice with EBV-infected B cells can produce tumors in a small animal model.13,14 In this model system, host cells are never infected with EBV, and tumorigenesis in the immunodeficient animal is inevitable if sufficient numbers of EBV-infected B cells are inoculated. In addition, the development of large B-cell lymphomas has been observed in nonobese diabetic SCID (NOD/SCID) mice reconstituted with human CD34+ cells after EBV infection in vivo.15 It should be considered, however, that the preponderance of human cells in these mice are B cells and that T cells are lacking.
We recently described the use of a different strain of immunodeficient Rag2−/− γc−/− mice in which transplanted human CD34+ cells gave rise to human T cells in addition to human B cells.16,17 We used this model to better define the strategy of EBV infection in lymphoid B cells and to compare results with different conditions of EBV infections in humans.
Materials and Methods
Cord Blood Samples
Human cord blood was obtained from healthy full-term newborns with written parental informed consent, with the approval of the local ethical board. CD34+ cells were enriched using immunomagnetic beads (Miltenyi Biotec, Gladbach, Germany), as described.16,18 Cells were either frozen or transplanted immediately.
Animal Model
Human CD34+ cell reconstituted mice were generated as described in accordance with the guidelines of the Institute for Research in Biomedicine, Bellinzona, animal facility.16,18 Rag2−/− γc−/− mice were originally kindly provided by M. Ito, Kawasaki, Japan. Fourteen mice were constituted with CD34+ cord blood cells obtained from four different donors. Twelve of them were infected at 12 to 20 weeks of age by intraperitoneal injection of 300 μl of B95-8 supernatant, containing 3.8 log10 (TCD50/ml)19 of EBV, and were subsequently sacrificed at 16 to 25 weeks of age. Two uninfected mice were used as controls. Percentages of human CD45+ cells, CD19+ B cells, and CD4+ and CD8+ cells were evaluated by fluorescence-activated cell sorting (FACSCalibur; Becton Dickinson Immunocytometry System, Mountain View, CA), as previously described (Table 1).16,18 Spleen and lymph nodes were collected from all mice and subjected to further evaluations.
Table 1.
Characteristics of Human Cord Blood Cell-Transplanted Mice
| Group | huCD45 PB engraftment %, age | Age at infection | Age at analysis | Spleen engraftment at analysis (FACS) | Spleen CD4/CD8 | Histology |
|---|---|---|---|---|---|---|
| 1 | 44.1% at 6 weeks | 12 weeks | 16 weeks | CD19: 59%; CD4: 4%; CD8: 8% | 1/2.2 | FH with GCs |
| 1 | 57.5% at 6 weeks | 12 weeks | 16 weeks | CD19: 49%; CD4: 5%; CD8: 13% | 1/2.6 | FH with GCs |
| 1 | 54.3% at 6 weeks | 12 weeks | 16 weeks | CD19: 57%; CD4: 3%; CD8: 20% | 1/6.6 | NDP |
| 1 | 55.9% at 6 weeks | 12 weeks | 16 weeks | CD19: 70%; CD4: 3%; CD8: 13% | 1/4.3 | NDP |
| 1 | 44.5% at 6 weeks | 12 weeks | 16 weeks | CD19: 76%; CD4: 3%; CD8: 10% | 1/3.3 | FH with GCs |
| 2 | 30% at 11 weeks | 12 weeks | 17 weeks | CD19: 2.94%; CD4: 2;64%; CD8: 10.9% | 1/4.2 | FH with GCs |
| 2 | 33.3% at 11 weeks | 12 weeks | 17 weeks | CD19: 5.83%; CD4: 7%; CD8: 9.31% | 1/1.3 | FH with GCs |
| 2 | 33% at 11 weeks | 12 weeks | 17 weeks | CD19: 23.2%; CD4: 1.93%; CD8: 17% | 1/8.8 | NDP |
| 3 | 51% at 9 weeks | 20 weeks | 25 weeks | CD19: 12.2%; CD4: 8.3%; CD8: 51.7% | 1/6.2 | NDP |
| 3 | 31% at 9 weeks | 20 weeks | 25 weeks | CD19: 13%; CD4: 6.3%; CD8: 29.5% | 1/4.7 | NDP |
| 4 | 29% at 7 weeks | 18 weeks | 25 weeks | CD19:15%; CD4: 3%; CD8: 30% | 1/10 | NDP |
| 4 | 13.6% at 7 weeks | 18 weeks | 25 weeks | CD19: 4%; CD4: 3.2%; CD8: 22% | 1/6.9 | FH with GCs |
| 4 | 8.4% at 7 weeks | Uninfected | 22 weeks | CD19: 1.3%; CD4: 8.5%; CD8: 3.8 | 1/0.4 | FH/few GCs |
| 4 | 12.7% at 7 weeks | Uninfected | 25 weeks | CD19: 0%; CD4: 2.4%; CD8: 0.5% | 1/0.2 | FH/few GCs |
Immunohistochemistry
Immunohistochemical studies were performed on 7- to 10-μm-thick consecutive tissue sections from each case, using microwave pretreatment of slides for antigen retrieval.20 A large panel of antibodies (Table 2), recognizing formalin-resistant epitopes of various antigens, was used in conjunction with the streptavidin-biotin method to visualize antibody binding.21 Double staining for LMP1 and EBNA2 as well as for EBNA2 and both CD20 and CD27 were also performed on consecutive sections as previously described.22
Table 2.
Panel of Antibodies Used for EBV Latent Gene Expression and Immunophenotyping of EBV-Infected Cells
| Antibodies | Source |
|---|---|
| Anti-CD3 | Neomarkers |
| Anti-CD4 | Menarini |
| Anti-CD8 | Neomarkers |
| Anti-CD20 | Neomarkers |
| Anti-IgM | DAKO |
| Anti-IgD | Neomarkers |
| Anti-CD21 | Neomarkers |
| Anti-CD30 | Neomarkers |
| Anti-CD27 | Novocastra |
| Anti-EBNA2 | DAKO |
| Anti-LMP1 | DAKO |
| Anti-ZEBRA | DAKO |
EBV RNAs in situ Hybridization
For in situ hybridization, 5-μm-thick sections were mounted and dewaxed. Two hundred μl of proteinase K (Boehringer-Mannheim, Mannheim, Germany), 3 μg/μl in 50 nmol/L Tris HCl, pH 6.0, were added for 30 minutes at 37°C. The slides were washed in pure water, dehydrated in ethanol, air-dried, and then incubated with 30 μl of EBV-oligonucleotides/fluorescein isothiocyanate complementary to the two nuclear EBER RNAs encoded by EBV (DAKO, Glostrup, Denmark) for 2 hours at 37°C. The slides were then washed and incubated with 150 μl of rabbit F(ab′) anti-fluorescein isothiocyanate/AP for 30 minutes. After being washed and buffered, slides were incubated with 10 ml of substrate solution containing 32 μl of 5-bromo-4-chloro-3-indolyl-phosphate (50 mg/ml) and 64 μl of nitroblue tetrazolium (50 mg/ml) for 2 hours at 37°C, and finally counterstained with fuchsine. EBV-positive sections from cases of Hodgkin’s disease served as positive controls, and EBV-negative lymphoid tissue provided negative controls. Slides negative for EBV RNAs control cases were tested for viability of total RNA using a poly d(T) probe.
Cell Counts
After the mice were sacrificed at 4, 5, and 7 weeks after infection, the number of EBERs, EBNA2-, and LMP1-expressing cells were determined on serial sections in cases characterized by follicular hyperplasia. The mean number of tagged cells in each case was obtained by counts performed in 10 randomly chosen high-power fields each comprising ∼96,162.5 μm2 (objective ×40, ocular ×10; Zeiss, Göttingen, Germany). Cell counts at 4, 5, and 7 weeks were analyzed using the Mann-Whitney nonparametric statistical test. A significance level of 95% (P < 0.05) was considered.
Laser Capture Microdissection (LCM)
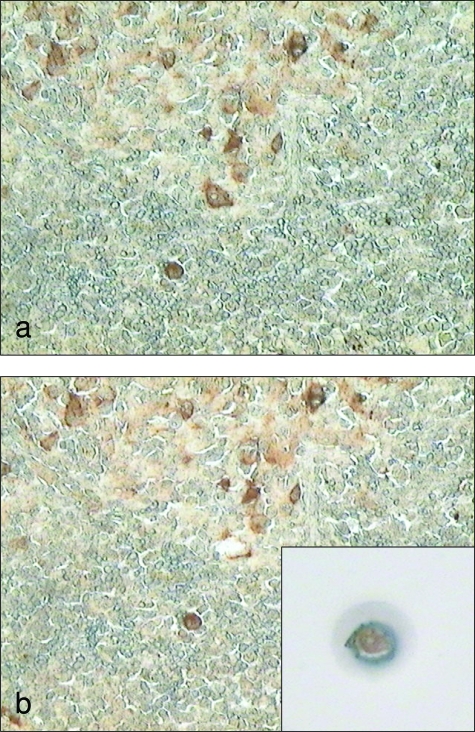
Among the 12 cases analyzed, four cases characterized by follicular hyperplasia and two cases characterized by nodular and diffuse proliferation were used for molecular analysis on single cells obtained by YCM. In particular, in cases characterized by follicular hyperplasia LMP1+, EBNA2+, and EBERs+ cells were microdissected from GCs, mantle, and interfollicular regions, respectively, in consecutive sections. In the cases showing nodular and diffuse lymphoid proliferation, double-positive LMP1/EBNA2 cells and only EBNA2+ cells were microdissected from nodular and diffuse areas (Figure 1, a and b). LCM was performed by means of an Arcturus PixCell II LCM system (MWG, Florence, Italy) on 3-μm-thick immunostained tissue taken from the previously described formalin-fixed paraffin-embedded tissue.22 Single cells were isolated and transferred to a polymerase chain reaction (PCR) test tube.
Figure 1.
LCM on EBNA2-positive EBV-infected B cells. These pictures show LCM on single EBNA2-positive cells. A snapshot of the whole field was performed before (a) and after (b) LCM. The single cell on the cap surface clearly shows the nuclear immunostaining for EBNA2 (b, inset). Original magnification: ×200 (a, b).
The single cells were dropped in 10 μl of PCR buffer (50 mmol/L KCl, 10mmol/L Tris-HCL, pH 8.4, 0.01% gelatin) containing 200 μg/ml of proteinase K (Qiagen, Hilden, Germany). The isolated cells were covered with 50 μl of mineral oil and digested at 37°C for 16 hours. Successful PCR from microdissected single cells was achieved in only 13.8% of cases with follicular hyperplasia and in 14% of cases with lymphoid proliferation. Microdissection was performed twice in different sections to collect more cells and confirm the results obtained.
PCR Amplification
PCR amplification of VH genes and direct sequencing of the PCR products were performed according to previously described methods.23 The IgH gene rearrangements of single cells LMP1+, EBNA2+, as well as LMP1+EBNA2+ and EBERs+, were amplified using a seminested PCR. Briefly, the first amplifications were performed using a consensus V region as a forward primer (FR2A), and a joining (J) consensus V region as a reverse primer (LJH). For re-amplification, the LJH primer was replaced by a nested consensus J region primer (VLJH), and 3 μl of the first round amplification product were used as a template. Primers (FR2A, LJH, VLJH) used for seminested PCR amplification were as follows: 5′-TGGRTCCGMCAGSCYYCNGG-3′ for FRIIA, 5′-TGAGGAGACGGTGACC-3′ for LJH, and 5′-GTGACCAGGGTNCCTTGGCCCCAG-3′ for VLJH. Both reactions were performed using AccuPrime high-fidelity TaqDNA polymerase (Invitrogen, Milan, Italy). To assess successful PCR amplification, 20 μl of each amplification reaction were separated by electrophoresis on a 2% agarose gel stained with ethidium bromide.
DNA Sequence Analysis
PCR products were subsequently sequenced in both directions using the re-amplification primers FR2A and VLJH. DNA sequence analysis was performed with an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA). Sequence alignments were performed by means of MEGA 3.1 software (www.megasoftware.net) (MEGA, Tempe, AZ). Only cases with a complete homology between both sequences were chosen for comparison with germline sequences from the ImMunoGeneTics information system (http://imgt.cines.fr) (ImMunoGeneTics, Montpellier, France) database. VH genes were considered mutated if they differed 2% or more from the corresponding germ line sequence.
Antigen Selection Analysis
The ratio of replacement to silent mutations (R/S) in the CDR2 and FWR3 regions was calculated, and a sequence was considered to be antigen-selected when the R/S ratio in the CDR2 region was higher than 2.9 and the R/S ratio in the FWR3 region was lower than 1.5.24 We also tested other types of calculations as described previously,25 but the calculation of a reliable P value failed in the cases with a very low number of somatic mutations. Therefore, the R/S calculation was considered the most appropriate for the cases in this study.
Results
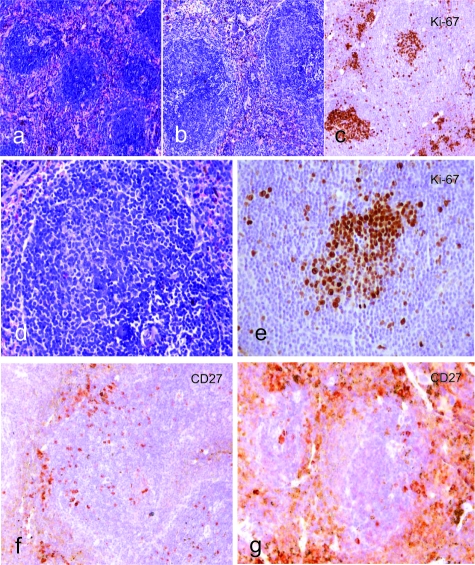
We analyzed the morphology of the lesions, the topographical distribution of the infected cells in lymphoid tissues, the type of EBV latency program, the immunophenotype of infected cells, and the immunoglobulin gene mutation status in EBV-infected cord blood cell-transplanted mice. The spleen and lymph nodes in control (uninfected) animals showed follicular hyperplasia with primary follicles and few GCs (Figure 2a). IgM+/IgD+ B cells prevailed in the inner part of the follicles, whereas a small number of CD27+ B cells were detected on the outermost surface (Figure 2f). T cells were predominantly restricted to the periarteriolar lymphatic sheet, although a few were scattered in the follicles.
Figure 2.
Comparison of lymphoid tissue morphology in uninfected and infected mice highlighted a different histological picture as well as CD27+ cell number and distribution. The white pulp of the spleen in uninfected mice showed follicular hyperplasia with primary follicles and few GCs (a, Giemsa). On the other hand, half of infected mice developed a follicular hyperplasia with florid GCs (b, Giemsa; d, Giemsa) as confirmed by Ki-67 immunostaining (c, d, e). Accumulation of CD27+ B cells at the outermost follicular periphery and in extrafollicular areas was observed in infected animals (g) as compared to the uninfected control mice (f). All images were acquired through Axiovision 4 software, by means of a Zeiss Axioskop 40 equipped with a Zeiss Axiocam H Rc. Original magnification: ×100 (a–c); ×200 (d–g).
Two outcomes of experimental infection were observed in the four groups of mice tested at different intervals after infection. One was characterized by follicular hyperplasia with florid GCs (Figures 2 and 3) and the other by nodular and diffuse lymphoid proliferation (Figure 4). Our results suggest that the outcome of EBV infection in Rag2−/− γc−/− cord blood-reconstituted mice does not depend on the source of CD34+ cord blood cells used and the level of engraftment of preinfection human peripheral blood, or the time from infection, but rather on the quality of the immune response against infected cells. Indeed, the only difference found among cases showing different morphology, immunophenotype, and molecular characteristics was the type of T-cell infiltration (Figure 5).
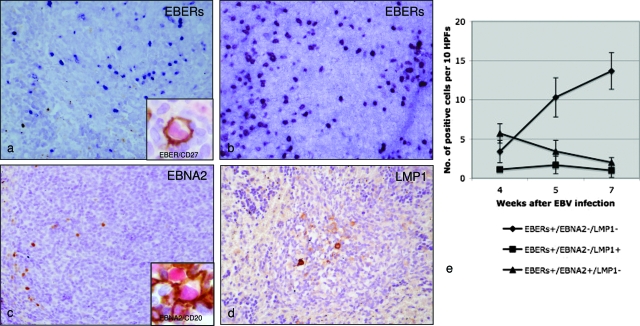
Figure 3.
Topographical distribution, latency gene expression, and cell counts of EBERs+-, EBNA2+-, and LMP1+-expressing cells at three different time points in cord-blood cell transplanted mice showing follicular hyperplasia. The comparison of in situ hybridization for EBERs performed at 5 and 7 weeks (a and b, respectively) showed an increase and relocalization of EBER-expressing cells in extrafollicular areas in mice sacrificed after 7 weeks. Double staining performed on serial sections for viral antigens revealed that these EBV-infected cells were either CD27+ (a, inset) and CD20+ and lack EBNA2 and LMP1 expression thus suggesting a type I latency program (b–d). d: A few CD20+-infected cells were also detected in the mantle zone and within the GCs (inset). They showed a staining consistent with a type IIb latency program (EBERs+/EBNA2+/LMP1−) in the mantle (c), whereas a viral phenotype consistent with a type IIa latency program (EBERs+/EBNA2−/LMP1+) was detected in GCs (d). e: Interestingly, we observed a statistically significant increase in EBERs+/EBNA2−/LMP1− extrafollicular cells in both mice analyzed between 4 and 5 weeks (Mann-Whitney test: P < 6.3 × 10−5) and mice analyzed between 5 and 7 weeks (Mann-Whitney test: P < 0.03) after infection. All images were acquired using Axiovision 4 software, by means of a Zeiss Axioskop 40 equipped with a Zeiss Axiocam H Rc. Original magnification: ×200 (a–d).
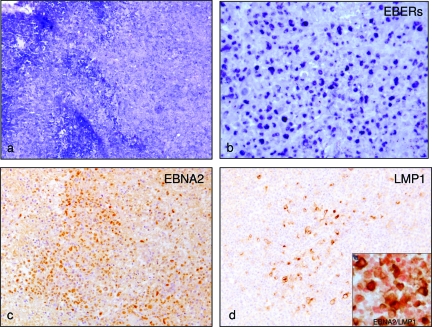
Figure 4.
Lymphoid tissue morphology, topographical distribution, and latent gene expression of EBV-infected cells in cord blood cell-transplanted mice showing nodular and diffuse proliferation. Nodular and diffuse lymphoid proliferation without presence of GCs was observed in half (n = 6) of the cases (a, Giemsa). Comparison of in situ hybridization for EBERs (b) with EBNA2 (c) and LMP1 staining (d) on serial sections highlighted that a fraction of the EBV-infected cells expressed a type III EBV latency program (EBERs+/EBNA2+/LMP1+) even though a considerable number of cells also showed a type IIb latency program (EBERs+/EBNA2+/LMP1−). This was also confirmed by double staining with EBNA2 and LMP1 (d, inset). All images were acquired using Axiovision 4 software, by means of a Zeiss Axioskop 40 equipped with a Zeiss Axiocam H Rc. Original magnification: ×100 (a); ×200(b–d).
Figure 5.
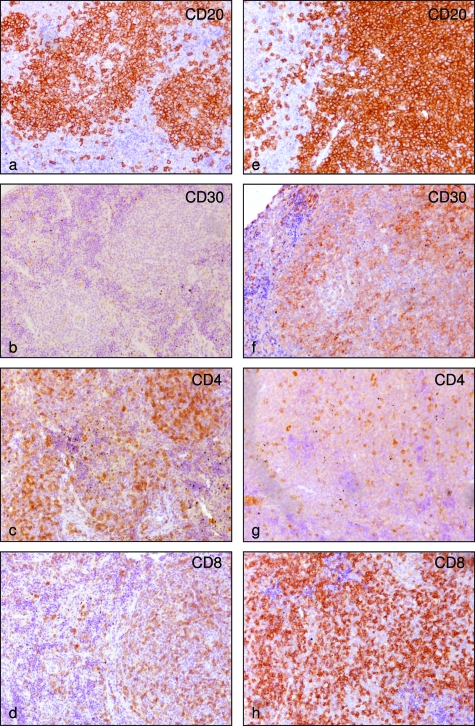
Immunophenotype of EBV-infected cells in cord blood cell-transplanted mice. a: CD20 immunostain highlighting follicular hyperplasia and scattered B cells in the interfollicular areas. b: Few CD30+ cells were identified in the GCs. c and d: The relative numbers of CD4+ and CD8+ cells appear normal (both 10X/0.25). e and f: On the contrary, EBV-infected cells in cases with nodular and diffuse proliferation were CD20+ (e) CD30+ (f, 10×/0.25). g and h: Moreover a marked preponderance of CD8+ T cells is obvious (both 10×/0.25). Original magnification: ×100 (all).
In particular, six cases were characterized by follicular hyperplasia (Figure 2b), with florid GCs (Figure 2, c–e) and few blast cells in the extrafollicular areas of the lymph nodes, as well as in the white pulp of the spleen. According to CD27+ B-cell distribution in human lymphoid organs,26,27 we found accumulations of CD27+ B cells at the outermost follicular periphery, outside a CD27− region corresponding to the mantle zone of splenic follicles (Figure 2g). In situ hybridization for EBERs showed that most of the EBV-infected cells (small to medium-sized lymphoid cells) were localized at the follicular periphery and scattered in the extrafollicular areas (Figure 3, a and b). Double staining performed on serial sections for viral antigens revealed that these EBV-infected cells were either CD27+ (Figure 3a, inset) and CD20+ and lack EBNA2 and LMP1 expression thus suggesting a type I latency program (Figure 3, b–d). A few CD20+-infected cells were also detected in the mantle zone and within the GCs (Figure 3c, inset). They showed a staining consistent with a type IIb latency program (EBERs+/EBNA2+/LMP1−) in the mantle (Figure 3c), whereas a viral phenotype consistent with a type IIa latency program (EBERs+/EBNA2−/LMP1+) was detected in the GCs (Figure 3d). No cells expressing ZEBRA antigen indicative of a productive lytic infection were detected. Interestingly, we observed a statistically significant increase in EBERs+/EBNA2−/LMP1− extrafollicular cells in both mice analyzed between 4 and 5 weeks (Mann-Whitney test: P < 6.3 × 10−5) and mice analyzed between 5 and 7 weeks (Mann-Whitney test: P < 0.03) after infection (Figure 3e). In these cases T cells were spread over the periarteriolar lymphatic sheet and scattered in the follicles. Among them, the relative distribution of CD4+ (Figure 5c) and CD8+ (Figure 5d) T cells appeared normal and was calculated to be ∼1:3 based on fluorescence-activated cell sorting analysis of spleen cells (Table 1).
Single cell immunoglobulin gene analysis of infected cells revealed the absence of somatic mutation in the mantle cells, suggesting that these are in fact naïve unmutated B cells, but also revealed the presence of mutations in EBV-infected cells in the GCs and in the interfollicular areas (Table 3). All sequences were unique, without evidence of clonal relationships. The average mutation frequency of EBV-infected cells in the GC and in extrafollicular areas was 1.7 and 3.5, respectively. Moreover, the estimation of antigen selection in the cells located in extrafollicular areas showed that the mutation pattern in their Ig VH genes suggests antigen-driven selection for expression of a functional B-cell receptor in three of seven cells. In fact, all rearrangements of those cells had a R/S in the CDR2 >2.9 and in FRWR3 <1.5. In these cases the cells preferentially rearranged genes of the VH3 family, the most represented VH gene family in the normal B-cell repertoire, without evidence of significant biased usage.24
Table 3.
Molecular Analysis of IgH Genes of Single EBV-Infected Cells in Cases Characterized by Follicular Hyperplasia
| Cells isolated | EBV latent gene expression | Total sequences | Mutated/total | AMF % (range) | Antigen selected/mutated | |
|---|---|---|---|---|---|---|
| MCs | 65 | EBERs+/EBNA2+/LMP1− | 10 | 0/10 | 0 | 0 |
| GCCs | 64 | EBERs+/EBNA2−/LMP1+ | 9 | 9/9 | 1.7 (5 to 9) | 0/9 |
| ECs | 85 | EBERs+/EBNA2−/LMP1− | 7 | 7/7 | 3.5 (7 to 18) | 3/7 |
The other six cases analyzed showed instead a nodular and diffuse lymphoid proliferation without the presence of GCs in both the spleen and the lymph nodes. The lymphoid proliferation was composed of polymorphous cells in some cases and in other cases of more monomorphous, large lymphoid cells with areas of necrosis (Figure 4a). The EBV-infected cells were large cells with immunoblastic morphology or Hodgkin-like morphology. Immunohistochemistry and in situ hybridization analysis on consecutive sections support a latency gene expression consistent with a type III EBV latency program (EBERs+/EBNA2+/LMP1+) (Figure 4, b–d). However, a considerable fraction of cells also express a type IIb latency program (EBERs+/EBNA2+/LMP1−), as confirmed by double staining for EBNA2 and LMP1 (Figure 4d, inset). The immunophenotype of EBV-infected cells was CD20+ (Figure 5e), CD30+ (Figure 5f), CD10−, BCL6−, CD27− (not shown). The CD4+/CD8+ T-cell ratio in these cases was clearly altered, with a preponderance of CD8+ T cells (Figure 5, g and h), and was calculated to be ∼1:7 based on fluorescence-activated cell sorting analysis of spleen cells (Table 1).
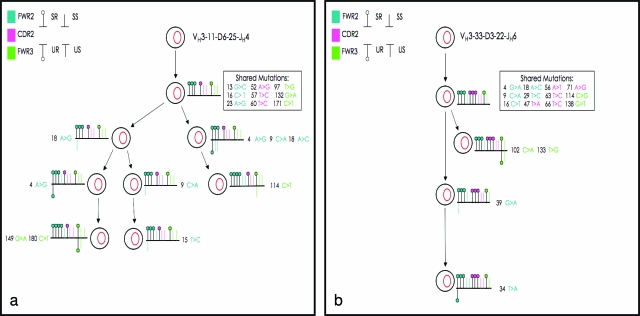
Molecular analysis of single EBERs+/EBNA2+/LMP1− cells showed that they were preferentially unmutated naïve B cells. All sequences were unique, without evidence of clonal relationships (Table 4). On the other hand, the immunoglobulin gene analysis of single EBERs+/EBNA2+/LMP1+ cells demonstrated that there was no preferential infection of unmutated or mutated B cells. In fact these infected cells showed either a high number of somatic mutations or no mutation (Table 4). In addition, we found oligo-clonal expansion of both unmutated and mutated infected EBERs+/EBNA2+/LMP1+ B cells. In particular, of the 20 productive VH gene rearrangements obtained in case 1, four were unique and three were shared by three different groups of cells comprising three, six, and seven cells, respectively. In case 2 the three VH gene rearrangements obtained were nonunique and belonged to three different groups, two of which were composed of four cells and one of seven cells. Interestingly, the same VH rearrangements could be identified in the nodular and diffuse areas, as well as on the different slides analyzed. Furthermore, intraclonal diversity was detected among the B cells carrying somatically mutated VH genes, suggesting an ongoing hypermutation process without signs of antigen selection (Figure 6, a and b). In both cases analyzed the cells again preferentially rearranged genes of the VH3 family (the most represented VH gene family in the normal B-cell repertoire), without evidence of significant biased usage.24
Table 4.
Molecular Analysis of IgH Genes of Single EBV-Infected Cells in Cases Characterized by Nodular and Diffuse Proliferation
| Case | Cells isolated | EBV latent gene expression | Total sequences | Mutated/total | AMF % (range)* | Clones identified/total | Clones with mutated V genes/total clones | Antigen selected/mutated | Intraclonal V gene diversity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | EBERs+/EBNA2+/LMP1− | 10 | 1/10 | 3.8 | 0/10 | 0/0 | 0/0 | No |
| 140 | EBERs+/EBNA2+/LMP+ | 20 | 7/20 | 5.1 (10 to 13) | 3/20 | 1/3 | 0/7 | Yes | |
| 2 | 47 | EBERs+/EBNA2+/LMP1− | 4 | 8.4 | 0/4 | 0/0 | 0/0 | No | |
| 110 | EBERs+/EBNA2+/LMP+ | 15 | 3/15 | 4.6 (13 to 14) | 3/15 | 1/3 | 0/3 | Yes |
Figure 6.
Clonal relationship between EBV-infected B cells in two cases with nodular and diffuse proliferation. IgH sequence analysis in the first case revealed a group of seven clonally related cells (a), whereas four clonally related cells were identified in the second case (b). In the diagram the putative progenitors are placed at the top, and the IgH gene rearrangement of the clonally related cells deriving from each progenitor indicated above. Shared point mutations and acquired unique mutations are indicated above and below the line, respectively. Vertical bars depict S mutations, and lollipops depict R mutations. S and R mutations occurring in FWR2, CDR2, and FWR3 are represented in blue, pink, and green colors, respectively.
Discussion
Here we have used Rag2−/− γc−/− mice transplanted with human CD34+ cord blood cells as newborns as an in vivo model to better define the strategy of EBV infection in human B cells and to compare with different conditions of EBV infection in humans. According to recent studies, EBV first infects naïve B cells and activates a growth program in these cells so that they can differentiate into resting memory B cells through the process of the GC reaction. However, the relation between such cells has been inferred from parallels with the biology of normal B cells and so far has not been demonstrated experimentally. In fact, EBV is undetectable in the self-renewing peripheral CD5+ or B1 cells, a subset that has not been through a GC, whereas no such restriction was observed in tonsillar B cells. Therefore, the virus has access to a range of B-cell subsets in the lymphoid tissues but it is tightly restricted to a specific long-lived compartment of B cells, ie, the IgD+, CD27+, and CD5− memory B cells in the periphery.28 This suggests that access to this compartment is essential to allow the growth-promoting latent genes to be switched off, to create a site of persistent infection that is neither pathogenetic nor a target for immunosurveillance. Here, in an experimental animal model, we provide independent, corroborating evidence that this occurs through the GC reaction and that it depends on T-cell response. In fact, in the four groups of mice tested, we found two outcomes of experimental infections, one characterized by follicular hyperplasia with GC formation and the other characterized by nodular and diffuse lymphoid proliferation without GC formation. Indeed, the only difference found among cases showing different morphology, immunophenotype, and molecular characteristics was the type of T-cell infiltration.
In cases characterized by follicular hyperplasia and a relatively normal CD4+ and CD8+ T-cell distribution, EBV-infected cells were scattered in the extrafollicular areas, whereas a few infected cells were also detected in the mantle zone and within the GCs. From the analysis of serial sections immunostained for viral antigens LMP1 and EBNA2 and molecular analysis of single EBV-infected cells, we found that EBERs+/EBNA2+/LMP1− cells in the follicle mantle did not carry somatic mutations in the immunoglobulin genes, suggesting that they are in fact naïve infected B cells. EBNA2 is the first latent protein expressed, driving the cell from G0 to G1 and activating the promoters necessary to produce all nine of the latent proteins expressed in the growth program.29,30 The result is that infected normal B cells become activated lymphoblasts and begin to proliferate. A fraction of these cells will be driven into GC reactions in which EBNA2 is turned off by methylation and the cell assumes a GC phenotype and expresses the default program.31,32 This is in line with our observation that only a few infected cells were also identified within the GC, showed latency gene expression consistent with a type IIa latency program (EBERs+/EBNA2−/LMP1+), and carried few mutations in the immunoglobulin genes. Finally, these infected cells may become resting memory B cells, migrate to the extrafollicular areas where they are found only to express EBERs, to be increased in number in late stages of infection, and to carry somatic mutations of immunoglobulin genes, as confirmed by our molecular studies. Similarities with latent EBV infection of B cells have been found in mice infected with MHV68,33,34 although the access of MHV68 to the memory B-cell compartment seems to be more reliant on conventional B-cell activation and differentiation pathways than is the case for EBV.35 In fact, numerous GC B cells positive for the virus can be found in such mice, unlike the situation with EBV in our models. However, it should be considered that because growth regulation by EBV is a self-regulating process, a large expansion of EBV-infected cells would not be expected in the GC. Alternatively our findings may depend on the analysis being performed at time points after infection. Even other possibilities cannot be completely excluded; for example that the virus can also directly infect some memory B cells. This has been reported in infectious mononucleosis, in which EBV has been found preferentially in B cells with somatically mutated V genes.36,37
In contrast, the other six cases analyzed showed a nodular and diffuse lymphoid proliferation without the presence of GCs in both the spleen and the lymph nodes. The lymphoid proliferation was composed of polymorphous cells in some cases and in others of more monomorphous, large lymphoid cells with areas of necrosis. The EBV-infected cells were large cells with immunoblastic morphology or Hodgkin’s-like morphology, similar to that observed in vivo in infectious mononucleosis or in lymphoproliferative disorders in immunocompromised patients. Intriguingly, in these cases the CD4+/CD8+ T cell ratio was clearly altered, with a preponderance of CD8+ T cells. However, these seem unable to control the expansion of infected cells, at least at the time points analyzed, probably reflecting immunosuppression in humans. Human T cells in Rag2−/− γc−/− mice mount some responses to in vivo EBV infection, as suggested by the preponderance of in situ CD8+ T cells reported here, the CD4+/CD8+ ratio inversion and ex vivo CD8+ T-cell proliferation reported previously.16 It should be considered that, as human T-cell development occurs on a mouse background in this system, insufficient T-cell responses might in part be a result of suboptimal T-cell selection and maintenance. Thus, it is important to determine the EBV epitope and MHC specificity of T cells in future studies.17
In these cases, we found a considerable fraction of cells expressing only EBNA2 (type IIb latency program EBERs+/EBNA2+/LMP1−) and carrying preferentially unmutated immunoglobulin genes without evidence of clonal expansion. In addition, cells co-expressing LMP1 and thus supporting latency gene expression consistent with a type III latency program (EBERs+/EBNA2+/LMP1+) were also identified. The immunoglobulin gene analysis of these cells showed that they were unmutated and mutated B cells with evidence of a clonal expansion. Furthermore, ongoing somatic hypermutations could be demonstrated among the B cells carrying somatically mutated VH genes. The proportion of naïve and pre-existing memory B cells in the lymphoid tissues of controls as compared to the infected animals further suggests that these mutated genotypes may arise in naïve cells activated into somatic hypermutation by EBV.
Because no GC structures were found in these cases, it can be argued that the virus can induce somatic hypermutation outside the GC and that the viral genes rather than the antigens provide the signals required to activate the mutation program. These results are in accordance with recent data showing the expression of somatic hypermutation-inducing molecules in human B cells infected with EBV.36 This may well represent a potential new mechanism for establishing a long-term carrier state. However, there is no evidence for any such events occurring in human tissues. In the study by Kurth and colleagues7 EBNA2-driven expansions were observed inside and outside GCs, but there was no ongoing mutation. From our data, the possibility that (some of) these cells had been infected inside GCs cannot be completely ruled out because GCs may vanish during the course of the infectious process (as occurs in infectious mononucleosis), or may be effaced by the proliferation of these infected cells. Yet, the EBV-infected cells did not show the immunophenotype either of GC or memory B cells, and no signs of antigen selection were found in their immunoglobulin genes.
A comprehensive understanding of how EBV persists in vivo may provide insights into the origin of EBV-associated diseases. In this study, we were able to identify all of the types of latent infections found in EBV-associated malignant lymphomas. In fact the infected cells in the extrafollicular areas showed latency gene expression consistent with a type I latency program (EBERs+/EBNA2−/LMP1−), as observed in resting memory B cells of EBV carrier individuals and in Burkitt lymphoma in vivo.37 On the other hand, the infected cells found in the mantle and around the GC expressed a viral phenotype consistent with a type IIb latency program (EBERs+/EBNA2+/LMP1−). This is an interesting finding because this type of EBV latency program has so far only been described in B-CLL cells infected with EBV in vitro38,39 and in human infectious mononucleosis tissues for EBV+ B cells in the GC.7 In addition, the few infected cells identified within the GC showed latency gene expression consistent with a type IIa latency program (EBERs+/EBNA2−/LMP1+), as is usually seen in patients with Hodgkin’s lymphoma.40 Finally, a viral phenotype consistent with a latency III program was observed in cases characterized by diffuse proliferation of large, pleomorphic B cells, similar to posttransplant lymphoproliferative disorders.
In conclusion, the data presented here gives evidence of the different strategies of EBV infection in B cells in vivo that probably corresponds to different conditions of EBV infections in humans, which depends on the efficacy of the immune response in controlling the expansion of EBV-infected cells. Furthermore, in future experiments, it will be interesting to test this model for EBV infection throughout extended periods of time and to assess the contribution of each EBV latent gene by means of mutated EBV strains. These experiments might allow us to dissect mechanisms responsible for EBV persistent infection and hopefully provide targets for higher tailored therapies.
Acknowledgments
We thank the staff of Ospedale San Giovanni (Bellinzona, Switzerland) for cord blood collection.
Footnotes
Address reprint requests to Lorenzo Leoncini, Department of Human Pathology and Oncology, Division of Pathological Anatomy, University of Siena, Via delle Scotte n°6, 53100 Siena, Italy. E-mail: leoncinil@unisi.it.
Supported in part by Ministero deII’Università e della Ricerca (to L.L.), the Fondazione Monte dei Paschi di Siena Foundation (to L.L.), the University of Siena (to L.L.), the Swiss National Science Foundation (grant 3100A0-102221 to M.G.M.), and the Bill and Melinda Gates Foundation (to M.G.M.).
Current address of R.T.: Department of Biomedicine, Division of Developmental and Molecular Immunology, University of Basel, Basel, Switzerland; and E.T.: Department of Pediatric Science, “G. Gaslini” Institute, Genova, Italy.
References
- Cohen JI. Clinical aspects of Epstein-Barr virus infection. Robertson ES, editor. Norfolk: Caister Academic Press,; Epstein-Barr Virus. 2005:pp 35–54. [Google Scholar]
- Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origin of the associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci USA. 2000;97:12250–12255. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth J, Spieker T, Wustrow J, Strickler GJ, Hansmann LM, Rajewsky K, Kuppers R. EBV-infected B-cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13:485–495. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- Kurth J, Hansmann ML, Rajewsky K, Kuppers R. Epstein-Barr virus-infected cells expanding in germinal centers of infectious mononucleosis patients do not participate in the germinal center reaction. Proc Natl Acad Sci USA. 2003;100:4730–4735. doi: 10.1073/pnas.2627966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, Crawford DH. The role of EBV in post-transplant malignancies: a review. J Clin Pathol. 2000;53:248–254. doi: 10.1136/jcp.53.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Hotchin NA, Allday MJ, Amlot P, Rose M, Yacoub M, Crawford DH. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990;49:944–953. doi: 10.1097/00007890-199005000-00022. [DOI] [PubMed] [Google Scholar]
- Levine AM. AIDS-related malignancies: the emerging epidemic. J Natl Cancer Inst. 1993;85:1382–1397. doi: 10.1093/jnci/85.17.1382. [DOI] [PubMed] [Google Scholar]
- Rowe M, Rowe DT, Gregory CD, Young LS, Farrel PJ, Rupani H, Rickinson AB. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gregory C, Sample C, Rowe M, Liebowitz D, Murray R, Rickinson A, Kieff E. Epstein-Barr virus latent membrane protein (LMP1) and nuclear protein 2 and 3C are effectors of phenotype changes in B lymphocytes: EBNA2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong GK, Gulley ML, Feng WH, Delecluse HJ, Holley-Guthrie E, Kenney SC. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease on a SCID mouse model. J Virol. 2005;79:13993–14003. doi: 10.1128/JVI.79.22.13993-14003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzari PL, de Totero D, Bolognesi A, Testoni N, Pileri S, Roncella S, Stein H, Gobbi M, Stirpe F. An Epstein-Barr virus-infected lymphoblastoid cell line (D430B) that grows in SCID-mice with the morphologic features of a CD30+ anaplastic large cell lymphoma, and is sensitive to anti-CD30 immunotoxins. Haematologica. 1999;84:988–995. [PubMed] [Google Scholar]
- Islas-Ohlmayer M, Padjett-Thomas A, Domiati-Saad R, Melcus MW, Cravens PD, Martin M, del P, Netto G, Garcia JV. Experimental infection of NOD/SCID mice reconstituted with human CD34+ cells with Epstein-Barr Virus. J Virol. 2004;78:13891–13900. doi: 10.1128/JVI.78.24.13891-13900.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26:537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Baenziger S, Tussiwand R, Schlaeper E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, Aguzzi A, Manz MG, Speck R. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/− γc−/− mice. Proc Natl Acad Sci USA. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DJ, Pope JH. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972;17:233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Leoncini L, Del Vecchio MT, Spina D, Megha T, Barbini P, Sabattini E, Pileri S, Tosi P, Kraft R, Laissue JA, Cottier H. Presence of the bcl-2 protein and apoptosis in non-Hodgkin lymphomas with diffuse growth pattern. Int J Cancer. 1995;61:826–831. doi: 10.1002/ijc.2910610614. [DOI] [PubMed] [Google Scholar]
- Falini B, Abdulaziz Z, Gerdes J, Canino S, Ciani C, Cordell JL, Knight PM, Stein H, Grignani F, Martelli MF, Mason DY. Description of a sequential staining procedure for double immunoenzymatic staining of pairs of antigens using monoclonal antibodies. J Immunol Methods. 1986;93:265–273. doi: 10.1016/0022-1759(86)90199-7. [DOI] [PubMed] [Google Scholar]
- Bellan C, De Falco G, Lazzi S, Micheli P, Vicidomini S, Schürfeld K, Amato T, Palumbo A, Bagella L, Sabattini E, Bartolommei S, Hummel M, Pileri S, Tosi P, Leoncini L, Giordano A. CDK9/CYCLIN T1 expression during normal lymphoid differentiation and malignant transformation. J Pathol. 2004;203:946–952. doi: 10.1002/path.1588. [DOI] [PubMed] [Google Scholar]
- Lazzi S, Bellan C, Tiacci E, Palummo N, Vatti R, Oggioni M, Amato T, Schurfeld K, Tonini T, Tosi P, Falini B, Leoncini L. IRTA1+ monocytoid B cell in reactive lymphadenitis show a unique topographic distribution and immunophenotype and a peculiar usage and mutational pattern of IgVH genes. J Pathol. 2006;209:56–66. doi: 10.1002/path.1944. [DOI] [PubMed] [Google Scholar]
- Klein U, Goossens T, Fischer M. Somatic hypermutation in normal and transformed B cells. Immunol Rev. 1998;162:261–280. doi: 10.1111/j.1600-065x.1998.tb01447.x. [DOI] [PubMed] [Google Scholar]
- Tamaru J, Hummel M, Marafioti T, Kalvelage B, Leoncini L, Minacci C, Tosi P, Wright D, Stein H. Burkitt’s lymphomas express VH genes with a moderate number of antigen-selected somatic mutation. Am J Pathol. 1995;147:1398–1407. [PMC free article] [PubMed] [Google Scholar]
- Steiniger B, Timphus EM, Barth PJ. The splenic marginal zone in humans and rodents: an enigmatic compartment and its inhabitants. Histochem Cell Biol. 2006;126:641–648. doi: 10.1007/s00418-006-0210-5. [DOI] [PubMed] [Google Scholar]
- Steiniger B, Timphus EM, Jacob R, Barth PJ. CD27+ B cells in human lymphatic organs: re-evaluating the splenic marginal zone. Immunology. 2005;116:429–442. doi: 10.1111/j.1365-2567.2005.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MA, Babcock GJ, Thorley-Lawson DA. EBV persistence involves strict selection of latently infected B cells. J Immunol. 2000;165:2975–2281. doi: 10.4049/jimmunol.165.6.2975. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Palmero I, Peters J, Farrell PJ. EBNA2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E, Rickinson AB. Epstein-Barr virus and its replication. Knipe DM, Howley Kieff PM, editors. New York: Lippincott Williams and Wilkins,; Fields Virology. (ed 4) 2001:pp 2511–2574. [Google Scholar]
- Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptotic-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–977. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GJ, Hochberg D, Thorley-Lawson DA. The expression pattern of Epstein-Barr virus latent gene in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- Olivadoti M, Toth LA, Weinberg J, Opp MR. Murine gammaherpesvirus68: a model for the study of Epstein-Barr virus infections and related diseases. Comp Med. 2007;57:44–50. [PubMed] [Google Scholar]
- Willer O, Speck SH. Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40. J Virol. 2005;79:2891–2899. doi: 10.1128/JVI.79.5.2891-2899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten DC, Sparks-Thissen RL, Kreher S, Speck SH, Virgin HW., IV An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection. J Virol. 2005;79:2573–2583. doi: 10.1128/JVI.79.4.2573-2583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epeldegui M, Ping Hung Y, McQuay A, Ambinder RF, Martinez-Maza O. Infection of human B cells with Epstein-Barr virus results in the expression of somatic hypermutation-inducing molecules and in the accrual of oncogene mutations. Mol Immunol. 2007;44:934–942. doi: 10.1016/j.molimm.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2004;101:239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Bandobashi K, Nagy N, Teramoto N, Gogolák P, Pokrovskaya K, Székely L, Björkholm M, Klein G, Klein E. Epstein-Barr virus can infect B-chronic lymphocytic leucemia cells but it does not orchestrate the cell cycle regulatory proteins. J Hum Virol. 2001;4:227–237. [PubMed] [Google Scholar]
- Teramoto N, Gogolak P, Nagy N, Maeda A, Kvarnung K, Björkholm T, Klein E. Epstein-Barr virus-infected B-chronic lymphocyte leukemia cell express the virally encoded nuclear proteins but they do not enter the cell cycle. J Hum Virol. 2000;3:125–136. [PubMed] [Google Scholar]
- Küppers R. Molecular biology of Hodgkin’s lymphoma. Adv Cancer Res. 2002;84:277–312. doi: 10.1016/s0065-230x(02)84009-x. [DOI] [PubMed] [Google Scholar]