GFP Tagging of Sieve Element Occlusion (SEO) Proteins Results in Green Fluorescent Forisomes (original) (raw)
Abstract
Forisomes are Ca2+-driven, ATP-independent contractile protein bodies that reversibly occlude sieve elements in faboid legumes. They apparently consist of at least three proteins; potential candidates have been described previously as ‘FOR’ proteins. We isolated three genes from Medicago truncatula that correspond to the putative forisome proteins and expressed their green fluorescent protein (GFP) fusion products in Vicia faba and Glycine max using the composite plant methodology. In both species, expression of any of the constructs resulted in homogenously fluorescent forisomes that formed sieve tube plugs upon stimulation; no GFP fluorescence occurred elsewhere. Isolated fluorescent forisomes reacted to Ca2+ and chelators by contraction and expansion, respectively, and did not lose fluorescence in the process. Wild-type forisomes showed no affinity for free GFP in vitro. The three proteins shared numerous conserved motifs between themselves and with hypothetical proteins derived from the genomes of M. truncatula, Vitis vinifera and Arabidopsis thaliana. However, they showed neither significant similarities to proteins of known function nor canonical metal-binding motifs. We conclude that ‘FOR’-like proteins are components of forisomes that are encoded by a well-defined gene family with relatives in taxa that lack forisomes. Since the mnemonic FOR is already registered and in use for unrelated genes, we suggest the acronym SEO (sieve element occlusion) for this family. The absence of binding sites for divalent cations suggests that the Ca2+ binding responsible for forisome contraction is achieved either by as yet unidentified additional proteins, or by SEO proteins through a novel, uncharacterized mechanism.
Keywords: Ca2+-dependent contractility, Composite plant, Fabaceae, Forisome, Phloem-specific protein, Sieve element occlusion (SEO) protein
Introduction
The phloem of the angiosperms contains tubular cells, so-called sieve elements (SEs) (Esau 1969). Adjacent SEs form continuous tubes as the cell walls between them are perforated; since these walls resemble sieves, they are referred to as sieve plates (Evert 1990). In the sieve tube network, photoassimilates are distributed throughout the plant body by pressure-driven mass flow (Evert 1982) at velocities of up to 0.2–0.4 mm s−1 (Windt et al. 2006).
While SEs lose most organelles including the nucleus during their differentiation, they also develop unique structures (Sjolund 1997). In addition to the prominent SE plastids (P-plastids), a multitude of phloem-specific proteins occur in the SEs of various dicotyledonous species (Sabnis and Sabnis 1995). The ultrastructure of these P-proteins has been scrutinized, leading to a classification of the proteins as amorphous, crystalline, filamentous, tubular or fibrillar (Evert 1990). However, their molecular composition remains mostly obscure. The best known P-proteins so far are PP1 and PP2 from Cucurbita maxima. PP1 is a filament-forming protein to which PP2, a dimeric lectin, binds covalently (Bostwick et al. 1992, Golecki et al. 1999). The two PPs are synthesized in companion cells (CCs) and transported into the SEs via the pore–plasmodesma units. PP2 subunits move with the assimilate stream and cycle between SEs and CCs (Dinant et al. 2003). Thus far, the function of PP1 and PP2 has eluded clarification. Similarly, the physiological role of the ‘crystalline P-proteins’ or ‘non-dispersive P-protein bodies’ found in several dicot taxa remains obscure (Behnke 1991). Their name, however, is aptly chosen since they invariably rest in a ‘crystalloid’, inert state within mature SEs.
SEs of the faboid legumes (subfamily Faboideae in the legume family, Fabaceae) contain elongate protein bodies called forisomes (‘gate-bodies’; Knoblauch et al. 2003). Forisomes consist of fibrils aligned in an orderly way with the protein body's long axis and were previously classified as ‘non-dispersive P-protein bodies’ (Behnke 1991). Ultrastructural studies led some authors to suggest that these protein bodies undergo a transition from a crystalloid state with co-aligned fibrils to a ‘slime-body’ state with irregularly dispersed fibrils during SE differentiation (Wergin and Newcomb 1970, Palevitz and Newcomb 1971). Others argued that the dispersal was an artifact attributable to turgor loss during tissue preparation for electron microscopy (Fisher 1975, Lawton 1978). More recently, we demonstrated that the transition is in fact a rapid and reversible conformational change in which forisomes shorten longitudinally while expanding radially. This anisotropic contraction is associated with a several-fold volume increase (Knoblauch et al. 2003, Peters et al. 2007a, Peters et al. 2008) which enables forisomes to form sieve tube plugs (Knoblauch et al. 2001). Cycling between the longitudinally contracted and expanded states can be induced in isolated forisomes by exchange of simple bathing media containing either Ca2+ or chelators. In vitro, reversible forisome swelling can also be evoked by non-physiologically high or low pH (Knoblauch et al. 2003). Plug formation by forisomes is triggered in vivo by plasma membrane leakage induced through injury or permeabilizing compounds, and by abrupt turgor changes imposed by osmotic shock (Knoblauch et al. 2001).
The elucidation of the molecular mechanisms controlling forisome action is paramount. This is not only because forisomes are the first case of P-proteins with a known physiological function, but also because forisomes represent a novel class of ATP-independent contractile proteins that is distinct from other Ca2+-driven actuators such as spasmonemes (Knoblauch and Peters 2004a). Therefore, forisomes attract interest from material scientists as a natural prototype of a proteinaceous smart material (Mavroidis and Dubey 2003, Knoblauch and Peters 2004b, Huck 2008). Forisomes can be isolated and purified en masse from plant tissue, providing ample material for protein biochemical studies (Knoblauch et al. 2003). Analyzing purified forisomes from Vicia faba and our new model species, Canavalia gladiata (Peters et al. 2007a), by SDS–PAGE, Noll (2005) and Fontanellaz (2006) found one major band of 70–80 kDa. This band could be further separated through various two-dimensional electrophoresis techniques into at least three proteins. Partial amino acid sequences were obtained from these three proteins that enabled the identification of two open reading frames (ORFs) encoding hypothetical proteins and one consensus sequence in the Medicago truncatula genome, which might represent forisome protein homologs of this species. More recently, we and collaborators have suggested that the promoter associated with one of the putative Medicago forisome genes is active specifically in immature SEs (Noll et al. 2007). In that paper, sequence data were provided for assumed homologs from three species (M. truncatula, V. faba and C. gladiata) of the putative forisome protein which was called FOR1. It has to be stressed, though, that there is no direct evidence available to date that any of the hypothetical Medicago proteins takes part in forisome formation.
Here we report the expression in V. faba and Glycine max of the three putative M. truncatula forisome proteins C-terminally linked to green fluorescent protein (GFP), achieved through the ex vitro composite plant approach (Collier et al. 2005). In both species, expression of each of the three constructs caused GFP fluorescence exclusively in forisomes. The green fluorescent forisomes were capable of generating sieve tube plugs similar to wild-type plugs. The three proteins shared about 45% identity on the amino acid level and showed significant similarity to hypothetical proteins from various plant taxa but not to known proteins, indicating that they represent a new gene family. Since FOR, the symbol we and collaborators had used previously (Noll et al. 2007), is already registered for an unrelated gene family in Arabidopsis (Mourad et al. 2006; Gene Class Symbol List, www.arabidopsis.org), we here suggest the acronym SEO (sieve element occlusion) for this family. This renaming is inevitable not only because duplicating gene names for non-orthologous genes in different plant species should be avoided (VandenBosch and Frugoli 2001), but also because our database analyses showed that Arabidopsis itself possesses at least one _SEO_-related gene.
Results
Forisome-related genes and proteins in Medicago truncatula
Forisomes purified from V. faba are made up of at least three proteins of between 70 and 80 kDa (Noll 2005, Fontanellaz 2006). Database searches for nucleic acid sequences that corresponded to oligopeptides derived from these proteins led to the identification of two ORFs in the M. truncatula genome, which probably encoded homologs of two of these proteins (Noll 2005). These ORFs had been found in the NCBI databases under the protein IDs ABE83058.2 (locus tag MtrDRAFT_AC148487g40v2) and ABE83059.1 (MtrDRAFT_AC148487g23v2). We verified the presence of marginally different alleles in the M. truncatula line cultivated in our lab. Since the proteins encoded by these genes appear to be constituents of the forisome sieve tube valve, we will refer to them as sieve element occlusion proteins and call the two genes SEO1 and SEO2. To date, no genomic sequence is available in the databases for the third forisome-derived protein. However, a Medicago consensus sequence (TC107121) matching some of the oligopeptides generated from this protein was identified. Using primers derived from this sequence, we verified the presence of the gene in our plant material and denoted it SEO3. The calculated sizes of the three proteins encoded by the SEO genes in our plants (SEO1, 647 amino acids, 74.9 kDa; SEO2, 675 amino acids, 77.5 kDa; SEO3, 700 amino acids, 80.4 kDa) were in good agreement with the results of SDS–PAGE analysis of purified forisomes.
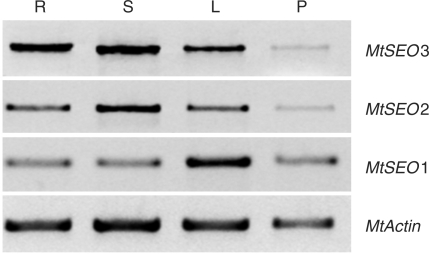
To see whether the three SEO genes actually were active in our Medicago plants, we tested for the presence of their mRNAs by reverse transcription–PCR (RT–PCR). Significant levels of the three mRNAs were found in bulk tissue preparations of all organs examined (roots, stems, leaves and pods; Fig. 1). At this stage, no physiological significance can be assigned to the apparently different levels of mRNA accumulation visible in Fig. 1. This is because differences between transcript levels of genes could be due to differences in the activity levels of the different primer sets under the PCR conditions applied. Moreover, variances between organs might be caused by different relative amounts of phloem in the bulk tissue preparations of the various organs. In any case, the M. truncatula genome contains three active genes whose protein products correspond to proteins detected in preparations of purified forisomes from another faboid legume, V. faba.
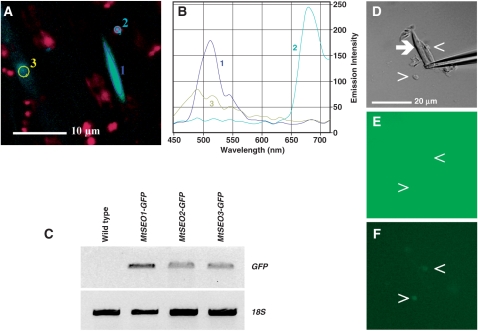
Fig. 1.
RT-PCR analysis of the expression of MtSEO1, MtSEO2 and MtSEO3 in wild-type Medicago truncatula. MtSEO1, MtSEO2 and MtSEO3 cDNAs were amplified from the total RNA of whole-organ preparations of roots (R), stems (S), leaves (L) and pods (P) with gene-specific primers. MtActin cDNA was amplified as an internal control. MtSEO genes are expressed in roots, stems, leaves and pods.
SEO protein primary structure in relation to known and hypothetical proteins
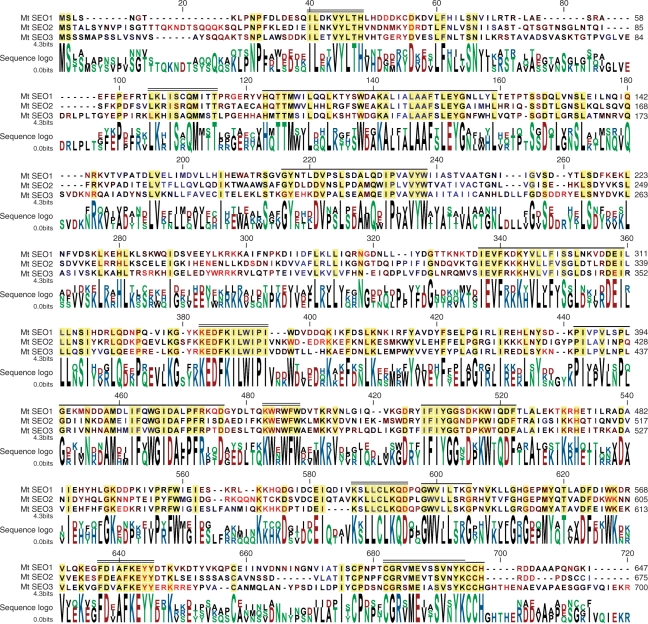
The sequence alignment of our _Medicago_-derived SEO proteins demonstrates a high degree of similarity between the proteins (Fig. 2). There are several highly conserved motifs, and the percentage of identical amino acids in paired alignments of the three sequences ranges from 41.8 to 50.0% (compare Supplementary Fig. S3). Moreover, overall hydrophobicity patterns (color-coded residue symbols in the aligned sequences in Fig. 2) are similar with the exception of the termini and the region around alignment position 566, and amino acid replacements tend to preserve polarity patterns within the conserved regions (color-coded sequence logo in Fig. 2). This evidence supports the idea that the three MtSEO genes in fact are members of the same gene family, and that their protein products have similar functions.
Fig. 2.
Amino acid sequence alignment of the three Medicago truncatula SEO proteins. Conserved residues appear on a yellow background. To highlight similarities between the hydrophobicity patterns of the sequences, running means (five amino acids block width) of hydrophobicity were calculated using the Kyte–Doolittle scale, and were color-coded on the residue symbols in the aligned sequences. On a continuous scale, red represents hydrophilic regions, black is intermediate, and blue symbolizes hydrophobic zones. The color-coding of the sequence logo represents polarity: red, negatively charged; blue, positively charged; green, polar, black, non-polar. The calculated molecular weights of the proteins shown are MtSEO1, 74.9 kDa; MtSEO2, 77.5 kDa; MtSEO3, 80.4 kDa. Double lines on top of the aligned sequences indicate motifs with a high degree of conservation in SEO proteins (SEO-specific motifs). Single lines mark sequences in which characteristic patterns of residues are conserved in SEO and SEO-related proteins (SEO-related motifs; compare Supplementary Figs. S2, S3).
Previously, we and collaborators have described three variants of a protein we called FOR1 from M. truncatula, V. faba and C. gladiata (accession Nos. ABV32455, ABV32454 and ABV32453, respectively; Noll et al. 2007). The sequences of MtFOR1 and MtSEO1 differ in only four residues (Supplementary Fig. S1); we therefore will regard them as products of two alleles of the same gene, MtSEO1 (in case the allelelic isoforms need to be distinguished, we will refer to the one first described as MtSEO1-1 and to the one characterized here as MtSEO1-2). The alignment of MtSEO1 and ‘FOR1’ sequences from different species (Supplementary Fig. S1) showed an even higher degree of similarity than that of the three MtSEO proteins (Fig. 2); identity scores of all possible pairwise alignments of SEO1/FOR1 sequences were between 54.6 and 58.4%, compared with 41.8–52.9% for sequence pairs in which one or both partners were not a SEO1/FOR1 protein (Supplementary Fig. S3). Thus, SEO1/FOR1 proteins form a subset within a more inclusive group which also contains MtSEO2 and MtSEO3. SEO1 also seems to exist in Pisum sativum (L.). A 519 amino acid segment of the protein (accession No. ACD45457) corresponding to alignment positions 106–642 in Fig. 2 has been deduced from an mRNA fragment (accession No. EU681253) isolated from pea plants (K. Subramanian and N. Tuteja, direct submission to the database). In pairwise alignments, the putative PsSEO1/PsFOR1 showed 62% identity with MtSEO1 on the amino acid level, 56% identity with MtSEO2, and 49% with MtSEO3. However, since the terminal regions of PsSEO1 including three conserved motifs are not known, we did not include the protein segment in the following analyses.
Protein BLAST searches using one of our three MtSEO sequences as queries did not yield significant similarities (arbitrarily defined here as E values <10−6) to known proteins except for the FOR1s of Noll et al. (2007). However, independently of which SEO protein was used as a query, the searches consistently produced the same set of hypothetical proteins of unknown function for which ORFs had been identified in various genome projects. In late 2007, this set comprised 29 sequences; 23 from Vitis vinifera, four from Arabidopsis thaliana, and one each from Plantago major and M. truncatula. To obtain a more detailed picture of structural similarities than that provided by the E values of the BLAST searches, we aligned each of these hypothetical proteins individually with each of the SEO sequences. As a result, we were able to distinguish two sets of conserved motifs in the SEO sequences. One set was strictly SEO specific (SEO-specific motifs, double black lines in Fig. 2) while the second one consisted of motifs that were also present in at least some of the hypothetical proteins (SEO-related motifs, single black lines in Fig. 2). Three of the hypothetical proteins (accession Nos. ABD32254 from M. truncatula, CAO14653 from V. vinifera and NP_186817 from A. thaliana) appeared particularly closely related to SEO proteins when similarities within the conserved SEO-related motifs rather than overall E values were considered. The alignment of the amino acid sequences of the three SEO-related proteins which were derived from the genomes of species from three orders revealed an unexpectedly high degree of similarity (Supplementary Fig. S2). When aligned pairwise, the SEO-related proteins showed identities between 45.2 and 50.5%, whereas the identity scores for pairs of one SEO/FOR sequence and one SEO-related protein ranged from 23.8 to 29.3% (Supplementary Fig. S3). Thus, a previously uncharacterized gene family, SEO, seems to exist exclusively in the faboid legumes; it shares similarities with a group of _SEO_-related genes found in several higher plant taxa including the faboid legumes. The SEO family has not been identified in an extensive in silico search for legume-specific genes (Graham et al. 2004) since most SEO members had not yet been included in the databases at that time.
SEO protein primary structure and Ca2+-driven forisome contractility
The responsiveness of forisomes to pH and Ca2+ should be reflected by structural features of the forisome proteins. In fact, SEO proteins contain more acidic and basic residues that are charged under physiological conditions than plant proteins do on average (SEO1, + 46%; SEO2, + 21%; SEO3, + 27%, based on the database included with the CLC Combined Workbench software) which may explain their pH sensitivity. The calculated pH dependencies of overall protein charge (Supplementary Fig. S4) appear to agree with pH-dependent conformational changes described previously (Knoblauch et al. 2003, Peters et al. 2008).
In addition to acidic residues, cysteine and histidine are frequently involved in metal binding. The contents of these amino acids in SEO1 and SEO2 correspond to the plant protein average; only SEO3 carries 48% more histidine than the average plant protein (as estimated by the CLC Combined Workbench software). Surprisingly, database searches for conserved domains (CDs) did not recognize potential binding sites for Ca2+ such as EF-hands (Zhou et al. 2006), or for other divalent cations, such as zinc-finger-like motifs (Ravasi et al. 2003). Through manual searches, we identified three motifs that resembled parts of known cation-binding sites, all in the SEO3 sequence. However, none of them seemed to exist in a sequence context appropriate for cation binding (Supplementary Fig. S5).
Having failed to detect the expected divalent cation-binding site, we performed searches for other CDs in the SEO and SEO-related sequences. Nineteen of the 29 hypothetical proteins that had been identified as possibly related to SEO proteins by BLAST searches contained motifs with some similarity (E < 10−3) to the TryX-NRX domain of the tryparedoxin (TryX) and nucleoredoxin (NRX) subfamily of disulfide oxidoreductases. The physiological roles of these oxidoreductases are mostly unclear, particularly in plants (Funato and Miki 2007). None of the SEO-related hypothetical proteins possessed the Cys-x-x-Cys motif that is essential for oxidoreductase function. Therefore, the similarity between some of the SEO-related hypothetical proteins and TryX-NRX oxidoreductases seems of little relevance with respect to the function of SEO proteins in forisomes.
Noll (2005) hypothesized that coiled-coil structures presumably present in MtSEO2 (MFOR_2 of Noll, 2005) might play a role in forisome function, whereas Fontanellaz (2006) discussed the potential of VfSEO1 (VFF1 of Fontanellaz 2006; VfFOR1 of Noll 2005) to form coiled-coil structures. We were unable to verify significant probabilities for coiled-coils to occur in SEO proteins using standard prediction software (Lupas 1996).
Expression of SEO–GFP in composite plants
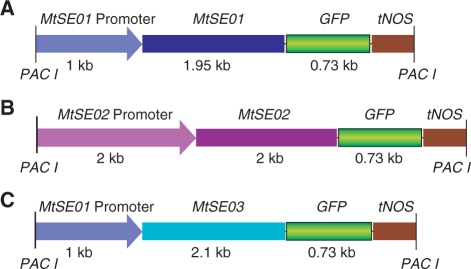
Since the amino acid sequences of the SEO proteins had not provided sound clues as to their function, we decided to study their involvement in forisome formation and function by expressing MtSEO–GFP constructs in transgenic plants. Following Noll et al. (2007), we cloned 1,000 bases upstream of MtSEO1 as the promoter region for MtSEO1 and MtSEO3. Since no promoter studies are available for p_MtSEO2_, we cloned a fragment of 2,050 bases, which frequently is sufficient to isolate the functional promoter, upstream of MtSEO2. Fusion with GFP gave rise to p_MtSEO1–MtSEO1–GFP_, p_MtSEO2–MtSEO2–GFP_ and p_MtSEO1–MtSEO3–GFP_ (Fig. 3). We used ex vitro composite plants which provide a time-saving alternative to stable transformants (Collier et al. 2005). Composite plants consist of wild-type shoots which develop transgenic roots from cut surfaces that have been exposed to Agrobacterium vectors. Hoping that forisome genes and proteins were sufficiently similar to function in many faboid legumes, we chose V. faba as a target species, mainly because its forisomes are much larger than those of Medicago, allowing for the detection of structural heterogeneity and the measurement of fluorescence emission spectra. Depending on the species, forisomes occur with or without tail-like protrusions (Lawton 1978). Since Vicia and Medicago forisomes lack tails, we cross-checked our results in G. max whose tailed forisomes are well characterized (Peters et al. 2008), expecting to obtain insights into possible roles for the three SEO proteins in tail formation.
Fig. 3.
Fusion protein _Pac_I cassettes. (A) MtSEO1 promoter-driven MtSEO1–GFP fusion gene. (B) MtSEO2 promoter-driven MtSEO2–GFP fusion gene. (C) MtSEO1 promoter-driven MtSEO3–GFP fusion gene.
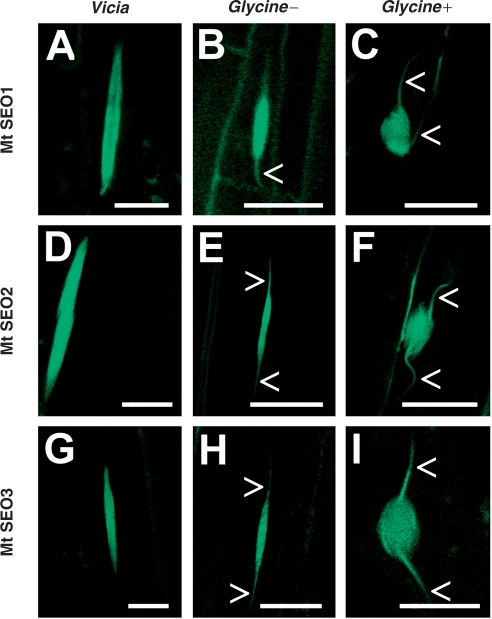
Both Vicia and Glycine showed good callus formation and a high percentage (∼80%) of transgenic roots which could easily be identified by the presence of ‘green fluorescent forisomes’ despite some background signal that probably was due to autofluorescence of phenolic compounds in the cell walls (Figs. 4 and 5A, B). Fluorescence was evenly distributed over and within the forisome main body, indicating that each of the three SEO proteins was involved in the formation of forisome filaments. The shape and size of fluorescent forisomes were indistinguishable from those in wild-type plants. Moreover, fluorescent forisomes formed sieve tube plugs by radial expansion in response to Ca2+, just like wild-type plugs (Fig. 4C, F, I). Glycine forisomes showed fluorescence in the main body as well as in the tails, although forisome tails do not react to Ca2+ (Peters et al. 2008) and differ ultrastructurally from the main body (Lawton 1978).
Fig. 4.
Functional ‘green fluorescent forisomes’ in Vicia faba and Glycine max composite plants transformed with MtSEO1–, MtSEO2– or MtSEO3–GFP fusions. In both species, the fluorescence caused by any of the three constructs was evenly distributed over the forisome body in the resting state when Ca2+ was absent (A, D and G for Vicia; B, E and H for Glycine). Forisome tails (arrowheads) in Glycine showed a similar fluorescence intensity to the forisome main body. Addition of calcium led to the formation of sieve tube plugs (Glycine +, in C, F and I), demonstrating that the GFP tag did not impair the ability of fluorescent forisomes to contract. Scale bars, 10 µm.
Fig. 5.
Test conducted to verify that the GFP signal co-localized with forisomes in transgenic roots was brought about by specific interactions of the recombinant GFP–SEO proteins with forisomes. (A, B) Analysis of emission spectra from a transgenic root of Vicia faba expressing MtSEO3–GFP. The fluorescence micrograph shows a green fluorescent forisome (1), the red chlorophyll emission signal originating from developing chloroplast in the light-exposed root (2) and the blue-green background signal occasionally observed (3). The zones marked in (A) are the areas in which the emission spectra shown in (B) were measured. The spectrum of the blue-green background signal is clearly distinct from the typical GFP spectrum emitted by the fluorescent forisome. It should be noted that we chose this particular analysis as an example because the intensity of the blue-green background emission was unusually high as compared with the GFP emission intensity. Roots transformed with MtSEO1–GFP and MtSEO2–GFP yielded similar results. (C) RT–PCR analysis of GFP expression in composite V. faba plants. Agarose gel showing RT–PCR products generated with primers amplifying a 700 bp portion of the GFP coding region in hairy roots expressing MtSEO1–GFP, MtSEO2–GFP and MtSEO3–GFP. Wild-type roots were used as a negative control; 18s rRNA cDNA was amplified as an internal control. (D, E, F) In vitro test for GFP binding by forisomes isolated from wild-type V. faba. A forisome (arrow) in a crude forisome preparation is kept in place by a glass micropipet that protrudes into the image from the right (D; differential interference contrast micrograph). After addition of free GFP, a homogenous green fluorescence signal was observed that appeared slightly increased in some cell debris particles (arrowheads) but not in the forisome (E; fluorescence micrograph). After washing with GFP-free medium (10-fold volume of the reaction chamber), a slight fluorescence remained on the debris particles but not on the forisome (F; fluorescence micrograph). Identical results were obtained in the absence and presence of calcium ions, implying that forisomes did not bind free GFP either in the longitudinally expanded or in the contracted state.
As expected, GFP fluorescence was never observed in forisomes of stem and leaf tissue of composite plants with transgenic roots, and neither did it occur in roots which did not express the fusion proteins. To confirm that the fluorescence observed was due to the expression of the GFP constructs, we performed RT–PCR on transgenic and non-transgenic roots as well as on leaves of composite plants to detect GFP mRNA. PCR products of roots containing fluorescent forisomes showed amplification whereas wild-type roots did not (Fig. 5C), and neither did leaves of wild-type and composite plants (data not shown). To corroborate this finding, we compared the emission spectra of fluorescent forisomes and other cell constituents. Only emission spectra of fluorescent forisomes in transgenic roots showed the characteristic peak at about 515 nm and the typical shape of the GFP5 spectrum (Fig. 5A, B).
To exclude the possibility that the GFP tagging of forisomes in composite plants was due to an unspecific binding of GFP to forisomes, we produced crude forisome preparations from wild-type V. faba and incubated them with free GFP for several minutes under microscopic observation. Accumulation of GFP fluorescence occurred on cell debris particles but never on forisomes, and no GFP fluorescence remained on forisomes when the GFP solution was replaced by an identical solution without GFP (Fig. 5D–F). Identical results were found in longitudinally expanded forisomes (–Ca2+) and in contracted (+Ca2+) forisomes in which much greater surface areas are available for any unspecific binding due to the decreased molecular order (Peters et al. 2007b) and increased mean distance between forisome fibrils (Pickard et al. 2006). As an additional control, we expressed p_MtSEO1–GFP_ in V. faba composite plants; a faint GFP signal could be detected within sieve elements but never in forisomes (data not shown). We concluded that the GFP tagging of forisomes in transgenic roots depended specifically on the presence of the SEO moiety of the GFP–SEO protein.
Fluorescent forisomes were capable of sieve element occlusion (Fig. 4). We verified Ca2+-dependent contractility of SEO–GFP forisomes in vitro. Crude forisome preparations were produced from V. faba composite plants, and individual forisomes were caused to contract and expand longitudinally by flushing the reaction chamber alternately with media containing either EDTA or Ca2+, both buffered to pH 7.3. In all three types of Vicia transformants (MtSEO1–GFP, MtSEO2–GFP and MtSEO3–GFP), fluorescent forisomes were reactive (Fig. 6). We submitted forisomes to up to 10 contraction/expansion cycles to see whether any decrease in fluorescence intensity or any change in the homogenous distribution of the fluorescence signal across the forisome could be detected; no such effects became evident. Since forisomes undergo significant structural changes during a contraction/expansion cycle (Knoblauch et al. 2001) which include the assembly/disassembly of highly ordered, dense molecular arrays (Peters et al. 2007b), and because the reversible volume increase of forisomes during the cycle implies an exchange of the interfibrillar medium (Pickard et al. 2006), these findings suggested that the three SEO–GFPs were tightly bound to, rather than loosely associated with the forisomes. We concluded that SEO proteins are components of functional forisomes which do not seem to be impaired by the presence of the additional GFP moiety.
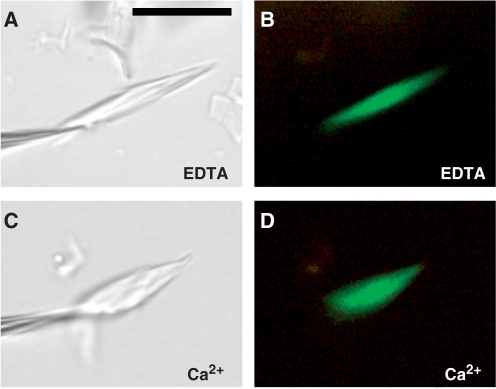
Fig. 6.
Calcium-induced contractility in a fluorescent forisome isolated from a transgenic Vicia faba root expressing MtSEO3–GFP. (A) and (C) bright-field micrographs; (B) and (D) green fluorescence emitted from GFP. The forisome is shown in Ca2+-free medium containing the chelator EDTA (A, B) and after the response to Ca2+ (C, D). It is held in place by a micropipet that is visible on the left in the bright-field images (A, C). Scale bar in (A), 10 µm (applies to all micrographs). Fluorescent forisomes from plants expressing MtSEO1–GFP and MtSEO2–GFP showed similar responses.
Discussion
SEO proteins are components of forisomes
When purified but still functional forisomes from V. faba are submitted to SDS–PAGE, they separate into at least three protein bands which are not easily distinguishable because of their overlapping positions in the gel; the estimated molecular weights of the three proteins are 70–80 kDa (Noll 2005, Fontanellaz 2006). In screens of publicly available databases for genomic sequences that corresponded to short partial amino acid sequences derived from the three Vicia forisome proteins, three ORFs in the M. truncatula genome were identified. We established that the three ORFs actually represented active genes in M. truncatula (Fig. 1). The calculated molecular weights were similar between the three proteins encoded by these genes (Fig. 2) and turned out to agree well with those established experimentally for the forisome proteins. The expression of fusion constructs of any of the three Medicago genes with GFP in V. faba as well as G. max composite plants led to the development of green fluorescent forisomes in transgenic roots (Fig. 4). The occurrence of fluorescent forisomes in an organ correlated with the presence of GFP mRNA in that organ (Fig. 5C). The fluorescence observed originated from GFP and was forisome specific, as typical GFP emission spectra could be recorded only from fluorescent forisomes but not from other cell or tissue components (Fig. 5A, B). Wild-type forisomes did not bind free GFP in vitro (Fig. 5D–F), demonstrating that the specific association of the GFP signal with forisomes in composite plants required the SEO moiety of the GFP–SEO construct. In planta, the fluorescent forisomes were reactive and capable of forming SEOs (Fig. 4). Fluorescent forisomes could be isolated and submitted to repeated contraction/expansion cycles during which they were flushed with large volumes of media. They did not show significant decreases in fluorescence intensity in the process (Fig. 6), indicating that the fluorophore was tightly bound to the forisome. The SEO proteins linked to GFP seemed to interact with fibrils throughout the forisome, as indicated by the homogenous distribution of the fluorescence signal (Figs. 4, 6). From this chain of evidence leading from purified functional forisomes to GFP-tagged transgenic ones, we conclude that the three proteins called SEO1, SEO2 and SEO3 are components of forisomes. At this stage, we cannot exclude that additional factors may exist which have eluded detection in the SDS–PAGE analyses of purified forisomes so far.
The amino acid sequences of the three SEO proteins share numerous conserved motifs arranged in an identical order (Fig. 2; Supplementary Fig. S1), and their GFP fusions yielded identical fluorescence patterns in composite plants (Fig. 4). Therefore, it seems possible that, rather than representing three distinct elements of the forisomes’ contractile machinery, the proteins are functionally redundant isoforms. The idea is supported by the fact that all Medicago genes and promoters worked equally in Vicia and Glycine. In this context, it seems relevant that forisomes can perform numerous contraction/expansion cycles in dilute artificial media that contain a few inorganic ions only (Knoblauch et al. 2003). No soluble organic compounds are required, no enzymatic activity must be maintained to enable energy input and no sophisticated, multicomponent regulatory mechanisms are essential for the contractility of forisomes. Because of this ‘low-tech’ mode of action, forisomes resemble artificial smart materials more closely than complex cellular actuators such as the actin–myosin system (Knoblauch and Peters 2004b, Huck 2008). It would not be surprising if this mechanistic simplicity corresponded to a similarly simple structure, generated by just one type of protein.
Ultrastructural studies demonstrated that forisomes in the longitudinally expanded resting state consist of fibrils that are longitudinally co-aligned with an almost crystalline degree of molecular order as expressed by the occurrence of a perfectly regular cross-striation pattern with 12 nm periodicity (Wergin and Newcomb 1970, Palevitz and Newcomb 1971, Lawton 1978). Polarization-microscopical investigations of non-fixed, functional forisomes supported the conclusion that the reversible self-assembly and disassembly of highly ordered molecular arrays has a role in forisome longitudinal expansion and contraction (Peters et al. 2007b). Therefore, it might have been expected that the linkage of GFP (size about 27 kDa) to an SEO protein (70–80 kDa) would interfere with forisome assembly and contractility. This was not the case (Figs. 4, 6). Either the high molecular regularity within forisomes still allows the inclusion of large, alien peptide chains, or the proportion of GFP-tagged SEO molecules among GFP-less wild-type SEO proteins was too small in the transgenic cells to evoke detectable effects—or both. Generally, forisomes are remarkably tolerant of treatments that normally destroy well-defined molecular structures. For example, the protocols for crude forisome preparations (Peters et al. 2007a) and for en masse purification of forisomes (Knoblauch et al. 2003) include between one and five cycles of slow freezing/thawing, but the capacity of forisomes for Ca2+-induced contraction does not seem affected by this treatment. Again, forisomes seem to resemble artificial smart materials of relatively simple chemical composition more closely than complex protein machineries.
With any of the three transgenes, G. max forisomes showed fluorescence in the main body as well as in the tails which are unresponsive to Ca2+ (Peters et al. 2007a, Knoblauch et al. 2001; see also Fig. 4C, F, I). Ultrastructurally, tails resemble the forisome main body, but the conspicuous 12 nm cross-striation is overlain by an additional 45 nm striation (Wergin and Newcomb 1970, Palevitz and Newcomb 1971, Lawton 1978). These facts create a conundrum: forisome tails and main bodies appear to consist of identical elements, but the arrangements of these elements seem to differ in ways that control Ca2+ responsiveness. Alternatively, an as yet unidentified component(s) required for contractility might be present in the forisome main body but not in the tails.
SEO protein primary structure and Ca2+ binding
We did not detect convincing sequence similarities between SEO proteins and any known divalent cation-binding domain. The absence of obvious Ca2+-binding motifs from putative forisome proteins might tempt us to conclude that SEO proteins are not directly involved in Ca2+-driven forisome contractility, despite the fact that forisomes purified for biochemical analysis, from which no components other than the three SEO proteins described here have been isolated yet, are fully reactive (Knoblauch et al. 2003). We are far from excluding the possibility that essential component(s) of forisomes, perhaps the one(s) responsible for cation binding, might still await detection. However, we also note that with an increasing number of well-characterized proteins, variants of calcium-binding structures have become known that would not have been classified correctly before their first publication (Asaoka et al. 2003, Rigden and Galperin 2004, Zhou et al. 2006). Quite conceivably, SEO proteins might possess Ca2+-binding sites which cannot be identified through sequence similarities to known motifs at this time.
A third possibility is that forisome Ca2+-binding sites might be formed by residues of more than one SEO protein molecule, thus remaining invisible in searches for conserved domains. However, Ca2+ binding causes forisome filaments to become organized in a less orderly way (Peters et al. 2007b) and to increase their average distances significantly (Pickard et al. 2006); the opposite would be expected if each Ca2+ ion would be bound by pairs or groups of SEO molecules.
A fourth possible explanation for the apparent absence of Ca2+-binding sites from supposedly Ca2+-binding proteins is that Ca2+ ions do not need to be tightly bound to the forisome to drive contraction. In previous studies, we used chelators to remove Ca2+ from forisomes in order to trigger longitudinal expansion. In fact, chelator-induced longitudinal expansion proceeds faster than Ca2+-induced contraction (Peters et al. 2007a, Peters et al. 2008). However, longitudinally contracted forisomes expand spontaneously when incubated in distilled water for several minutes (Schwan et al. 2007). It appears that chelators merely increase the rate of Ca2+ ion loss from forisomes which is significant at low external Ca2+ levels even in the absence of chelators. This opens up the interesting possibility that forisome proteins are not Ca2+ binding in a strict sense, but rather provide the structural matrix for a Donnan phase in which certain ion species are specifically accumulated. If such a model turns out to be correct, it will be yet another parallel between forisomes and artificial smart materials (de Rossi and Osada 1999, Huck 2008).
Incidentally, the lack of obvious Ca2+-binding motifs in the SEO protein sequences accords with our previous conclusion that forisomes are unrelated mechanisticly and phylogeneticly to the second known type of Ca2+-driven cellular actuators, the spasmonemes of sessile ciliates (Knoblauch and Peters 2004a). Spasmonemes contain spasmin which binds Ca2+ in well-defined EF-hands (Maciejewski et al. 1999).
Forisome evolution
The evolution of forisomes in only one subfamily of higher plants provides an opportunity to study the development of a mechanism of contractility on the molecular level, within a well-defined taxonomic context. The identification of three SEO proteins as forisome components represents an important and potentially crucial step. Available data provide no basis for a phylogenetic hypothesis of forisome evolution yet, but the forisome-specific SEO gene family within a greater group of SEO-related genes now has been circumscribed (Fig. 2; Supplementary Figs. S1–S3). Knowledge of the genomic sequences corresponding to conserved SEO-specific and SEO-related amino acid motifs will facilitate the discovery of additional family members, as will the impending completion of the genome projects of the model legumes M. truncatula and Lotus japonicus (Sato et al. 2007, Ané et al. 2008). In three orders of higher plants, SEO-related hypothetical proteins exist that are more similar to each other than they are to SEO proteins (Supplementary Figs. S2, S3). This supports our hypothesis that forisome proteins evolved in the faboid legumes from unknown precursors that are widely distributed in the angiosperms. Current work focuses on the establishment of the actual presence of these hypothetical proteins in plants, as we anticipate that the elucidation of their physiological roles will provide decisive clues to the evolution of forisome-based sieve tube gating mechanisms.
Materials and Methods
Plant material
Vicia faba L. (Witkiem major, Nunhem Zaden, Haelen, The Netherlands) and M. truncatula Gaertn. cv Jemalong were grown in a greenhouse (14 h photoperiod, 300–400 µmol photons m−2 s−1; 20°C/15°C). Glycine max L. cv Hutcheson (University of Missouri Delta Center) was grown in growth chambers under a 16 h photoperiod (400 µmol photons m−2 s−1; 26°C/18°C). For M. truncatula RNA extraction, roots, stems, leaves and pods were harvested in liquid nitrogen from 3-month-old plants and stored at –80°C until use.
Isolation of SEO genes
Based on the Medicago bacterial artificial chromosome (BAC) clone sequence Mt AC148487 and the consensus expressed sequence tag (EST) TC107121, specific primers MtSEO1 forward 5′ATGTCATTGTCCAATGGAACTAAAC 3′, MtSEO1 reverse 5′TATCTTGCCATTCTGTGGAGCAGCAG3′, MtSEO2 forward 5′ATGTCCACTGCATTGTCCTATAATG3′, MtSEO2 reverse 5′AATGCAGCAACTATCTGGATCATCA3′, MtSEO3 forward 5′ATGTCGTCTTCAATGGCGCCATCTTC3′, and MtSEO3 reverse 5′AGACCTTTTCTCAATCTGAACAAAA3′ were designed to isolate the full-length cDNAs of SEO1, SEO2 and SEO3 from the M. truncatula line used in our lab by RT–PCR. RT–PCR was performed using total RNA from M. truncatula obtained according to Pélissier and Tegeder (2007), treated with Turbo DNase I (Ambion, Austin, TX, USA), and reverse-transcribed by M-MLV Reverse transcriptase (Promega, Madison, WI, USA). RT–PCR products were cloned into pGEM-T-easy vector (Promega) and sequenced for verification.
Isolation of SEO promoter regions
The promoter regions of MtSEO1 and MtSEO2 were PCR amplified based on the sequences in the M. truncatula BAC clone (MtAC148487) available on the National Center for Biotechnology Information database, containing both genes as well as their putative promoters. Medicago truncatula genomic DNA was extracted as described previously (Collier et al. 2005), and PCR was carried out using specific primers containing restriction sites for _Kpn_I and _Xho_I (pMtSEO1 forward 5′TTCGGTACCTGGATACATTAACTTTAATATATC3′, pMtSE01 reverse 5′TTCGCTCGAGCATATTGATAAATTCAACTTTAGGC3′, pMtSEO2 forward 5′TTCGGGTACCAACACAATTGAATTGCAACC3′, pMtSEO2 reverse 5′TTCGCTCGAGGATGATTTGTTTATAAATTAATAAG3′) with 0.2 µg of genomic DNA in a 50 µl reaction buffer (0.2 mM dNTPs, 0.5 µM forward primer, 0.5 µM reverse primer, 1 × Taq buffer and 2.5 U of High Fidelity Platinum Taq DNA polymerase, Invitrogen, Carlsbad, CA, USA), using the protocol: 5 min at 95°C; 37 cycles of 30 s at 95°C, 45 s at 50°C, 4 min at 68°C; and 10 min at 68°C. The resulting PCR product was cut with _Kpn_I/_Xho_I and cloned into the vector AKK 1408 (gift from Dr. Christopher G. Taylor, Donald Danforth Plant Science Center, St Louis, MO, USA) and sequenced for confirmation.
Construction of plant transformation vectors
All cloning was done using the Modular Binary Construct System (gift from Dr. Christopher G. Taylor). The forisome cDNAs SEO1, SEO2 and SEO3 were subcloned by PCR into a modified AKK 1435 vector, containing the GFP reporter gene. The promoters p_MtSEO1_ and p_MtSEO2_ were then cloned into the SEO gene-containing AKK 1435 vectors, to produce the constructs p_MtSEO1–MtSEO1–GFP_, p_MtSEO2–MtSEO2–GFP_ and p_MtSEO2–MtSEO2–GFP_ (Fig. 3). As a control, the promoter p_MtSEO1_ was cloned into the 1435 vector to construct the p_MtSEO1–GFP_ fusion gene. The promoter fusion protein cassettes were subcloned with Pac_I into the binary vector AKK 1426B. The 1426–p_MtSEO1–MtSEO1–GFP, 1426–p_MtSEO2–MtSEO2–GFP_, 1426–p_MtSEO1–MtSEO3–GFP_ and 1426–p_MtSEO1–GFP_ vectors were electroporated into Agrobacterium rhizogenes (strain NCPPB 2659; gift from Dr. Christopher G. Taylor).
Plant transformation
To analyze the function of the three M. truncatula genes in planta, transcriptional fusions of the promoter region with the forisome genes and GFP were introduced into V. faba and G. max using the ex vitro composite plant induction method, as described by Collier et al. (2005), with modifications: after initiation of the root teratoma, plants were removed from the rockwool cubes (Fibrgro®, Hummert International, Earth City, MO, USA) and transferred to water, which led to improved rates of root formation and growth, especially for V. faba.
RT–PCR analysis
Analysis of organ-specific expression of SEO genes in M. truncatula. Total RNA from wild-type M. truncatula organs (roots, stems, leaves and pods) was extracted as detailed before (Pélissier and Tegeder 2007). Total RNA (2 µg) was treated with Turbo DNase I (Ambion) at 37°C for 20 min. First-strand cDNA synthesis was carried out with M-MLV Reverse transcriptase, according to the manufacturer's instructions (Promega). MtSEO1, MtSEO2 and MtSEO3 cDNAs were each amplified using two different pairs (A and B) of gene-specific primers: MtSEO1 forward A (5′CTTACTCAAAAATGGAGGTGG3′), MtSEO1 reverse A (5′GTCTCCTTTCACCTGTATTCC3′), MtSEO1 forward B (5′CTCATCAGATCAACTTGTTAACTC3′) and MtSEO1 reverse B (5′GAATCTATTTGCCATTTGCTTAGC3′); MtSEO2 forward A (5′GACGCGTGGCACAGCAGAATG3′), MtSEO2 reverse A (5′CTCCTTAACCACATCAGAGAGAC3′), MtSEO2 forward B (5′GATATTGGTTACCCGCCTATTTTG3′), and MtSEO2 reverse B (5′ACTGTAACATGACGCCCTCTAC3′); MtSEO3 forward A (5′CCCTTGGCTGGAATAAGGTTGA3′), MtSEO3 reverse A (5′ATCTGAAACAAAACCACCACTCTC3′), MtSEO3 forward B (5′CTGGTTATGAGCCTCCTATCCG3′), and MtSEO3 reverse B (5′CTTACACTGGAAGCACGCTTGAC3′). Reverse transcription-negative (no enzyme) controls were performed to monitor for contamination with genomic DNA. Medicago truncatula actin cDNA (accession No. TC107326) was amplified using forward primer (5′GCTGTCCTCTCCCTCTATGC3′) and reverse primer (5′CAATGTTGCCGGGTACAGATCC3′) as an internal standard. Reverse transcription-negative (no enzyme) controls were performed to monitor for contamination with genomic DNA. PCR was carried out with 1 µl of the reverse transcription reaction in a 50 µl reaction (0.2 mM dNTPs, 0.2 µM forward primer, 0.2 µM reverse primer, 1 × Taq buffer and 2.5 U of Taq polymerase; Promega), using the protocol: 5 min at 95°C; 30 cycles of 30 s at 95°C, 45 s at 57°C, 1 min at 72°C; and 5 min at 72°C, and 30 cycles of amplification for MtActin, 32 cycles for MtSEO3 and MtSEO2, and 35 cycles for MtSEO1.
Analysis of GFP expression in V. faba composite plants. As a control for GFP expression in V. faba plants, total RNA from V. faba transgenic hairy roots was isolated and used for reverse transcription as described above. Total RNA from wild-type V. faba roots was used as a control. GFP cDNA was amplified using forward (5′GAGAAGAACTTTTCACTGGA3′) and reverse (5′TGTATAGTTCATCCATGCCA3′) primers. Reverse transcriptase-negative (no enzyme) controls were performed to monitor for contamination with bacterial DNA. The 18s rRNA cDNA was utilized as an internal standard and was amplified using specific forward (5′ATTCTATGGGTGGTGGTGC3′) and reverse (5′CCATCCAATCGGTAGGAGC3′) primers. PCR was carried out as described above using 35 cycles.
Analysis of sequences
Analyses of amino acid sequences, such as alignments, searches for conserved domains etc., were performed using tools and resources publicly available through the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/) and the software package CLC Combined Workbench v.3.6 (CLC bio USA, Cambridge, MA, USA). In addition, the web-based programs CalPred (http://www.juit.ac.in/calpred/application.html) and COILS (http://www.ch.embnet.org/software/COILS_form.html) were used to search for potential EF-hand domains and coiled-coil regions, respectively. For details on the phylogenetic analysis, see Supplementary Fig. S3.
Confocal laser-scanning microscopy of green fluorescent forisomes
Young transgenic roots from V. faba and G. max were harvested about 1 week after teratoma-derived root primordia became visible. Fresh hand sections were incubated in calcium medium (1 mM CaCl2, 100 mM KCl, 10 mM HEPES pH 7.3) or calcium-free medium (10 mM EDTA, 100 mM KCl, 10 mM HEPES pH 7.3) to observe the longitudinally contracted, plug-forming or the longitudinally expanded, free-flow state of forisomes, respectively. Images were taken with a Zeiss 510 META confocal laser scanning microscope (Zeiss, Wetzlar, Germany) using the 488 nm argon laser excitation wavelength. The emitted light was passed through a 510–570 nm bandpass filter.
Fluorescence emission spectra of GFP-tagged forisomes and other cell components were determined with the META detector of the Zeiss 510 confocal laser scanning microscope. GFP5 was excited with a 405 nm diode laser. Emitted light was quantified between 449 and 716 nm in blocks of 10.7 nm of wavelength, each evaluated by a separate photomultiplier of the META detector.
Crude forisome preparations and in vitro observations
To characterize the Ca2+-dependent contractility of transgenic forisomes in vitro, crude forisome preparations were produced from V. faba as detailed before (Knoblauch et al. 2003). Forisomes were observed in calcium medium and calcium-free medium (as above) with a Leica DM LFSA (Leica Microsystems, Wetzlar, Germany) upright microscope equipped with water immersion lenses not corrected for coverslips (HCX Plan-APOU-V-I series) and a differential interference contrast (DIC) system. GFP was detected with an I3 filter block. Media were exchanged using a custom-built flow-through system. Images were taken with a Leica DFC 300 FX cooled CCD camera.
To test for unspecific GFP binding by forisomes, wild-type forisomes were isolated and observed as described above, and were exposed to free GFP (recombinant GFP, Clontech Inc., Mountain View, CA, USA) in the presence and absence of Ca2+.
Supplementary data
Supplementary data are available at PCP Online.
Supplementary Material
[Supplementary Data]
Acknowledgments
We gratefully acknowledge technical support from Washington State University's Franceschi Microscopy and Imaging Center, and thank Valerie Lynch-Holm, Christine Davitt, Laura Rarig, Chuck Cody (all from Washington State University) and Joan Taing (Indiana/Purdue University Fort Wayne) for technical assistance. We are particularly grateful to Antje Eggers (Hannover, Germany) and Jeeranan Silakot (Petchaboon, Thailand) for letting us evaluate unpublished data. Thanks to Natalie Walter for critical reading of the manuscript.
Glossary
Abbreviations:
BAC
bacterial artificial chromosome
CC
companion cell
CD
conserved domain
FOR
forisome-related protein
GFP
green fluorescent protein
NRX
nucleoredoxin
ORF
open reading frame
PP
phloem-specific protein
RT–PCR
reverse transcription–PCR
SE
sieve element
SEO
sieve element occlusion
TryX
tryparedoxin.
References
- Ané J-M, Zhu H, Frugoli J. Recent advances in Medicago truncatula genomics. Int. J. Plant Genomics. 2008 doi: 10.1155/2008/256597. Article ID 256597, doi:10.1155/2008/256597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka T, Ando T, Meguro T, Yamato I. Development of a structure-based protein function prediction method: calcium binding protein. Chem. Biol. Inform. J. 2003;3:96–113. [Google Scholar]
- Behnke HD. Nondispersive protein bodies in sieve elements: a survey and review of their origin, distribution and taxonomic significance. IAWA Bull. 1991;12:143–175. [Google Scholar]
- Bostwick DE, Dannenhoffer JM, Skaggs MI, Lister RM, Larkins BA, Thompson GA. Pumpkin phloem lectin genes are specifically expressed in companion cells. Plant Cell. 1992;4:1539–1548. doi: 10.1105/tpc.4.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R, Fuchs B, Walter N, Lutke WK, Taylor CG. Ex vitro composite plants: an inexpensive, rapid method for root biology. Plant J. 2005;43:449–457. doi: 10.1111/j.1365-313X.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- de Rossi D, Osada Y. Berlin: Springer; 1999. Polymer Sensors and Actuators. [Google Scholar]
- Dinant S, Clark AM, Zhu Y, Vilaine F, Palauqui JC, et al. Diversity of the superfamily of phloem lectins (phloem protein 2) in angiosperms. Plant Physiol. 2003;131:114–128. doi: 10.1104/pp.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. Stuttgart: Borntraeger; 1969. The Phloem. [Google Scholar]
- Evert RF. Sieve tube structure in relation to function. Bio-Science. 1982;32:789–795. [Google Scholar]
- Evert RF. Dicotyledons. In: Behnke HD, Sjolund RD, editors. Sieve Elements. Berlin: Springer; 1990. pp. 103–137. [Google Scholar]
- Fisher DB. Structure of functional soybean sieve elements. Plant Physiol. 1975;56:555–569. doi: 10.1104/pp.56.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanellaz ME. Cloning and molecular characterization of vff1 gene encoding forisomes of Vicia faba. Doctoral Thesis (supervisor: R. Fischer), Rheinisch-Westfälische Technische Hochschule, Aachen, Germany. 2006 http://darwin.bth.rwth-aachen.de/opus3/volltexte/2007/2087/pdf/Fontanellaz_Maria.pdf.
- Funato Y, Miki H. Nucleoredoxin, a novel thioredoxin family member involved in cell growth and differentiation. Antiox. Redox Signal. 2007;9:1037–1057. doi: 10.1089/ars.2007.1550. [DOI] [PubMed] [Google Scholar]
- Graham MA, Silverstein KAT, Cannon DSB, VandenBosch KA. Computational identification and characterization of novel genes from legumes. Plant Physiol. 2004;135:1179–1197. doi: 10.1104/pp.104.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golecki B, Schulz A, Thompson GA. Translocation of structural P proteins in the phloem. Plant Cell. 1999;11:127–140. doi: 10.1105/tpc.11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck WTS. Responsive polymers for nanoscale actuation. Mater. Today. 2008;11:24–32. [Google Scholar]
- Knoblauch M, Noll GA, Müller T, Prüfer D, Schneider-Hüther I, et al. ATP-independent contractile proteins from plants. Nat. Mater. 2003;2:600–603. doi: 10.1038/nmat960. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, Peters WS. Forisomes, a novel type of Ca2+-dependent contractile protein motor. Cell Motil. Cytoskel. 2004a;58:137–142. doi: 10.1002/cm.20006. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, Peters WS. Biomimetic actuators: where technology and cell biology merge. Cell. Mol. Life Sci. 2004b;61:2497–2509. doi: 10.1007/s00018-004-4158-0. [DOI] [PubMed] [Google Scholar]
- Knoblauch M, Peters WS, Ehlers K, van Bel AJE. Reversible calcium-regulated stopcocks in legume sieve tubes. Plant Cell. 2001;13:1221–1230. doi: 10.1105/tpc.13.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton DM. P protein crystals do not disperse in uninjured sieve elements in roots of runner bean (Phaseolus multiflorus) fixed with glutaraldehyde. Ann. Bot. 1978;42:353–361. [Google Scholar]
- Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- Maciejewski JJ, Vacchiano EJ, McCutcheon SM, Buhse HE., Jr Cloning and expression of a cDNA encoding a Vorticella convallaria spasmin: an EF-hand calcium-binding protein. J. Eukaryot. Microbiol. 1999;46:165–173. doi: 10.1111/j.1550-7408.1999.tb04601.x. [DOI] [PubMed] [Google Scholar]
- Mavroidis C, Dubey A. From pulses to motors. Nat. Mater. 2003;2:573–574. doi: 10.1038/nmat973. [DOI] [PubMed] [Google Scholar]
- Mourad GS, Snook BM, Prabhaker JT, Mansfield TA, Schultes NP. A fluoroorotic acid-resistant mutant of Arabidopsis defective in the uptake of uracil. J. Exp. Bot. 2006;57:3563–3573. doi: 10.1093/jxb/erl107. [DOI] [PubMed] [Google Scholar]
- Noll GA. Molekularbiologische Charakterisierung der Forisome. Doctoral Thesis (in German; supervisor: A.J.E. van Bel), Justus-Liebig Universität, Gießen, Germany. 2005 http://geb.uni-giessen.de/geb/volltexte/2007/4805/pdf/NollGundula-2005-12-12.pdf.
- Noll GA, Fontanellaz ME, Rueping B, Ashoub A, van Bel AJE, et al. Spatial and temporal regulation of the forisome gene for1 in the phloem during plant development. Plant Mol. Biol. 2007;65:285–294. doi: 10.1007/s11103-007-9217-0. [DOI] [PubMed] [Google Scholar]
- Palevitz BA, Newcomb EH. The ultrastructure and development of tubular and crystalline P protein in the sieve elements of certain papilionaceous legumes. Protoplasma. 1971;72:399–426. [Google Scholar]
- Pélissier HC, Tegeder M. PvUPS1 plays a role in source–sink transport of allantoin in French bean (Phaseolus vulgaris) Funct. Plant Biol. 2007;34:282–291. doi: 10.1071/FP06277. [DOI] [PubMed] [Google Scholar]
- Peters WS, Knoblauch M, Warmann SA, Pickard WF, Shen AQ. Anisotropic contraction in forisomes: simple models won't fit. Cell Motil. Cytoskel. 2008;65:368–378. doi: 10.1002/cm.20266. [DOI] [PubMed] [Google Scholar]
- Peters WS, Knoblauch M, Warmann SA, Schnetter R, Shen AQ, Pickard WF. Tailed forisomes of Canavalia gladiata: a new model to study Ca2+-driven protein contractility. Ann. Bot. 2007a;100:101–109. doi: 10.1093/aob/mcm080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters WS, Schnetter R, Knoblauch M. Reversible birefringence suggests a role for molecular self-assembly in forisome contractility. Funct. Plant Biol. 2007b;34:302–306. doi: 10.1071/FP06281. [DOI] [PubMed] [Google Scholar]
- Pickard WF, Knoblauch M, Peters WS, Shen AQ. Prospective energy density in the forisome, a new smart material. Mater. Sci. Eng. 2006;C 26:104–112. [Google Scholar]
- Ravasi T, Huber T, Zavolan M, Forrest A, Gaasterland T, et al. Systematic characterization of the zinc-finger-containing proteins in the mouse transcriptome. Genome Res. 2003;13:1430–1442. doi: 10.1101/gr.949803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden DJ, Galperin MY. The DxDxDG motif for calcium binding: multiple structural contexts and implications for evolution. J. Mol. Biol. 2004;343:971–984. doi: 10.1016/j.jmb.2004.08.077. [DOI] [PubMed] [Google Scholar]
- Sabnis DD, Sabnis HM. Phloem proteins: structure, biochemistry and function. In: Iqbal M, editor. The Cambial Derivatives, Encyclopedia of Plant Anatomy, Vol. 9. Berlin: Borntraeger; 1995. pp. 271–292. [Google Scholar]
- Sato S, Nakamura Y, Asamizu E, Isobe S, Tabata S. Genome sequencing and genome resources in model legumes. Plant Physiol. 2007;144:588–593. doi: 10.1104/pp.107.097493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan S, Fritzsche M, Cismak A, Heilmann A, Spohn U. In vitro investigation of the geometric contraction behavior of chemo-mechanical P-protein aggregates (forisomes) Biophys. Chem. 2007;125:444–452. doi: 10.1016/j.bpc.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Sjolund RD. The phloem sieve element: a river runs through it. Plant Cell. 1997;9:1137–1146. doi: 10.1105/tpc.9.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBosch KA, Frugoli J. Guidelines for the genetic nomenclature and community governance for the model legume Medicago truncatula. Mol. Plant-Microbe. Interact. 2001;14:1364–1367. doi: 10.1094/MPMI.2001.14.12.1364. [DOI] [PubMed] [Google Scholar]
- Wergin WP, Newcomb EH. Formation and dispersal of crystalline P protein in sieve elements of soybean (Glycine max L.) Protoplasma. 1970;71:365–388. [Google Scholar]
- Windt CW, Vergeldt FJ, de Jager PA, van As H. MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ. 2006;29:1715–1729. doi: 10.1111/j.1365-3040.2006.01544.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang W, Kirberger M, Lee H-W, Ayalasomayajula G, Yang JJ. Prediction of EF-hand calcium-binding proteins and analysis of bacterial EF-hand proteins. Proteins. 2006;65:643–655. doi: 10.1002/prot.21139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplementary Data]