Cell Adhesion Receptors in Mechanotransduction (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 1.
Published in final edited form as: Curr Opin Cell Biol. 2008 Jun 24;20(5):551–556. doi: 10.1016/j.ceb.2008.05.005
Abstract
Integrins and cadherins are tri-functional: they bind ligands on other cells or in the extracellular matrix, connect to the cytoskeleton inside the cell, and regulate intracellular signaling pathways. These adhesion receptors therefore transmit mechanical stresses and are well positioned to mediate mechanotransduction. Studies of cultured cells have shown that both integrin- and cadherin-mediated adhesion are intrinsically mechanosensitive. Strengthening of adhesions in response to mechanical stimulation may be a central mechanism for mechanotransduction. Studies of developing organisms suggest that these mechanisms contribute to tissue level responses to tension and compression, thereby linking morphogenetic movements to cell fate decisions.
Introduction
The importance of mechanical force to development, differentiation, normal physiology and various disease states is well established. Current attention is focused on the molecular mechanisms used by cells and tissues to sense and respond to changes in their physical microenvironments. As might be expected from their roles as receptors that both transmit mechanical forces and regulate a myriad of intracellular signaling pathways, cadherin and integrin dependent adhesive specializations have emerged as critical components of the cellular tension-sensing apparatus. Here we discuss recent work about how these receptors participate in the propagation of mechanical signals that regulate a variety of cell and tissue behaviors. The importance of cell adhesion and mechanotransduction is also considered in the context of cell differentiation, embryonic development and tissue morphogenesis.
Integrins as mechanosensors
Integrins have been implicated in a remarkable range of mechanotransduction phenomena, including cellular responses to stretch, elevated hydrostatic pressure, fluid shear stress and osmotic forces [1]. In the case of stretch, cells on elastic substrata are subject to strain delivered through their adhesive contacts, thus, the involvement of integrins is obvious. In the other systems, the requirement for integrins in force transmission is not obvious and evidence suggests their roles are indirect.
Integrins in initiation of mechanotransduction
Numerous studies have shown that increasing the force on an adhesion, either by increasing cellular contractility or by external application of force, strengthens and enlarges the adhesions (reviewed in [2]). This mechanism appears to govern maturation of small focal complexes into larger focal adhesions as well as govern focal adhesion dynamics. At least two mechanisms have been identified. First, increasing tension on integrins leads to rapid (10's of sec) recruitment of vinculin, zyxin and probably other focal adhesion components. Second, over minutes to 10's of minutes, tension triggers conformational activation of a subset of cells’ unoccupied integrins, which induces their binding to the ECM proteins on the substratum [3]. These mechanisms probably adjust adhesion strength to the requirements of the environment.
Although likely, there is no direct evidence that the first type of rapid adhesion strengthening initiates cytoplasmic signaling to promote gene expression, etc. However, conformational activation and new binding via the second mechanism mediates activation of JNK in response to stretching of elastic substrata [3]. Interestingly, JNK activation is seen in cells adherent to only a subset of ECM proteins, consistent with this model. Multiple studies have shown that applying strain to adherent cells triggers activation of focal adhesion kinase and c-src [1]. These events occur over 10's of minutes and correlate with increased integrin recruitment and clustering in focal adhesions. Inhibiting these kinases blocks multiple downstream events, such as activation of MAP kinases and cell cycle progression. These data therefore support the idea that focal adhesion strengthening triggers signaling.
The molecular mechanisms that govern focal adhesion strengthening are not understood, however, mechanotransduction is generally thought to involve protein domains that undergo conformation changes under force [4]. The adapter protein p130Cas was found to mediate activation of Rap1 in response to force [5]*. Application of strain to purified p130Cas was found to increase the susceptibility of its substrate domain tyrosines to kinases; phosphorylation created SH2 sites that recruited Crk and the Rap1 GEF C3G to activate Rap1. Though not explored in that study, Rap1 can induce integrin conformational activation through its effector RIAM, which binds directly to talin [6]. Thus the p130Cas-Rap1 pathway could mediate adhesion strengthening through integrin activation as described above.
Responses to stretch play crucial roles in regulation of heart function. Contractility of the heart is regulated on a subsecond time scale by the stretch due to filling volume (Starling's Law) [7]. Over weeks to years, the heart remodels to maintain efficient pumping in the face of elevated pressure. In heart failure, compensatory remodeling cannot keep up with demand and pathological remodeling leads to decreased output. Several proteins that localize to costamers in the intact organ and focal adhesions in cultured cells have been implicated in adaptive remodeling and heart failure. Deletion of the gene for melusin does not block development of the heart or function under normal conditions [8]. However, when challenged by pressure overload, melusin−/− mice develop dilated cardiomyopathy, essentially a failure to undergo adaptive hypertrophy. Melusin−/− hearts did not activate GSK3βand Akt in response to pressure overload, suggesting that a defect in mechanotransduction mediates later heart failure.
Integrin linked kinase (ILK) has also been implicated in heart failure [9]**. ILK deletion in mice causes early embryonic death, which precludes analysis of heart function. However, zebrafish embryos survive longer with heart defects. A mutation that blocks expression of two mechanosensitive genes, atrial natriuretic factor and VEGF, was identified as a point mutation in ILK that blocked both ILK binding to β-parvin and putative kinase activity. This mutation, anti-sense suppression of ILK or β-parvin do not inhibit initial heart formation or function but cause failure at later times.
Distinguishing a structural role from a role in mechanotransduction per se is a major challenge in these systems. The fact that adhesion strengthening, a form of mechanotransduction, contributes to mechanical stability further complicates the analysis. However, in fibroblasts, integrin linked kinase (ILK) is required for the rapid (5 min) activation of RhoA in response to cyclic stretch and the subsequent induction of tenascin-C expression, even though stretch activation of Akt and Erk are unaffected [10]. These data lend support to the notion that ILK contributes to transduction and not only structure.
Integrins as intermediates in mechanotransduction
Paradoxically, integrins are also implicated in mechanotransduction in other systems where they are not force-bearing elements. Osmotic stress responses allow cells to adjust to both changes in extracellular osmolarity and to stimuli such as insulin that that alter in ion transport [11]. Osmotic stress triggers activation of FAK and src, which require adhesion to a substratum and are blocked by anti-integrin antibodies [12]. Pulling directly on β1 integrins with magnetic beads triggers opening of the same chloride channels as does cell swelling. Osmotic stress opening of stretch-activated channels that transport Ca2+ is modulated by the matrix on which cells are plated and by soluble integrin agonists [13].
Cells also respond to hydrostatic pressure. A small increase (15 Torr) increased cell adhesiveness, activated src and FAK, and induced phosphorylation of α-actinin on a critical tyrosine [14]. In chondrocytes, “pressure-induced strain” that involves higher pressure (120 Torr) and very low strain (0.31%) stimulated FAK phosphorylation and K+ channel activity, leading to hyperpolarization [15]. These events were blocked by antibodies to integrin α5β1 or CD47, or by suppression of CD47 expression. CD47, also known as integrin associated protein, physically associates with α5β1 in these cells. In other systems, CD47 also activates integrins to increase cell adhesion, which may be related (below).
Fluid shear stress acting upon endothelial cells is a major determinant of vascular morphology and function [16]. It has been proposed that the force of fluid flow must be transferred to cell-cell and cell matrix contacts that serve as anchor points to prevent cell detachment [17]. However, forces from fluid flow are 100−1000 times less than typical traction forces and many of the responses are specific to endothelial cells. Yet, once again, flow activates FAK and src, and many responses to flow are integrin-dependent. There is now strong evidence that integrins undergo conformational activation in response to flow [18] but activation occurs downstream of a complex of proteins at cell-cell junctions ([19]; see next section). Newly activated integrins then bind to ECM proteins beneath the cell and initiate a wave of signaling. Importantly, because different integrins signal differently, the subendothelial ECM modulates responses to flow. While some pathways (eg. eNOS activation) are unaffected, ECs on proteins typical of inflamed or injured tissue like fibronectin and fibrinogen support flow activation of inflammatory pathways such as NF-κB and PAK [20,21]. By contrast, cells plated on basement membrane proteins fail to activate these pathways. These effects correlate with patterns of ECM staining and inflammatory markers in vivo.
The flow system provides a paradigm that may explain the involvement of integrins in other situations. The evidence is weaker for stretch, osmotic stress and hydrostatic pressure, but suggests that these forces trigger activation of integrins to increase cell adhesiveness, which leads to new binding and signaling. Mechanical stresses might also trigger increased integrin clustering, possibly through cytoskeletal reorganization [22]. This model could explain how so many different mechanical stimuli can share common integrin-dependent pathways. Of course, each type of mechanical response has unique outputs as well.
Cadherins as mechanotransducers
Though weaker than for integrins, the data suggest that cadherin-dependent cell-cell junctions exhibit similar force-dependent behavior. When E-cadherin-expressing cells contact one another, Rho activation [23] and myosin II regulatory light chain (MLC) phosphorylation increase [24]. Both myosin light chain kinase (MLCK) and ROCK contribute to MLC phosphorylation, and, together with myosin II, are required for proper junction formation [24]. Careful microscopic analysis of initial junction formation revealed that Rho is activated toward the outer edges of the forming cell-cell contact and that myosin-dependent contractility expands the contact zone by pulling outward [25]*. Cadherins also appear to undergo conformation changes that modulate their homophilic adhesiveness [26], similar to integrins.
Plating cells on surfaces coated with cadherin extracellular domains triggered cell spreading and clustering of cadherins into linear streaks at the ends of actin bundles, closely resembling focal adhesions [24,27]. Cells adhering via N-cadherin exerted traction forces on the substratum that were only moderately lower than forces exerted through integrins [28]. Both cadherin clustering and adhesion strength were dependent on myosin II. Myosin-dependent cadherin clustering on surfaces uniformly coated with ligand strongly suggests cellular mechanisms that promote local positive cooperativity. Adhesion strengthening under force is one such mechanism.
Myosin VI has also been implicated in the late stage of junction maturation [29]*. This myosin is a minus end directed motor previous implicated in vesicular transport. However, in this system, E-cadherin surface expression was unaffected. Instead, myo VI was recruited to junctions during the late phase of maturation. It also contributed to adhesion strength on cadherin extracellular domain surfaces. Myosin VI mediated the recruitment of vinculin, which mediated these effects. Whether vinculin recruitment is specifically force-dependent as it is in focal adhesions is unknown but the parallel is intriguing. Taking these data together, cadherins appear to possess the features needed for force-dependent adhesion strengthening similar to integrins. Whether this actually happens has not been definitively demonstrated but data are supportive.
Cadherins have also been implicated in mechanotransduction in specialized systems. Inner ear hair cells contain apical villi that are connected by a filament called the tip link [30]. Bending by sound waves causes relative displacement of long and short villi, creating tension that triggers ion channel opening. Tip links are essential for this process and mutations in cadherins 23 and 15 that localize to these structures cause deafness. Recent work established that these cadherins undergo heterophilic end-to-end binding to form the tip link [31]**. They are therefore essential for formation of the structure that transmits force to stretch-activated channels.
VE-cadherin, the classical cadherin specific to vascular endothelium, has been implicated in responses to both flow and stretch. As mentioned above, ECs are highly sensitive to the rather modest force exerted by flowing blood on their apical surface. The flow sensor that initiates conformational activation of integrins is comprised of PECAM-1, VE-cadherin and VEGFR2 [19]. However, the role of VE-cadherin in this system appears to be as an adapter rather than direct force transducer. Indeed, cadherin homophilic binding was not required. Instead, force was transduced or transmitted by PECAM-1. Interestingly, stretching endothelial monolayers stimulates cell cycle progression in a VE-cadherin-dependent manner [32]. A role for PECAM-1 was not excluded, however, unlike flow, an antibody that blocks VE-cadherin homophilic adhesion inhibited DNA synthesis. Interestingly, Rac was activated by stretch and was required for cell cycle progression. Thus, it seems likely that VE-cadherin mediates effects of stretch in this system.
Adhesion Dependent Mechanotransduction and Gene Expression
Morphogenetic processes during embryonic development generate forces both locally and on adjacent tissues. Resultant mechanical signals may be mediated by both integrin-ECM and cadherin-dependent adhesions. However, direct evidence linking specific adhesive contacts to mechanical signals has only recently begun to emerge.
Regulation of Stem Cell Lineage Commitment by Mechanical Forces
Investigations of stem cell lineage commitment and specification provide prime examples of mechanical signaling in developmental processes. Human mesenchymal stem cells (MSCs) can differentiate into a range of anchorage-dependent cell types. Plating density alone, which varies the area cells have to spread, can affect MSC differentiation [33]. McBeath and colleagues [34] demonstrated that these constraints on cell shape likely contribute to MSC lineage specification. Micropatterned substrates composed of fibronectin “islands” of defined size were used to constrain the area and shape occupied by single MSC cells. Under these conditions, cells on smaller islands became adipocytes whereas cells on larger islands that spread to cover larger areas progressed to an osteogenic lineage. Importantly, Rho/ROCK activity and acto-myosin generated tension were required for specification of osteogenic differentiation in highly spread cells, highlighting the importance of mechanical cues in driving cell fate decisions [34]. These results fit well with studies showing that cell contractility and traction forces increase as spread area increases [35], suggesting that higher forces mediate the effect of spread area on differentiation.
Recent work suggests that the elastic properties of the substrate can also profoundly influence MSC lineage specification [36]**. In these studies, soft substrates promote neurogenic differentiation, intermediate substrates promote myogenic differentiation and the stiffest substrates promoted an osteogenic phenotype. In each case, the elastic modulus of the substrates matched the properties of the relevant tissue in vivo. Again, inhibition of acto-myosin contractility shifted differentiation toward the neurogenic pathway [36], consistent with the idea that cell generated force is the crucial variable. While these studies illustrate the importance of cell-ECM contact (i.e., fibronectin and collagen I, respectively), the role of cell-cell adhesive contacts has not been addressed.
Mechanotransduction in Embryogenesis
Mechanical information may also inform cell fate decisions during embryogenesis. Studies of Drosophila development [37] showed that physical compression (equivalent to 10% uniaxial lateral deformation) of embryos at the cellular blastoderm stage dramatically expanded the expression of the transcription factor Twist. Twist is normally expressed on the ventral side of the embryo at this stage but when constrained for as little as 5 minutes, was observed in all cells. Twist transcriptional activation occurred within 10 minutes and required translocation of Armadillo/β-catenin to the nucleus. Furthermore, anterior expression of Twist in unperturbed embryos requires germ band extension, where normal mesoderm movements exert pressure on anterior cells. Nuclear localization of β-catenin is also seen in this setting. Thus, mechanical forces may be part of the normal developmental program that regulates Twist expression. It is not yet clear how embryos sense mechanical force but a mechanism involving dynamic associations of cadherin/catenin complexes is attractive.
Adhesion-Dependent Mechanotransduction and Morphogenesis
Mechanical forces also likely play key roles in controlling cell and tissue morphogenetic movements such as the extensive rearrangements during convergent extension [38,39]. A longstanding problem in development has been how dissociated early embryonic cells reaggregate and sort out according to their germ layer origins [40]. This sorting behavior may be relevant to understanding cell intercalation movements during convergent extension and other rearrangements. The differential cell adhesion hypothesis [41] has been the predominant model but the importance of cortical surface tension was recently recognized [42]. Krieg and colleagues [43]* used atomic force microscopy to measure both adhesive strength and cell-cortex tension in individual zebrafish gastrula cells from the 3 primary germ layers. They found that differences in actomysoin contractility and cortical tension among germ layer cells were critical for cell sorting behaviors and suggested that differential adhesion is alone insufficient to drive sorting. However, there is evidence from other systems that integrin or cadherin-mediated adhesion can control cortical tension or cortical cytoskeletal organization [23,44]. Thus, adhesion receptors may contribute to cortical tension as well as the purely adhesive component of cell sorting.
We have considered only a sampling of developmentally significant processes that are most likely to involve direct force application through adhesive contacts. However, many other events may operate indirectly through adhesion-dependent mechanical linkages. Dynamic mechanical stresses in processes as disparate as fluid sheer stress, intravascular and interstitial flow, hydrostatic pressure and gravity may each contribute to adhesive mechanotransduction signaling.
Conclusions
Cell culture models have provided strong evidence that multiple classes of cell adhesion receptors participate in mechanotransduction. Evidence suggests that adhesions strengthen under applied force, which is crucial for stable adhesion. By promoting recruitment of new components, strengthening leads to activation of signaling pathways. However, if strengthening were universal, adhesions would always be long lived and cell migration or other tissue rearrangements would be difficult. Thus, adhesion strengthening and subsequent signaling must be regulated. How cells control these mechanisms is an important question for future investigation.
Developmental models have begun to yield insights into the role of forces in regulating cell fate and patterning decisions in embryos. As in cell culture, adhesion receptors have emerged as prominent players in these processes. Their exact roles in mechanotransduction, however, are poorly understood. Cooperation between integrins and cadherins is suggested by many studies. These receptors interact with a common cytoskeletal network and it is likely that adhesive strengthening in response to force on cell-matrix adhesions can influence cadherin adhesion and signaling, and vice versa. However, definitive evidence for coordinated mechanotransduction in vivo is lacking, as is information about mechanism. New methods for assaying local signaling events, for detecting protein unfolding under force and for analyzing patterns of gene expression will help establish both the universality of mechanical force as a regulator of gene expression and elucidate specific pathways and mechanism in individual developmental processes.
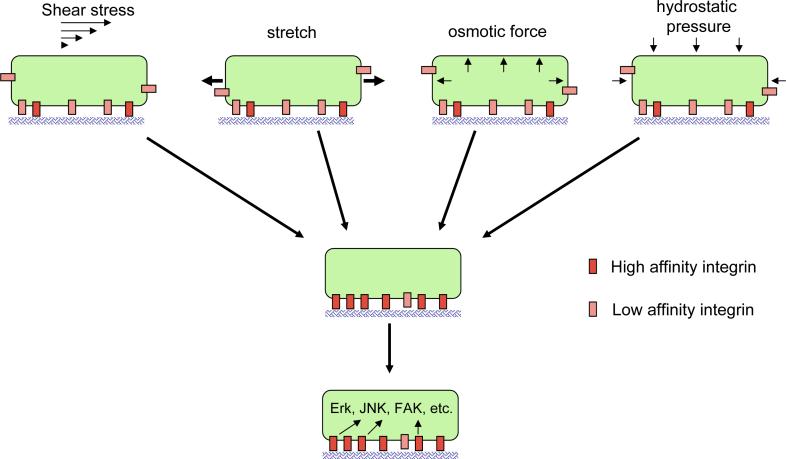
Figure 1. Model for common pathways in mechanotransduction.
Multiple types of mechanical stimuli may trigger conformational activation of low affinity integrins, leading to increased binding to ECM proteins and subsequent activation of signaling pathways. This model may explain why very different types of mechanical stimuli share responses such as FAK and src activation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 2.Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2006;85:165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 4.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 5.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [*This paper shows that stretching the p130cas substrate domain promotes phosphorylation by fyn, leading to recruitment of adapters and activation of Rap1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, et al. Reconstructing and Deconstructing Agonist-Induced Activation of Integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Tavi P, Laine M, Weckstrom M, Ruskoaho H. Cardiac mechanotransduction: from sensing to disease and treatment. Trends Pharmacol Sci. 2001;22:254–260. doi: 10.1016/s0165-6147(00)01679-5. [DOI] [PubMed] [Google Scholar]
- 8.Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, et al. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- 9.Bendig G, Grimmler M, Huttner IG, Wessels G, Dahme T, Just S, Trano N, Katus HA, Fishman MC, Rottbauer W. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 2006;20:2361–2372. doi: 10.1101/gad.1448306. [**This paper uses genetic screens in zebrafish to elucidate a pathway by which ILK, β-parvin and downstream genes promote force-dependent remodeling of the heart during development] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maier S, Lutz R, Gelman L, Sarasa-Renedo A, Schenk S, Grashoff C, Chiquet M. Tenascin-C induction by cyclic strain requires integrin-linked kinase. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamcr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Schliess F, Haussinger D. Osmosensing by integrins in rat liver. Methods Enzymol. 2007;428:129–144. doi: 10.1016/S0076-6879(07)28007-3. [DOI] [PubMed] [Google Scholar]
- 12.Browe DM, Baumgarten CM. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, et al. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24:498–504. doi: 10.1007/s00774-006-0716-x. [DOI] [PubMed] [Google Scholar]
- 14.Craig DH, Haimovich B, Basson MD. Alpha-actinin-1 phosphorylation modulates pressure-induced colon cancer cell adhesion through regulation of focal adhesion kinase-Src interaction. Am J Physiol Cell Physiol. 2007;293:C1862–1874. doi: 10.1152/ajpcell.00118.2007. [DOI] [PubMed] [Google Scholar]
- 15.Orazizadeh M, Lee HS, Groenendijk B, Sadler SJ, Wright MO, Lindberg FP, Salter DM. CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model. Arthritis Res Ther. 2008;10:R4. doi: 10.1186/ar2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [*This paper demonstrates that the extracellular matrix beneath endothelial cells modulates activation of inflammatory pathways in response to fluid flow] [DOI] [PubMed] [Google Scholar]
- 17.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzima E, Pozo MAD, Shattil SS, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. Identification of a mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 20.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Op. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 23.Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 24.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [*This paper carries out a detailed microscopic analysis of cell-cell junction formation to reveal roles for Rho, Rac and the actin cytoskeleton] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 28.Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, Ladoux B. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 29.Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–540. doi: 10.1083/jcb.200612042. [*This paper shows that myosin VI is required for maturation of cell-cell junctions via recruitment of vinculin] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holme RH, Steel KP. Hair cell function - it's all a matter of organization. Trends Mol Med. 2001;7:138. doi: 10.1016/s1471-4914(01)01932-3. [DOI] [PubMed] [Google Scholar]
- 31.Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [**This study identifies a heterophilic adhesive interaction between cadherin 23 and protocadherin 15; this interaction leads to formation of the tip link filament that transmits forces in the inner ear] [DOI] [PubMed] [Google Scholar]
- 32.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101:e44–52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 33.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 34.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 35.Wang N, Ostuni E, Whitesides GM, Ingber DE. Micropatterning tractional forces in living cells. Cell Motil Cytoskeleton. 2002;52:97–106. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- 36.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [** This study used substrates of defined stiffness to demonstrate the importance of the elasticity of the extracellular matrix in directing mesenchymal stem cell commitment and differentiation] [DOI] [PubMed] [Google Scholar]
- 37.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 38.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 39.Green JB. Davidson LA: Convergent extension and the hexahedral cell. Nat Cell Biol. 2007;9:1010–1015. doi: 10.1038/ncb438. [DOI] [PubMed] [Google Scholar]
- 40.Tepass U, Godt D, Winklbauer R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr Opin Genet Dev. 2002;12:572–582. doi: 10.1016/s0959-437x(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 43.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [* This study demonstrated that cortical surface tension as well as differential cell adhesion meidates cell sorting in germ layer organization using dissociated zebrafish gastrula cells] [DOI] [PubMed] [Google Scholar]
- 44.Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]