Biology of claudins (original) (raw)
Abstract
Claudins are a family of tight junction membrane proteins that regulate paracellular permeability of epithelia, likely by forming the lining of the paracellular pore. Claudins are expressed throughout the renal tubule, and mutations in two claudin genes are now known to cause familial hypercalciuric hypomagnesemia with nephrocalcinosis. In this review, we discuss recent advances in our understanding of the physiological role of various claudins in normal kidney function, and in understanding the fundamental biology of claudins, including the molecular basis for selectivity of permeation, claudin interactions in tight junction formation, and regulation of claudins by protein kinases and other intracellular signals.
Keywords: tight junction, paracellular, permeability
claudins were first purified and identified by Mikio Furuse in the tight junction laboratory of the late Shoichiro Tsukita (33). Their name was derived from the Latin word “claudere,” which means to close, because it was anticipated that these proteins might constitute the tight junctional barrier. It soon became apparent that the claudins are part of a large multigene family (75). A role for claudins in regulating paracellular permeability became evident when Simon et al. (91) cloned the gene responsible for familial hypercalciuric hypomagnesemia with nephrocalcinosis (FHHNC), a renal tubule disorder characterized by failure of paracellular divalent cation transport in the thick ascending limb of Henle (TALH). They found that FHHNC was caused by mutations in paracellin-1, which is now considered to be claudin-16. In the past few years, research in this area has proliferated, and several comprehensive general reviews of this have recently been published (89, 99, 103). In this article, we focus on reviewing recent discoveries regarding the biology of claudins and their implications for understanding renal tubule function and human renal diseases.
Integral Membrane Proteins of the Tight Junction
Epithelial cells are interconnected by sets of cell-cell contacts, of which the tight junction, a network of anastomosing parallel strands at the apical end of the lateral membrane, is known to form the principal barrier to paracellular diffusion. The tight junction complex is formed by several components: membrane-spanning proteins, whose extracellular domains reach into the paracellular space, like claudins, occludin, tricellulin, junctional adhesion molecules (JAM), and the coxsackie/adenovirus-associated receptor (CAR); scaffolding proteins that link membrane proteins to the actin cytoskeleton, like ZO-1, ZO-2, and ZO-3; and other cytosolic proteins with signaling functions, such as transcription factors, kinases and phosphatases (for a more detailed review, see Refs. 17 and 38). Barrier formation and restriction of paracellular transport require interaction between the tight junction and solutes that permeate through the paracellular space and thus, by definition, must involve the membrane protein components of the tight junction.
All of the tight junction membrane proteins can mediate cell-cell adhesion (Table 1), and overexpression or disruption of them can have gross effects on paracellular permeability. For example, overexpression of occludin (10, 72) and CAR (20) increase transepithelial electrical resistance (TER), while antibodies against JAM-A inhibit the recovery of TER after exposure to low calcium (70, 71). However, occludin and JAM-A do not form tight junction fibrils when expressed in fibroblasts that lack tight junctions (32, 54). Neither paracellular permeability nor tight junction morphology is significantly affected by occludin knockout in mice (87) or knockdown in cell lines (117). Tricellulin is confined mostly to the vertically oriented tight junction strands of tricellular contacts (49). Furthermore, since occludin, tricellulin, JAMs, and CAR-like genes exist as a limited number of different isoforms (Table 1), they are unlikely to be able to account for the diversity of paracellular permeability characteristics observed for different epithelia. Thus none of these proteins is likely to determine paracellular permselectivity. Instead, this role is now thought to be fulfilled by claudins.
Table 1.
Role of integral membrane proteins of the tight junction
| Gene Family | Transmembrane Domains | No. of Isoforms | Mediates Cell-Cell Adhesion? | Polymerize Into Tight Junction Fibrils? |
|---|---|---|---|---|
| Claudin | 4 | ≥24 Genes | Yes (62) | Yes (36) |
| Occludin | 4 | 1 Gene with several splice variants | Weakly* | No |
| Tricellulin | 4 | 1 Gene with several splice variants | Unknown | Unknown |
| JAM-A/B/C | 1 | 3 Genes | Yes | No |
| CAR/ESAM/JAM4 | 1 | 3 Genes | Yes | No |
Structure of Claudins
Functional regions of the claudin protein.
Claudins constitute a protein family of 24 members in mammals, with a molecular mass ranging from 20 to 27 kDa. Hydropathy plots suggest that claudins bear four transmembrane domains, like occludin and tricellulin, but they do not show any sequence similarity to either of these proteins. Claudins have a short intracellular NH2-terminal sequence (∼7 residues), a large first extracellular loop (∼52 residues) and shorter second extracellular loop (16–33 residues), and a cytoplasmic COOH-terminal sequence that varies considerably in length between different isoforms (21–63 residues) (103).
The first extracellular loop contains charged amino acids, of which some are conserved in different claudin isoforms. As discussed in detail below, there is considerable evidence that the first extracellular loop lines the paracellular pathway and determines the charge selectivity of paracellular transport. Two highly conserved cysteines are expressed in the first extracellular loop of all claudins and potentially form an intramolecular disulfide bond to stabilize protein conformation. However, this hypothesis has not been tested so far.
The functions of the second extracellular loop are less understood. Recent studies by Piontek et al. (81) in claudin-5-transfected HEK cells suggest a role in the formation of tight junction strands via _trans_-interaction. Based on molecular modeling, they hypothesize that the second extracellular loop is folded in a helix-turn-helix motif and forms dimers between claudins of opposing cell membranes via hydrophobic interaction of conserved aromatic residues. The second extracellular loop of some claudin isoforms has also been shown to act as a receptor for Clostridium perfringens enterotoxin, an attribute that could potentially be used in barrier modulation and drug delivery (31).
The COOH-terminal tail shows the greatest structural heterogeneity between different claudin isoforms, suggesting that it contributes to isoform-dependent paracellular selectivity based on differences in protein targeting and regulation. It contains a PDZ-binding motif that enables claudins to directly interact with the tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3 (53), with MUPP1 (40) and with PATJ (85). The interaction with cytoplasmic scaffolding proteins like ZO-1 indirectly links claudins to the actin cytoskeleton and most likely stabilizes them at the tight junction (77). The cytoplasmic tail upstream of the PDZ is required for targeting of claudins to the tight junctional complex (86) and a determinant of protein stability (105). As discussed in more detail below, claudin localization and function are also regulated by phosphorylation of the cytoplasmic tail, a target of serine/threonine and tyrosine kinases. Other posttranslational modifications of claudins involve palmitoylation, which is required for targeting of claudin-14 to the plasma membrane and hence efficient insertion into tight junctions (106).
Homotypic and heterotypic claudin interactions.
The structure of claudin-based paracellular pores is still largely unknown, but it seems likely to be composed of claudin oligomers. This is supported by some limited evidence. Claudin-4 protein has been observed to migrate as oligomers (up to hexamers) when solubilized in perfluoro-octanoic acid (73). The second extracellular domain of claudin-5 is able to form dimers in vitro (14). Finally, fluorescence resonance transfer studies show that there is a close spatial association between claudins within the same cell membrane (14). Thus claudins likely interact to form oligomers both within the same cell and across adjacent cells.
Native epithelial cells typically express multiple claudin isoforms. This raises the question of whether different claudin isoforms can interact to form heteropolymers. Such heterotypic interaction could potentially occur in two ways: between claudins of the same cell membrane (side-by-side interaction) or between claudins of opposing cell membranes (head-to-head interaction). Studies using coexpression of multiple isoforms in fibroblasts by Furuse et al. (36) suggest that different claudin isoforms can be coincorporated into the same tight junction strands. However, analysis of such cells by coimmunoprecipitation of claudins showed that side-by-side interactions are in fact restricted to specific combinations of isoforms (e.g., claudins-3 and -5) but not others (23). Heterotypic head-to-head interactions between claudins of opposing membranes seem also to be limited to specific combinations of claudins (Table 2). Daugherty et al. (24) investigated this phenomenon in detail and found that claudins-1 and -4 and claudins-3 and -4 do not undergo heterotypic head-to-head interaction, while claudins-1 and -3 are compatible and interact with each other. This was surprising because the extracellular domains of claudins-3 and -4 are very similar. Experiments on chimeras and mutant proteins showed that the first and second extracellular loops as well as an unknown motif beyond the extracellular domains determine head-to-head compatibility. These studies are important because they raise the possibility that paracellular pores could exist that are heteromers of different claudin isoforms and hence have permeability properties distinct from those of the individual claudins.
Table 2.
Head-to-head interaction between different claudin isoforms
| Claudin | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | + (34) | − (34) | + (24, 34), − (23) | − (24) | − (23) |
| 2 | − (34) | + (34) | + (34) | NT | NT |
| 3 | + (24, 34), − (23) | + (24,34) | + (34) | − (24) | + (23, 24) |
| 4 | − (24) | NT | − (24) | + (24) | − (24) |
| 5 | − (23) | NT | + (23, 24) | − (24) | + (24, 81) |
Physiological Function of Claudins
The role of individual claudins has been investigated in three ways; by overexpression or downregulation of claudins in epithelial cells, by knockout of claudin genes in mice, and by the study of the phenotype of human diseases due to claudin mutations (see below). The data provide considerable evidence that claudins determine the selectivity of paracellular transport and probably do so by forming aqueous paracellular pores through the tight junction that allow selective passage of small solutes and ions, as postulated by Claude (19).
Overexpression and knockdown of claudins in epithelial cell lines.
Numerous studies of the functional properties of claudin-overexpressing cells and more recently of cells with knockdown of claudin (43) have now been performed (see Table 1 in Ref. 8 for a recent comprehensive summary). The predominant role of claudins appears to be regulation of paracellular selectivity to small ions. Most claudins (claudins-1, -4, -5, -8, -11, and -14) have been observed to increase the transepithelial resistance (TER) of the host cell line, usually by decreasing cation permeability. Some claudins (-2, -10b, -15) decrease TER by acting as cation pores. Claudin-10a (108) and possibly claudin-7 (43) are the only claudin isoforms so far that have been found to have significant anion pore properties. An important caveat of such studies is that the phenotype can depend on the background permeability of the host cell line. For example, in Madin-Darby canine kidney (MDCK) II cells which are highly Na+ permeable, claudins-4 and -11 decrease Na+ permeability (_P_Na) and claudins-2 and -15 have no effect, while in LLC-PK1 cells, which are relatively Na impermeable, claudins-2 and -15 increase _P_Na while claudins-4 and -11 have no effect (101). Since all epithelial cells endogenously express multiple claudins, host cell-dependent differences in the functional effect of transfected claudins probably depend on which endogenous claudins are already present, as has been shown for claudin-8 (7). It is important to recognize that a claudin that acts as a selective pore to one population of ions must, by definition, constitute a relative barrier to all other ions; that this function is manifest may depend on the host cell. For example, Hou et al. (43) used small interfering (si) RNA to knock down endogenous claudins in epithelial cells and found that claudin-4 and claudin-7 act as cation barriers in MDCKII cells while forming anion-selective pores in LLC-PK1 cells (43).
The function of several claudins remains highly controversial. For example, claudin-16 (paracellin-1) and claudin-19, which are the culprit genes in FHHNC (see below), were originally proposed to be divalent cation pores. Indeed, overexpression studies of claudin-16 by Ikari et al. (47) seemed to show that it increases Mg2+ and Ca2+ transport, while decreasing _P_Na. However, Ca2+ transport was strikingly asymmetrical (exclusively apical-to-basal), inhibited by Mg2+, and only slightly increased above control levels, raising some doubt as to whether it was truly paracellular or specific. By contrast, Kausalya et al. (57) found that claudin-16 modestly increased Mg2+ permeability (_P_Mg) without changing _P_Na, and Hou et al. (44) found a marked increase in _P_Na with only a minor increase in _P_Mg. Similarly, while we have found that claudin-19, when expressed in MDCKII cells, decreases permeability to Na+, Ca2+, and Mg2+ (6), Hou et al. (45) found that claudin-19 transfection into LLC-PK1 cells reduced only Cl− permeability (_P_Cl).
Molecular basis of charge selectivity.
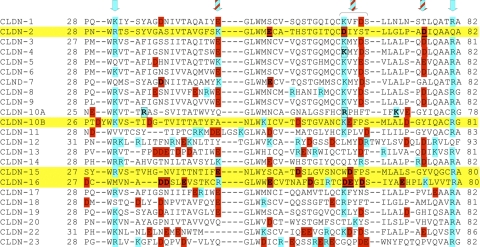
The structural elements that determine charge selectivity reside on the first extracellular loop. By domain swapping between claudin-2 and claudin-4, Colegio et al. (21) showed that the first extracellular loop confers the selectivity properties of the claudin. Figure 1 shows an alignment of the amino acid sequences of the first extracellular domains of claudins, summarizing the data on residue function derived from published mutagenesis studies. The predominant method of study, spearheaded by Anderson's group, has been to perform charge-reversing site-directed mutagenesis of charged residues in this region (22, 108). This approach has generally yielded multiple positions at which mutations can contribute to charge selectivity in a roughly additive manner. Colegio et al. (22) first showed that K65 in claudin-4 is involved in constituting the cation barrier, while D55 and E64 but not E46 in claudin-15 play a role in cation permeation. Furthermore, mutation of R32 and R59 decreases anion selectivity in claudin-10a while mutation of K66 has no effect on protein function (108). Note that R59 in claudin-10a corresponds to K65 in claudin-4. The importance of the first extracellular loop in constituting the selectivity filter was also confirmed by Alexandre et al. (2), who found that mutation of charged residues of the first loop in claudin-7 but not the second loop affects charge selectivity.
Fig. 1.
Alignment of the 1st extracellular domains of claudins. Acidic residues are highlighted in red, basic residues in blue. Claudins that have been shown to act as cation-selective pores are marked with yellow. Arrows indicate conserved regions that contain only positively charged residues (blue arrows) or both positively and negatively charged residues (striped arrows). Boxes indicate positions at which mutations have been shown to decrease or abolish claudin function; residues that have been mutated without loss of function are underlined. Data are from Refs. 3, 22, 44, and 108 except for claudin-2 (our unpublished observations).
A potential concern with charge-reversing mutations is that they introduce an artificial electrostatic environment that is not normally present. Thus while these mutations clearly indicate amino acid positions that either line the pore or are close enough to the pore opening to influence permeation, they do not necessarily inform on the normal physiological location of the ion selectivity filter. We have tried to address this by performing charge-neutralizing mutations in the first extracellular domain of claudin-2 and have identified one position, D65, that appears to be the major contributor to an intrapore Na+-binding site (Yu AS and Angelow S, unpublished observations). Interestingly, other claudins that are known to increase cation permeation, claudin-10b, -15, and -16, also express negatively charged amino acids at this key position and/or next to it while most other claudin isoforms express a positively charged lysine at this position, suggesting that this conserved region may play an important role in determining charge selectivity and facilitating cation permeation. Hou et al. (44), however, found that mutation of negatively charged amino acids in the homologous region of claudin-16 had no effect on Na+ permeation while abolishing negative charges further upstream partly affected cation selectivity. Thus a consistent picture in all claudins for the location of the selectivity filter does not yet exist.
Size selectivity of claudins.
Van Itallie et al. (107) have used polyethylene glycol oligomers to probe the size of claudin-based paracellular pores. The paracellular pathway for these neutral solutes was found to consist of a size-dependent component with an apparent diameter of ∼8 Å and a size-independent component that allowed a small but finite permeability to molecules of at least 14-Å diameter. Claudin-2 overexpression increased the size-dependent component, suggesting that it forms pores of ∼8-Å diameter. Consistent with this, we have used diffusion potential measurement of permeability to organic cations of varying sizes to determine the apparent size of the claudin-2 pore for cations permeation and find that it is ∼7.5 Å (Yu AS and Angelow S, unpublished observations). There are currently no data on the sizes of pores formed by other claudin isoforms.
Expression and Localization of Claudins in the Kidney
Claudin expression and function in renal tubules.
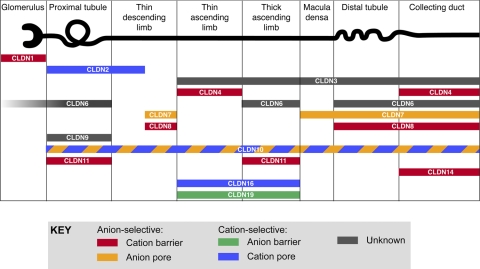
The majority of claudins whose expression patterns have been studied are expressed in the kidney (59). Not surprisingly, most are expressed at the tight junction of renal tubule epithelial cells. Each claudin gene exhibits a unique nephron segment pattern of expression, and each nephron segment expresses multiple claudins (Fig. 2). It is believed that the particular combination of claudins determines the unique paracellular permeability properties of each nephron segment. However, the role of renal tubular claudins can be inferred for only a few isoforms. For example, claudin-2, which forms high-conductance cation pores (4, 34), is expressed in the proximal tubule and early thin descending limb (28, 59) and is likely responsible for the substantial amount of paracellular Na+ reabsorption in these segments. In contrast, claudins-4 and -8 function primarily as cation barriers (102, 116) and are expressed in the distal nephron (59, 116), where they likely protect against the dissipation of transtubular Na+, K+, and H+ gradients established by transcellular active transport. In addition, claudins can undergo alternative splicing. Thus, claudin-10 is spliced to yield two variants, 10a and 10b, that have different extracellular domains and different functional properties (108). Claudin-10a, which is more anion selective, is concentrated in renal tubules in the cortex, while 10b is more cation selective and is more highly expressed in the medulla.
Fig. 2.
Localization and putative function of claudin proteins in mammalian kidney. Localization data were summarized from the following studies: claudins-1, -3,- 4, and -11 (59), claudin-2 (28), claudin-6 (1, 6, 118), claudins-7 and -8 (68), claudin-9 (1), claudin-10 (108), claudin-14 (12), claudin-16 (61, 91), and claudin-19 (6, 61). Tubule expression of claudins-6 and -9 are only found in neonatal kidney (1). Many of these claudins have not been rigorously examined in all nephron segments, so the data shown are not comprehensive. Macula densa claudin expression is from our own unpublished results. Claudins-5 and -15 are confined to endothelial cells of the vasculature and glomeruli (59) and so are not shown. Claudins-12, -18, and -20 to -24 have not yet been examined. Claudins are color coded according to their putative charge selectivity and permeability. Figure modified from Angelow S, Yu AS. Curr Opin Nephrol Hypertens 16: 459–464, 2007 (copyright Lippincott, Williams & Wilkins, 2007).
Claudins are not only confined to the intercellular junction of epithelia but can sometimes be found at other subcellular locations. A striking example is claudin-7, which is predominantly basolateral in the connecting tubule and collecting duct (68), as it is in airway, intestinal, and epididymal epithelia (23, 42, 51). At least in the epididymis it is not polymerized into strands (51). The function of basolateral claudins is not well understood. They could serve as a reserve pool of claudins that could be recruited to the junction, or perhaps subserve some other function such as cell-cell or cell-matrix adhesion. Possibly consistent with a role in adhesion, claudin-7 was recently shown to directly interact with the basolateral cell-cell adhesion molecule EpCAM (63). Interestingly, claudin-7 has also been reported to be distributed intracellularly in a punctate distribution in rabbit thin limbs of Henle's loop (37), but its role there is unknown.
Extratubular claudin expression.
Claudins are found in the kidney at locations other than the tubule epithelium. Claudins-5 and -15 are known to be predominantly endothelial claudins and are indeed expressed in the endothelia of renal blood vessels (59). Claudin-1 has been detected in parietal epithelial cells of Bowman's capsule (59). The parietal epithelium consists of two types of cells: squamous cells, which have multiple tight junction strands and are believed to function primarily as a barrier to leakage of the primary filtrate (98), and cuboidal cells, which seem to be an extension of the adjacent proximal convoluted tubule epithelium and are likely to be leaky (109). Since claudin-1 is not found in the adjacent proximal tubule, it is probably expressed in the squamous cells. The podocyte slit diaphragm resembles a modified tight junction and may express low levels of claudin-6 (118). Importantly, podocytes exhibit true tight junctions during development (82), a pattern that is recapitulated during injury (64). Both claudin-6 and TM4SF10, a closely related protein that is part of the PMP22/EMP/claudin gene superfamily, are expressed at podocyte junctions during development (15, 118). Claudin-6 is also upregulated at podocyte junctions with protamine sulfate treatment and in puromycin aminogylcoside nephrosis (118), while claudin-3 is upregulated in podocytes of nephrin-knockout mice (25).
Claudins can also be developmentally regulated. mRNA of claudins-6, -9, and -13 is expressed in neonatal mouse kidney but disappears by adulthood (1). In the neonate, claudin-6 protein was found to be expressed in the proximal tubule, TALH, distal tubule, and collecting duct, while claudin-9 mRNA was detectable in isolated proximal tubules.
Claudin Regulation
The barrier properties of claudins can potentially be influenced by environmental cues, developmental changes, and physical disruption of cell-cell contacts. Functional regulation can occur at the level of posttranslational modification, e.g., phosphorylation, and at the level of gene expression. Direct studies of renal or renal cell claudin regulation are few, but many of the findings in nonrenal models are potentially generalizable to the renal tubule epithelium.
Phosphorylation of claudins.
There is evidence from a number of studies that claudin function can be regulated acutely by phosphorylation, in some cases contributing to increased barrier function of the tight junction and in other cases having the opposite effect. For example, PKA-mediated phosphorylation of claudin-3 in an ovarian cancer cell line was associated with a decrease in TER (26), but PKA-dependent phosphorylation of claudin-5 in cultured blood-brain barrier endothelial cells treated with cAMP increased TER (52). As is true for all phosphorylation sites identified in claudins to date, claudin-3 phosphorylation by PKA occurred in the COOH-terminal cytoplasmic domain, on Thr192 (26). A putative PKA consensus phosphorylation site was identified at Thr207 in the COOH-terminal domain of claudin-5 (52), but this has yet to be confirmed. Another reported target of PKA phosphorylation is claudin-16. In the study by Ikari et al. (48), claudin-16 acted as a Mg2+-selective pore when heterologously expressed in MDCKII cells. This effect was associated with constitutive phosphorylation of claudin-16 and was reversed by adding inhibitors of PKA and adenylate cyclase, or by introducing the point mutation Ser217Ala. Dephosphorylated claudin-16 moved out of the membrane and into the lysosome, suggesting that PKA-mediated phosphorylation at Ser217 is important for localization to the tight junction.
PKC is known to regulate epithelial and endothelial barrier function, with reports linking various isoforms of PKC to both assembly and disassembly of the tight junction (5, 9, 18). Two studies report direct phosphorylation of claudins by PKC. In the first study, overexpression of claudin-1 on the right side of chick embryos randomized the direction of heart looping, an event in embryonic development that normally departs from the bilateral symmetry in an evolutionarily conserved direction (90). Mutating the predicted PKC phosphorylation target, Thr206, to alanine abolished this randomization, suggesting that appropriately localized claudin-1 expression is important for developing correct heart looping and that PKC phosphorylation of Thr206 is important for this function. However, these results did not include in vitro phosphorylation data or potential effects of kinase inhibitors. In the second study, claudin-4, endogenously expressed in an ovarian cancer cell line, was phosphorylated in response to phorbol ester-induced activation of PKC (27). Additional effects of phorbol ester treatment include reduction in tight junction strength and altered claudin-4 localization. Experiments with a panel of PKC inhibitors and siRNA suggested that the PKCε isozyme is responsible for the observed effects, and heterologous expression of mutated claudin-4 proteins in an ovarian cancer cell line lacking endogenous claudin-4 points to residues Thr189 and Ser194 as the targets of PKCε phosphorylation.
WNK1 (with no lysine[K] 1) and WNK4 are serine/threonine kinases that have been linked to pseudohypoaldosteronism type II (PHAII), a hereditary form of human hypertension coupled with hyperkalemia (113). WNK4 shows staining in the tight junctions of distal convoluted tubules and the collecting duct, suggesting that dysregulation of paracellular electrolyte handling in these segments of the nephron may be a factor contributing to PHAII (113). Heterologous expression of WNK4 in MDCKII cells was associated with increased paracellular Cl− permeability and phosphorylation of endogenous claudins-1 and -4 as well as heterologously expressed claudins-2 and -3 (115). Introducing a PHAII-causing mutation into WNK4 further enhanced both of these effects. A combination of in vitro phosphorylation experiments and coimmunoprecipitation of WNK4 and claudins, expressed in COS7 cells, led the authors to conclude that the increased phosphorylation of claudins observed with PHAII-mutated WNK4 resulted from a stronger interaction with claudins rather than enhanced intrinsic kinase activity. Another group confirmed the electrophysiological observations that heterologous expression of WNK4 in MDCKII cells increased Cl− permeability and that this effect was further enhanced by introducing a PHAII-causing mutation into WNK4 (55). In the renal epithelial cell line LLC-PK1, heterologously expressed WNK4 was reported to coimmunoprecipitate with endogenous claudin-7 (97). By in vitro phosphorylation studies, it was shown that WNK4 phosphorylated claudin-7 on Ser206 and that a PHAII-causing mutation in WNK4 enhanced this phosphorylation. In a background of overexpressed claudin-7, which increases the TER of LLC-PK1 cells, WNK4 lowered TER by increasing paracellular Cl− permeability, an effect that was further enhanced by a PHAII-causing mutation in WNK4. Taken together these studies suggest that WNK4 regulates paracellular Cl− permeability by phosphorylation of claudins and that PHAII may be the result of a gain-of-function mutation in WNK4, producing a chloride shunt in the distal convoluted tubule that promotes abnormal paracellular NaCl reabsorption (hypertension) and inhibits K+ secretion (hyperkalemia). WNK1 does not localize to the tight junction, but in a study where WNK1 was heterologously expressed in MDCKII cells the effects were similar to those reported by Yamauchi and colleagues (80) for WNK4, including increased paracellular Cl− permeability and phosphorylation of claudin-4. This report lends further support to the notion that chloride shunting is a contributing factor to the PHAII phenotype, regardless of which WNK isoform that is affected.
Aldosterone regulation of claudin-4 was examined in the cortical collecting duct cell line RCCD2 (67). Aldosterone was found to induce phosphorylation of claudin-4 at threonine residues within 20 min, and this was associated with decreased TER and increased 125I flux, suggesting that the anion permeability of claudin-4 may have been stimulated.
Other kinases reported to phosphorylate claudins include MAP kinase, Rho kinase, and EphA2. (30, 96, 114).
Regulation of claudin expression.
An important process associated with changes in claudin expression is epithelial-to-mesenchymal transition (EMT), which is involved in embryonic development, repair of epithelial injury including renal tubulointerstitial injury, and potentially in tumorigenesis. In cultured mouse mammary epithelial cells, overexpression of the transcription factor Snail induces EMT together with downregulation of the adherens junction protein E-cadherin (11, 16), and the tight junction proteins occludin and claudins-3, -4, and -7 (50). Claudin genes (as well as E-cadherin and occludin) are known to contain E-box motifs in their promoter to which Snail can directly bind and repress transcription. Interestingly, another report suggests that Snail downregulates claudin-1 without affecting transcription, suggesting that it might also regulate claudin translation (79).
Claudin expression can also be altered in response to events such as renal ischemia. Microarray-based analysis of differential gene expression in experimental murine renal ischemia-reperfusion injury identified significant upregulation of the genes for claudins -1, -3, and -7 and a small downregulation of claudin-2 (58).
Two circulating growth factors, HGF and EGF, have similar effects when applied to low-resistance MDCKII cells in culture. They both cause an increase in TER associated with reduced claudin-2 expression and activation of ERK1/2 (69, 92). EGF also increased expression of claudins-1, -3. and -4, which may have contributed to the increased TER (92). The involvement of ERK1/2 was confirmed by the observation that an ERK1/2 inhibitor abolished the HGF-mediated effects and that transfection of constitutively active ERK1/2 into MDCKII cells had the same effect as HGF treatment (69). Interestingly, the high-resistance strain of MDCK cells, MDCK I, was shown to have high levels of active ERK1/2 and low levels of claudin-2 expression. Adding an ERK1/2 inhibitor reversed both of these and lowered TER, effectively converting the cells to the phenotype of the low-resistance strain (69). Taken together, these studies suggest that the signaling pathway that converges on ERK1/2 may be important in determining renal paracellular permeability characteristics.
Role of Claudins in Human Diseases
Claudin-16 mutations and FHHNC.
The only inherited renal disease attributable to claudin mutations so far is FHHNC, an autosomal recessive disease characterized by renal Mg2+ wasting, hypercalciuria, and nephrocalcinosis (84), which usually leads to chronic renal failure. Rodriguez-Soriano et al. (84) first proposed that FHHNC might be due to a defect in tubular reabsorption in the TALH. This was based on the magnitude of the observed increase in fractional excretion of Mg2+, which could only be accounted for by a defect in the TALH and the fact that linked transport of Ca2+and Mg2+ is characteristic of the TALH. Blanchard et al. (13) subsequently demonstrated that FHHNC patients are unable to further increase their fractional excretion of Mg2+ and Ca2+ in response to the loop diuretic furosemide while having a preserved natriuretic response, thus confirming that there is a selective defect in divalent cation reabsorption in the TALH.
In 1999, Simon et al. (91) showed that FHHNC was caused by mutations in a gene they called paracellin-1, which we now know as claudin-16. Their study reported that claudin-16 was expressed in the tight junctions of the TALH. It was also noted at the time that the first extracellular domain of claudin-16 has a disproportionately large number of acidic residues. At least 30 different claudin-16 mutations have now been reported in families with FHHNC (13, 56, 60, 65, 66, 76–78, 88, 91, 94, 95, 100, 110, 111). Several are predicted to prematurely truncate the protein, and many have been shown to cause defects in trafficking of the protein to the plasma membrane (44, 57, 60). These findings suggested that claudin-16 might function as a divalent cation-selective paracellular pore and that FHHNC could be due to loss-of-function mutations that would be expected to abolish paracellular _P_Ca and _P_Mg in the TALH.
As discussed in the previous section, the in vitro studies of claudin-16 permeability properties have yielded conflicting results, and the idea that claudin-16 by itself forms a Mg2+ pore is not well supported by the available evidence (44, 48, 57). There are two alternative models that have been proposed to explain the pathogenesis of the disease. The first is that claudin-16 must heteromultimerize with another claudin isoform to form a functional Mg2+ pore. Consistent with this, there is indeed a second claudin gene that is mutated in FHHNC, claudin-19, and it does interact with claudin-16, but so far there is no evidence that this reconstitutes a functioning Mg2+ pore (45) (see below).
The second model, put forward by Goodenough and colleagues (46), is that claudin-16 really functions primarily as a paracellular Na+ pore. This is supported by recent analysis of claudin-16 RNAi knockdown mice, which reproduce the human phenotype of renal Ca2+ and Mg2+ wasting and nephrocalcinosis (46). In studies using in vitro perfused TALH tubules from these mice, the _P_Na/_P_Cl ratio was found to be decreased twofold with no change in _P_Na/_P_Mg or transtubular resistance, suggesting that the major defect is a decrease in _P_Na in the TALH. The reason this is thought to cause Ca2+ and Mg2+ wasting is as follows. The TALH generates a spontaneous lumen-positive transepithelial voltage that drives passive reabsorption of Ca2+ and Mg2+ via the paracellular pathway. This voltage is generated by two components (41): 1) an electrogenic component that is now known to be due to electroneutral Na+-K+-Cl− reabsorption across the apical membrane coupled with apical K+ recycling through ROMK and basolateral Cl− exit through ClC-K channels; and 2) a dilution potential that is due to the reabsorption-induced transepithelial NaCl concentration gradient. Because the paracellular permeability of the tubule is normally cation selective (_P_Na/_P_Cl ∼2 in the mouse), a lumen-positive diffusion potential develops. The relative contribution of these two voltage components varies, depending on tubular flow. At high flows there is minimal dilution potential because any concentration gradient is washed out, but under low-flow conditions quite a large NaCl gradient and hence substantial lumen-positive NaCl dilution potential can develop (83). If claudin-16 contributes to paracellular _P_Na, then loss of this would be predicted to abolish the dilution potential component and hence substantially reduce the transepithelial voltage, which is the main driving force for paracellular Ca2+ and Mg2+ reabsorption.
Claudin-19 mutations and FHHNC.
FHHNC exhibits genetic heterogeneity. In 2006, a second locus was identified, CLDN19, which encodes claudin-19 (61). Mutations in claudin-19 were found primarily in Spanish families and are associated with a similar phenotype to that due to claudin-16 mutations, with the exception that there was also a high prevalence of significant ocular abnormalities, including macular colobomata, nystagmus, and myopia. Claudin-19 is normally expressed at high levels in the retina (61), but why it causes these ocular disorders is unknown.
The fact that mutations in claudins-16 and -19 cause the same disease suggests the possibility that they may function in the same pathway. Indeed, claudins-16 and -19 completely colocalize in the TALH and thin ascending limbs of Henle (6, 61). Overexpression of claudin-19 alone has yielded conflicting results (6, 45). However, when claudins-16 and -19 are coexpressed, they interact in a yeast two-hybrid system, suggesting that they may form heteromultimers (45). Coexpression of claudins-16 and -19 increased _P_Na/_P_Cl more than either one alone, suggesting that this may be important for generating the dilution potential in the TALH.
Role of claudins in nonrenal diseases.
Claudins are mutated in a number of nonrenal disorders. Claudin-1 is expressed in the skin, where it is essential for the epidermal water barrier (35), and in cholangiocytes of the bile duct. Mutations in claudin-1 cause neonatal sclerosing cholangitis with ichthyosis (39). Claudin-14 probably plays a role in the cation-restrictive barrier that maintains normal endolymph ionic concentration, bathing the outer hair cells of the cochlea (12), and mutations in it cause nonsyndromic deafness (112). Claudins are also cell surface receptors for epithelial pathogens. Claudins-3 and -4 are receptors for C. perfringens enterotoxin (93), while claudins-1, -6. and -9 are coreceptors for cellular entry of the hepatitis C virus (29, 119). Finally, changes in claudin expression are often associated with epithelial cancers and may potentially play a role in their pathogenesis (74).
Summary and Future Directions
The biology of claudins is a rapidly evolving field, and many intriguing questions remain unanswered. Although it had been assumed that the reason there are ≥24 isoforms of claudin is that each one has distinct permeability properties, this has not turned out to be the case. Most of the ones that have been studied so far primarily regulate small inorganic ion permeability and are nonspecifically cation- or anion-selective. This raises the possibility that claudins may have other roles. There is increasing evidence that many claudins are not only at the tight junction but found along the entire basolateral membrane. This suggests that they may be involved in cell-matrix interactions or have scaffolding functions similar to the tetraspanins in organizing basolateral membrane proteins.
The structure of claudin-based pores and barriers remains unknown, and biophysical studies of claudin pore behavior remain rudimentary. The manner in which claudins interact and the role of heterotypic as opposed to homotypic interactions are poorly defined. A crystal structure for claudin is therefore keenly awaited.
There is also very little knowledge of the role of claudins in renal tubule physiology. Knockout mice have been generated for only a handful of claudin genes, and their renal phenotype has not been examined rigorously (or in most cases at all). It is likely that hormones and signals that regulate renal tubule transport may act in part by regulating paracellular permeability through claudins. This is supported by early studies suggesting that mineralocorticoids and WNK kinases regulate claudins. The finding that claudins may be expressed in podocytes under certain circumstances raises the interesting possibility that they may also be involved in maintaining the integrity of the glomerular filtration barrier.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-062283.
REFERENCES
- 1.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol 291: F1132–F1141, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl− permeability. Biochem Biophys Res Commun 357: 87–91, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci, 2005. [DOI] [PubMed]
- 4.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Andreeva AY, Piontek J, Blasig IE, Utepbergenov DI. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int J Biochem Cell Biol 38: 222–233, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol 293: F166–F177, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 215: 147–159, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens 16: 459–464, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol 123: 293–302, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 12: 2049–2061, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59: 2206–2215, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci 63: 505–514, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruggeman LA, Martinka S, Simske JS. Expression of TM4SF10, a claudin/EMP/PMP22 family cell junction protein, during mouse kidney development and podocyte differentiation. Dev Dyn 236: 596–605, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta 1778: 588–600, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Citi S Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol 117: 169–178, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claude P Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J Membr Biol 39: 219–232, 1978. [DOI] [PubMed] [Google Scholar]
- 20.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA 98: 15191–15196, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 284: C1346–C1354, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283: C142–C147, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285: L1166–L1178, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem 282: 30005–30013, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Doné SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K. Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 73: 697–704, 2008. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem 280: 26233–26240, 2005. [DOI] [PubMed] [Google Scholar]
- 27.D'Souza T, Indig FE, Morin PJ. Phosphorylation of claudin-4 by PKCepsilon regulates tight junction barrier function in ovarian cancer cells. Exp Cell Res 313: 3364–3375, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 281: F966–F974, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446: 801–805, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Fujibe M, Chiba H, Kojima T, Soma T, Wada T, Yamashita T, Sawada N. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res 295: 36–47, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett 476: 258–261, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci 109: 429–435, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147: 891–903, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Mariscal L, Namorado Mdel C, Martin D, Sierra G, Reyes JL. The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle's loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant 21: 2391–2398, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778: 729–756, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, Lyonnet S, De Prost Y, Munnich A, Hadchouel M, Smahi A. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology 127: 1386–1390, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 277: 455–461, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F432–F442, 1981. [DOI] [PubMed] [Google Scholar]
- 42.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 6: 581–588, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem 281: 36117–36123, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118: 5109–5118, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem 282: 17114–17122, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Ikari A, Hirai N, Shiroma M, Harada H, Sakai H, Hayashi H, Suzuki Y, Degawa M, Takagi K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem 279: 54826–54832, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Suzuki Y, Degawa M, Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci 119: 1781–1789, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171: 939–945, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116: 1959–1967, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Inai T, Sengoku A, Hirose E, Iida H, Shibata Y. Claudin-7 expressed on lateral membrane of rat epididymal epithelium does not form aberrant tight junction strands. Anat Rec (Hoboken) 290: 1431–1438, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res 290: 275–288, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147: 1351–1363, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 154: 491–497, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl− permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci USA 101: 14877–14882, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang JH, Choi HJ, Cho HY, Lee JH, Ha IS, Cheong HI, Choi Y. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis associated with CLDN16 mutations. Pediatr Nephrol 20: 1490–1493, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J Clin Invest 116: 878–891, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int 64: 480–492, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Konrad M, Hou J, Weber S, Dotsch J, Kari JA, Seeman T, Kuwertz-Broking E, Peco-Antic A, Tasic V, Dittrich K, Alshaya HO, von Vigier RO, Gallati S, Goodenough DA, Schaller A. CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 19: 171–181, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S. Ca2+-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr Biol 9: 1035–1038, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW, Weitz J, Zoller M. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res 5: 553–567, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 141: 805–816, 1992. [PMC free article] [PubMed] [Google Scholar]
- 65.Kutluturk F, Temel B, Uslu B, Aral F, Azezli A, Orhan Y, Konrad M, Ozbey N. An unusual patient with hypercalciuria, recurrent nephrolithiasis, hypomagnesemia and G227R mutation of paracellin-1. An unusual patient with hypercalciuria and hypomagnesemia unresponsive to thiazide diuretics. Horm Res 66: 175–181, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Kuwertz-Broking E, Frund S, Bulla M, Kleta R, August C, Kisters K. Familial hypomagnesemia-hypercalciuria in 2 siblings. Clin Nephrol 56: 155–161, 2001. [PubMed] [Google Scholar]
- 67.Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol 289: C1513–C1521, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 286: F1063–F1071, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Lipschutz JH, Li S, Arisco A, Balkovetz DF. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J Biol Chem 280: 3780–3788, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci 113: 2363–2374, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem 279: 16254–16262, 2004. [DOI] [PubMed] [Google Scholar]
- 72.McCarthy KM, Skare IB, Stankewich MC. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci 12: 218–227, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morin PJ Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 65: 9603–9606, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96: 511–516, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller D, Kausalya PJ, Bockenhauer D, Thumfart J, Meij IC, Dillon MJ, van't Hoff W, Hunziker W. Unusual clinical presentation and possible rescue of a novel claudin-16 mutation. J Clin Endocrinol Metab 91: 3076–3079, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Muller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet 73: 1293–1301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muller D, Kausalya PJ, Meij IC, Hunziker W. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: blocking endocytosis restores surface expression of a novel claudin-16 mutant that lacks the entire C-terminal cytosolic tail. Hum Mol Genet 15: 1049–1058, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci 117: 1675–1685, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Ohta A, Yang SS, Rai T, Chiga M, Sasaki S, Uchida S. Overexpression of human WNK1 increases paracellular chloride permeability and phosphorylation of claudin-4 in MDCKII cells. Biochem Biophys Res Commun 349: 804–808, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 22: 146–158, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978. [PubMed] [Google Scholar]
- 83.Rocha AS, Kokko JP. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest 52: 612–623, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez-Soriano J, Vallo A, Garcia-Fuentes M. Hypomagnesaemia of hereditary renal origin. Pediatr Nephrol 1: 465–472, 1987. [DOI] [PubMed] [Google Scholar]
- 85.Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem 277: 27501–27509, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol 83: 135–144, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141: 397–408, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanjad SA, Hariri A, Habbal ZM, Lifton RP. A novel PCLN-1 gene mutation in familial hypomagnesemia with hypercalciuria and atypical phenotype. Pediatr Nephrol 22: 503–508, 2007. [DOI] [PubMed] [Google Scholar]
- 89.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213–C1228, 2004. [DOI] [PubMed] [Google Scholar]
- 90.Simard A, Di Pietro E, Young CR, Plaza S, Ryan AK. Alterations in heart looping induced by overexpression of the tight junction protein claudin-1 are dependent on its C-terminal cytoplasmic tail. Mech Dev 123: 210–227, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999. [DOI] [PubMed] [Google Scholar]
- 92.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem 279: 3543–3552, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 147: 195–204, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staiger K, Staiger H, Haas C, Thamer C, Risler T, Machicao F, Haring HU. Hypomagnesemia and nephrocalcinosis in a patient with two heterozygous mutations in the CLDN16 gene. J Nephrol 20: 107–110, 2007. [PubMed] [Google Scholar]
- 95.Tajima T, Nakae J, Fujieda K. Two heterozygous mutations of CLDN16 in a Japanese patient with FHHNC. Pediatr Nephrol 18: 1280–1282, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of claudin-4 and mediates paracellular permeability. J Biol Chem 280: 42375–42382, 2005. [DOI] [PubMed] [Google Scholar]
- 97.Tatum R, Zhang Y, Lu Q, Kim K, Jeansonne BG, Chen YH. WNK4 phosphorylates ser(206) of claudin-7 and promotes paracellular Cl− permeability. FEBS Lett 581: 3887–3891, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Taugner R, Boll U, Zahn P, Forssmann WG. Cell junctions in the epithelium of Bowman's capsule. Cell Tissue Res 172: 431–446, 1976. [DOI] [PubMed] [Google Scholar]
- 99.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol 14: 531–536, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Turkmen M, Kasap B, Soylu A, Bober E, Konrad M, Kavukcu S. Paracellin-1 gene mutation with multiple congenital abnormalities. Pediatr Nephrol 21: 1776–1778, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Van Itallie C, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285: F1078–F1084, 2003. [DOI] [PubMed] [Google Scholar]
- 102.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006. [DOI] [PubMed] [Google Scholar]
- 104.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci 110: 1113–1121, 1997. [DOI] [PubMed] [Google Scholar]
- 105.Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol 199: 29–38, 2004. [DOI] [PubMed] [Google Scholar]
- 106.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci 118: 1427–1436, 2005. [DOI] [PubMed] [Google Scholar]
- 107.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci, 2008. [DOI] [PubMed]
- 108.Van Itallie CM, Rogan S, Yu AS, Seminario-Vidal L, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288–F1299, 2006. [DOI] [PubMed] [Google Scholar]
- 109.Ward AM Tubular metaplasia in Bowman's capsule. J Clin Pathol 23: 472–474, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weber S, Hoffmann K, Jeck N, Saar K, Boeswald M, Kuwertz-Broeking E, Meij II, Knoers NV, Cochat P, Sulakova T, Bonzel KE, Soergel M, Manz F, Schaerer K, Seyberth HW, Reis A, Konrad M. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet 8: 414–422, 2000. [DOI] [PubMed] [Google Scholar]
- 111.Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 12: 1872–1881, 2001. [DOI] [PubMed] [Google Scholar]
- 112.Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Friedman TB. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104: 165–172, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol 172: 521–533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci USA 101: 4690–4694, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in MDCK cells augments the paracellular barrier to cation permeation. J Biol Chem 278: 17350–17359, 2003. [DOI] [PubMed] [Google Scholar]
- 117.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288: C1231–C1241, 2005. [DOI] [PubMed] [Google Scholar]
- 118.Zhao L, Yaoita E, Nameta M, Zhang Y, Cuellar LM, Fujinaka H, Xu B, Yoshida Y, Hatakeyama K, Yamamoto T. Claudin-6 localized in tight junctions of rat podocytes. Am J Physiol Regul Integr Comp Physiol (First published March 26, 2008). doi: 10.1152/ajpregu.00862.2007. [DOI] [PubMed]
- 119.Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J, Song X, Ding M, Deng H. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol 81: 12465–12471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]