Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures (original) (raw)
. Author manuscript; available in PMC: 2008 Nov 3.
Published in final edited form as: Osteoporos Int. 2008 Mar 29;19(9):1283–1290. doi: 10.1007/s00198-008-0582-6
Abstract
Summary
We determined the prevalence of vitamin D deficiency and lower extremity function in women with hip fractures. Women with extremely low vitamin D levels had reduced lower extremity muscle function and increased falls 1 year later. Ensuring vitamin D sufficiency after a hip fracture may improve function and reduce falls.
Introduction
Hip fractures are the most devastating of fractures, commonly leading to loss of independent ambulation and living. In this retrospective analysis we determined the prevalence of vitamin D deficiency in women with hip fractures and the association between 25-hydroxyvitamin D [25(OH)D] levels and functional impairment one year later.
Methods
One hundred ten community-dwelling women with hip fractures were recruited from Boston, MA (n= 30) and Baltimore, MD (n=80) before 1998 and 25(OH)D levels were measured by radioimmunoassay. In a subset of women from Baltimore, a performance measure of the lower extremities using the lower extremity gain scale (LEGS) was measured at 2, 6, and 12 months. Falls, grip strength, chair rise time, walking speed, and balance were also determined.
Results
Vitamin D insufficiency defined as a 25(OH)D ≤32 ng/mL was present in 96% of the women with hip fractures and 38% had extremely low levels ≤9 ng/mL. At 1 year post-fracture, compared to women with a 25(OH) D >9 ng/mL, those with 25(OH)D ≤9 ng/mL had poorer LEGS performance (p<0.0001) and higher fall rates, without group differences in grip strength or balance.
Conclusion
Vitamin D sufficiency may have important effects on lower extremity function following hip fractures, without excessive healthcare costs.
Keywords: Falls, Fracture, Grip strength, Hip, Muscle function, Vitamin D
Introduction
Hip fractures increase exponentially with age and are associated with progressive loss of muscle mass and functional capacity [1–3]. More than half of subjects with hip fractures lose the ability to walk independently; there is markedly increased morbidity and mortality in women and men with hip fractures [2, 4, 5]. Because the number of patients with hip fractures is expected to increase several-fold in the next 20 to 50 years, it is important to understand what factors contribute to morbidity associated with hip fractures with the goal of optimizing bone health and functional independence after hip fractures [6].
In addition to the well-established role for vitamin D in calcium homeostasis, recent data show that vitamin D is important for muscle function. For example, there are receptors for vitamin D on muscle cells that decrease with advancing age [7]. Data from a cross-sectional analysis of lower extremity function in older women enrolled in the civilian United States National Health and Nutrition Examination Survey (NHANES III) showed an association between very low 25-hydroxyvitamin D [25(OH)D] concentrations, a measure of steady-state vitamin D, and reduced ability to walk 8 feet and perform sit-to-stand tests [8]. Although vitamin D deficiency is often present in the elderly with hip fractures [9, 10], the effects of low vitamin D levels on long-term functional recovery following hip fractures are not known. The objectives of this study were 1) to evaluate the prevalence of vitamin D deficiency in community-dwelling women with hip fractures according to current boundaries of vitamin D sufficiency, and 2) to test the hypothesis that very low 25(OH)D levels at the time of fracture are associated with impaired lower extremity function and increased falls monitored longitudinally for 1 year after a hip fracture.
Methods
Study population
One hundred and ten community-dwelling women between 59 and 95 years of age with hip fractures were enrolled: 30 women in Boston, Massachusetts, and 80 women in Baltimore, Maryland. Women with high-energy fractures or not community dwelling at the time of fracture were excluded from these studies. The Boston cohort of 30 white women with new hip fractures and no other secondary causes for osteoporosis was part of a larger study of 98 women, including 68 control women admitted for joint replacement for non-inflammatory arthritis. Subjects were recruited from the community between 1995 and 1998 and provided information regarding lifestyle, reproductive factors, calcium intake, and physical activity as previously reported [9]. The Baltimore cohort of 80 white women who presented with fresh hip fractures was part of a larger study of 205 patients recruited between 1992 and 1995. A complete description of how they were recruited and serum analyses that were performed was already reported on this population [1]. In the study presented here, all women who provided serum for the vitamin D assay (i.e., n=80) are included. Subjects provided information regarding pre-fracture functioning; their co-morbidity information was collected through medical chart review. The 80 subjects from Baltimore included in this report were younger than the other patients not providing serum samples for vitamin D (mean age 79.7±8.0 versus 81.9±7.5 years, p=0.05) and had fewer co-morbid conditions [mean modified Charlson comorbidity score (described below) of 1.78±1.64 versus 2.10±1.49, p=0.16].
Vitamin D levels
In both cohorts, serum samples were obtained within 10 days post fracture and stored 25(OH)D levels were measured using radioimmunoassay (DiaSorin, Stillwater, Minn). Although 25(OH)D assays show variability among laboratories, this assay accurately reflects administered vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), and results are reported to correspond well with HPLC values [11, 12]. The normal range for that assay until recently was indicated to be 9 to 53 ng/mL. The inter-assay and intra-assay reproducibility for that assay were 12 to 14% and 8.7 to 10%, respectively [13].
There has been a redefinition of the boundaries that describe low vitamins D levels. Data show that calcium absorption is optimal, PTH levels are not elevated, and fracture risk is reduced at 25(OH)D levels greater than 75–80 nmol/L (30 to 32 ng/mL) [10, 14–17]. In this paper we describe vitamin D status in hip fracture patients according to the recently defined boundaries for vitamin D deficiency and sufficiency, information that was not included in our previous publications [1, 9].
Thus, vitamin D “sufficiency” in our cases of hip fractures is defined as a 25(OH)D level >80 nmol/L or 32 ng/mL. Although vitamin D “deficiency” was defined previously as 25(OH)D levels of 37.5 nmol/L (15 ng/mL) [18] or lower, some experts now define vitamin D deficiency as a 25(OH)D level of ≤50 nmol/L (20 ng/mL) [17, 19]. Because 25(OH)D level ≤22.5 nmol/L (9 ng/mL) represents a level below which osteomalacia and impaired muscle function may be present, we analyzed muscle function according to this extremely low level of 25(OH)D [8]. The very low vitamin D levels in this subset at that time made it possible to assess the effects of extreme vitamin D deficiency on longitudinal measures of muscle function in the Baltimore cohort as described below.
Intact PTH levels were measured by immunoradiometric assay (IncStar Corporation, Stillwater, MN) at 3, 10, 60, 180, and 365 days post fracture. As reported previously, consistent with the presence of vitamin D deficiency presented here, PTH levels rose gradually after fracture and were significantly elevated at 365 days compared with all other time points (P<0.0001) [1, 2]. Baseline samples for 25(OH)D levels were stored and measured in the same assays at the end of the study to reduce inter-assay variability. According to the study protocol, the investigators provided recommendations on the management of osteoporosis according to the National Osteoporosis Foundation (NOF) guidelines including calcium, vitamin D and physical activity. Bone density results were provided to patients and their primary care providers.
These studies were reviewed and approved by the Institutional Review Boards of the respective institutions and informed consent was obtained from each of the participants or a designated proxy.
Functional measures
Longitudinal functional testing after hip fracture was performed in the women enrolled in the Baltimore Hip Study during (or within 10 days of) hospitalization and at 2, 6, and 12 months post-fracture [20].
Lower extremity gain scale
Lower extremity performance was measured with the lower extremity gain scale (LEGS) [2]. The LEGS is a performance-based measure that focuses on clinically relevant aspects of lower extremity functioning for hip fracture subjects. The LEGS score is a composite measure of nine lower extremity functions, which include walking 3 meters (~10 feet), climbing up and down four stairs, rising from a chair, putting a sock and shoe on the affected leg, reaching for an item on the floor from a sitting position, and getting on/off the toilet. A clinically meaningful difference in the LEGS scores for a population in this age range has been shown to be 2–3 points [2].
Walking speed
The time needed to walk up to 3 meters (10 feet) at normal speed was measured to compute a walking speed (m/sec). Walking speeds were categorized by quartiles derived from published sources for normal elderly persons. In addition, 1 point was added to the score for subjects able to perform without needing assistance (e.g., a cane or walker). The scale ranges from 0 to 5, with higher scores indicative of better walking speed.
Chair rise time
The amount of time in seconds required to stand up from a chair with a level seat 0.42 m (1.38 feet) from the floor without using arms was assessed. The time was measured in seconds and categorized by quartiles derived from unpublished sources [3] for persons who had not fractured a hip. In addition, 1 point was added to the score for subjects able to rise without using their arms. The scale ranges from 0 to 5, higher score representing faster rising.
Grip strength
Strength was measured with a JAMAR Hydraulic Hand Dynamometer. After a practice test, scoring was based on the mean of the second and third tests.
Falls
Falls were recorded in a diary for the 80 women with hip fractures and summarized in patient and proxy follow-up surveys at 1, 2, 4, 6, 9, and 12 months post-fracture. Subjects were classified as fallers if they fell one or more times in the year post-fracture.
Balance
Balance was measured at 6 months by monitoring the length of time subjects could stand with eyes closed.
Co-morbidity
A modified Charlson type co-morbidity index was constructed from information derived from abstraction of medical charts. The index was developed by summing points awarded for each co-morbid condition based on the following scheme: 1 point for myocardial infarction, congestive heart failure, DVT or peripheral vascular disease, dementia or disorientation/confusion/delirium, COPD, arthritis, ulcers, or diabetes; 2 points for cancer or stroke; 3 points for cirrhosis. The score, thus, ranges from 0–15 with higher score indicating poorer health status. The resulting summary scores observed for these subjects ranged from 0 to 7 points, with a mean of 1.79 points (SD=1.63).
Pre-fracture functioning
Patients self-report of the number of activities of daily living (ADLs) in which they were impaired (needed equipment or human assistance or were unable to perform) was summarized to create an index of pre-fracture functioning. The ADLs included were walking 10 feet or across the room; walking one block; climbing five steps; getting into/out of a car; transferring into/out of bed; rising from an armless chair; putting on pants; putting on shoes and socks; getting into the bath; taking a bath; getting on to/off of the toilet; and reaching for an item on the ground from a sitting position.
Statistical analyses
Longitudinal data were analyzed with Proc Mixed in SAS version 8. An unstructured covariance matrix was specified and robust standard error terms were used. The analyses were adjusted for baseline ADLs dependence, age, summary score of co-morbidity, body mass index, and baseline levels of the dependent variables.
Results
The mean ages (±SD) for the Boston and Baltimore cohorts were 77.9±9.2 and 79.7±8.0 years, respectively. The median 25(OH)D levels were 32.5 nmol/L and 25.5 nmol/L (13.0 and 10.2 ng/mL), respectively. In the combined groups, vitamin D deficiency (defined as a 25(OH)D level of ≤50 nmol/L (20 ng/mL) was present in 85% of the subjects (Table 1). Extremely low 25(OH)D levels, defined as ≤22.5 nmol/L (9 ng/mL), were present in 38% of the women. The 80 women who were enrolled for longitudinal studies were classified on the basis of 25(OH)D levels equal to or less than or greater than 22.5 nmol/L (9 ng/mL), the lower limit of the assay when this study was performed (Table 2). There was significantly greater co-morbidity in the group with 25(OH)D levels ≤22.5 nmol/L (9 ng/mL) and fewer subcapital fractures (p=0.02), but other baseline characteristics were comparable.
Table 1.
Frequency of boundaries of 25-Hydroxyvitamin D level
| Extremely low ≤22.5 nmol/L (≤9 mg/mL) n (%) | Deficiency ≤50 nmol/L (≤20 ng/mL) n (%) | Insufficiency ≤80 nmol/L (≤32 ng/mL) n (%) | Sufficiency >80 nmol/L (>32 ng/mL) n (%) | |
|---|---|---|---|---|
| Combined groups | 42 (38%) | 94 (85%) | 106 (96%) | 4 (4%) |
| Boston | 10 (33%) | 23 (77%) | 27 (90%) | 3 (10%) |
| Baltimore | 32 (40%) | 71 (89%) | 79 (99%) | 1 (1%) |
Table 2.
Baseline characteristics of the subset of 80 women who had functional parameters measured up to 1 year after a hip fracture (m ± SD)
| Measure | All Subjects (n=80) | 25(OH)D ≤22.5 nmol/L [≤9 ng/mL (n=32)] | 25(OH)D >22.5 nmol/L [>9 ng/mL (n=48)] | p-value |
|---|---|---|---|---|
| a25-hydroxy Vitamin D (ng/mL) | 30.75 (21.5) | 15.25 (5.5) | 36.58 (22.0) | <0.001 |
| Age in years | 79.7 (8.0) | 80.8 (7.3) | 78.9 (8.4) | 0.32 |
| Pre-fracture | 1.64 (1.65) | 1.83 (1.83) | 1.51 (1.53) | 0.42 |
| Comorbidity | 1.79 (1.63) | 2.31 (1.96) | 1.44 (1.27) | 0.02 |
| BMI (kg/m2) | 23.5 (4.1) | 23.1 (4.5) | 23.8 (4.0) | 0.54 |
| Subcapital fracture | 53.8% | 37.5% | 65.2% | 0.02 |
| Arthroplasty | 47.4% | 46.7% | 47.8% | 0.92 |
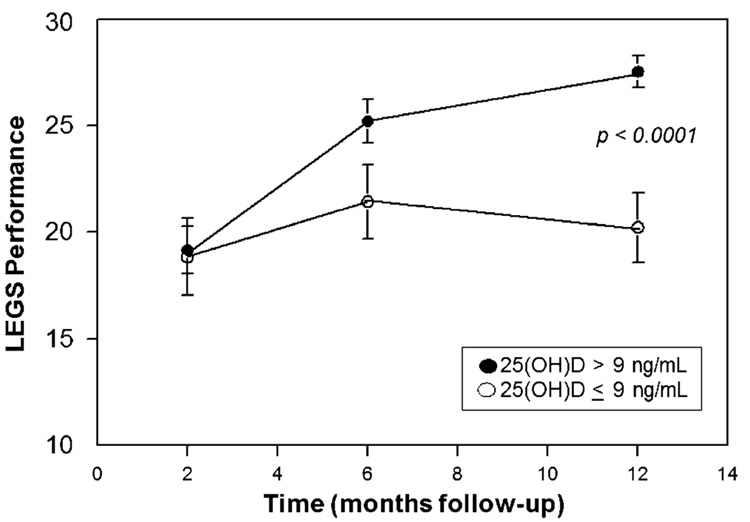
At 2, 6, and 12 months post-fracture, functional LEGS parameters, chair rise time, and grip strength were measured in a subset of between 44 to 55 subjects. Subjects with baseline 25(OH)D >22.5 nmol/L (9 ng/mL) showed an increase in LEGS performance with time after fracture whereas those with lower levels of 25(OH)D did not (P<0.0001, Fig. 1). There was also a significant difference in the chair stand time subscale at 6 months (P<0.05, Table 3). There were no differences, at any time, in grip strength or other measures of upper limb function (Table 3).
Fig. 1.
Effect of vitamin D status on lower extremity gain scale (LEGS, M ± SEM), measured at intervals following hip fracture. Subjects were classified on the basis of serum 25(OH)D levels on admission for hip fracture
Table 3.
Longitudinal analyses of other performance measures function of 25(OH)D levels a
| Longitudinal analysis (months) | |||
|---|---|---|---|
| Performance measure | 2 months | 6 months | 12 months |
| Chair subscale | |||
| 25(OH)D ≤ 22.5 nmol/L | 2.83 (0.26) | 2.56 (0.30) | 2.73 (0.24) |
| 25(OH)D > 22.5 nmol/L | 3.00 (0.12), ns | 3.32 (0.11), p<0.05 | 3.21 (0.14), ns |
| Grip strength (kg) | |||
| 25(OH)D ≤ 22.5 nmol/L | 16.30 (0.69) | 17.06 (0.84) | 16.53 (0.89) |
| 25(OH)D > 22.5 nmol/L | 17.74 (0.60), ns | 16.86 (0.76), ns | 16.90 (0.89), ns |
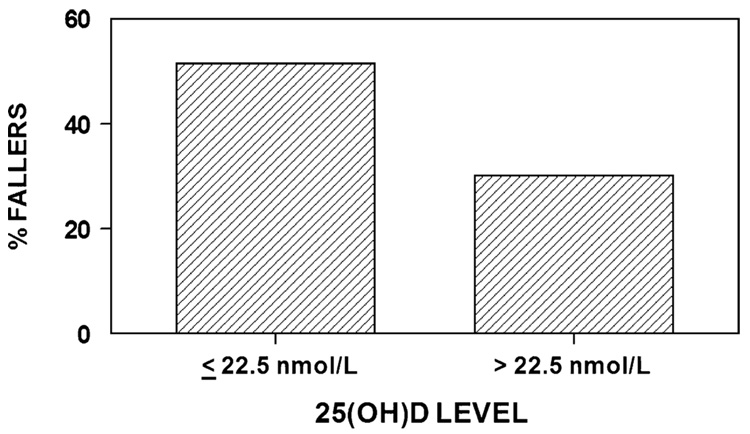
Baseline 25(OH)D levels were compared in those who fell once or more in the year post-fracture compared with those having no falls in that time interval. As shown in Fig. 2, women with 25(OH)D levels ≤22.5 nmol/L (9 ng/mL) were more likely to fall than those with levels greater than 22.5 nmol/L (9 ng/mL) (51.5% versus 30.0%, chi square with 1 df=3.88, p=0.049). In addition, balance times for the task “standing with eyes closed” were tested at 6 months. There was no difference between the two groups; for subjects with a very low 25(OH)D level at baseline (≤22.5 nmol/L) (9 ng/mL), mean standing time was 29.0 seconds; for those with 25(OH)D levels >22.5 nmol/L (9 ng/mL) the mean time was 28.3 seconds (p=0.68.)
Fig. 2.
Falls were more common in the group of patients who had vitamin D levels in the low range. Subjects with 25(OH)D levels ≤22.5 nmol/L (≤9 ng/mL) were more likely to fall than those with levels greater than 22.5 nmol/L (>9 ng/mL) (51.5% versus 30.0%, chi square with 1 df=3.88, p=0.049). The mean (SD) baseline 25(OH)D levels for fallers was 26.50 (13.50) versus non-fallers 33.75 (24.75)
Discussion
Hip fractures increase exponentially with age and are associated with impaired function, disability, and increased mortality. This study shows that according to the current thresholds for low vitamin D levels, 85% of the women admitted with hip fractures in Boston and Baltimore had vitamin D deficiency defined as a 25(OH)D level less than 50 nmol/L (20 ng/mL), and only 4% of these women had 25(OH)D levels in the sufficient range of >80 nmol/L (32 ng/mL). Extremely low levels of vitamin D of ≤22.5 nmol/L (≤9 ng/mL) were present in 38% of the women at the time of fracture; these extremely low vitamin D levels were associated with impaired lower extremity function and elevated risk of falls one year post-fracture, features that were not explained by differences in balance. To our knowledge, this is one of the first studies to report that very low levels of vitamin D at the time of fracture are associated with impaired lower extremity function one year later.
Vitamin D-deficiency and insufficiency have been linked to the risk of an increase in PTH levels, which can adversely affect the skeleton. Although thresholds for vitamin D deficiency defined as the level at which PTH levels increase may be affected by calcium intakes, vitamin D deficiency is well established in patients with low bone mass, elderly residents of nursing homes, centarians, and hospitalized subjects with co-morbid medical conditions [13, 18, 21, 22]. Previous data from the United States and Europe indicate that vitamin D deficiency is underdiagnosed in adults with hip fractures [9, 10]. Despite the prevailing data that vitamin D deficiency is preventable and treatable, <29% of subjects with hip fractures are treated with calcium and vitamin D or other therapies to reduce fractures [23, 24]. Although to our knowledge there are no long-term studies, a short-term study in a rehabilitation hospital in Italy showed a positive association between 25(OH)D levels in hip fracture subjects and functional recovery assessed by the Barthel Index score [25]. According to our data, after one year the composite LEGS score was markedly lower and the number of falls was greater in women with extremely deficient baseline 25(OH)D levels. Although this was not a randomized or an intervention study, our data suggest that a higher vitamin D level at the time of fracture is associated with better lower extremity task performance and a reduced likelihood of falling during the year following a hip fracture. These results could be explained by the following biologic mechanism: it is possible that the very low 25(OH)D levels did not provide sufficient substrate for the kidney to convert 25(OH)D to 1,25 dihydroxyvitamin D, which binds to 1,25 dihydroxyvitamin D receptors in muscle. The renal 1-hydroxylase, however, is activated by low levels of calcium and phosphorus or high concentrations of parathyroid hormone. Thus, patients with severe vitamin D deficiency may have normal, low, or even high 1, 25 dihydroxyvitamin D levels. There is increasing interest in the autocrine and paracrine effects of vitamin D and the intracellular conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D in many tissues [26, 27]. Therefore, the reduced substrate availability in hip fracture patients and low intra-cellular conversion of 25-hydroxyvitamin D to 1, 25 dihydroxyvitamin D may potentially contribute to their muscle weakness, impaired lower extremity muscle function, and increased falls.
Recent data suggest a possible link between vitamin D deficiency and musculoskeletal function. Vitamin D deficiency may lead to loss of type II muscle fibers, and thereby to atrophy of proximal muscles with an increased risk of falling and fractures [28]. In addition, other studies indicate an association between 25(OH)D levels and lower extremity functional parameters. In a cross-sectional analysis of subjects enrolled in NHANES III study who were 60 years or older, those with 25(OH)D levels between 40 and 94 nmol/L (16.0 to 37.7 ng/mL) had better lower extremity muscle function (walking speed and chair rise) than those with lower levels [8].
Vitamin D deficiency is also associated with musculo-skeletal pain and dysfunction, which may be improved with vitamin D replacement. In a study of 150 patients with musculoskeletal pain, 93% of the patients had vitamin D deficiency with a 25(OH)D level of <50 nmol/L (20 ng/mL). In a population survey among 349 elderly participants, Mowe et al. found reduced muscle function in the subjects with low vitamin D levels [29]. In that study, vitamin D levels were positively associated with arm muscle strength, ability to climb stairs, physical activity and the absence of fall occurrences. In another study 6-month treatment with a vitamin D analog (alphacalcidol) led to significant improvements (compared with controls) in isometric knee extensor strength and an increase in walking distance [30]. A randomized study in elderly women indicated that supplementation with vitamin D (800 IU/d) and calcium compared with calcium alone lead to a 49% reduction in falls and improved musculoskeletal function in only 3 months [31]. In addition, a meta-analysis of randomized controlled studies concluded that vitamin D supplementation significantly reduces the risk of falls among older healthy individuals by 22% [32]. Thus, on the basis of the available data and those presented here, low vitamin D levels may lead to impaired muscle function and increased risk of falls, which may be reversible.
Several but not all studies show an association between vitamin D therapy and reduced risk of fractures. A randomized controlled trial in older British subjects of 100,000 IU of vitamin D3 every 4 months reported reduction of fractures with restoration of vitamin D levels to 75 nmol/L (30 ng/mL) [33]. In the large Women’s Health Initiative Calcium and Vitamin D trial, postmenopausal women with average calcium intakes of ~1150 mg/day were randomized to 1000 mg of calcium carbonate with 400 units of vitamin D versus placebo. According to an intention to treat analysis, calcium and vitamin D supplementation did not reduce the risk of hip fractures. However, adherent women who took at least 80% of their supplements had a 29% reduction in hip fracture; and in women 60 years of age and older there was a significant 21% reduction in hip fractures [34]. In another study, elderly ambulatory women who were institutionalized with mean 25(OH)D levels of ~22.5 nmol/L (9.0 ng/mL), supplementation with calcium and vitamin D3 led to a decrease in secondary hyperparathyroidism and a 43% reduction in hip fractures [10]. However, a recent randomized, placebo-controlled trial (Randomized Evaluation of Calcium or Vitamin D) found no difference in the incidence of new fractures among participants allocated to calcium (1000 mg) and those who were not and participants allocated to vitamin D3 (800 IU/D) and those who were not [35]. Low compliance was a limitation of that study, with 54.5% of subjects taking tablets at 24 months and the achieved 25 (OH)D level was only 62 nmol/L (25 ng/mL), which is below the 25(OH)D level associated with reduced fracture risk of >75 to 80 nmol/L (30 to 32 ng/mL) [16].
The significance of our study relates to the needed care for patients with hip fractures who are at high risk for reduced functional recovery and mortality. Extremely low vitamin D levels are common in hip fracture patients and they may lead to impaired lower extremity functional recovery and increased falls. We have put in place hip fracture care pathways at Brigham and Women’s Hospital and the University of Maryland to transform the clinical care of patients with hip fractures [36, 37]. A limitation of our study is that, because only a small fraction of women with a hip fracture had a 25(OH)D level that was in the “sufficient range” (greater than 80 nmol/L or 32 ng/mL), we could not assess changes in functional performance over a wide range of 25(OH)D levels. Second, longitudinal performance measures were studied in 44 to 55 subjects and the possibility of residual confounding cannot be eliminated, although longitudinal analyses were adjusted for age, a summary score of co-morbidity, body mass index, and baseline activities of daily living. In addition, we do not have important information on the re-fracture rate in these women with low vitamin D levels. Finally, we do not have information about vitamin D treatment after this study was completed.
At the present time, the standard of care nationally does not include supplementation of hip fracture subjects with vitamin D and calcium. Correction of vitamin D deficiency may be a key factor in functional benefits of rehabilitative programs, fracture repair, and deriving maximal benefits from potent inhibitors of bone resorption and anabolic agents. Definitive tests of the independent or combined effects of vitamin D on lower extremity function, fall prevention, and other adverse outcomes of hip fracture need to await randomized clinical trials in which administration of therapeutic doses of vitamin D to correct vitamin D insufficiency are evaluated following a hip fracture. Until more information is available, the current clinical implications from this and other recent studies for hip fracture patients are as follows: 1) vitamin D levels should be measured in patients with hip fractures and low vitamin D levels should be treated with appropriate doses of vitamin D; 2) changes in hospital wide-policies are necessary to ensure adequate calcium and vitamin D supplementation/replacement; 3) definitive treatment of the underlying osteoporosis or metabolic bone disease should be systematically and routinely implemented. There are national efforts through the Center for Medicare and Medicaid Services in the United States that seek to improve fracture care. A new 2007 and 2008 initiative through the Physician Quality Reporting Initiative (PQRI) includes osteoporosis care and management as a quality measure for adults with fractures [38].
In summary, there is a high prevalence of vitamin D deficiency in community-dwelling American women with hip fractures. Measurement of vitamin D-deficiency is easily preventable and treatable. Correction of vitamin D deficiency in fracture patients with pharmacological doses of vitamin D may potentially improve lower extremity function and hip fracture repair and reduce the risk of falls, which are commonly associated with hip fractures. However future prospective trials are necessary to demonstrate the long-term effects of vitamin D repletion at the time of hip fracture on lower extremity muscle function, falls, and the refracture rates. Until those data are available, supplementation with vitamin D of 800–1000 units/day, or much higher doses in the setting of vitamin D deficiency may be critical for the prevention of falls and fractures in the elderly.
Acknowledgments
We thank Natalie Glass, B.A., M.A. for her assistance in manuscript preparation.
Dr. Jay Magaziner and Dr. Meryl LeBoff had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
All Funding Sources Funding support, in part by: NIH R01 AG13519, NIH R01 AG 12271, and R01 25015 (MSL, JG), the NCRR, General Clinical Research Center (Brigham and Women’s Hospital), and R37 G09901 and R01 AG18668 CWH (WH, JY, JM). Support also was provided by the Claude D. Pepper Older Americans Independence Center (OAIC) P60 AG12583 (WH, JM).
Footnotes
Conflict of interest statement Dr. LeBoff works on active research projects that are supported by the National Institutes of Health; she has an unrestricted Center of Excellence education grant from Abbott and served as a consultant for an advisory board dinner for Eli Lilly and Company. At the time of the study, Dr. LeBoff served on the Scientific Advisory Board for Incstar, which manufactured a vitamin D assay, but she is no longer a member of the board. Incstar did not provide any funding for this study, and the study is not about any technology that is owned by or contractually obligated to Incstar in any way. Dr. Magaziner consults for Merck and has active research projects that are supported by the National Institutes of Health, Merck, and Novartis Pharmaceuticals. He also has lectured for Pfizer and consulted for Amgen and GTx, Inc. in the past year. All other authors have no conflicts of interest.
The present study evaluates the impact of extremely low vitamin D levels on functional recovery from hip fractures over 1 year post-fracture according to validated assessments of lower extremity muscle function. In addition, we also determined the associations between extremely low vitamin D levels at the time of hip fracture and measures of grip strength, falls, balance, and body composition.
Contributor Information
M. S. LeBoff, Skeletal Health and Osteoporosis Center and Bone Density Unit, Endocrine, Diabetes, and Hypertension Division, Brigham and Women’s Hospital, 221 Longwood Ave, Boston, MA 02115, USA, e-mail: mleboff@partners.org
W. G. Hawkes, Division of Gerontology, Department of Epidemiology and Preventive Medicine, School of Medicine, University of Maryland Baltimore, 660 W. Redwood Street, Suite 200, Baltimore, MD 21201, USA, email: whawkes@epi.umaryland.edu
J. Glowacki, Department of Orthopedic Surgery, Brigham and Women’s Hospital and Harvard Medical School, 221 Longwood Avenue, Boston, MA 02115, USA e-mail: jglowacki@partners.org
J. Yu-Yahiro, Department of Orthopedics, The Union Memorial Hospital, 3333 N. Calvert Street, Suite 400, Baltimore, MD 21218, USA, e-mail: Janet.Yu.Yahiro@Medstar.Net
S. Hurwitz, Center for Clinical Investigation, Brigham and Women’s Hospital, Harvard Medical School, 1620 Tremont St., One Brigham Circle, Boston, MA 02120, USA, e-mail: hurwitz@hms.harvard.edu
J. Magaziner, Division of Gerontology, Department of Epidemiology and Preventive Medicine, School of Medicine, University of Maryland Baltimore, 660 W. Redwood Street, Suite 200, Baltimore, MD 21201, USA, e-mail: jmagazin@epi.umaryland.edu
References
- 1.Fox KM, Magaziner J, Hawkes WG, et al. Loss of bone density and lean body mass after hip fracture. Osteoporos Int. 2000;11(1):31–35. doi: 10.1007/s001980050003. [DOI] [PubMed] [Google Scholar]
- 2.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000 Sep;55(9):M498–M507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Harris TB, Fox KM, et al. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci. 2000 Aug;55(8):M434–M440. doi: 10.1093/gerona/55.8.m434. [DOI] [PubMed] [Google Scholar]
- 4.Magaziner J, Lydick E, Hawkes W, et al. Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health. 1997 Oct;87(10):1630–1636. doi: 10.2105/ajph.87.10.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehren LE, Hawkes WG, Orwig DL, Hebel JR, Zimmerman SI, Magaziner J. Gender differences in mortality after hip fracture: the role of infection. J Bone Miner Res. 2003 Dec;18(12):2231–2237. doi: 10.1359/jbmr.2003.18.12.2231. [DOI] [PubMed] [Google Scholar]
- 6.Office of the Surgeon General. Bone Health and Osteoporosis: A Report of the Surgeon General. United States Department of Health and Human Services, ed. 2004:6. [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004 Feb;19(2):265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥60 y. Am J Clin Nutr. 2004 Sep;80(3):752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 9.LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. Jama. 1999 Apr 28;281(16):1505–1511. doi: 10.1001/jama.281.16.1505. [DOI] [PubMed] [Google Scholar]
- 10.Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002 Mar;13(3):257–264. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 11.Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: No easy task. J Clin Endocrinol Metab. 2004 Jul;89(7):3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 12.Kolatkar NS, LeBoff MS. Analytic challenges in monitoring vitamin D therapy. Am J Clin Pathol. 2007 Mar;127(3):472–473. [PubMed] [Google Scholar]
- 13.Haden ST, Fuleihan GE, Angell JE, Cotran NM, LeBoff MS. Calcidiol and PTH levels in women attending an osteoporosis program. Calcif Tissue Int. 1999 Apr;64(4):275–279. doi: 10.1007/s002239900618. [DOI] [PubMed] [Google Scholar]
- 14.Heaney RP. Calcium absorption. J Bone Miner Res. 1989 Oct;4(5):795–796. doi: 10.1002/jbmr.5650040521. [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP. Vitamin D: how much do we need, and how much is too much? Osteoporos Int. 2000;11(7):553–555. doi: 10.1007/s001980070074. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005 Jul;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005 Jun;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 18.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990 Jun;51(6):1075–1081. doi: 10.1093/ajcn/51.6.1075. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006 Jul;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Wehren LE, Hawkes WG, Hebel JR, Orwig DL, Magaziner J. Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci. 2005 Jan;60(1):80–84. doi: 10.1093/gerona/60.1.80. [DOI] [PubMed] [Google Scholar]
- 21.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998 Mar 19;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 22.Passeri G, Pini G, Troiano L, et al. Low vitamin D status, high bone turnover, and bone fractures in centenarians. J Clin Endocrinol Metab. 2003 Nov;88(11):5109–5115. doi: 10.1210/jc.2003-030515. [DOI] [PubMed] [Google Scholar]
- 23.Harrington JT, Broy SB, Derosa AM, Licata AA, Shewmon DA. Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum. 2002 Dec 15;47(6):651–654. doi: 10.1002/art.10787. [DOI] [PubMed] [Google Scholar]
- 24.Simonelli C, Chen YT, Morancey J, Lewis AF, Abbott TA. Evaluation and management of osteoporosis following hospitalization for low-impact fracture. J Gen Intern Med. 2003 Jan;18(1):17–22. doi: 10.1046/j.1525-1497.2003.20387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A. Muscle mass and functional recovery in women with hip fracture. Am J Phys Med Rehabil. 2006 Mar;85(3):209–215. doi: 10.1097/01.phm.0000200387.01559.c0. [DOI] [PubMed] [Google Scholar]
- 26.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005 Jul;289(1):F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 27.Bouillon R. Vitamin D: from photosynthesis, metabolism, and action to clinical applications. In: DeGroot LJ JJd., editor. Endocrinology. Philadelphia: [Google Scholar]
- 28.Sato Y, Inose M, Higuchi I, Higuchi F, Kondo I. Changes in the supporting muscles of the fractured hip in elderly women. Bone. 2002 Jan;30(1):325–330. doi: 10.1016/s8756-3282(01)00645-7. [DOI] [PubMed] [Google Scholar]
- 29.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999 Feb;47(2):220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 30.Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA. Muscle strength, functional mobility and vitamin D in older women. Aging (Milano) 2000 Dec;12(6):455–460. doi: 10.1007/BF03339877. [DOI] [PubMed] [Google Scholar]
- 31.Bischoff-Ferrari HA, Conzelmann M, Dick W, Theiler R, Stahelin HB. Effect of vitamin D on muscle strength and relevance in regard to osteoporosis prevention. Z Rheumatol. 2003 Dec;62(6):518–521. doi: 10.1007/s00393-003-0561-4. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. Jama. 2004 Apr 28;291(16):1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomized double blind controlled trial. Bmj. 2003 Mar 1;326(7387):469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006 Feb 16;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 35.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomized Evaluation of Calcium Or vitamin D, RECORD): a randomized placebo-controlled trial. Lancet. 2005 May 7–13;365(9471):1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 36.Glowacki J, LeBoff MS, Kolatkar NS, Thornhill TS, Harris MB. Importance of vitamin D in hospital-based fracture care pathways. J Nutr Health Aging. 2008 doi: 10.1007/BF02982657. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streeten EA, Mohamed A, Gandhi A, et al. The inpatient consultation approach to osteoporosis treatment in patients with a fracture. Is automatic consultation needed? J Bone Joint Surg Am. 2006 Sep;88(9):1968–1974. doi: 10.2106/JBJS.E.01072. [DOI] [PubMed] [Google Scholar]
- 38.CMS. Physician Quality Reporting Initiative. 2007 September 7; http://www.cms.hhs.gov/pqri/