Molecular basis for a link between complement and the vascular complications of diabetes (original) (raw)
Abstract
Activated terminal complement proteins C5b to C9 form the membrane attack complex (MAC) pore. Insertion of the MAC into endothelial cell membranes causes the release of growth factors that stimulate tissue growth and proliferation. The complement regulatory membrane protein CD59 restricts MAC formation. Because increased cell proliferation characterizes the major chronic vascular complications of human diabetes and because increased glucose levels in diabetes cause protein glycation and impairment of protein function, we investigated whether glycation could inhibit CD59. Glycation-inactivation of CD59 would cause increased MAC deposition and MAC-stimulated cell proliferation. Here, we report that (i) human CD59 is glycated in vivo, (ii) glycated human CD59 loses its MAC-inhibitory function, and (iii) inactivation of CD59 increases MAC-induced growth factor release from endothelial cells. We demonstrate by site-directed mutagenesis that residues K41 and H44 form a preferential glycation motif in human CD59. The presence of this glycation motif in human CD59, but not in CD59 of other species, may help explain the distinct propensity of humans to develop vascular proliferative complications of diabetes.
Humans are particularly prone to develop proliferative micro- and macrovascular disease that mediates some of the most common long-term complications of diabetes mellitus (1). These vascular complications of diabetes are caused by elevated blood glucose levels over long periods of time (2). Understanding the molecular mechanisms that link hyperglycemia and the vascular proliferative disease in humans is essential for designing adequate animal models and therapeutic strategies for a condition that represents a leading cause of morbidity and mortality in the adult population.
Glycation is considered a major pathophysiological mechanism causing tissue damage in diabetic subjects (3). Glycation involves the reaction of glucose and/or other reducing sugars with amino groups in proteins, resulting in the formation of a Schiff base or aldimine. This labile adduct can tautomerize via the Amadori rearrangement to the more stable ketoamine. The function of a glycated protein may be impaired if an amino group affected by glycation is in, or close to, its active site. For example, glycation of the β chains of hemoglobin gives rise to the glycated hemoglobins (HbA1), in which responsiveness to 2,3-diphosphoglycerate is decreased and oxygen affinity increased (4). Also, glycation of the major thrombin inhibitor of the coagulation system, antithrombin III, decreases its affinity for heparin, possibly contributing to the hypercoagulable state associated with diabetes (5).
Even though proteins contain many surface amino groups, only a few are preferentially glycated. This intriguing observation was explained when the identification of glycated amino groups in proteins with known three-dimensional structure revealed that glycation preferably occurs at amino groups that are either close to an imidazole moiety or part of a lysine doublet. Proximity (≈5 Å) of an amino group to an imidazole moiety is the strongest predictor of susceptibility to glycation (6). This site specificity of protein glycation is the consequence of localized acid–base catalysis of the aldimine/ketoamine tautomerization (7, 8).
Reports of increased deposition of the membrane attack complex of complement (MAC) in blood vessels and kidneys of diabetic patients (9, 10) suggest that there may be a link between complement activation and the development of chronic proliferative diabetic complications. Indeed, the MAC stimulates proliferation of fibroblasts and smooth muscle, mesangial, and other cells (11, 12), in part by releasing growth factors such as basic fibroblast growth factor and platelet-derived growth factor from MAC-targeted endothelium (13). The MAC also induces increased synthesis of extracellular matrix proteins by mesangial cells (14). Thus, increased MAC deposition in diabetic tissues may induce the release of growth factors that would stimulate cell proliferation in the vascular wall and contribute to the development of vascular proliferative disease. In the kidneys, MAC-induced vascular proliferation and expansion of the extracellular matrix may contribute to the characteristic glomerulosclerosis of diabetic nephropathy (15).
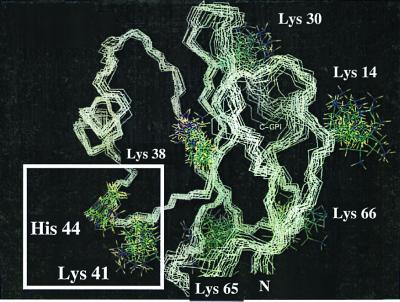
Increased MAC deposition in diabetes is well documented but the underlying mechanism is poorly understood. Autologous MAC deposition is normally restricted because cells express complement regulatory membrane proteins such as DAF and CD59, which limit complement activation and MAC formation. In particular, CD59 restricts MAC assembly by interacting with the terminal complement proteins C8 and C9, thus preventing C9 polymerization (16). To explain increased MAC deposition in diabetes, we reasoned that human CD59 could be inactivated by glycation. Indeed, the NMR structure of human CD59 (17) reveals that lysine-41 (K41) should be susceptible to glycation because of its critical proximity to the only histidine, histidine-44 (H44), in the protein (Fig. 1). Furthermore, the fact that K41 is adjacent to tryptophan-40 (W40), a conserved amino acid that is essential for CD59 function (18, 19), suggests that glycation of K41 may hinder the activity of CD59.
Figure 1.
NMR structure of the protein backbone of human CD59. The figure shows the 20 lowest energy structures of human CD59 with all lysine side chains and H44 (PDB Id: 1CDQ) (17). The structures were superimposed for the backbone of the β turn 41–44. The square highlights the K41–H44 glycation motif. K41 is within 5.91 ± 1.44 Å of the D1 imidazolic nitrogen of H44.
Here we report that (i) in vitro glycation of human CD59 inhibits its homologous restriction activity, (ii) replacement by site-directed mutagenesis of either K41 or H44 abolishes the sensitivity of human CD59 to glycation–inactivation, (iii) glycation of CD59 in human RBC (hRBC) increases their sensitivity to MAC-mediated lysis, (iv) glycation of human umbilical vein endothelial cells (HUVEC) renders them more sensitive to MAC-mediated growth factor release, and (v) glycated CD59 is present in human urine, indicating that CD59 is glycated in vivo.
Importantly, the H44 residue of human CD59 is not present in any other species in which CD59 has been sequenced. We propose that the presence of the glycation motif K41–H44 in human CD59 provides a possible molecular explanation for the propensity of humans to develop the combination of vascular complications that characterize human diabetes.
Methods
Cell Culture and Transfections.
HUVEC were grown in DMEM, and Chinese hamster ovary cells (CHO) were grown in Ham's/F12 medium (10% FCS, 37°C). Transfections were performed by using calcium phosphate precipitation methods.
CD59 Purification.
CD59 from human urine was isolated by anion exchange chromatography by using a DEAE protein-Pak 8-h column (Waters) as described (20). CD59 from hRBC butanol extracts and CHO lysates was purified by immunoaffinity chromatography as described (21) by using the monoclonal rat anti-human CD59 Ab YTH53.1 (Serotec). Protein concentration was estimated by the microBCA protein assay (Pierce). Western blots were performed on proteins separated by SDS/PAGE by using the Supersignal detection system (Pierce).
Site-Directed Mutagenesis.
Plasmid pK562-3 containing the cDNA of human CD59 was obtained from the American Type Culture Collection. Site-directed mutagenesis to replace residue K41 or H44 with glutamine (Gln) was performed by using the Altered Sites II system (Promega). The mutagenic primers used were 5′-GTC GTT GAA ATT ACA ATG CTC AAA CTG CCA ACA CTT-3′ for the Gln-41 substitution, and 5′-GTT GAA ATT GCA CTG CTC AAA CTT CCA-3′ for the Gln-44 mutation. Successful mutagenesis was confirmed by sequencing.
Expression, Purification, and Functional Activity Assays.
Wild-type (WT) and mutant CD59 cDNAs were subcloned into the mammalian expression vector pSVK3 (Amersham Pharmacia) and transfected into CHO together with the selection marker pBABE, which confers resistance to puromycin. Expression and functionality of WT and mutant CD59 in the puromycin-resistant clones were tested by Western blot analysis, by immunohistochemistry in nonpermeabilized cells by using the YTH53.1 Ab and a Texas red-conjugated secondary Ab, and by a functional dye release assay that measures protection against human complement, as described (22).
Activity of purified CD59 and its mutants before and after glycation was determined by a hemolysis protection assay using guinea pig erythrocytes (GPE) exposed to the human MAC, as described (13, 11). GPE are very sensitive to human complement: ≈10−3 unit of human C5b6 plus 1 μg/ml of purified C7, C8, and C9 (Advanced Research Technologies, San Diego, CA) is required to lyse 50% of a GPE cell suspension, whereas 1 unit is the amount of C5b6 required to lyse 50% of an hRBC suspension under similar conditions. The protective activity against human MAC conferred to GPE by purified human CD59 was calculated as the difference between the percentage lysis of the unprotected GPE minus the percentage lysis of the CD59-protected cells.
In Vitro Glycation.
Immunoaffinity-purified human CD59 (10–30 μg/ml) was incubated for different time intervals at 37°C in 0.5 M of either reducing monosaccharides (glucose, ribose, or glucose 6-phosphate) or the nonglycating sorbitol.
Incorporation of CD59 into GPE.
To assess CD59 incorporation into GPE, purified CD59 was iodinated with 125I (DuPont/NEN) by using Iodo-Beads (Pierce). Iodination of CD59 did not affect either its activity or its sensitivity to glycation–inactivation. After incubation of GPE with 125I-CD59, the cells were washed and lysed, and the incorporated radioactivity was measured in a γ counter. The number of CD59 molecules incorporated per GPE was calculated from the specific activity of the 125I-CD59 preparation (150 cpm/ng) and the hematocrit of the GPE suspension.
Glycation of hRBC.
A 10% hRBC suspension was incubated with or without ribose (50 mM) for 48 h at room temperature, followed by 20 min of incubation with cyanoborohydride (NaBH3CN, 20 mM). Cell volume was then adjusted by the nystatin procedure (23), and the osmotic fragility of the cell suspension was measured by standard procedures. Glycated hRBC were used only when their average cell volume and osmotic fragility were similar to those of control cells.
Glycation of HUVEC.
Confluent second passage HUVEC were incubated with or without ribose (50 mM) for 24 h (37°C, 5% CO2) followed by 20 min of incubation with NaBH3CN (20 mM). Cells were then exposed to purified human terminal complement proteins to form the MAC, and aliquots of conditioned media were separated to test for mitogenic activity in indicator quiescent 3T3 cells, all as described (13).
Results
Glycation Inhibits the Activity of Human CD59.
The NMR structure of human CD59 shown in Fig. 1 predicts that the protein is susceptible to glycation. To assess the effect of glycation on the activity of CD59, human CD59 was purified from hRBC by immunoaffinity chromatography, and then incubated with or without glucose (0.5 M). CD59 is a glycosylphosphatidylinositol (GPI)-anchored membrane protein. Because immunoaffinity-purified CD59 retains the GPI linker, it can be attached to cell membranes and assayed for protective activity against human complement. To this end, purified CD59 was incorporated into GPE and exposed to increasing concentrations of human MAC formed with purified terminal complement components C5b6, C7, C8, and C9.
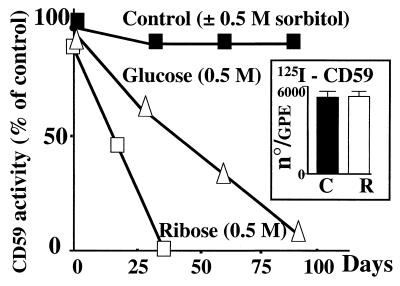
Exposure of CD59 to glucose resulted in a progressive decrease of its activity; it lost approximately 40% of its protective capacity in 30 days and 85% in 90 days (Fig. 2). In contrast, incubation of CD59 for the same period and under identical conditions with nonglycating sorbitol (0.5 M) resulted in minimal loss of CD59 activity. Incubation of CD59 with the faster glycating agent ribose (0.5 M, Fig. 2) or glucose 6-phosphate (not shown) completely abrogated CD59 activity in ≈30 days. This faster rate of CD59 inactivation by ribose compared with glucose reflects the higher proportion of the reactive open-chain carbonyl form in ribose because the rate of glycation of proteins is critically influenced by the equilibrium between the open and ring structures of the sugar (24). Phosphate enhances the rate of protein glycation because it catalyzes the Amadori rearrangement (25). Purified CD59 exposed to ribose (0.5 M) lost 50% of its initial activity in ≈15 days when incubated in low phosphate (5 mM) and in 2 days when incubated in high phosphate (60 mM). Faster rates of inactivation were also observed during incubations with glucose in high phosphate medium (not shown). The effect of phosphate accelerating the rate of CD59 inactivation by glycating agents indicates that glycation with Amadori product formation is responsible for the functional inhibition of CD59. These experiments demonstrate that glycation progressively abrogates the complement inhibitory activity of human CD59.
Figure 2.
Glycation abrogates the homologous restriction activity of human CD59. A 10% cell suspension of GPE was incubated with immunoaffinity-purified hRBC CD59 previously exposed for different time intervals to glucose (▵, 0.5 M) or ribose (□, 0.5 M), or to nonglycating sorbitol (■, 0.5 M). The sensitivity of GPE to human MAC was then tested by using purified human C5b6 and C7, C8, and C9. Each point represents the mean of triplicate determinations (SEM smaller than data points). Representative of three experiments with comparable results. (Inset) Number of CD59 molecules incorporated per GPE, determined with 125I-CD59 before (C) and after (R) glycation with ribose.
The hemolytic protection assay with GPE used for functional activity depends critically on the insertion of CD59 into the GPE cell membranes. To ascertain that the observed loss of CD59 activity in the GPE protection assay was due to CD59 inactivation and not to impaired insertion into the GPE membrane, incorporation of nonglycated and glycated purified CD59 into GPE was measured by using 125I-CD59. Even though 125I-CD59 exposed to 0.5 M ribose completely lost activity, the number of CD59 molecules that were incorporated per GPE was not affected by glycation (Fig. 2, Inset). Thus, glycated CD59 loses its anticomplement activity without losing its ability to incorporate into GPE membranes.
During prolonged exposure of proteins to glycating sugars, the ketoamine has the potential to form advanced glycation end products (AGE). To explore whether AGE formation could explain the observed inactivation of CD59, glycation was performed in the presence of aminoguanidine, an inhibitor of AGE formation (25). Aminoguanidine did not affect CD59 activity in either the presence or the absence of the glycating agents (data not shown). These results indicate that AGE formation does not mediate the inactivation of CD59 depicted in Fig. 2. This interpretation was confirmed by immunoblot analysis of glycated CD59 with two anti-AGE Abs (kindly provided by R. Bucala) that did not recognize glucose-inactivated CD59 (not shown). Thus, glycated CD59 is most likely inactivated because of Amadori product formation. This conclusion is strongly supported by the findings that (i) the rate of inactivation of CD59 by glucose or ribose is proportional to the rate of protein glycation by the respective sugar, and is enhanced by phosphate, and (ii) inactivated CD59 loses immunoreactivity to anti-AGE Abs. Further, immunoblotting of glycated CD59 by an anti-hexitol–lysine Ab that specifically recognizes the reduced ketoamine moiety in glycated proteins directly demonstrates Amadori product formation (26). This Ab recognized immunoaffinity-purified hRBC CD59 only after exposure to a glycating agent (see Fig. 5B).
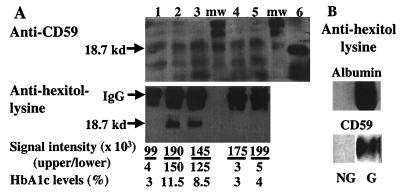
Figure 5.
Glycated CD59 in human urine. (A) Urine samples from diabetic (lanes 2 and 3) and nondiabetic (lanes 1, 4, and 5) subjects were concentrated by ultrafiltration and separated by anion exchange chromatography, and fractions were dot-blotted for the presence of CD59 with anti-CD59-specific Ab. CD59-positive fractions were pooled and immunoprecipitated with the HC1 anti-CD59-specific Ab. Immunoprecipitates were spun down and boiled for 30 min; then the immunocomplexes were separated by SDS/PAGE and immunoblotted with the monoclonal YTH53.1 anti-CD59 Ab (A, Upper), and with the anti-hexitol–lysine Ab (A, Lower). The Upper samples were separated by using a 20-cm-long gel that resolved the multiple CD59 bands (mw = molecular weight markers). The Lower samples were separated by using a minigel. Immunoaffinity-purified hRBC CD59 was included in the Upper gel as a control (lane 6). The signal intensity of the immunoblot bands marked by the arrows was quantified by BIOQUANT image analysis software. HbA1c levels were measured at the clinical laboratory of The Joslin Diabetes Center by HPLC. (B) Immunoblot of glycated (G) and nonglycated (NG) albumin (Upper) and immunoaffinity-purified hRBC CD59 (Lower) with anti-hexitol–lysine Ab.
Mapping of the Ab epitopes of CD59 has shown that neutralizing Abs such as the YTH53.1 bind to a region that includes W40 and residues adjacent or in close proximity to W40, and that the HC1 Ab binds to an epitope that is far away from W40 and the glycation domain K41–H44 (18). Western blot analysis of glycated human CD59 showed that it lost functional activity in parallel with immunoreactivity toward neutralizing YTH53.1 but not toward HC1 Ab (data not shown). These results indicate that CD59 is glycated close to or at the active site responsible for both inhibition of MAC formation and binding of neutralizing antibodies.
Site-Directed Mutagenesis of K41 and H44 in Human CD59 Abrogates Its Sensitivity to Glycation–Inactivation.
The prediction that the glycation motif K41–H44 renders the human protein sensitive to glycation-induced inactivation was further tested by site-directed mutagenesis. Mutant CD59 in which either K41 or H44 was replaced with a glutamine (CD59–Gln-41 and CD59–Gln-44, respectively) were expressed in CHO cells. The expression and membrane localization of the CD59 mutants were confirmed by immunocytochemistry using the anti-human CD59 YTH53.1 Ab (Fig. 3A) and by Western blot analysis (not shown). Both mutants preserved CD59 activity because they protected CHO cells from human complement-mediated lysis (not shown).
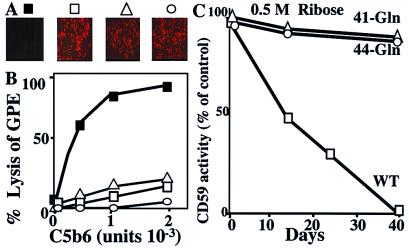
Figure 3.
Site-directed mutagenesis of K41 or H44 abrogates the sensitivity of human CD59 to glycation–inactivation. (A) WT and CD59–Gln-41 or CD59–Gln-44 mutants were expressed in CHO cells. Expression was confirmed by immunocytochemistry by using the anti-human CD59 monoclonal Ab YTH53.1 and fluorescence (Texas red)-labeled anti-rat IgG secondary Ab: ■, transfection vector only; □, WT CD59; ▵, Gln-41 mutant CD59; and ○, Gln-44 mutant CD59. (B) Recombinant WT and mutant CD59 were immunoaffinity purified from CHO cells, and their activity was tested in the GPE hemolytic assay (symbols as in A). (C) Activity of immunoaffinity-purified WT and mutant CD59s before and after glycation with ribose for different time intervals. The points represent the mean of triplicate determinations (SEM smaller than the data points). Representative of three experiments with comparable results.
Recombinant WT, CD59–Gln-41 and CD59–Gln-44 proteins were purified by affinity chromatography from transfected CHO cells, and the activity of the purified proteins was measured in the GPE hemolytic assay. The WT and both mutant CD59 proteins prevented lysis of GPE by human MAC (Fig. 3B). To assess the sensitivity of the recombinant proteins to glycation–inactivation, they were incubated with 0.5 M ribose at 37°C, and their activity was measured after different time intervals in the GPE assay. Fig. 3C shows that the recombinant WT CD59 was inactivated at a rate comparable to that of the protein purified from hRBC (Fig. 2). In contrast, neither the CD59–Gln-41 nor the CD59–Gln-44 mutant was inactivated by glycation. The decreased sensitivity to glycation–inactivation observed in both CD59 mutants confirms that both K41 and H44 residues are part of a glycation site responsible for glycation–inactivation of human CD59.
Functional Consequences of CD59 Glycation.
The functional consequence of CD59 glycation–inactivation was determined in two assays: MAC-induced lysis of hRBC and growth factor release from HUVEC.
Because hRBC and HUVEC cannot be maintained in high glucose for long periods of time without compromising their viability and because short glycation times were not enough to allow for accumulation of sufficient stable ketoamine-bound carbohydrate, experiments in these cells required the addition of NaBH3CN to stabilize the aldimine adduct. Although similar stabilization of the aldimine can be obtained with high phosphate (8), NaBH3CN was chosen to avoid changes in cell volume and membrane potential, two variables that influence the cells' sensitivity to MAC-induced lysis.
Glycation Increases MAC-Induced Lysis of RBC.
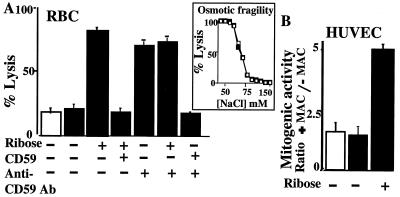
In hRBC and platelets, MAC deposition is under the regulatory control of CD59 (27, 28). To test whether glycation increases the sensitivity of hRBC to human MAC-induced lysis, hRBC were exposed to ribose for 48 h followed by short NaBH3CN reduction. This treatment increased the sensitivity of hRBC to human MAC-mediated lysis (Fig. 4A). Exposure of hRBC to NaBH3CN alone did not make them more sensitive to MAC-induced lysis. This different sensitivity to MAC-induced lysis observed in glycated vs. nonglycated cells could not have arisen from differences in cell volume because the osmotic fragility of the glycated and nonglycated cells was comparable (Fig. 4A, Inset).
Figure 4.
Glycation increases the sensitivity of hRBC to MAC-mediated lysis and of HUVEC to MAC-induced release of growth factors. (A) hRBC were incubated without or with 50 mM ribose for 48 h at room temperature followed by reduction with NaBH3CN (open column, cells not exposed to NaBH3CN; filled columns, cells exposed to NaBH3CN). Cell volume was adjusted in glycated and nonglycated control cells, and aliquots of cells were separated for rescue with immunoaffinity-purified human CD59 (4 μg) or incubation with neutralizing anti-CD59 Ab (YTH53.1), before exposure to purified human C5b6, C7, C8, and C9 to form the MAC. The last column to the right represents cells that were first exposed to the anti-CD59 Ab and then rescued with affinity-purified human CD59. (Inset) Similar osmotic fragility of control (□) and glycated (■) cells after volume adjustment. (B) HUVEC were incubated with or without 50 mM ribose (24 h, 37°C), followed by reduction with NaBH3CN. Cells were then exposed to purified human C5b6, C7, C8, and C9 to form the MAC, and the mitogenic activity in the conditioned medium was measured as in ref. 13. Results are expressed as the ratio of mitogenic activity released into the conditioned media in the presence or absence of MAC.
Because CD59 specifically restricts MAC formation, these results strongly suggest that glycation inhibits CD59 function. To test this hypothesis, glycated hRBC were exposed to MAC before and after incubation with affinity-purified human CD59. Addition of purified CD59 rescued the glycated hRBC because it fully restored their resistance to MAC-induced lysis (Fig. 4A). Addition of purified CD59 to nonglycated hRBC did not change their sensitivity to MAC-induced lysis (not shown). Furthermore, a neutralizing anti-CD59 Ab markedly increased MAC-induced lysis in control but not in glycated hRBC, and Ab-treated cells were rescued by exposure to purified human CD59 (Fig. 4A). Together, these experiments indicate that glycation decreases the activity of human CD59 in cell membranes, as it does in the purified protein.
Glycation Increases MAC-Induced Growth Factor Release from HUVEC.
Insertion of human MAC into the membrane of HUVEC induces release of growth factors such as basic fibroblast growth factor and platelet-derived growth factor without affecting their viability (13). To investigate the effect of glycation on the sensitivity of HUVEC to MAC-induced growth factor release, HUVEC were exposed to 50 mM ribose for 24 h before exposure to the MAC. Then, the mitogenic activity of the conditioned media was determined. Exposure of HUVEC to ribose followed by short NaBH3CN reduction significantly increased the MAC-induced mitogenic activity in the conditioned medium (Fig. 4B). Exposure to the glycating agent or to NaBH3CN did not affect the mitogenic activity in the conditioned media of HUVEC not exposed to the MAC; and exposure to NaBH3CN alone did not affect MAC-induced growth factor release. The increased growth factor release was not caused by MAC-induced lysis because there was no difference between the lactate dehydrogenase activity in conditioned media of MAC-treated and control cells (data not shown). These results indicate that glycation increases MAC-induced growth factor release from endothelium.
Human Urine Contains Glycated CD59.
To test whether glycation of CD59 occurs in humans, the soluble form of CD59 normally excreted in urine (29) was analyzed for glycation by using the anti-hexitol–lysine Ab that specifically recognizes the reduced ketoamine in glycated lysine residues (kindly provided by N. Taniguchi) (30). Concentrated human urine from normoglycemic and hyperglycemic (diabetic) individuals was separated by anion exchange chromatography, and CD59-positive fractions (dot-blotted with the YTH53.1 anti-human CD59 Ab) were pooled and immunoprecipitated with the HC1 anti-human CD59 Ab (kindly provided by S. Meri). This Ab was used for immunoprecipitation because it binds to an epitope distant from the K41–H44 putative glycation motif of CD59 (18) and, in preliminary experiments, recognized both glycated and nonglycated CD59 (see below).
SDS/PAGE separation of the original pooled fractions before and after immunoprecipitation and of the immunoprecipitates, followed by immunoblot analysis with anti-CD59 Ab, confirmed that the 19-kDa immunoprecipitated protein was CD59 because (i) it was recognized by a specific monoclonal anti-CD59 Ab, (ii) it ran exactly as CD59 purified from hRBC (included as a positive control; Fig. 5, lane 6), and (iii) an anti-CD59 positive 19-kDa protein present in the original pooled urine fractions was depleted by immunoprecipitation (not shown).
The relative amount of CD59 contained in each immunoprecipitate was estimated by immunoblot analysis with anti-CD59 Abs of serial immunoprecipitate dilutions. Then, aliquots of the immunoprecipitates containing equal amounts of CD59 were separated by SDS/PAGE and immunoblotted with both anti-CD59 and anti-hexitol–lysine Abs (Fig. 5A). Immunoblotting with anti-hexitol–lysine Ab (Fig. 5A, Lower) showed that CD59 was glycated in the urine of diabetic but not or minimally in the urine of nondiabetic subjects. The specificity of the anti-hexitol–lysine Ab for glycated proteins is documented in Fig. 5B. The figure also shows that the anti-hexitol–lysine Ab recognizes purified hRBC CD59 only after glycation.
The fraction of urine CD59 that is glycated was estimated by measuring the intensity of the immunoblot signal from the blots shown in the Upper and Lower panels of Fig. 5A. The results show that a significant fraction of human CD59 is glycated in the urine of diabetic subjects with high levels of glycated hemoglobin but not in the urine of non-diabetics. This identification of glycated CD59 in human urine conclusively establishes in vivo glycation of CD59. The results in a limited number of subjects suggest that glycation of human CD59 in urine may correlate with the levels of glycated hemoglobin (Fig. 5A), a clinically relevant issue that deserves further investigation.
Discussion
The experimental evidence reported here conclusively shows that the complement regulatory protein CD59 is subject to glycation in vivo. Using an anti-hexitol–lysine-specific Ab, glycated CD59 was identified in the urine of diabetic patients (Fig. 5). Glycation affects CD59 function (Fig. 2), as predicted from the finding that CD59 contains a preferential glycation motif in or very close to its active site (Fig. 1). In intact cells, glycation–inactivation of CD59 increases their sensitivity to MAC-induced phenomena, such as growth factor release from endothelial cells (Fig. 4). Release of growth factors from endothelium and mesangial cells contributes to the development of atherosclerosis and proliferative renal diseases (31, 32). The results of this work raise the possibility that, in diabetes, a fraction of CD59 molecules in endothelium and other cells might be inhibited by glycation, and that this fraction would increase with poor glycemic control. Glycation–inactivation of endothelial CD59 would increase the sensitivity of the diabetic endothelium to MAC-induced release of growth factors and cytokines and thereby contribute to the development of vascular complications of the disease. Release of growth factors from macrophages activated by AGEs has been postulated as a glycation-related mechanism contributing to the development of the proliferative complications of diabetes (33). We propose that increased MAC-induced release of growth factors from endothelium as a consequence of glycation–inactivation of CD59 would act synergistically with macrophage-dependent mechanisms to produce the long-term vascular complications and glomerulosclerosis that characterize human diabetes.
Glycation of urinary CD59 may correlate with the levels of glycated hemoglobin that are indicative of glycemic control (Fig. 5). In its GPI-anchored membrane form, CD59 is found in blood, endothelial, and epithelial cells. Both soluble (34, 35) and lipid-tailed forms attached to small vesicle microparticles have been found in human urine (36), but the origin of urinary CD59 has not been elucidated (37). It has been proposed that membrane-anchored CD59 may be shed from blood, endothelial, and epithelial cells in the form of small membrane vesicles or after cleavage of the anchor by plasma phospholipase C or D (37). Soluble CD59 without a lipid tail is hydrophilic and small enough to be filtered through the kidney glomeruli and excreted into the urine. In the experiment depicted in Fig. 5, urinary CD59 was immunoprecipitated from concentrated urine samples. Even though both diabetic subjects (lanes 2 and 3 in Fig. 5) had some degree of proteinuria, increased urine protein concentration in the diabetic subjects could not account for the increased amount of glycated CD59 because an equal amount of CD59 was loaded for SDS-PAGE separation and immunoblotting. Indeed, immunoblot analysis of immunoprecipitates from concentrated urine verified comparable amounts of CD59 in all samples (Fig. 5, Upper). Structural analysis of the glycans and GPI-anchors in CD59 molecules, which vary in different tissues, indicate that urinary CD59 may originate from multiple sources (37), although the apparent absence of _O_-glycans in urinary CD59 suggests that little or no urinary CD59 originates from hRBC (38). The absence of protein turnover in hRBC allows glycation to proceed to a significant extent when these cells are exposed to high plasma glucose for long periods of time, as is the case of HbA1c and some hRBC membrane proteins (39). Increased amounts of glycated CD59 in the urine of diabetics may originate from an increased fraction of RBC CD59 filtered through the glomeruli or from endothelial and/or epithelial cell sources. In these cells, the extent of CD59 glycation would be determined by both the turnover and the glycation rates of CD59 in vivo. It has been documented that GPI-linked proteins like CD59 can turn over very slowly (40), a feature that could contribute to the sensitivity of GPI-anchored CD59 to glycation–inactivation in diabetes. In addition, the rate of glycation–inactivation of CD59 observed in hRBC or HUVEC (Fig. 4) was faster than the inactivation of the soluble purified protein (Fig. 2). This observation may reflect different rates of CD59 glycation–inactivation in its natural membrane environment vs. in solution. Different rates of ligand binding to proteins in membranes as compared with proteins in solution have been established (41). In the cell membrane, interaction with membrane phospholipids and/or other proteins, as well as the geometry of its packing, may increase the rate of CD59 glycation, as compared with that of the soluble protein glycated in vitro. Indeed, it has been proposed that the multiple glycans found in GPI-anchored CD59 would restrict the conformational space available to the protein in the membrane, influence the geometry of its packing, and stabilize an exposed surface location for the active site that is centered on W40 (38). In view of these results, it is important to determine the tissue of origin of glycated CD59 in diabetic urine, to evaluate the rate of CD59 glycation in vivo, and to document the presence of glycated CD59 in diabetic endothelium.
Glycation increased the sensitivity of hRBC to MAC-mediated lysis, most likely by inactivation of CD59. This interpretation is supported by the results depicted in Fig. 4A, in which (i) purified nonglycated CD59 rescued glycated hRBC, restoring protection against MAC-mediated lysis, and (ii) a neutralizing anti-CD59 Ab that effectively increased MAC sensitivity in normal control cells did not affect the sensitivity of glycated hRBC. Remarkably, a shortened RBC half-life, an increased reticulocyte count indicative of hemolysis, and an abnormally high number of platelet-derived microvesicles have been documented in diabetic patients (42, 43). Furthermore, reticulocytosis, abnormal platelet function, and thrombosis are hematological complications of diabetics that correlate with the levels of glycated hemoglobin and reverse with normalization of hyperglycemia (44). A similar syndrome of hemolysis and thrombosis is seen in the disorder paroxysmal nocturnal hemoglobinuria, which is a complement-mediated disease characterized by deficiency of GPI-anchored proteins, including CD59, in the membrane of most blood cells (45, 46). We postulate that the previously unexplained hematological abnormalities seen in diabetes could be complement-mediated as a consequence of glycation–inactivation of CD59.
At the molecular level, glycation–inactivation of CD59 occurs because the protein contains a preferential glycation motif formed by residues K41 and H44 in close proximity to the active site of CD59. That glycation of CD59 occurs at a lysine residue is demonstrated by the recognition of glycated CD59 by the hexitol–lysine-specific Ab (Fig. 5). Also, the site-directed mutagenesis experiments (Fig. 3) demonstrate that K41 is part of the glycation–inactivation site. Interestingly, K41 is adjacent to residue W40, which has been identified by mutational analysis as essential for inhibition of MAC formation by CD59 (18, 19). We propose that steric hindrance introduced by the bulkiness of the sugar glycating K41 interferes with C8 and C9 binding on the surface of CD59 and is responsible for the observed glycation-induced functional inhibition of human CD59.
Nephropathy, retinopathy, and accelerated atherosclerosis are the characteristic long-term proliferative complications of diabetes. Animal models of diabetes exhibit some of these complications, but no animal model reproduces them in the combination and extent seen in human diabetes (1). We have demonstrated by site-directed mutagenesis that H44 is required for glycation–inactivation of human CD59. Remarkably, the H44 residue of human CD59 is not present in other species in which CD59 has been sequenced (Table 1). It is tempting to postulate that the existence of a preferential glycation site in human CD59 contributes to the peculiar sensitivity of humans to the development of proliferative complications of chronic hyperglycemia.
Table 1.
Alignment of CD59 amino acid sequences from different species around residue W40
| Species | Residue at position | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | |
| Human | K | C | W | K | F | E | H | C | N | F |
| Mouse | R | C | W | K | Q | S | D | C | H | G |
| Rat | Q | C | W | R | F | S | D | C | N | A |
| Baboon | Q | C | W | K | F | A | N | C | N | F |
| African green monkey | Q | C | W | K | F | A | N | C | N | E |
| Owl monkey | R | C | W | K | F | E | D | C | T | F |
| Squirrel monkey | R | C | W | K | F | D | D | C | S | F |
| Pig | R | C | W | R | F | D | E | C | N | F |
| Marmoset | R | C | W | K | F | E | D | C | T | F |
Acknowledgments
We thank Dr. D. C. Tosteson and Dr. V. Dzau for continuous support and Dr. M. Tosteson for useful comments on the manuscript. We thank Dr. R. Bucala for kindly providing the anti-AGE antibodies, Dr. N. Taniguchi for the anti-hexitol–lysine Ab, and Dr. S. Meri, for the HC1 Ab. We also thank Dr. M. Fletcher and Dr. D. Neuhaus for their help with the structure of CD59. This work was supported in part by National Institutes of Health Grant DK52855.
Abbreviations
AGE
advanced glycation end products
GPE
guinea pig erythrocytes
GPI
glycosylphosphatidylinositol
MAC
membrane attack complex of complement
hRBC
human RBC
HUVEC
human umbilical vein endothelial cell
CHO
Chinese hamster ovary
WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Duhault J, Koenig-Berard E. Therapie. 1997;52:375–384. [PubMed] [Google Scholar]
- 2.Nathan D M. Ann Intern Med. 1996;124:86–89. doi: 10.7326/0003-4819-124-1_part_2-199601011-00002. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M, Vlassara H, Cerami A. Ann Intern Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 4.McDonald M J, Bleichman M, Bunn H F. J Biol Chem. 1979;254:702–707. [PubMed] [Google Scholar]
- 5.Ceriello A, Giugliano D, Quatraro A, Stante A, Consoli G, Dello Russo P, D'Onofrio F. Diabete Metab. 1987;13:16–19. [PubMed] [Google Scholar]
- 6.Flückiger R, Strang C J. Protein Sci. 1995;4:186A. [Google Scholar]
- 7.Iberg N, Flückiger R. J Biol Chem. 1986;261:13542–13545. [PubMed] [Google Scholar]
- 8.Watkins N G, Neglia-Fisher C I, Dyer D G, Thorpe S R, Baynes J W. J Biol Chem. 1987;262:7207–7212. [PubMed] [Google Scholar]
- 9.Weiss J S, Sang D N, Albert D M. Cornea. 1990;9:131–138. [PubMed] [Google Scholar]
- 10.Falk R J, Sisson S P, Dalmasso A P, Kim Y, Michael A F, Vernier R L. Am J Kidney Dis. 1987;9:121–128. doi: 10.1016/s0272-6386(87)80089-6. [DOI] [PubMed] [Google Scholar]
- 11.Halperin J A, Taratuska A, Nicholson-Weller A. J Clin Invest. 1993;91:1974–1978. doi: 10.1172/JCI116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torzewski J, Oldroyd R, Lachmann P, Fitzsimmons C, Proudfoot D, Bowyer D. Arterioscler Thromb Vasc Biol. 1996;16:673–677. doi: 10.1161/01.atv.16.5.673. [DOI] [PubMed] [Google Scholar]
- 13.Benzaquen L R, Nicholson-Weller A, Halperin J A. J Exp Med. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner C, Braunger M, Beer M, Rother K, Hansch G M. Exp Nephrol. 1994;2:51–56. [PubMed] [Google Scholar]
- 15.Ziyadeh F N. Am J Kidney Dis. 1993;22:736–744. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya H, Sims P J. J Biol Chem. 1992;267:13675–13680. [PubMed] [Google Scholar]
- 17.Fletcher C M, Harrison R A, Lachmann P J, Neuhaus D. Structure. 1994;2:185–199. doi: 10.1016/s0969-2126(00)00020-4. [DOI] [PubMed] [Google Scholar]
- 18.Bodian D L, Davis J S, Morgan B P, Rushmere N K. J Exp Med. 1997;185:507–516. doi: 10.1084/jem.185.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Abagyan R, Dong S, Gilbert A, Nussenzweig V, Tomlinson S. J Exp Med. 1997;185:745–753. doi: 10.1084/jem.185.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies A, Simmons L, Hale G, Harrison R A, Tighe H, Lachmann P J, Waldemann H. J Exp Med. 1989;170:637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Den Berg C W, Harrison R A, Morgan B P. Immunology. 1993;78:349–357. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Rollins S A, Maher S E, Bothwell A L, Sims P J. J Biol Chem. 1991;266:13418–13422. [PubMed] [Google Scholar]
- 23.Halperin J A, Brugnara C, Kopin A S, Ingwall J, Tosteson D C. J Clin Invest. 1987;80:128–137. doi: 10.1172/JCI113037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunn H F, Higgins P J. Science. 1981;213:222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 26.Myint T, Hoshi S, Ookawara T, Miyazawa N, Suzuki K, Taniguchi N. Biochim Biophys Acta. 1995;1272:73–79. doi: 10.1016/0925-4439(95)00067-e. [DOI] [PubMed] [Google Scholar]
- 27.Wiedmer T, Hall S E, Ortel T L, Kane W H, Ross W F, Sims P J. Blood. 1993;82:1192–1196. [PubMed] [Google Scholar]
- 28.Lachmann P J. Immunol Today. 1991;12:312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- 29.Nakano Y, Noda K, Endo T, Kobata A, Tomita M. Arch Biochem Biophys. 1994;311:117–126. doi: 10.1006/abbi.1994.1216. [DOI] [PubMed] [Google Scholar]
- 30.Myint T, Hoshi S, Ookawara T, Miyazawa N, Suzuki K, Taniguchi N. Biochim Biophys Acta. 1995;1272:73–79. doi: 10.1016/0925-4439(95)00067-e. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 32.Couser W G. Nephrol Dial Transplant. 1998;13:10–15. doi: 10.1093/ndt/13.suppl_1.10. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee M, Cerami A, Vlassara H. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 34.Watts M J, Dankert J R, Morgan E P. Biochem J. 1990;265:471–477. doi: 10.1042/bj2650471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalman L S, Brothers M A, Muller-Eberhard H J. J Immunol. 1989;143:1943–1947. [PubMed] [Google Scholar]
- 36.Lehto T, Honkanen E, Teppo A M, Meri S. Kidney Int. 1995;47:1403–1411. doi: 10.1038/ki.1995.197. [DOI] [PubMed] [Google Scholar]
- 37.Meri S, Lehto T, Sutton C W, Tyynela J, Baumann M. Biochem J. 1996;316:923–935. doi: 10.1042/bj3160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudd P M, Morgan B P, Wormald M R, Harvey D J, van den Berg C W, Davis S J, Ferguson M A, Dwek R A. J Biol Chem. 1997;272:7229–7244. doi: 10.1074/jbc.272.11.7229. [DOI] [PubMed] [Google Scholar]
- 39.Miller J A, Gravallese E, Bunn H F. J Clin Invest. 1980;65:896–901. doi: 10.1172/JCI109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Censullo P, Davitz M A. Semin Immunol. 1994;6:81–88. doi: 10.1006/smim.1994.1012. [DOI] [PubMed] [Google Scholar]
- 41.Ellory J C, Willis J S. Biochim Biophys Acta. 1976;443:301–305. doi: 10.1016/0005-2736(76)90512-5. [DOI] [PubMed] [Google Scholar]
- 42.Giugliano D. Diabetologia. 1982;22:223. doi: 10.1007/BF00283761. (lett.). [DOI] [PubMed] [Google Scholar]
- 43.Nomura S, Suzuki K, Katsura K, Xie G L, Miyazaki Y, Miyake T, Kido H, Kagawa H, Fukuhara S. Atherosclerosis. 1995;116:235–240. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 44.Peterson C M, Jones R L, Koenig R J, Melvin E T, Lehrman M L. Ann Intern Med. 1977;86:425–429. doi: 10.7326/0003-4819-86-4-425. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura J, Murakami Y, Kinoshita T. Am J Hematol. 1999;62:175–182. doi: 10.1002/(sici)1096-8652(199911)62:3<175::aid-ajh7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Halperin J A, Nicholson-Weller A. Complement Inflammation. 1989;6:65–72. doi: 10.1159/000463072. [DOI] [PubMed] [Google Scholar]
- 47.Powell M B, Marchbank K J, Rushmere N K, van den Berg C W, Morgan B P. J Immunol. 1997;158:1692–1702. [PubMed] [Google Scholar]