Motor Learning Induces Astrocytic Hypertrophy in the Cerebellar Cortex (original) (raw)
. Author manuscript; available in PMC: 2008 Oct 29.
Published in final edited form as: Behav Brain Res. 2007 Jan 25;178(2):244–249. doi: 10.1016/j.bbr.2006.12.022
Abstract
Motor skill learning, but not mere motor activity, is associated with an increase in both synapse number and glial cell volume within the cerebellar cortex. The increase in synapse number has been shown to persist for at least four weeks in the absence of continued training. The present experiment similarly examined how a prolonged interruption in training affects the training-induced increase in astrocytic volume. Adult female rats were randomly allocated to either an Acrobatic motor learning Condition (AC) or a Motor Control condition (MC). The AC animals were trained to traverse a complex series of obstacles and each AC animal was pair matched with an MC animal that traversed an obstacle-free runway. These groups were further assigned to one of three training conditions. Animals in the EARLY condition were trained for ten consecutive days, animals in the DELAY condition received the same ten days of training followed by a 28 day period without training, and animals in the CONTINUOUS condition were trained for the entire 38 days. Unbiased stereological techniques were used to determine that AC animals had a significantly greater volume of astrocytes per Purkinje cell in the cerebellar paramedian lobule than the MC animals, a difference which was reduced (and not statistically detectable) among animals in the DELAY condition. These findings demonstrate that learning triggers the hypertrophy of astrocytic processes and furthermore that, unlike learning-induced synaptogenesis, astrocytic growth is reduced in the absence of continued training.
Keywords: Bergmann Glia, Cerebellum, Motor Training, Astrocyte, Plasticity
INTRODUCTION
The brain is remarkably plastic in response to experience, and a growing body of work is continuing to elaborate the ways by which experience can modify the morphology of neurons, glial cells, myelination, and the brain’s vascular elements [reviewed by 35]. Motor skill learning is an experience that induces synaptogenesis in higher-order brain regions involved in motor learning, such as the motor cortex [31,32] and cerebellum [2,8,33,34]. This proliferation of synapses is not merely the result of increased locomotor activity, as animals that engage in unskilled motor movements (such as on a running wheel) do not show any changes in the number of synapses in these brain areas [8,32–34].
Synapses consist of three components including the pre- and post-synaptic elements and the surrounding astroglial ensheathment [see 53]. Synaptic and astrocytic membranes are often separated by as little as 10 nm, creating a distinct synaptic micro-environment that is highly influenced by astrocytic function. Because astrocytes can regulate concentrations of various neurotransmitters (including glutamate) and ions within this environment, astrocytic function can dramatically modulate the efficacy of synapses [4,37,40], including those on Purkinje cells in the cerebellar cortex [46,47]. In addition to releasing and sequestering various neurotransmitters, astrocytes themselves express membrane receptors for glutamate, GABA, a variety of voltage-dependent ion channels [reviewed by 52], and multiple purinergic receptors [19], such that the communication between astrocytes and neurons at the synapse is routinely bidirectional. For instance, synaptic activity can trigger a calcium spike in nearby astrocytes that spreads from cell to cell [16,18] and conversely, electrical stimulation of astrocytes can induce a rise in cytosolic calcium of neighboring neurons [30,39]. In the cerebellum, parallel fiber stimulation induces plasticity in Bergmann glia extrasynaptic currents [5], and stimulation of Bergmann glia has relatively long-lasting consequences for excitatory postsynaptic currents in Purkinje cells [9,11]. The morphology of astrocytes is also sensitive to changes in neuronal activity; for instance, hypertrophy of astrocytic processes has been observed following the induction of long term potentiation [54] and kindling [22].
Several experiments have suggested complementary experience-driven changes in glial and synaptic morphology. In the visual cortex, for example, glial atrophy has been observed following developmental visual deprivation [23,38], a manipulation which similarly reduces synapse number [17,55]. Conversely, the increase in synapse number observed following housing in a complex environment [10,49] is associated with glial cell proliferation [1], increased surface density of GFAP positive astrocytic processes [29,44] and increased astrocytic ensheathment of synapses [28]. Finally, using a motor learning paradigm that had previously been shown to increase synapse number within the cerebellar cortex [8], Anderson and colleagues [3] demonstrated that the increased synapse number was accompanied by a proportional increase in the volume of glia per Purkinje cell (so the volume of glia per synapse was maintained). In contrast, glial volume was unchanged in rats that exercised in a running wheel or on a treadmill over the same period, demonstrating that, as with the previously observed synaptic changes, the change in glial volume was not attributable to locomotor activity alone. We have shown that the learning-dependent increase in synapse number within the cerebellar cortex persists in the absence of continued training for at least four weeks [34]. In the present study, we similarly examined the persistence of increased glial volume within the cerebellar cortex one month after the cessation of training.
MATERIALS AND METHODS
Behavioral Training
Forty-eight female Long Evans hooded rats (age 3–4 months) were randomly assigned to either an Acrobatic Condition (AC) or a Motor Control group (MC). Animals in the AC group were trained to traverse an elevated obstacle course consisting of ropes, ladders, chains, and parallel bars requiring substantial motor coordination to complete. The time to traverse each obstacle on each trial was recorded. Each AC animal was pair matched with an MC animal which was forced to traverse a flat, obstacle free runway equal in length to the AC task. If an AC animal stopped at any point during a task, it was immediately given a gentle prod to the hindquarters by the experimenter. When an AC animal received a prod, an experimenter would also simultaneously prod the paired MC animal such that both animals spent equal amounts of time on their respective training apparati and received comparable amounts of handling. Animals from both groups were then further assigned to one of three training conditions. In the EARLY condition (AC n=8, MC n=8), rats were trained for ten consecutive days, while animals in the DELAY condition (AC n=8, MC n=8) received an identical ten days of training followed by a 28 day period without training. On the last day of the delay period, these animals were given two trials on the task in order to determine whether the delay period affected task performance. Finally, animals in the CONTINUOUS condition (AC n=8, MC n=8) were trained for the entire 38 consecutive days. All animals were sacrificed within fifteen minutes of their final training session. Synaptic changes in the cerebellar cortex of these animals have been described in a previous report [34].
Tissue Preparation
Following training, animals received a lethal overdose of pentobarbital (100 mg/kg), were transcardially perfused with 2% paraformaldehyde/2.5% glutaraldehyde in 0.1 M phosphate buffer (pH = 7.4), and brains were removed and post-fixed in that solution overnight at 4°C. Approximately ten blocks (300 μm, sagittal sections) were taken through one hemisphere of the paramedian lobule (PML) of the cerebellum using a vibratome. Sections were washed in cacodylate buffer (0.1 M), postfixed in 2% osmium tetroxide/1.5% potassium ferrocyanate in 0.1 M cacodylate buffer for 2 hours, stained en block with 2% uranyl acetate for 45 minutes, and then gradually dehydrated in alcohol, transferred into propylene oxide, and embedded in Medcast resin. All tissue samples were coded with respect to experimental condition prior to data analysis; one animal (in the EARLY MC group) was excluded from the anatomical analysis due to inadequate tissue perfusion.
Stereological Methods
Volume of Molecular Layer Per Purkinje Cell
Approximately 80 serial sections (1μm) were taken through a randomly chosen PML tissue block from each animal using a diamond histo-knife (Diatome) and an ultramicrotome. These sections were mounted on chrom alum gelatin coated slides and stained with Toluidine blue. Purkinje cell density was quantified with the physical disector [24,45], using a computer-assisted microscope and software package for unbiased stereology (Phokus on Stereology). This method involves comparing two serial sections, the Reference section and the Lookup section. Within randomly placed, unbiased counting frames of a known area, the number of nucleoli (each Purkinje cell having only one) which are present in the Reference section but not the Lookup section (Q−) is determined. The volume of tissue through which the cells are counted (Vdis) is given by:
Where Aframe is the area of the counting frame and H is the section thickness multiplied by the number of sections. The neuronal density (NvPcell) is then determined by:
This method allows for an accurate estimation of Purkinje cell density [8,34] which is unbiased with respect to cell size and shape [24,45]. The volume of the molecular layer per Purkinje cell could then be determined as:
Astrocytic Volume Fraction
Following the 1μm sectioning, a small pyramid was trimmed into the molecular layer of the cerebellar cortex using the 1μm thick Toluidine blue stained sections as a guide. Several 70 nm sections were then taken using an ultramicrotome (Reichert Ultracut S) and estimates of astrocytic volume fraction were obtained from six electron micrographs (print magnification: 30,000x) taken at random positions within these sections. Astrocytic profiles were first identified on the micrographs by their sparse cytoplasm, lack of form, and their tendency to follow the profiles of other cellular processes [3,41]. An acetate sheet containing a number of equally spaced points (1.93 cm intervals) was then placed over each micrograph (see Fig. 1) and astrocytic volume fraction (Vv) was determined by dividing the number of points falling within astrocytic profiles by the total number of points falling on the micrograph [3,21]. The volume of astrocytes/Pcell was then determined by:
Fig. 1.
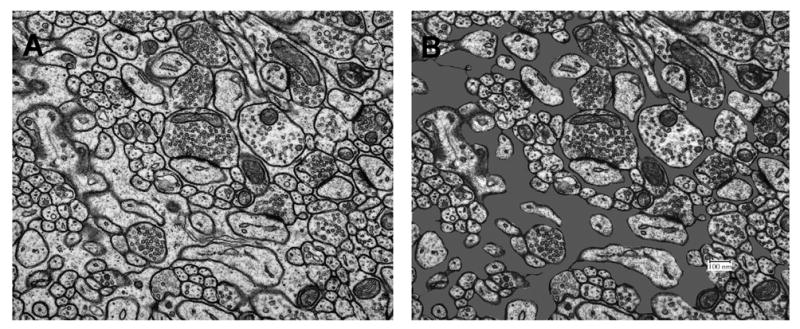
A. Electron micrograph taken within the molecular layer of the cerebellar paramedian lobule. B. The same micrograph is shown with glial profiles shaded in gray. Astrocytic volume fraction was estimated by overlaying a grid of equi-distant points, counting the total number of points (line intersections) located within astrocytic profiles, and then dividing by the total number of points on the micrograph (see text).
Vastro per Pcell=(VV)(Vmol per Pcell)
Possible changes in the astrocytic volume per synapse were also explored (using synapse per Pcell data from these animals [34]) by determining:
Vastro per Synapse=Vastro per PcellSynapses per Pcell
RESULTS
Behavioral Measures
The behavioral performance of these animals has been previously reported [34]. Briefly, an analysis of variance (ANOVA) revealed that the mean time/trial/task significantly decreased as training progressed for animals in all three conditions of the AC group (EARLY (F7,72= 14.24, p<.001); DELAY (F7,80=23.31, p<.001); CONTINUOUS (F7,296= 32.54, p<.001)) (see Fig. 2A, modified from [34]. The training-related reduction in time required to complete this task reflects a progressive decrease in the number of foot faults (errors) committed by the animals [32]. In the present experiment, the DELAY condition animals had a mean time/trial following four weeks without training that was comparable to that of animals in the CONTINUOUS condition, indicating that these animals’ skills persisted in the absence of training.
Fig. 2.
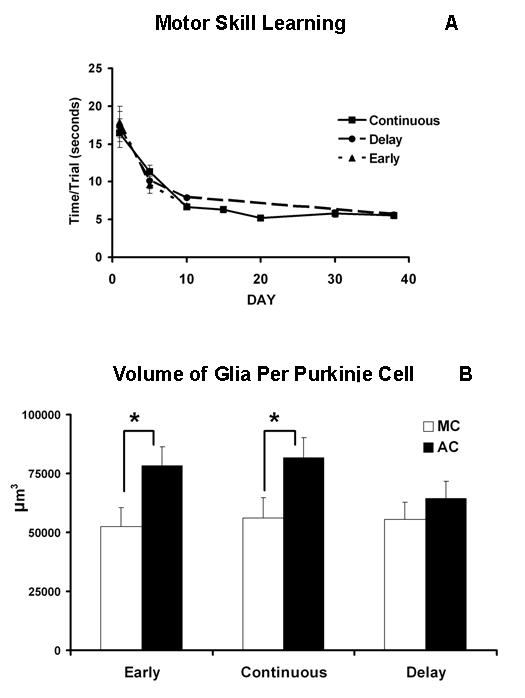
A. Mean time/trial/task (±SEM) on the motor learning apparatus. Despite a four week break from training, animals in the DELAY condition performed as well as animals in the CONTINUOUS condition (reprinted from Kleim et al., 1997). B. Motor learning induces hypertrophy of astrocytes in the cerebellar cortex. The volume of astrocytes per Purkinje cell (± SEM) within the cerebellar paramedian lobule was greater in AC animals compared to MC animals (p<.01).
Anatomical Measures
A 2 × 3 ANOVA with GROUP (AC, MC) and CONDITION (EARLY, CONTINUOUS, DELAY) as between subjects factors revealed that AC animals had a greater volume of astrocytes per Purkinje cell (F1,41= 8.1, p<.01; Fig. 2B) and greater volume of the molecular layer per Purkinje cell (F1,41= 55.9, p<.0001; Table 1) than did MC animals. Additionally, GROUP interacted with CONDITION to influence the volume of the molecular layer per Purkinje cell (F2,41= 3.5, p<.05). Because this experiment was designed to test specific comparisons of interest (i.e., the stability of astrocytic plasticity in the DELAY condition), planned comparisons were made between AC and MC animals in the three conditions, using the least significant differences method. This analysis revealed that, as expected, AC animals had greater astrocytic volume per Purkinje cell than MC animals in both the EARLY (49% difference, p<.05) and CONTINUOUS (45% difference, p<.05) conditions (Fig. 3). In contrast, the difference between AC and MC animals in the DELAY condition was much lower (16%) and did not reach statistical significance (p=.47) (Fig. 3). The volume of the molecular layer per Purkinje cell was greater in AC animals than MC animals of all three conditions, although this difference was also smallest among animals in the delay condition (EARLY 19% p<.0001; CONTINUOUS 32% p<.0001; DELAY 13% p<.01). Neither astrocytic volume fraction nor the volume of astrocytes per synapse differed between any two groups (Tables 2 and 3).
Table 1. VOLUME OF MOLECULAR LAYER PER PURKINJE CELL (mm3).
Motor learning increased the volume of molecular layer per Purkinje cell (±SEM) within the paramedian lobule.
| GROUP | EARLY | CONTINUOUS | DELAY |
|---|---|---|---|
| AC* | 490338 ± (15958) | 527034 ± (11724) | 474052 ± (14562) |
| MC | 410345 ± (17583) | 399692 ± (12760) | 420009 ± (12892) |
Table 2. ASTROCYTIC VOLUME FRACTION.
Astrocytic volume fraction (±SEM) within the molecular layer of the paramedian lobule was not influenced by motor learning.
| GROUP | EARLY | CONTINUOUS | DELAY |
|---|---|---|---|
| AC | .163 ± (.024) | .154 ± (.018) | .135 ± (.017) |
| MC | .130 ± (.022) | .142 ± (.020) | .132 ± (.015) |
Table 3. VOLUME OF ASTROCYTES PER SYNAPSE (mm3).
Volume of glia per synapse within the paramedian lobule was not influenced by motor learning.
| GROUP | EARLY | CONTINUOUS | DELAY |
|---|---|---|---|
| AC | .432 ± (.054) | .437 ± (.045) | .345 ± (.042) |
| MC | .414 ± (.046) | .365 ± (.030) | .373 ± (.035) |
DISCUSSION
The present findings demonstrate that astrocytes, like their neuronal partners, are morphologically sensitive to experience. In the paramedian lobule of the cerebellum, motor learning increased the volume of both astrocytes and the molecular layer per Purkinje cell. These results are consistent with previous work demonstrating that motor learning but not mere motor activity is associated with an increase in astrocytic volume per Purkinje cell within the cerebellar cortex [3]. Although the volume of the molecular layer per Purkinje cell increased following motor learning, the fraction of that volume occupied by astrocytic processes was maintained, resulting in an increase in astrocytic volume per Purkinje cell. The volume of astrocytes per Purkinje cell was significantly greater in the AC animals as compared to the MC animals after either 10 or 38 consecutive days of training. In contrast, the increase in astrocytic volume observed in the AC animals after 10 days of training was reduced and statistically undetectable following 28 days without training. These results indicate that the training-induced increase in the volume of astrocytes per Purkinje cell does not persist in the absence of continued training. In contrast, the training-induced increase in synapse number per Purkinje cell was found (in the same animals) to remain stable for at least four weeks after training was discontinued [34]. We have previously shown that this increase is specific to parallel fiber synapses rather than climbing fiber synapses [33].
Astrocytic processes were found to occupy approximately 15% of the molecular layer of the cerebellar cortex, a figure which is consistent with previous estimates [3]. This represents a much greater proportion of the neuropil than is found in other brain regions including the visual cortex [28] and hippocampus [22], suggesting that astrocytes may have a more prominent functional role in the cerebellum. The vast majority of glial profiles in the cerebellar cortex belong to the Bergmann fibers originating from the cell bodies of the Golgi epithelial cells located within the Purkinje cell layer, although a small proportion originate from Fanana cells and the protoplasmic astrocytes; fibers from these three cell types cannot be reliably distinguished from one another in electron micrographs [42]. The specialized Bergmann astrocytes extend highly arborized processes with lamellate appendages that wrap around Purkinje cell spines and ensheathe synapses, often completely (see Fig. 1). These appendages, although irregular-appearing, are actually organized into distinct “microdomains”, each consisting of a long, thin stalk connected to a mitochondria-containing head, suggesting that each microdomain is metabolically independent [20]. Furthermore, stimulation of parallel fibers has been shown to increase intracellular calcium in distinct compartments along Bergmann glial cell processes (which correspond in size to the anatomically-described microdomains); blocking synaptic neurotransmitter release prevents this effect [20]. Thus the physical arrangement of microdomains on Bergmann glial cell processes ensheathing Purkinje cell synapses does not appear to be conducive for the propagation of Ca2+ waves either within the cell (beyond the microdomain) or between neighboring glial cells, as is known to occur elsewhere in response to synaptic activity [reviewed by 43]. Rather, this organization may serve to minimize synaptic cross-talk by protecting each synapse from the non-specific influences of ions or neurotransmitters diffusing from neighboring synapses, and at the same time increase synaptic efficacy for the ensheathed synapses.
The dual role of limiting glutamate spillover between synapses and increasing synaptic efficacy for ensheathed synapses appears to be largely achieved by glial glutamate transporters. Bergmann glia abundantly express GLAST and (to a lesser degree) GLT-1, especially on portions of the cell membrane that face excitatory Purkinje cell synapses [13]; in fact, the presence and proper functioning of these transporters appears to be responsible for maintaining the single climbing fiber per Purkinje cell ratio [25,46,47]. (For a discussion of the different roles of glial and neuronal glutamate transporters at Purkinje cell synapses see Takayasu et al. [47]). The functional interdependence of Bergmann glia and Purkinje cells is further demonstrated by the findings that stimulation of either parallel or climbing fibers evokes currents in Bergmann glial cells [7,14], that parallel fiber stimulation induces plasticity in Bergmann glia extrasynaptic currents [5,6], and that stimulation of Bergmann glia in turn alters excitatory postsynaptic currents in Purkinje cells [9,11].
Although the mechanisms underlying the increase in astrocytic volume per Purkinje cell observed to accompany learning in the present study are uncertain, one likely possibility is that the activity of newly formed Purkinje cell synapses stimulates astrocytic growth. In cultured hippocampal astrocytes, for instance, bath application of glutamate induces filopodia extension [15]. As with most other astrocytes, Bergmann glia possess receptors for glutamate, GABA, and voltage-dependent ion channels, although they appear to lack voltage-gated Ca2+ channels [reviewed in 52]. Of particular interest are the AMPA-type glutamate receptors they express, which are unusual in that they lack the GluR2 (GluR-B) subunit and as a result are highly permeable to Ca2+ [12]. The function conveyed by this type of AMPA receptor was left to speculation until recently, when it was discovered that conversion of these receptors to Ca2+-impermeable receptors by viral-mediated transfer of the GluR2 gene resulted in a retraction of cultured Bergmann glial cell processes and that, conversely, overexpression of Ca2+-permeable AMPA receptors caused a dramatic elongation of processes by these cells [27]. Because the adenovirus had a much higher affinity to glial cells than to neurons, it was possible to replicate the first of these findings in vivo by injections into the molecular layer of the rat cerebellar cortex [26]. Expression of the GluR2 gene by Bergmann glia (and the resulting impermeability of their AMPA receptors to Ca2+) caused these cells to retract processes that formerly ensheathed synapses on Purkinje cell dendritic spines (detected using quantitative electron microscopic methods similar to those of the present study) and at the same time abated the clearance of synaptically released glutamate [26]. Interestingly, this treatment also resulted in the multiple innervation of Purkinje cells by climbing fibers. Therefore, expression of Ca2+-permeable AMPA receptors by Bergmann glia is essential for proper structural and functional relationships between these cells and the Purkinje cells with which they are so intimately connected.
The interdependence of the morphology of Bergmann glial processes and synaptic activity suggests that the learning-induced increase in astrocytic processes observed here is related to the learning-induced synaptogenesis which we have previously observed [34]. Although glia are functionally sensitive to changes in the level of neuronal energy metabolism [48], it is unlikely that the increase in glial volume associated with motor learning is merely the result of increased metabolic demand. Rats experiencing extensive physical exercise, sufficient to induce an increase in vasculature within the cerebellar cortex [8], do not show significant increases in glial volume in comparison to inactive controls [3]. In the present study, behavioral skill was largely maintained in the absence of continued training. Because increases in synapses are maintained for up to four weeks following the cessation of behavioral training [34], whereas we report here that changes in astrocytic volume are not, it appears that the demonstration of learned motor skill depends more upon synapses than upon ancillary glial change. It is as if glial cell hypertrophy, which is initially induced in association with the formation of new synapses, depends upon the continued use of those new synapses in order to be maintained. Alternatively, the function of glial cell hypertrophy in the cerebellum could be to assist in learning-induced synaptogenesis (and thus it would not be required to persist beyond the period of synaptogenesis), since it has been shown that in some systems soluble factors released by astrocytes are required to induce the formation of new synapses [e.g., 36, reviewed by 50,51]. The preserved glial hypertrophy in the group of AC animals that continued training for 38 consecutive days—without a further increase in synaptogenesis beyond that observed in animals trained for 10 days only—however, could argue against this interpretation. In any case, the learning-induced hypertrophy of glial cells does not appear to be required to maintain the synapses formed in response to learning.
Experience has historically been believed to be encoded primarily through adaptations in brain structure and function involving changes in synapse number. The results from this and other experiments demonstrate that the learning-dependent addition of synapses is accompanied by an increase in the volume of glial material. Unlike the changes in synapse number, however, the increase in glial volume does not persist in the absence of continued training.
Acknowledgments
We thank Amity Carrubba, Jennifer Drew, and Enali Kleim for assistance in training the animals and printing the micrographs, the Beckman Institute Optical Visualization Facility for use of their stereology system and the University of Illinois Center for Electron Microscopy for the use of their facilities. This work was supported by AG10154, MH35321, the Illinois-Eastern Iowa District of Kiwanis International Spastic Paralysis Research Foundation, the Retirement Research Foundation and an NSERC fellowship. J.A.M. supported by HD07333.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman J, Das GD. Autoradiographic Examination of the Effects of Enriched Environment on the Rate of Glial Multiplication in the Adult Rat Brain. Nature. 1964;204:1161–3. doi: 10.1038/2041161a0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BJ, Alcantara AA, Greenough WT. Motor-skill learning: changes in synaptic organization of the rat cerebellar cortex. Neurobiol Learn Mem. 1996;66:221–9. doi: 10.1006/nlme.1996.0062. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- 4.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–42. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy TC, Ogden D. Short-term plasticity of Bergmann glial cell extrasynaptic currents during parallel fiber stimulation in rat cerebellum. Glia. 2005;52:325–35. doi: 10.1002/glia.20248. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy TC, Ogden D. Long-term depression of neuron to glial signalling in rat cerebellar cortex. Eur J Neurosci. 2006;23:581–6. doi: 10.1111/j.1460-9568.2005.04588.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci U S A. 1997;94:14821–5. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bordey A, Sontheimer H. Modulation of glutamatergic transmission by bergmann glial cells in rat cerebellum in situ. J Neurophysiol. 2003;89:979–88. doi: 10.1152/jn.00904.2002. [DOI] [PubMed] [Google Scholar]
- 10.Briones TL, Klintsova AY, Greenough WT. Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Research. 2004;1018:130–5. doi: 10.1016/j.brainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Brockhaus J, Deitmer JW. Long-lasting modulation of synaptic input to Purkinje neurons by Bergmann glia stimulation in rat brain slices. J Physiol. 2002;545:581–93. doi: 10.1113/jphysiol.2002.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–70. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–20. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 14.Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502(Pt 2):335–50. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornell-Bell AH, Thomas PG, Smith SJ. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia. 1990;3:322–34. doi: 10.1002/glia.440030503. [DOI] [PubMed] [Google Scholar]
- 16.Corvalan V, Cole R, de Vellis J, Hagiwara S. Neuronal modulation of calcium channel activity in cultured rat astrocytes. Proc Natl Acad Sci U S A. 1990;87:4345–8. doi: 10.1073/pnas.87.11.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cragg BG. The development of synapses in kitten visual cortex during visual deprivation. Exp Neurol. 1975;46:445–51. doi: 10.1016/0014-4886(75)90118-1. [DOI] [PubMed] [Google Scholar]
- 18.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–40. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 19.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–43. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 21.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–94. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 22.Hawrylak N, Chang FL, Greenough WT. Astrocytic and synaptic response to kindling in hippocampal subfield CA1. II. Synaptogenesis and astrocytic process increases to in vivo kindling. Brain Res. 1993;603:309–16. doi: 10.1016/0006-8993(93)91253-o. [DOI] [PubMed] [Google Scholar]
- 23.Hawrylak N, Greenough WT. Monocular deprivation alters the morphology of glial fibrillary acidic protein-immunoreactive astrocytes in the rat visual cortex. Brain Res. 1995;683:187–99. doi: 10.1016/0006-8993(95)00374-y. [DOI] [PubMed] [Google Scholar]
- 24.Howard CV, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Springer; New York: 1998. [Google Scholar]
- 25.Huang H, Bordey A. Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J Neurosci. 2004;24:5659–69. doi: 10.1523/JNEUROSCI.1338-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–9. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- 27.Ishiuchi S, Tsuzuki K, Yamada N, Okado H, Miwa A, Kuromi H, Yokoo H, Nakazato Y, Sasaki T, Ozawa S. Extension of glial processes by activation of Ca2+-permeable AMPA receptor channels. Neuroreport. 2001;12:745–8. doi: 10.1097/00001756-200103260-00026. [DOI] [PubMed] [Google Scholar]
- 28.Jones TA, Greenough WT. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiol Learn Mem. 1996;65:48–56. doi: 10.1006/nlme.1996.0005. [DOI] [PubMed] [Google Scholar]
- 29.Jones TA, Hawrylak N, Greenough WT. Rapid laminar-dependent changes in GFAP immunoreactive astrocytes in the visual cortex of rats reared in a complex environment. Psychoneuroendocrinology. 1996;21:189–201. doi: 10.1016/0306-4530(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 30.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 31.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 32.Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–35. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998;69:274–89. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- 34.Kleim JA, Vij K, Ballard DH, Greenough WT. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. J Neurosci. 1997;17:717–21. doi: 10.1523/JNEUROSCI.17-02-00717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 37.Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 38.Muller CM. Dark-rearing retards the maturation of astrocytes in restricted layers of cat visual cortex. Glia. 1990;3:487–94. doi: 10.1002/glia.440030607. [DOI] [PubMed] [Google Scholar]
- 39.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–71. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 40.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–6. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 41.Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Springer Verlag; New York: 1974. pp. 288–321. [Google Scholar]
- 42.Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Springer Verlag; New York: 1974. pp. 288–321. [Google Scholar]
- 43.Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005;38:375–82. doi: 10.1016/j.ceca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Research. 1991;540:273–8. doi: 10.1016/0006-8993(91)90517-y. [DOI] [PubMed] [Google Scholar]
- 45.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 46.Takatsuru Y, Takayasu Y, Iino M, Nikkuni O, Ueda Y, Tanaka K, Ozawa S. Roles of glial glutamate transporters in shaping EPSCs at the climbing fiber-Purkinje cell synapses. Neurosci Res. 2006;54:140–8. doi: 10.1016/j.neures.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Takayasu Y, Iino M, Kakegawa W, Maeno H, Watase K, Wada K, Yanagihara D, Miyazaki T, Komine O, Watanabe M, Tanaka K, Ozawa S. Differential roles of glial and neuronal glutamate transporters in Purkinje cell synapses. J Neurosci. 2005;25:8788–93. doi: 10.1523/JNEUROSCI.1020-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–85. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- 50.Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–16. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- 51.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–61. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 52.Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 53.Volterra A, Magistretti P, Haydon PG. The Tripartite Synapse: Glia in Synaptic Transmission. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- 54.Wenzel J, Lammert G, Meyer U, Krug M. The influence of long-term potentiation on the spatial relationship between astrocyte processes and potentiated synapses in the dentate gyrus neuropil of rat brain. Brain Res. 1991;560:122–31. doi: 10.1016/0006-8993(91)91222-m. [DOI] [PubMed] [Google Scholar]
- 55.Winfield DA. The postnatal development of synapses in the visual cortex of the cat and the effects of eyelid closure. Brain Res. 1981;206:166–71. doi: 10.1016/0006-8993(81)90110-4. [DOI] [PubMed] [Google Scholar]