DAP12 Couples c-Fms Activation to the Osteoclast Cytoskeleton by Recruitment of Syk (original) (raw)
. Author manuscript; available in PMC: 2009 Aug 8.
Summary
We examined the mechanism by which M-CSF regulates the cytoskeleton and function of the osteoclast, the exclusive bone resorptive cell. We show that binding of M-CSF to its receptor c-Fms generates a signaling complex comprising phosphorylated DAP12, an adaptor containing an immunoreceptor tyrosine-based activation motif (ITAM) and the non-receptor tyrosine kinase Syk. c-Fms tyrosine 559, the exclusive binding site of c-Src, is necessary for regulation of DAP12/Syk signaling. Deletion of either of these molecules yields osteoclasts that fail to reorganize their cytoskeleton. Retroviral transduction of null precursors with wild type or mutant DAP12 or Syk reveals that the SH2 domain of Syk and the ITAM tyrosine residues and transmembrane domain of DAP12 mediate M-CSF signaling. Our data provide genetic and biochemical evidence that uncovers, an epistatic signaling pathway linking the receptor tyrosine kinase c-Fms to the immune adaptor DAP12 and the cytoskeleton.
Introduction
Osteoclasts, which are the exclusive resorptive cells of the skeleton, differentiate within the bone environment from members of the macrophage lineage under the control of M-CSF and RANK ligand (RANKL) (Boyle et al., 2003; Teitelbaum and Ross, 2003). M-CSF binds to c-Fms, a transmembrane receptor tyrosine kinase (RTK), and induces its auto-phosphorylation at seven tyrosine residues within the cytoplasmic tail (Pixley and Stanley, 2004). Following M-CSF binding, several Src homology 2 (SH2) domain-containing molecules are recruited to c-Fms and initiate signaling cascades which promote cell proliferation, differentiation and cytoskeletal reorganization (Pixley and Stanley, 2004).
DAP12 and the related molecule FcRγ are transmembrane signaling adaptors that contain cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs) (Barclay et al., 2002; Lanier and Bakker, 2000; Vivier et al., 2004). Both associate constitutively with a large family of immunoreceptors that are expressed on the surface of NK (Lanier et al., 1998; Takaki et al., 2006) and myeloid cells, including macrophages (Hamerman et al., 2005; Mocsai et al., 2006; Nakahashi et al., 2007), granulocytes (Looney et al., 2006; Mocsai et al., 2006), dendritic cells (Tomasello et al., 2000) and osteoclasts (Faccio et al., 2003b; Koga et al., 2004; Mocsai et al., 2004). DAP12 or FcRγ signaling is initiated by binding of ligands, present on adjacent cells and largely of unknown identity, to their cognate immunoreceptor.
Engagement of a DAP12-associated receptor induces tyrosine phosphorylation of the ITAM by Src family kinases (Lanier et al., 1998; Tomasello et al., 1998). The phosphorylated ITAM recruits the protein tyrosine kinases Syk or ZAP70, triggering their activation (Barclay et al., 2002; Lanier and Bakker, 2000; Vivier et al., 2004). Syk is a 72-kDa protein that is essential for integrin-mediated cytoskeleton organization in neutrophils (Mocsai et al., 2002), macrophages (Vines et al., 2001), platelets (Obergfell et al., 2002) and osteoclasts (Mocsai et al., 2004; Zou et al., 2007). However, whether the DAP12-Syk pathway is involved in M-CSF mediated cytoskeletal remodeling in the osteoclast is unknown.
M-CSF regulates osteoclast cytoskeletal reorganization in a c-Src dependent manner (Insogna et al., 1997). The fact that in other circumstances Src kinases phosphorylate ITAM proteins, resulting in activation of Syk and its effectors, prompted us to ask if ITAM containing adaptor proteins are involved in M-CSF signaling that mediates organization of the osteoclast actin cytoskeleton. Here we confirm that the ITAM-containing adaptor DAP12 is activated by M-CSF and that this pathway plays a central role in transducing signals from c-Fms to the osteoclast cytoskeleton.
Results
DAP12 deficient osteoclasts are defective in cytoskeletal reorganization and function
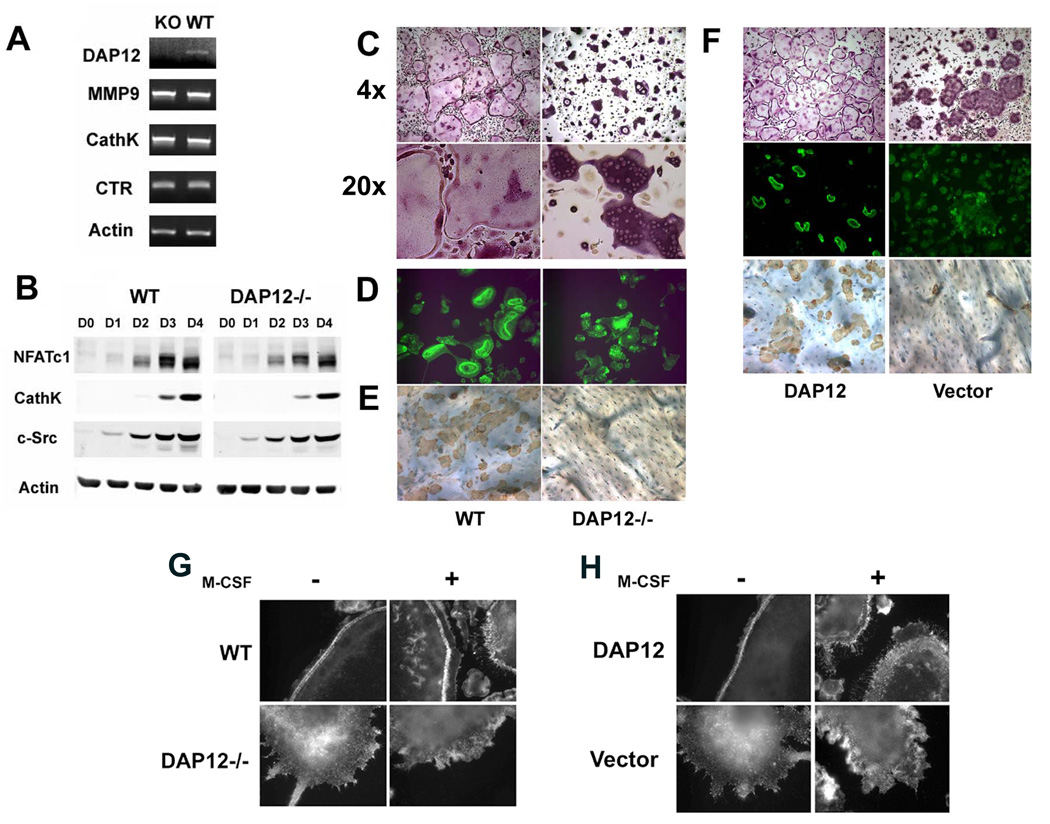
DAP12 deficient BMMs, like their wild-type (WT) counterparts, mature into tartrate-resistant acidic phosphatase (TRAP)-expressing, multinucleated cells. TRAP positive cells appear by day 2 and both genotypes became multinucleated on day 3 (Figure S1). Confirming no difference in commitment to the osteoclast lineage, expression of osteoclastogenic markers by WT and DAP12−/− cells is indistinguishable with time of culture (Figures 1A and B). On the other hand, while WT polykaryons form sheets of characteristic osteoclasts, those lacking DAP12 do not spread (Figure 1C).
Figure 1. DAP12 Deficiency Results in Abnormal Osteoclast Function In Vitro.
A) BMMs derived from WT or DAP12−/− mice were cultured with RANKL and M-CSF for 5 days after which the cells were analyzed for MMP9, cathepsin K and calcitonin receptor expression by RT-PCR. B) BMMs were cultured with RANKL and M-CSF (RANKL) for the indicated number of days. As control, cells were maintained in M-CSF (M-CSF) alone for 4 days. Osteoclast differentiation markers were determined by immunoblot. C) BMMs derived from WT or DAP12−/− mice were cultured with RANKL and M-CSF for 5 days after which the cells were stained for TRAP. Magnification is as shown. D–E) BMMs derived from WT or DAP12−/− mice were cultured with RANKL and M-CSF on bone slices for 6 days. D) Actin-ring formation was determined by immunofluorescence following FITC-phalloidin staining. E) After six days, osteoclasts were removed and the bones stained with HRP-labeled wheat germ agglutinin (WGA) to visualize resorption lacunae. F) Mature osteoclasts generated from DAP12 null precursors following retroviral transduction with either WT DAP12 or empty pMX vector, were grown on plastic dishes or bone slices. Cells were stained for multinucleation with TRAP, FITC-phalloidin for actin rings and wheat germ agglutinin for resorption pits. G) WT and DAP12 −/− osteoclasts were stimulated for 5 min with M-CSF (100 ng/ml) or vehicle alone, fixed, and subjected to immunofluorescence analysis with FITC-phalloidin to detect actin organization. H) DAP12−/− osteoclasts, transduced with either WT DAP12 or pMX vector, were treated as in A.
These data are in keeping with our previous observations that failure of differentiated osteoclasts to spread is indicative of a disorganized cytoskeleton (Faccio et al., 2005; McHugh et al., 2000; Zou et al., 2007). To determine if this is so in the context of DAP12 deficiency, we visualized the actin cytoskeleton of WT and DAP12−/− osteoclasts generated on bovine bone by staining the cells with FITC-phalloidin. Consistent with their abnormal shape (Figure 1C), osteoclasts lacking DAP12 are incapable of forming actin rings (Figure 1D), reflecting a deranged cytoskeletal organization. DAP12−/− osteoclasts are unable to degrade bone as shown by absence of resorptive lacunae (Figure 1E), a result that is in keeping with their dysfunctional cytoskeleton. The failure of DAP12-deficient osteoclasts to spread, form actin rings and resorb bone is normalized completely by retroviral reconstitution of DAP12 null precursors with WT DAP12 (Figure 1F).
Having established that DAP12 is essential for osteoclast cytoskeletal organization, we asked how this occurs, focusing on the role of M-CSF, which organizes the cytoskeleton of the resorptive cell (Faccio et al., 2007; Golden and Insogna, 2004). As shown in Figure 1G, 5 minutes of M-CSF treatment of WT osteoclasts induces rapid podosome redistribution from a peripheral belt to the cell surface, yielding numerous membrane ruffles and lamellipodia, whereas DAP12 deficient cells fail response to M-CSF treatment. Retroviral transduction of wild-type DAP12 in DAP12 null cells rescues the effect of M-CSF induced actin remodeling (Figure 1H).
DAP12 Mediates Syk Activation by M-CSF
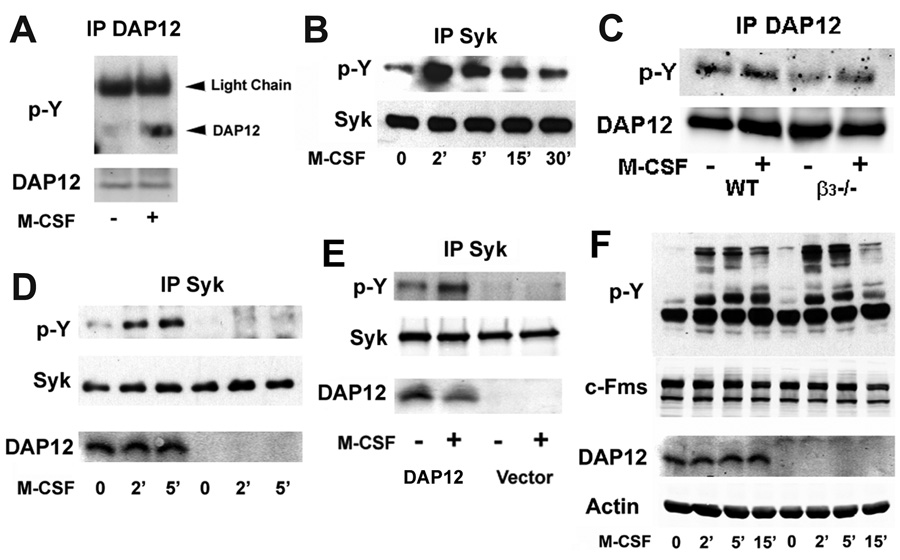
Turning to the biochemical basis for the role of DAP12 in regulating cytoskeletal changes in osteoclasts in response to M-CSF, we find that the cytokine stimulates DAP12 phosphorylation in mature polykaryons (Figure 2A), indicating that DAP12 is involved in M-CSF-induced signals that might regulate cytoskeletal organization in response to the cytokine.
Figure 2. DAP12 Mediates M-CSF-Induced Syk Phosphorylation.
A) WT BMMs were cultured with RANKL and M-CSF for 2 days. Pre-osteoclasts were starved of cytokines for 6 hours and then treated with M-CSF (100 ng/ml) for 5 minutes, after which DAP12 tyrosine phosphorylation was assessed by immunoblot. B) WT pre-osteoclasts were treated as in A with M-CSF (100 ng/ml) for the indicated times. Tyrosine phosphorylation of Syk was measured by immunoblot. C) WT and β3 null osteoclasts were starved of cytokines and then exposed to M-CSF (100 ng/ml) for 5 minutes. DAP12 phosphorylation and expression was determined by immunoblot. D) WT and DAP12−/− pre-osteoclasts were treated as in A with M-CSF (100 ng/ml) for the indicated times. The level of Syk tyrosine phosphorylation and DAP12 expression in whole cell lysates were determined by immunoblot. E) DAP12−/− pre-osteoclasts, transduced with either WT DAP12 or pMX vector, were starved of all cytokines for 6 hours and then treated with M-CSF (100 ng/ml) for 5 minutes. The level of Syk tyrosine phosphorylation and DAP12 expression in whole cell lysates were determined by immunoblot. F) WT and DAP12−/− BMMs treated with M-CSF (100 ng/ml) for indicated time after which the level of phosphorylated tyrosine in whole cell lysates was assessed by immunoblot.
M-CSF organization of the osteoclast cytoskeletal requires c-Src (Insogna et al., 1997), which in other cells phosphorylates the two tyrosine (Y) residues in the DAP12 ITAM motif. In consequence, the non-receptor tyrosine kinase Syk binds to tyrosine-phosphorylated DAP12 via its tandem SH2 domains, resulting in activation of downstream molecules which regulate the osteoclast cytoskeleton (Faccio et al., 2005). Furthermore, the functional and cytoskeletal reorganization defects of DAP12 deficient OCs are similar to those lacking Syk (Zou et al., 2007), raising the possibility that Syk and DAP12 collaborate in mediating M-CSF-induced cytoskeletal reorganization. In fact, like its effect on DAP12, M-CSF also phosphorylates Syk robustly in osteoclasts (Figure 2B).
We reported previously that regulation of the osteoclast cytoskeleton by ligation of the integrin αvβ3 requires the presence of DAP12 (Zou et al., 2007). Moreover, Mocsai et al. found that integrin activation results in DAP12 phosphorylation (Mocsai et al., 2006). These observations raised the possibility that the integrin is an intermediate in the pathway by which M-CSF modulates actin reorganization. To test this hypothesis we generated osteoclasts in which the β3 integrin subunit had been deleted genetically, exposed these cells to M-CSF and examined phosphorylation of DAP12. Figure 2C shows that activation of DAP12 is unaltered in osteoclasts that do not express αvβ3, demonstrating that the heterodimer has no role in linking the RTK c-Fms and the DAP12/Syk phosphorylation.
In other cells, the ITAM motif of DAP12 recruits Syk, leading to its activation. To determine if M-CSF-induced Syk activation requires DAP12 we assessed the ability of the cytokine to phosphorylate the non-receptor tyrosine kinase in WT and DAP12 deficient osteoclasts. As shown in Figure 2D, absence of DAP12 totally prevents M-CSF-induced Syk activation and this defect is rescued by reconstitution of the mutant cells with DAP12 (Figure 2E). Excluding the possibility that all M-CSF signaling is abolished in DAP12 deficient cells, c-Fms activation and total cell lysate tyrosine phosphorylation in osteoclasts treated with M-CSF is unaltered in the absence of DAP12 (Figure 2F and Figure S2). Hence, DAP12 is required for M-CSF-induced Syk activation, which in turn is essential for osteoclast function (Zou et al., 2007).
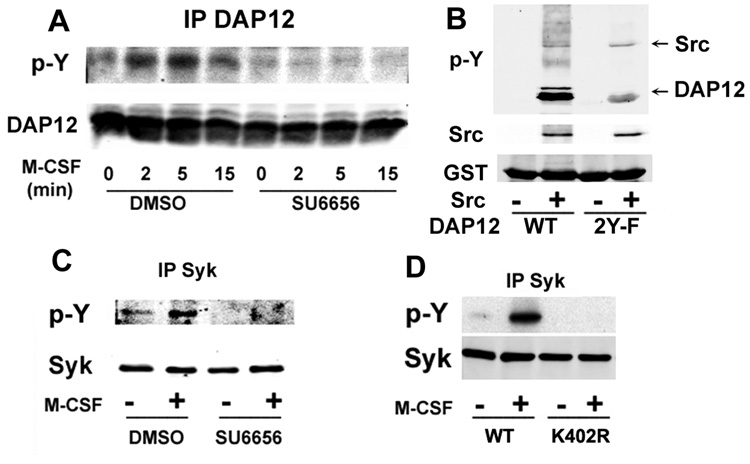
c-Src Mediates M-CSF-induced DAP12 and Syk Phosphorylation
Since Src family kinases phosphorylate ITAM proteins in NK cells, we speculated they may do the same to DAP12 after M-CSF engagement in osteoclasts. Thus, we pretreated osteoclasts with the Src kinase inhibitor SU6656 and stimulated the cells with M-CSF. Cell lysates were immunoprecipitated with anti-DAP12 antibody, followed by western blot with the anti-phosphotyrosine antibody 4G10. As shown in Figure 3A, M-CSF-induced DAP12 phosphorylation is totally blocked by the inhibitor. To examine if Src phosphorylates DAP12 directly, we performed an in vitro kinase assay. As shown in Figure 3B, we find that activated Src phosphorylates GST-tagged wild-type DAP12. However, ITAM-tyrosine mutant (2YF) phosphorylation of DAP12 by Src is markedly reduced, suggesting the Src acts mainly to phosphorylate the tyrosines within the ITAM motif of DAP12. Furthermore, 293 cells transfected with DAP12 and wild-type Src contain phosphorylated DAP12 while cells transfected with DAP12 and kinase inactive Src (K295R and Y416F) do not (data not shown). Since DAP12 induces Syk activation through its phosphorylated ITAM motif in other cell types, we examined Syk phosphorylation following M-CSF treatment in the osteoclast. Accordingly, SU6656 prevents M-CSF-induced Syk activation in osteoclasts (Figure 3C) and M-CSF-induced association of Syk and DAP12 (Figure S3).
Figure 3. c-Src Mediates M-CSF-Induced Syk Phosphorylation.
A) WT pre-osteoclasts, pretreated with SU6656 (2 µM) or DMSO for 20 minutes, were treated with M-CSF (100 ng/ml) for the indicated time. Tyrosine phosphorylation in DAP12 immunoprecipitates was determined by immunoblot. B) GST-DAP12 (20µg) was incubated at 30 °C for 1 h with 30 ng of Src. Proteins were separated in 10% sodium dodecyl sulfate polyacrylamide gels and immunoblotted with antibodies to assess DAP12 phosphorylation and levels of c-Src and GST-DAP12. C) WT pre-osteoclasts, pretreated with SU6656 (2 µM) or DMSO for 20 minutes, were treated with M-CSF (100 ng/ml) for 5 minutes. Tyrosine phosphorylation in Syk immunoprecipitates was determined by immunoblot. D) Syk−/− pre-osteoclasts, transduced to express either WT or kinase inactive Syk (K402R), were treated with M-CSF (100 ng/ml) for 5 minutes. The level of Syk tyrosine phosphorylation was measured by immunoblot.
Syk must be phosphorylated to activate its downstream targets. Typically Syk undergoes auto-phosphorylation when bound to the ITAM domain of immune response receptors (Pitcher and van Oers, 2003), but we reported that this is not so in the context of the integrin αvβ3 in osteoclasts, where c-Src serves as the kinase for Syk (Zou et al., 2007). To determine if M-CSF promotes Syk autophosphorylation, we transduced WT or kinase-inactive Syk (Syk K402R) into Syk−/− BMMs, which were then used to generate pre-OCs. The cells were treated with M-CSF, and tyrosine-phosphorylated Syk was measured. M-CSF induced Syk phosphorylation is abolished in Syk−/− pre-osteoclasts expressing kinase-inactive Syk (Figure 3D). Hence, c-Fms occupancy differs from that of αvβ3 in that it promotes auto-phosphorylation of Syk after its recruitment to DAP12.
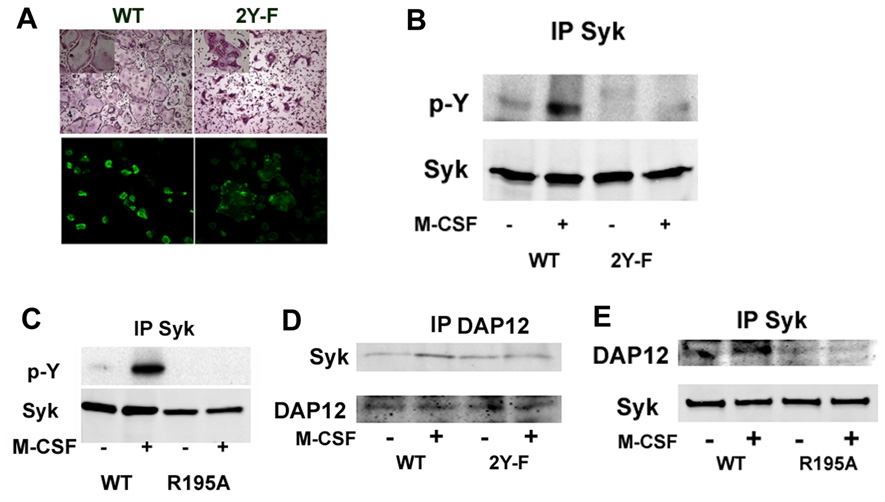
M-CSF Signaling Requires the Syk SH2 Domain and the DAP12 ITAM Motif
Phosphorylation of DAP12 ITAM tyrosine residues provides docking sites for the tandem Src homology 2 (SH2) domains of Syk, resulting in activation of the kinase. We asked therefore if the two tyrosine moieties in the DAP12 ITAM motif and the tandem SH2 domains of Syk are essential for osteoclast function. Thus, we retrovirally transduced DAP12−/− BMMs with WT DAP12 or that in which the ITAM tyrosines at positions 92 and 103 had been mutated to phenylalanine (2YF). While WT DAP12 normalizes the DAP12−/− osteoclast cytoskeleton, the 2YF mutant fails to do so (Figure 4A). Furthermore, M-CSF phosphorylates Syk in cells expressing WT but not 2YF DAP12 (Figure 4B), indicating that the two tyrosine residues in the ITAM motif are required for Syk recruitment and activation and subsequent cytoskeletal organization. We also transduced Syk−/− BMMs with WT or R195A Syk, which inactivates the C-terminal SH2 domain and find that M-CSF phosphorylates the WT protein but not that containing the mutant (Figure 4C). To confirm the interaction between the SH2 domain of Syk and the ITAM motif of DAP12, we assayed their interaction in Syk−/− cells transduced with either wild-type Syk or Syk SH2 mutant (R195A) and in DAP12−/− cells transduced with either wild-type DAP12 or 2YF DAP12. As shown in Figures 4D and E, association of DAP12 with Syk is detected only when WT DAP12 and Syk are co-expressed after M-CSF treatment while mutation in either the ITAM motif of DAP12 (2YF) or SH2 domain of Syk (R195A) abrogates binding. Thus, the SH2 domain of Syk is required for its interaction with the ITAM motif of DAP12.
Figure 4. M-CSF Signaling Requires the Syk SH2 Domain and the DAP12 ITAM Motif.
A) Mature osteoclasts were generated from DAP12 null precursors following transduction with either WT DAP12 or the 92/103YY-FF (2YF), double mutant on plastic dishes or bone slices. Cells were stained with TRAP, or FITC-phalloidin. B) Pre-osteoclasts, generated as in A), were stimulated with M-CSF (100 ng/ml) for 5 minutes and the level of Syk tyrosine phosphorylation was measured by immunoblot. C) Syk−/− pre-osteoclasts, transduced with either WT Syk or the C-terminal SH2 domain mutant Syk (R195A), were stimulated with M-CSF (100 ng/ml) for 5 minutes. The level of Syk tyrosine phosphorylation was measured by immunoblot. D) DAP12−/− pre-osteoclasts, transduced with either HA-tagged WT DAP12 or the ITAM double tyrosine mutant DAP12 (2YF), were stimulated with M-CSF (100 ng/ml) for 5 minutes. Total cell lysate was immunoprecipitated with anti-HA antibodies. Immunoprecipitates were probed by Western blotting for Syk. E) Syk−/− pre-osteoclasts, transduced with either HA tagged Syk or the C-terminal SH2 domain mutant Syk (R195A), were stimulated with M-CSF (100 ng/ml) for 5 minutes. Total cell lysate was immunoprecipitated with anti-HA antibodies. Immunoprecipitates were probed by Western blotting for DAP12.
DAP12 alone can couple c-Fms to the actin cytoskeleton
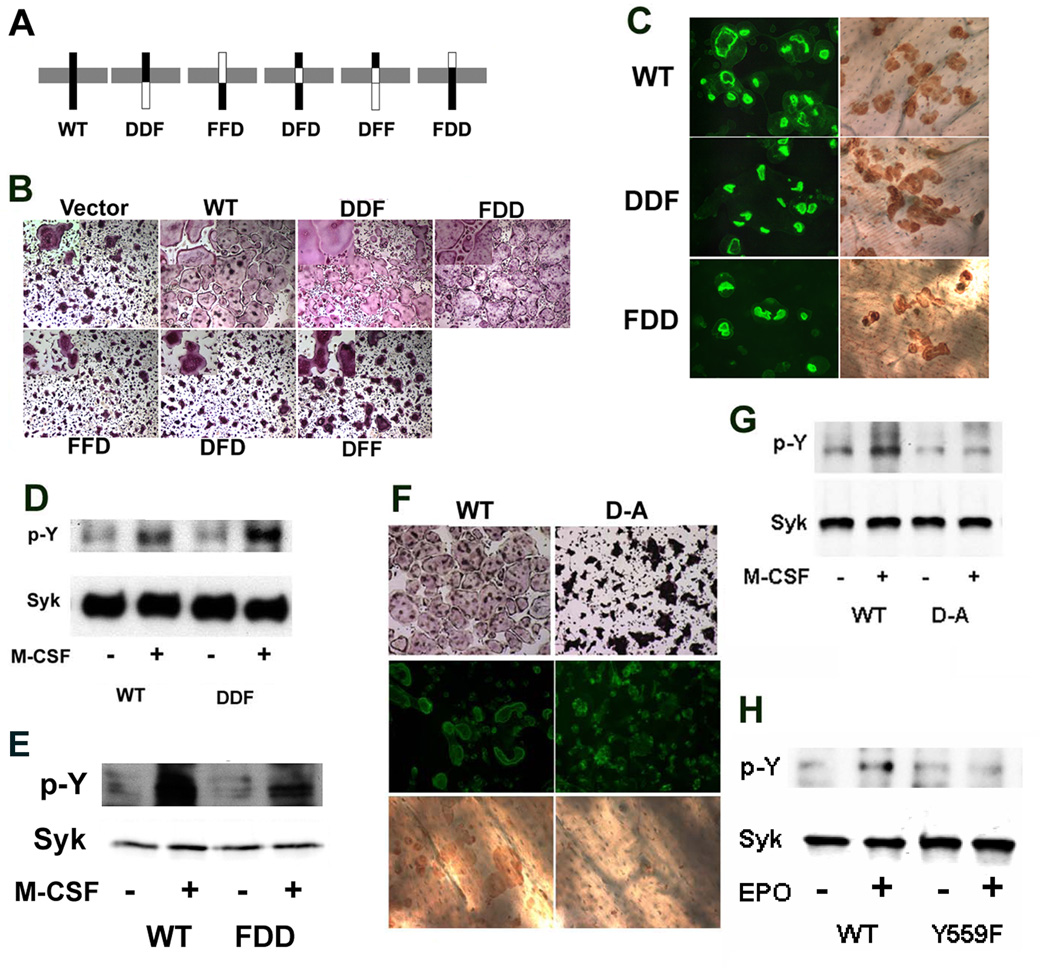
While osteoclasts and their precursors express both DAP12 and FcRγ, our results indicate that loss of DAP12 alone is sufficient to impair M-CSF signaling. On the other hand, deletion of both DAP12 and FcRγ, but not either molecule alone, results in severe osteopetrosis (Koga et al., 2004) suggesting they transmit redundant signals, particularly as the cytoplasmic tails of each adaptor each contain ITAM motifs that recruit Syk (Lanier and Bakker, 2000). To test this hypothesis, we constructed a number of DAP12 (D)-FcRγ (F) chimeras (Figure 5A) and retrovirally transduced them into DAP12 deficient BMMs, which were used to generate osteoclasts. When the DAP12 intracellular or extracellular domain is replaced by its FcRγ counterpart (DDF or FDD) the resulting chimera rescues DAP12 deficient osteoclast spreading, actin ring formation and bone resorption as effectively as WT (Figures 5B and C). Consistent with the fact that DAP12−/− osteoclasts expressing DDF and FDD organize their cytoskeleton in response to M-CSF, the same mutants rescue Syk phosphorylation following exposure to the cytokine (Figure 5D–E). Hence, signals emanating from the cytoplasmic tail of either DAP12 or FcRγ (DDF and WT) act redundantly to regulate the osteoclast cytoskeleton in response to M-CSF. Since the short extracellular domains of DAP12 and FcRγ each contain two cysteine residues involved in receptor dimerization and likely do not contribute to ligand binding (Lanier and Bakker, 2000; Lanier et al., 1998), we conclude that they are interchangeable (FDD and WT).
Figure 5. The DAP12 Transmembrane Domain is Important for Organization of the OC Cytoskeleton.
A) Scheme of DAP12 and FcRγ chimeras. B) Mature DAP12−/− osteoclasts, transduced to express either WT DAP12 or the chimeras in A) were generated and stained for TRAP activity. C) Mature DAP12−/− osteoclasts, transduced to express either WT DAP12 or DDF or FDD chimeras, were generated on bone slices for 6 days and stained with FITC-phalloidin and peroxidase-conjugated WGA followed by DAB. D) and E) DAP12−/− pre-osteoclasts, transduced to express either WT DAP12 or the indicated chimeras, were treated with M-CSF (100 ng/ml) for 5 minutes. Tyrosine phosphorylation of Syk immunoprecipitates was tested by immunoblot. F) Mature DAP12−/− osteoclasts, transduced to expressed either WT DAP12 or DAP12 D52A mutant, were generated and stained for TRAP activity, or with FITC-phalloidin and peroxidase-conjugated WGA-DAB. G) DAP12−/− pre-osteoclasts, transduced to express either WT DAP12 or D52A mutant, were treated with M-CSF (100 ng/ml) for 5 minutes and levels of Syk tyrosine phosphorylation were assessed by immunoblot. H) Wild type pre-osteoclasts, transduced with either WT EpoR-Fms or Y559F mutant EpoR-Fms chimeras, were stimulated with human erythropoietin (100 ng/ml) for 5 minutes. The level of Syk tyrosine phosphorylation was measured by immunoblot.
In contrast to the previous results, presence of the transmembrane domain of FcRγ in place of DAP12 (FFD, DFD, and DFF) fails to rescue osteoclast spreading (Figure 5B) or stimulate Syk activation (Figure S4). This result is not surprising, since each ITAM-bearing adaptor interact with its unique receptor within the transmembrane region via distinct charge-based interactions (Ravetch and Kinet, 1991; Underhill and Goodridge, 2007). Thus, interaction between DAP12 and its receptor requires formation of a salt bridge within the plasma membrane by approximation of a negatively-charged residue (aspartic acid) in DAP12 with a positively charged lysine in the receptor (Lanier et al., 1998). Given this model, we mutated DAP12 aspartate 52 to alanine (A) and retrovirally transduced this protein and its WT counterpart into DAP12 deficient cells. While WT DAP12 induces OC function, the charge mutant fails to rescue OC spreading, actin-ring formation, bone resorption and M-CSF-induced Syk phosphorylation (Figures 5F and G), confirming that DAP12 is activated by interaction within its transmembrane domain with a receptor such as TREM2 or SIRP1β.
Using a chimeric receptor approach we found that M-CSF-mediated cytoskeletal organization requires recruitment of a multimeric signaling complex containing c-Src, c-Cbl, PI3K and Grb2, an event involving Y559, Y697 and Y721 in the c-Fms cytoplasmic domain (Faccio et al., 2007). To identify the individual c-Fms cytoplasmic domain tyrosine residues which activate the DAP12/Syk pathway we utilized the same chimeric strategy, transducing WT BMMs with retroviral vectors coding for the external domain of the erythropoietin receptor linked to the transmembrane and cytoplasmic regions of c-Fms. Individual vectors contained all seven Y/F mutations known to transmit c-Fms signals. WT c-Fms cytoplasmic tail and kinase-inactive c-Fms (K602M) served as positive and negative controls. Puromycin-selected cells were used to generate osteoclasts, which were cytokine-starved prior to treatment with recombinant erythropoietin. Absence of only Y559 in the cytoplasmic tail of c-Fms, which we have demonstrated is not a kinase dead receptor (Takeshita et al., 2007) leads to complete blockade of Syk phosphorylation (Figure 5H). In contrast, all other single Y mutants continue to activate Syk (data not shown).
Discussion
Specific receptor tyrosine kinases promote actin remodeling and motility (Feldner and Brandt, 2002; Wu and Luo, 2005) which in the case of osteoclasts follow activation of c-Fms (Insogna et al., 1997). We determined the mechanism by which M-CSF occupancy of c-Fms organizes the osteoclast cytoskeleton and we find, unexpectedly, that this process involves immunoreceptor-like adaptors. This is the first report that a receptor tyrosine kinase activates immunoreceptor-associated proteins such as DAP12. Our results reveal that 1) activation of c-Fms leads to phosphorylation of DAP12; 2) DAP12 is essential for osteoclast cytoskeleton reorganization and function; 3) M-CSF activates Syk downstream of DAP12 and both molecules are important for osteoclast cytoskeleton reorganization; 4) M-CSF signaling requires the SH2 domains of Syk, the ITAM tyrosine residues of DAP12, and the transmembrane domain of DAP12 and 5) c-Fms tyrosine 559 is necessary to regulate DAP12/Syk signaling.
c-Fms and the integrin αvβ3 both organize the osteoclast cytoskeleton and do so in a collaborative manner sharing common effector molecules (Faccio et al., 2003a; Ross and Teitelbaum, 2005). Since αvβ3 mediates its cytoskeletal organizing effects via DAP12 (Zou et al., 2007) we asked if the same is true for c-Fms. Similar to occupancy of the integrin, M-CSF regulates the osteoclast cytoskeleton by utilizing c-Src (Ross and Teitelbaum, 2005) whose family of kinases phosphorylate ITAM motifs in other cell types (Lanier et al., 1998; Tomasello et al., 1998). Importantly, using osteoclasts lacking αvβ3, we show that the integrin plays no role in coupling activation of c-Fms and DAP12.
c-Fms signaling depends on autophosphorylation of intracellular tyrosine residues (Pixley and Stanley, 2004). Because of the presence of endogenous receptor, it was not possible previously to determine the components of c-Fms, which mediate specific events in osteoclasts. We overcame this difficulty by developing chimeric receptors consisting of the external domain of the erythropoietin receptor (EpoR) and the transmembrane and intracellular domains of c-Fms. WT BMMs expressing this receptor differentiate into authentic osteoclasts with exposure to RANKL and erythropoietin (Feng et al., 2002). Using this approach has permitted us to delineate the role of specific c-Fms residing tyrosine residues in osteoclast formation and function. In the present study, we establish that mutation of only c-Fms Y559 blunts DAP12 downstream phosphorylation. Importantly, this residue represents the sole c-Src binding site in the c-Fms cytoplasmic domain (Faccio et al., 2007; Takeshita et al., 2007), in keeping with the hypothesis that c-Src mediates M-CSF induced phosphorylation of the ITAM protein. This conclusion is strengthened by the fact that the c-Src inhibitor SU6656 arrests M-CSF-induced DAP12 phosphorylation.
DAP12 and the related molecule FcRγ, which each signal through an ITAM domain, are adaptors for transmembrane receptors activated by unknown ligands. These adaptors are required for normal bone homeostasis because mice lacking both develop osteopetrosis due to the inability of osteoclasts to resorb bone (Kaifu et al., 2003; Koga et al., 2004). Our data suggest that a major role of DAP12 in osteoclasts involves organization of the cytoskeleton, as opposed to differentiation of precursors. Suggesting an analogous role in other cells types, Takahashi et al reported that DAP12 activation regulates microglial cytoskeleton reorganization resulting in increased phagocytosis (Takahashi et al., 2005) and its phosphorylation prompts filipodia and lamellipodia formation in macrophages (Hayashi et al., 2004). Similarly, DAP12−/− BMMs differentiate into TRAP-expressing osteoclasts, albeit with an abnormal morphology, including failure to spread and form actin rings (Faccio et al., 2003b), which, in other circumstances, are central to optimal bone resorptive activity. As a result of these cytoskeletal abnormalities, DAP12−/− osteoclasts have markedly diminished capacity to excavate bone pits. The molecular mechanisms underlying these observations were undefined prior to our studies.
Our findings of normal osteoclast maturation in the absence of DAP12 are seemingly in conflict with those of Koga et al and Moscai and coworkers (Koga et al., 2004; Mocsai et al., 2004), who claim failure of osteoclastogenesis in this circumstance. On the other hand, Kaifu et al (Kaifu et al., 2003) demonstrate DAP12-deficient osteoclasts generated in vitro are similar in appearance to those we report and Mocsai reports normal expression of osteoclast differentiation markers in these cells (Mocsai et al., 2004). We propose therefore that failure to recognize the appearance of DAP12−/− osteoclasts may reflect difficulty in identifying multinucleation in the crenated phenotype.
Syk is a non receptor tyrosine kinase that is recruited to ITAM motifs in proteins such as DAP12 and FcRγ (McVicar and Burshtyn, 2001). In the context of macrophages/osteoclasts, DAP12 associates with TREM2 and SIRPβ1, while FcRγ associates with OSCAR (Koga et al., 2004). Since DAP12 and FcRγ contain ITAM motifs and Syk is crucial to regulating the osteoclast cytoskeleton (Zou et al., 2007), we examined its relationship to M-CSF. Consistent with M-CSF phosphorylation of DAP12, the cytokine also activates Syk. Moreover, mutation of either the Syk SH2 domain or DAP12 ITAM motif disrupts Syk activation, confirming that Syk is recruited to DAP12.
Finally, deficiency of DAP12 in osteoclasts abolishes Syk activation completely. Since these same cells express FcRγ we studied the selectivity of ITAM-containing receptors in M-CSF signaling. We generated several DAP12-FcRγ chimeras in which the DAP12 transmembrane domain, intracellular domain, or extracellular domain were replaced by the corresponding region from FcRγ. Interestingly, the chimeric adaptor in which the intracellular domain of DAP12 is replaced by the FcRγ (DDF) completely rescues DAP12−/− osteoclast function and M-CSF induced Syk phosphorylation, indicating that the DAP12 ITAM motif and that of FcRγ recruit similar or identical complexes that signal to the cytoskeleton. Consistent with the fact that the extracellular domain of DAP12 is not involved in ligand binding due to its short amino acid sequence, replacing the extracellular domain of DAP12 with that of FcRγ (FDD) also rescues DAP12 function in DAP12 null cells. In contrast, a chimeric DAP12/FcRγ receptor that contains the FcRγ transmembrane substitution (DFD) is nonfunctional in either osteoclastogenesis or Syk phosphorylation, since the mutant will not be activated. In similar fashion, a DAP12 transmembrane charge mutant that is unable to associate with DAP12-associated receptors also fails to restore osteoclast function. We also find that in NIH3T3 cells DAP12 associates with Syk only if DAP12-associated receptors, such as TREM2, are also present (data not shown), confirming that DAP12 function requires activation by associated receptors and plays a central role in M-CSF signaling in osteoclasts. Finally, since mice lacking FcRγ have unaltered DAP12-mediated signaling they have no bone phenotype and their osteoclasts generated in vitro spread normally (Mocsai et al., 2004) and our unpublished data.
This exercise provides genetic and biochemical evidence of an epistatic pathway linking the receptor tyrosine kinase c-Fms to organization of the osteoclast cytoskeleton. M-CSF induction of c-Fms phosphorylation leads to recruitment and activation of c-Src (Faccio et al., 2007), which phosphorylates DAP12 directly. Alternatively, the substrate for the proto-oncogene may be a co-associating receptor, such as TREM2 or SIRP1β, or one of the several unidentified ligands, which activate these receptors. At this time, we cannot distinguish between these three possibilities.
Our data suggest a model in which phosphorylation of DAP12 ITAM, in osteoclasts recruits Syk via its SH2 domain, resulting in autophosphorylation of the kinase. Signals downstream of DAP12 depend on the selectivity of the transmembrane domain, while its ITAM motif can be replaced by that of the FcRγ receptor. Phosphorylated Syk ultimately mediates the cytoskeletal organization and actin-ring formation which occurs when M-CSF occupies its receptor, thereby prompting functional bone resorption.
Experimental Procedures
Mice
DAP12−/− (C57BL/6 background) and Syk+/− (129/SV background) mice were described previously (Clements et al., 1998; Kaifu et al., 2003; Turner et al., 1995; Zou et al., 2007). All animals used in these experiments were 4–8 weeks old and housed in the animal care unit of Washington University School of Medicine, where they were maintained according to guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experimentation was approved by the Animal Studies Committee of Washington University School of Medicine.
Because of perinatal lethality of Syk−/− mice, we generated bone marrow chimeras by transplanting Syk−/− fetal liver cells into lethally irradiated WT recipients. Briefly, Syk−/− and littermate Syk+/? fetal liver cells were obtained from E15–E17 embryos from timed mating of Syk+/− breeders. Bone marrow chimeras were generated by intravenous injection of unfractionated fetal liver cells into 6–8 week old, lethally-irradiated congenic recipient wild type mice. Chimeras were used as a source of BMMs 4–8 weeks after bone marrow transplantation.
Macrophage isolation and osteoclast culture
Primary BMMs were prepared as described previously (Faccio et al., 2003b) with slight modification. Marrow was extracted from femora and tibiae of 6- to 8-wk-old mice with α-MEM and cultured in α-MEM containing 10% inactivated fetal bovine serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin (α-10 medium) with 1:10 CMG condition media (Takeshita et al., 2000) in bacterial plastic. Cells were incubated at 37°C in 6% CO2 for 3 days and then washed with PBS and lifted with 1x trypsin/EDTA (Invitrogen, Carlsbad, CA) in PBS. A total of 5×103 cells were cultured in 200 µl α-MEM containing 10% heat inactivated FBS with 100 ng/ml GST-RANKL and 40 ng/ml of mouse recombinant M-CSF in 96-well tissue culture plates, some containing sterile bone slices. Cells were fixed and stained for TRAP activity after 5 days in culture, using a commercial kit (Sigma 387-A, St. Louis, MO). For actin ring staining, cells were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, rinsed in PBS, and immunostained with Alexa 488 phalloidin (Molecular Probes). To quantitate resorption lacunae, cells were removed from bone slices with mechanical agitation. Bone slices were incubated with peroxidase-conjugated wheat germ agglutinin (Sigma) for 1 hour and stained with 3, 3'-diaminobenzidine (Sigma). For pre-osteoclast generation, 1.5 × 106 BMMs were plated per 10-cm tissues culture dish and cultured in 40ng/ml M-CSF and 100ng/ml GST-RANKL for 2–3 days.
Plasmids and Retroviral transduction
Mouse full length DAP12- with a FLAG tag was cloned by RT-PCR with primers: forward TTGGATCCTTCATGGGGGCTCTGGAGCCC; reverse AAGCGGCCGCTCACTTGTCGTCATCGTCTTTGTAGTCTCTGTAATATTGCCTCTG. The PCR product was introduced into the BamH1 and Not1 sites of the pMX-puro vector (Onishi et al., 1998). The DAP12 92/103YY-FF (2YF) mutant was generated using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). We used standard molecular biological methods to construct cDNAs coding for 5 chimeric proteins: DDF, consisting of the external and transmembrane domain of murine DAP12 linked to the intracellular domain of murine FcRγ; DFD, consisting of the external and intracellular domain of murine DAP12 linked by the transmembrane domain of murine FcRγ; FDD, consisting of the external domain of murine FcRγ linked to the transmembrane and intracellular domain of murine DAP12; DFF, consisting of the external domain of murine DAP12 linked to the transmembrane and intracellular domain of murine FcRγ and FFD, consisting of the external and transmembrane domain of murine FcRγ linked to the intracellular domain of murine DAP12. All relevant clones were sequenced to ensure they coded for the appropriate protein fragments. Wild type human Syk, its C-terminal SH2 domain mutant (R195A) and Kinase dead (K402R) Syk mutants, gifts of Dr. Shattil, were sub-cloned into the BamH1 and Xho1 sites of a retroviral vector in which the puromycin resistance sequence was replaced with one coding for blastocidin resistance; for selected immunoprecipitation studies an HA tag was added at the C-terminus of Syk. Chimeric EpoR/c-Fms receptors containing individual Tyr to Phe mutations were constructed and cloned into the pMX-puro retrovirus vector as described previously (Feng et al., 2002). Wild type or mutant DAP12, Syk and EpoR/c-Fms in retrovirus vectors, were transiently transfected into Plat-E packaging cells using FuGENE 6 Transfection Reagent (Roche). Virus was collected 48 h after transfection. BMMs were infected with virus for 24 h in the presence of 100ng/ml M-CSF and 4 µg/ml polybrene (Sigma). Cells were selected in the presence of M-CSF and 2 µg/ml puromycin (Sigma) or 1 µg/ml blasticidin (Calbiochem) for 3 days prior to use as osteoclast precursors.
Western blotting and immunoprecipitation
Cultured cells were washed twice with ice-cold PBS and lysed in RIPA buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, and 1x protease inhibitor mixture (Roche). After incubation on ice for 10 min, cell lysates were clarified by centrifugation at 15,000 rpm for 10 min. Forty micrograms of total lysates were subjected to 8 or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Filters were blocked in 0.1% casein in PBS for 1 h and incubated with primary antibodies at 4°C overnight followed by probing with fluorescence-labeled secondary antibodies (Jackson Lab). Proteins were detected with the Odyssey Infrared Imaging System (LI-COR Biosciences). The source of antibodies is as follows: rabbit anti-DAP12 polyclonal antibody from Exalpha Biological Inc (Watertown MA); rabbit anti-DAP12 serum for immunoprecipitation was kindly provided by Dr. Takai (Tohoku University, Japan); mouse anti-Syk monoclonal antibody from Abcam (Cambridge, MA); anti-phosphotyrosine mAb 4G10 from Upstate (Charlottesville VA); mAb 327, directed against the c-Src protein and full-length c-Src cDNA were gifts of Dr. A. Shaw (Department of Pathology, Washington University School of Medicine, St. Louis, MO); anti Src p-Y416 antibody and rabbit anti-SLP-76 antibody from Cell Signaling (Beverly MA); rabbit anti-Syk (N-19) was from Santa Cruz; Rabbit anti-HA antibody was purchased from Covance (Princeton, New Jersey). For immunoprecipitation, BMMs were cultured with M-CSF and RANKL in culture dishes for 2–3 days. Cells were starved in αMEM without serum for 3–4 hours and treated with recombinant M-CSF for the indicated times. Cells were washed in cold PBS, lysed on ice in RIPA lysis buffer (10 mM Tris, 150 mM NaCl, 1% NP40, 0.2% sodium deoxycholate, 1 mM EDTA, 2 mM Na3VO4, 10 mM NaF plus protease inhibitors cocktail (Roche)) and eight hundred micrograms of protein were incubated with 2 µg primary antibody, at 4°C overnight with rotation. Protein A or G agarose (Sigma) beads were then added and incubated with rotation for 3 h at 4°C. Beads were washed three times in lysis buffer and boiled in 2x sodium dodecyl sulfate sample buffer for 5 min. After centrifugation, proteins were separated by 8 or 12% sodium dodecyl sulfate polyacrylamide gels and western blotted as described above.
In Vitro Phosphorylation of DAP12
The cytoplasmic tail of wild-type or 2YF DAP12 was cloned into pGEX vector (Amersham Biosciences, Piscataway, NJ), expressed in BL21star E. coli (Invitrogen), and purified by using glutathione-Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ). Recombinant human Src expressed and purified from Sf9 cells was purchased from Upstate Biotechnology (Lake Placid, NY) and used for in vitro phosphorylation of GST-WT DAP12 and GST-2YF DAP12. GST-DAP12 (20µg) was incubated at 30 °C for 1 h with 30 ng of Src in 100 µl of reaction buffer. The reaction buffer included 20 mM Tris·HCl pH 7.4, 10 mM MgCl2, 1 mM MnCl2, 0.2 mM ATP, 1 mM DTT. The enzyme reaction was terminated by boiling in 2x sodium dodecyl sulfate sample buffer for 5 min. Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gels, transferred to PVDF membrane, and immunoblotted using antibodies as described above.
Supplementary Material
01
Acknowledgements
We wish to thank Dr. Toshiyuki Takai (Tohoku University, Japan) for DAP12 knock-out mice and anti-DAP12 serum, Dr. Sanford Shattil (University of California, San Diego) for the Syk plasmids, and Paulette Shubert for assistance in preparing this manuscript. This study was supported by grants from the National Institutes of Health AR046852 (FPR). AR032788, AR046523 (SLT), and 1 F30 AG302802 (JLR)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
References
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- Faccio R, Takeshita S, Colaianni G, Chappel JC, Zallone A, Teitelbaum SL, Ross FP. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-Y559/697/721. J. Biol. Chem. 2007;282:18991–18999. doi: 10.1074/jbc.M610937200. [DOI] [PubMed] [Google Scholar]
- Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the αvβ3 integrin collaborate during osteoclast differentiation. J. Clin. Invest. 2003a;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat. Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J. Cell. Biochem. 2003b;90:871–883. doi: 10.1002/jcb.10694. [DOI] [PubMed] [Google Scholar]
- Feldner JC, Brandt BH. Cancer cell motility--on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp. Cell Res. 2002;272:93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- Feng X, Takeshita S, Namba N, Wei S, Teitelbaum SL, Ross FP. Tyrosines 559 and 807 in the cytoplasmic tail of the M-CSF receptor play distinct roles in osteoclast differentiation and function. Endocrinology. 2002;143:4868–4874. doi: 10.1210/en.2002-220467. [DOI] [PubMed] [Google Scholar]
- Golden LH, Insogna KL. The expanding role of PI3-kinase in bone. Bone. 2004;34:3–12. doi: 10.1016/j.bone.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Ohnishi H, Okazawa H, Nakazawa S, Ikeda H, Motegi S, Aoki N, Kimura S, Mikuni M, Matozaki T. Positive regulation of phagocytosis by SIRPbeta and its signaling mechanism in macrophages. J. Biol. Chem. 2004;279:29450–29460. doi: 10.1074/jbc.M400950200. [DOI] [PubMed] [Google Scholar]
- Insogna KL, Sahni M, Grey AB, Tanaka S, Horne WC, Neff L, Mitnick M, Levy JB, Baron R. Colony-stimulating factor-1 induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J. Clin. Invest. 1997;100:2476–2485. doi: 10.1172/JCI119790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Bakker ABH. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today. 2000;21:611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J. Clin. Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicar DW, Burshtyn DN. Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Sci. STKE. 2001;2001:RE1. doi: 10.1126/stke.2001.75.re1. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–548. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- Nakahashi C, Tahara-Hanaoka S, Totsuka N, Okoshi Y, Takai T, Ohkohchi N, Honda S, Shibuya K, Shibuya A. Dual assemblies of an activating immune receptor, MAIR-II, with ITAM-bearing adapters DAP12 and FcRgamma chain on peritoneal macrophages. J. Immunol. 2007;178:765–770. doi: 10.4049/jimmunol.178.2.765. [DOI] [PubMed] [Google Scholar]
- Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, Lowell CA, Shattil SJ. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Nosaka T, Misawa K, Mui AL-F, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutive active STAT5 mutant that promotes cell proliferation. Mol. Cell. Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher LA, van Oers NSC. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Kinet JP. Fc receptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Ross FP, Teitelbaum SL. αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki R, Watson SR, Lanier LL. DAP12: an adapter protein with dual functionality. Immunol. Rev. 2006;214:118–129. doi: 10.1111/j.1600-065X.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Faccio R, Chappel JC, Zheng L, Feng S, Weber JD, Teitelbaum SL, Ross FP. c-Fms tyrosine 559 Is a major mediator of M-CSF-induced proliferation of primary macrophages. J. Biol. Chem. 2007;282:18980–18990. doi: 10.1074/jbc.M610938200. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000;15:1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Desmoulins P-O, Chemin K, Guia S, Cremer H, Ortaldo J, Love P, Kaiserlian D, Vivier E. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Olcese L, Vely F, Geourgeon C, Blery M, Moqrich A, Gautheret D, Djabali M, Mattei M-G, Vivier E. Gene Structure, Expression Pattern, and Biological Activity of Mouse Killer Cell Activating Receptor-associated Protein (KARAP)/DAP-12. J. Biol. Chem. 1998;273:34115–34119. doi: 10.1074/jbc.273.51.34115. [DOI] [PubMed] [Google Scholar]
- Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Goodridge HS. The many faces of ITAMs. Trends. Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Vines CM, Potter JW, Xu Y, Geahlen RL, Costello PS, Tybulewicz VL, Lowell CA, Chang PW, Gresham HD, Willman CL. Inhibition of beta 2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity. 2001;15:507–519. doi: 10.1016/s1074-7613(01)00221-7. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Wu J, Luo H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr. Opin. Hematol. 2005;12:292–297. doi: 10.1097/01.moh.0000166497.26397.9f. [DOI] [PubMed] [Google Scholar]
- Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VLJ, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the αvβ3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01