Claudin-1 and claudin-2 expression are elevated in inflammatory bowel disease and may contribute to early neoplastic transformation (original) (raw)
. Author manuscript; available in PMC: 2009 Oct 1.
Published in final edited form as: Lab Invest. 2008 Aug 18;88(10):1110–1120. doi: 10.1038/labinvest.2008.78
Abstract
Patients with inflammatory bowel disease (IBD) are at increased risk of developing colorectal adenocarcinoma. The factors that result in IBD-associated carcinogenesis are not understood. We hypothesized that altered expression of intestinal epithelial tight junction proteins might contribute to neoplastic progression. Semi-quantitative immunohistochemical staining of human biopsies was used to assess expression of the tight junction proteins claudin-1, claudin-2, claudin-4, and occludin in IBD, IBD-associated dysplasia, acute, self-limited colitis (ASLC), and sporadic adenomas. Claudin-1 and claudin-2 expression was elevated in active IBD, adenomas, and IBD-associated dysplasia, but not ASLC. In contrast, claudin-4 expression was elevated in both active IBD and ASLC. Occludin expression was similar to control in all cases. Importantly, in IBD, claudin-1 and claudin-2 expression correlated positively with inflammatory activity. To investigate mechanisms underlying altered claudin expression, β-catenin activation was assessed as nuclear localization. Like claudin-1 and claudin-2, β-catenin was markedly activated in IBD, dysplasia, IBD-associated dysplasia, but only slightly activated in ASLC. Taken together, these data suggest that β-catenin transcriptional activity is elevated in chronic injury and that this may contribute to increased claudin-1 and claudin-2 expression. We speculate that increased claudin-1 and claudin-2 expression may be involved in early stages of transformation in IBD-associated neoplasia.
Keywords: claudin-1, claudin-2, claudin-4, colon cancer, dysplasia, inflammatory bowel disease, occludin
INTRODUCTION
The intestinal epithelial barrier is established by a single layer of cells. These are held together by intercellular junctions that include desmosomes, adherens junctions, and tight junctions. The seal that regulates paracellular permeability is established by the tight junction, which is composed of transmembrane proteins, such as claudins and occludin, and cytosolic proteins, such as ZO-1. When examined by freeze-fraction electron microscopy, tight junctions are visualized as a network of anastamosing strands within the cell membranes. The number and complexity of these strands is decreased in inflammatory bowel disease (IBD), and this correlates with reductions in barrier function.1 The molecular correlates of this reduced barrier function are only beginning to be understood.2-5
While barrier dysfunction in IBD is incompletely understood,2 the increased inflammatory cytokine content of mucosa affected by IBD may play a critical role.3, 6-9 For example, cytokines have been shown to modify expression and distribution of tight junction proteins.5 Both IL-13 and TNF have been reported to increase claudin-2 expression.3, 4, 8 TNF also downregulates expression10 and promotes endocytosis of occludin.6, 11, 12 In addition to effects on barrier function, some data also suggest that these proteins may also regulate signaling, gene expression, or other aspects of cellular pathophysiology.13, 14
Claudin expression is altered in a variety of pre-neoplastic conditions and frank neoplasms.15-19 For example, claudin-4 is elevated in pancreatic adenocarcinoma20 and claudin-3 and claudin-4 are elevated in Barrett's esophagus21 and some gastric adenocarcinomas.15 As might be expected, claudin protein expression is also correlated with tumor differentiation.22 Several studies have evaluated tight junction protein expression in colonic adenocarcinoma, and most have reported increased expression of claudin-113, 23-25 and claudin-2.24, 26, 27 Claudin-1 expression is a positive prognostic indicator and correlates inversely with tumor grade, lymphovascular invasion, and patient survival.25 While the mechanism whereby claudins participate in tumorigenesis is poorly understood, it is interesting to note that β-catenin and claudin-1 co-localize in the nucleus of many metastatic colonic adenocarcinomas,28 and genetic overexpression of claudin-1 increases metastatic potential of tumor cell lines.13 Conversely, inhibition of claudin-1 expression reduces β-catenin/Tcf/lef signaling in vitro.13 Together with claudin-2 promoter activation by β-catenin,29 these data suggest that the interplay between claudins and the APC/β-catenin pathway may contribute to colonic neoplasia.
Although claudin expression and distribution has been studied extensively in IBD,3, 4, 8 the relationship between claudin modifications and IBD-associated neoplasia has not been examined. However, it is well-established that patients with IBD are at increased risk of developing colorectal carcinoma.30-32 Emerging data suggest that the risk of cancer in IBD may be related to the severity of inflammation,33, 34 suggesting that chronic injury may accelerate or otherwise contribute to neoplastic transformation. To define molecular mechanisms underlying this process, we asked if the altered expression and distribution of claudin proteins described in IBD was related to IBD-associated neoplasia. To test this we evaluated the expression of claudin-1, claudin-2, and claudin-4 in human biopsies of patients with IBD and IBD-associated dysplasia, as well as in sporadic non-IBD-associated adenomas. Claudin-1 and claudin-2 were elevated in sporadic adenomas, IBD, and IBD-associated dysplasia. Nuclear localization of β-catenin, a correlate of β-catenin activation, was increased in all conditions where claudin-1 and -2 upregulation occurred. In contrast, expression of claudin-1 and claudin-2 and nuclear localization of β-catenin were not affected in acute, self-limited colitis (ASLC). These data suggest that claudin-1 and claudin-2 expression may be regulated by β-catenin in chronic, but not acute, inflammatory conditions. Furthermore, the data indicate a potential association between increased claudin-1 and claudin-2 expression and elevated neoplastic risk in IBD.
MATERIAL AND METHODS
Patient Material
All patient materials for this study were obtained under a protocol approved by The University of Chicago Institutional Review Board. Formalin-fixed, paraffin-embedded tissue from biopsy and colectomy specimens used in this study included 16 specimens from 15 IBD patients with colonic epithelial dysplasia (12 ulcerative colitis (UC) and 3 Crohn's colitis). Biopsies from 12 patients were selected from biopsies deemed to have ASLC based on the presence of normal crypt architecture and acute lamina propria inflammation and the absence of abnormal crypt architecture, crypt atrophy, mixed lamina propria inflammation, basal plasmacytosis, basal lymphoid aggregates, and basal lymphoid hyperplasia35-38. All cases of ASLC were reviewed by at least three GI pathologists and, for a case to be included, all three had to agree that there were no features of IBD. In addition, no ASLC patients had history of IBD or developed biopsy-demonstrated in IBD in 1-2 year interval since the biopsy. Sporadic adenomas and invasive carcinomas were from patients without IBD who underwent colectomy for adenocarcinoma (36 patients). Control specimens were collected from the margins of these colectomies.
Tissue Microarray and Sectioning
Tissue microarrays containing 36 sporadic adenomas, normal adjacent colonic mucosa from each adenoma, and 3 samples of invasive carcinoma were prepared from paraffin tissue blocks. For each area, a minimum of two tissue cylinders with a diameter of 1.5 mm were arrayed into a recipient block with a manual tissue microarrayer (Beecher Instruments, Sun Prairie, Wisconsin, USA). The recipient tissue microarray block was cut into 4 μm thick sections for analysis by immunohistochemistry. After sectioning the array block, between 32 and 36 cores were available for staining on each slide. For the remaining 28 cases of ASLC and IBD that were not included in the array, tissue was immunostained using traditional paraffin sections.
Immunohistochemical Staining
Immunostaining was performed on 4 μm thick, formalin fixed, paraffin embedded tissue sections mounted on positively charged X-tra slides (Surgipath, Richmond, IL). Paraffin sections were deparaffinized in xylene, rehydrated, and washed in Tris-buffered saline. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in methanol for 5 minutes. Non-specific binding sites were blocked using non-serum Protein Block (DAKO, Carpinteria, CA) for 20 minutes. Antigen retrieval for monoclonal anti-claudin-2 (clone 12H12, Invitrogen, Carlsbad, CA), anti-claudin-4 (clone 3E2C1, Invitrogen), and monoclonal anti-β-catenin (clone 14, BD Biosciences, San Jose, CA) staining was carried out by heating sections in citrate Buffer (pH=6) for 15 minutes in a microwave oven. Sections to be stained with polyclonal anti-claudin-1 (JAY.8, Invitrogen) were heat antigen-retrieved in EDTA buffer (pH=9). For polyclonal rabbit anti-occludin (catalog number 71-1500, Invitrogen) staining, antigen was retrieved by combined enzymatic digestion with 0.04 % protease solution in distilled water followed by heat retrieval in citrate buffer (pH=6) for 15 minutes in a microwave oven. Tissue sections were incubated for 1 hour at room temperature in humidity chamber with primary antibodies diluted to 2.5 μg/ml (claudin-1 and occludin), 5 μg/ml (claudin-2 and claudin-4), and 1.25 μg/ml (β-catenin) using antibody diluent buffer (DAKO, Carpinteria, CA). After 3 washes, slides were incubated for 30 minutes with goat anti-rabbit or anti-mouse immunoglobulin conjugated to a horseradish peroxidase-labeled polymer (EnvisionTM+ System, DAKO, Carpinteria, CA). After washing slides were developed for 5 min with 3-3'-diaminobenzidine chromogen and counterstained with hematoxylin.
Analysis of immunohistochemical staining and inflammatory activity
Staining intensity of all immunostains was scored semi-quantitatively from 0 to 3 by two separate observers. When scores between the two observers were discordant, a consensus was achieved by conference at a two-headed microscope. Hematoxylin and eosin-stained slides were graded for inflammation from 0 to 3 using the criteria shown in Table 1.
Table I.
Method of grading active inflammation
| Activity Grade | Features |
|---|---|
| Grade 0 (normal) | no features of acute or chronic injury |
| Grade 1 (inactive) | architectural distortion, increased lamina propria lymphs, no activity |
| Grade 2 (active) | increased lamina propria granulocytes with or without intraepithelial granulocytes but without crypt abscesses |
| Grade 3 (active) | crypt abscesses or erosions |
Student's t-test was used to determine differences in expression between tissue groups. Pearson correlation was used to determine correlations between degrees of active inflammation and expression. For t-tests and Pearson correlation, statistical significance was taken as p ≤ 0.05.
RESULTS
Patient Population
The patient population for this study included 15 patients with IBD and dysplasia, 12 patients with ASLC, 36 patients with sporadic adenomas, and 3 cases of invasive colonic adenocarcinoma. Medications, at the time of biopsy or resection, were diverse within this patient population and precluded meaningful statistical analysis. Specimens were distributed throughout the right and left colon and there was no apparent association between location and tight junction protein expression. Patient information is summarized in Table 2. Inflammatory activity has been associated with risk of neoplasia in UC, and we have previously noted that expression of myosin light chain kinase, a regulator of tight junction permeability in colitis39, correlates with histologic inflammatory activity.40 Thus, the inflammatory activity of all specimens was graded semi-quantitatively (Table 3). All control tissue was free of active inflammation (grade 0). Sporadic adenomas often had increased intraepithelial or lamina propria granulocytes. In the three cases of carcinoma included in the tissue microarray, active inflammation was severe (grade 3). Intraepithelial granulocytes, with or without erosions, were the norm in ASLC.
Table II.
Patient Demographics
| Sporadic | Total IBD | ASLC | |||
|---|---|---|---|---|---|
| Adenoma† | UC | CD | |||
| Number of patients | 36 | 12 | 3 | 15 | 12 |
| Age mean (range), years | 68 (47-88) | 53 (28-74) | 47 (21-68) | 52 (21-74) | 42 (1-79) |
| Female:male | 14:22 | 5:7 | 2:1 | 7:8 | 6:6 |
| Family history IBD, percent (n) | 56% (9) | 100% (1) | 60% (10) | ||
| Average disease duration, years (n) | 26 (12) | 8 (1) | 25 (13) | ||
| Smoking history, percent (n) | 27% (11) | 0% (2) | 23% (13) | ||
| Location | |||||
| Right colon | 67% (24) | 33% (4) | 67% (2) | 40% (6) | 42% (5) |
| left colon | 33% (12) | 67% (8) | 33% (1) | 60% (9) | 58% (7) |
| Current therapy (n‡) | (7) | (1) | (8) | ||
| prednisone | 2 | 0 | 2 | ||
| sulfasalazine | 2 | 0 | 2 | ||
| belladonna alkaloids | 1 | 0 | 1 | ||
| mesalamine | 0 | 1 | 1 | ||
| 6-mercaptopurine | 1 | 0 | 1 | ||
| balsalazide disodium | 1 | 0 | 1 |
Table III.
Inflammatory grades of samples analyzed
| Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Control | 36 | ||
| IBD | 4 | 7 | 5 |
| ASLC | 8 | 4 |
Claudin-1 and claudin-2 expression is increased in active IBD
Expression of claudin-1, claudin-2, claudin-4, and occludin was assessed in control tissues and those involved by IBD. Claudin-1 did not localize specifically to the region of the tight junction, but was present along the lateral membrane and also detected weakly within cytoplasmic granules of crypt colonocytes in control tissue (Fig. 1). Expression was significantly increased within epithelia adjacent to lymphoid aggregates, where claudin-1 was localized to cytoplasmic granules and colonocyte lateral membranes (Fig 1; P<0.05).
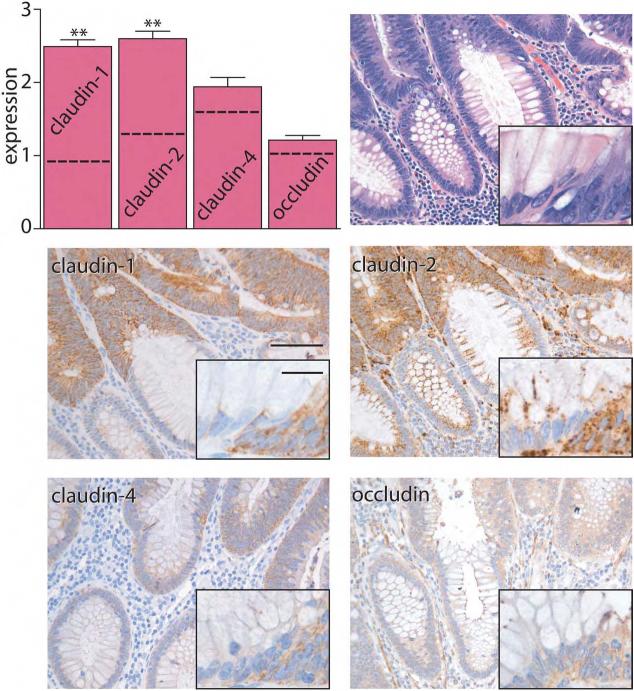
Figure 1. Claudin expression is elevated in IBD.
Immunohistochemical staining in control and IBD was scored semi-quantitatively from 0-3 and compared with inflammation grade. Inflammatory grades 1-3 are from patients with IBD. Control tissue (grade 0) is from the normal margins from patients without IBD who underwent resection for large adenoma or carcinoma. Expression of claudin-1 and claudin-2 is increased in an inflammation-dependent manner, while claudin-4 expression is more variably increased. Occludin expression was constant in all conditions. Comparison of expression in IBD to control tissue: *, P≤0.05; ***, P≤0.001. Scale bar =200μm, inset scale bar = 20 μm.
Claudin-1 expression in inactive IBD was similar to that in normal colon. In contrast, expression of claudin-1 was significantly increased when inflammatory activity was present (P<0.001). These increases in claudin-1 expression were progressive, with greater expression correlated with inflammatory activity grade in IBD (r = 0.75, P<0.001). In addition, active inflammation was associated with greater localization of claudin-1 to lateral membranes (Fig. 1). Claudin-1 expression was similar in colonic mucosa from UC and Crohn's disease (CD) patients.
In contrast to claudin-1, claudin-2 expression in surface colonocytes was most intense in the apical cytoplasm and tight junction region. Claudin-2 expression in crypt epithelium was punctate and confined to lateral cell membranes and cytoplasm (Fig. 1). There was no change in claudin-2 expression or localization in mucosa near lymphoid aggregates and expression in inactive IBD was similar to control tissue (Fig. 1). Like claudin 1, claudin-2 was increased in IBD when active inflammation was present (P<0.05), but was not increased in IBD with grade 1 inflammatory activity. Claudin-2 expression correlated positively with claudin-1 expression (r = 0.626, P<0.001) and correlated with disease activity (r = 0.451, P<0.01). Increased claudin-2 expression was noted in lateral membranes, cytoplasmic granules, and diffusely within the cytoplasm (Fig. 1). Claudin-2 expression was similar in cases of UC and CD.
Claudin-4 was detected primarily in lateral membranes of surface and crypt colonocytes (Fig. 1). In some cases of inactive IBD there was an increase in lateral membrane accumulation of claudin-4 that did not reach statistical significance (Fig. 1; P=0.15). Claudin-4 expression was enhanced in the presence of grade 2 inflammatory activity, where intense membranous staining was present (P< 0.05 vs. control). However, claudin-4 expression was variable, and not significantly increased compared to control, in IBD with grade 3 inflammatory activity. There was no difference in claudin-4 expression when UC and CD cases were compared.
Of the tight junction proteins studied, occludin demonstrated the greatest localization to the region of the tight junction. However, weak cytoplasmic staining was also present. Occludin expression and localization in both inactive and active IBD were similar to that in control tissue (Fig. 1).
Claudin-1 and claudin-2 expression are not increased in ASLC
Claudin-4 expression is increased in IBD with grade 2 inflammatory activity. In contrast, expression of claudin-1 and claudin-2 is increased in active IBD, but not inactive disease. Thus, increased expression of the claudin-1 and claudin-2 may be related to IBD or, alternatively, merely to the presence of active inflammation. To discriminate between these possibilities, we studied claudin expression in cases of ASLC. Claudin-1 expression was similar to control, non-inflamed tissue in 9 of 12 cases of ASLC cases and was scored as 2 or greater in only 3 cases. This was only slightly greater than claudin-1 expression in control tissues (Fig. 2), where one of 33 cases received a score of 2 and none were scored as 3 (P<0.05). Importantly, claudin-1 expression in ASLC was significantly lower than in cases of active IBD, in which 9 of 12 cases were scored 2 or greater (P<0.005). Expression of claudin-2 in ASLC was limited and was not significantly different from control (Fig. 2). Claudin-4 expression in ASLC with grade 2 inflammation was increased to an extent similar to that observed in IBD with grade 2 inflammatory activity (Fig. 2; P<0.001). Taken together, it appears that the overall expression of claudin-1, claudin-2, and claudin-4 are differently-regulated in ASLC relative to IBD. In particular, the data suggest that the increases in claudin-4 expression observed in IBD may be related to active inflammation. Although claudin-1 and claudin-2 expression are modified by active inflammation, this regulation in IBD is distinct from the regulation in ASLC.
Figure 2. Claudin-1 and claudin-2 expression are not elevated in ASLC.
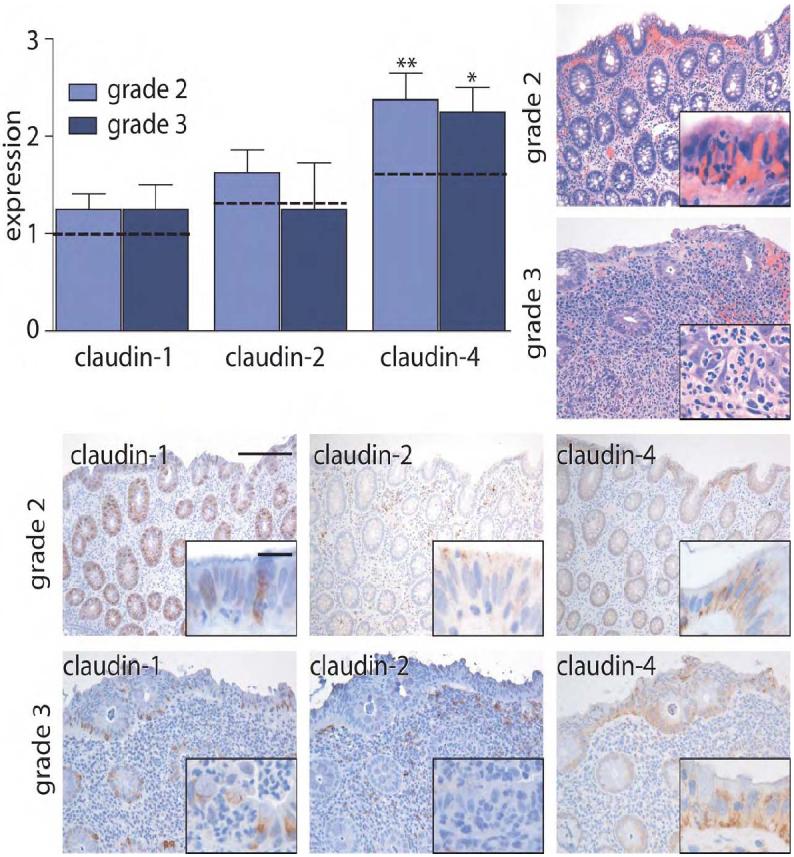
Immunohistochemical staining in grade 2 and grade 3 ASLC was scored semi-quantitatively from 0-3. Expression in control tissue (grade 0) is indicated by dashed lines. In contrast to the observations in IBD specimens, claudin-1 and claudin-2 expression are not significantly increased in ASLC. *, P≤0.05; **, P≤0.01. Scale bar =200 μm, inset scale bar = 20 μm.
Expression of claudin-1 and claudin-2 is increased in sporadic adenomas
While increased tight junction protein expression has been assessed in colorectal adenocarcinoma, there are no reports examining expression in pre-invasive dysplastic lesions. We therefore studied expression of claudin-1, claudin-2, claudin-4, and occludin in sporadic adenomas. As in invasive cancers, claudin-1 and claudin-2 expression was increased in adenomas (Fig. 3; P<0.001). Claudin-1 was localized to lateral membranes of dysplastic colonocytes while claudin-2 was concentrated within apical cytoplasmic granules in dysplastic colonocytes. However, increased claudin-1 and claudin-2 expression, which was seen in over 90% of sporadic adenomas, was the most remarkable change. We also looked at staining of 3 representative cases of invasive carcinoma and observed similar strong positive staining (claudin-1=2.00 ± 0.00, claudin-2=2.33±0.33). Claudin-4 and occludin expression and distribution were not significantly different between control, adenoma, or invasive carcinoma tissue.
Figure 3. Claudin-1 and claudin-2 expression are elevated in sporadic adenomas.
Immunohistochemical staining in sporadic adenomas was scored semi-quantitatively from 0-3. Claudin-1 and claudin-2 expression are significantly increased in dysplasia relative to nondysplastic tissue (dashed lines). **, P≤0.01. Scale bar =80 μm, inset scale bar = 20 μm.
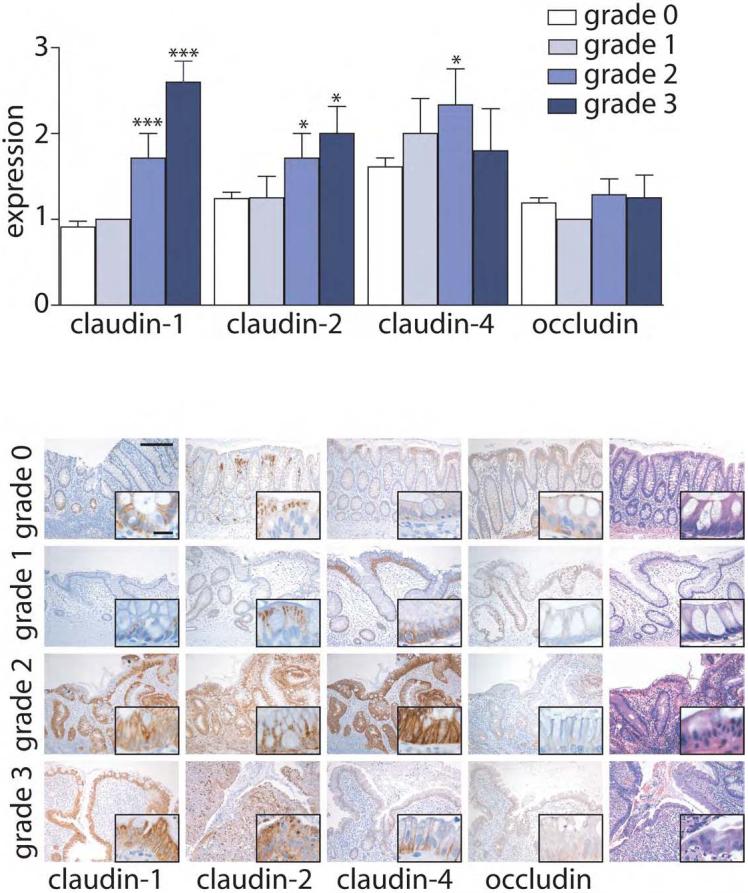
Claudin-1 and claudin-2 expression is increased in IBD-associated dysplasia
While IBD confers an increased risk of colorectal carcinoma, the mechanisms of neoplasia in IBD patients may differ from those in patients with sporadic cancer. We therefore asked, given the increased expression of claudin-1 and claudin-2 in both IBD and sporadic adenomas, whether the same alterations would be present in IBD-associated dysplasia. Claudin-1 and claudin-2 expression in IBD-associated dysplasia was increased relative to nondysplastic IBD (P<0.005) and was similar to that of sporadic adenomas (Fig. 4). In contrast, claudin-4 and occludin were only slightly increased and not significantly different from non-dysplastic IBD tissue. There were no differences between low and high grade dysplasia for any of these tight junction proteins. There were also no differences in between UC- and CD-associated dysplasia.
Figure 4. Claudin-1 and -2 expression are elevated in IBD-associated dysplasia.
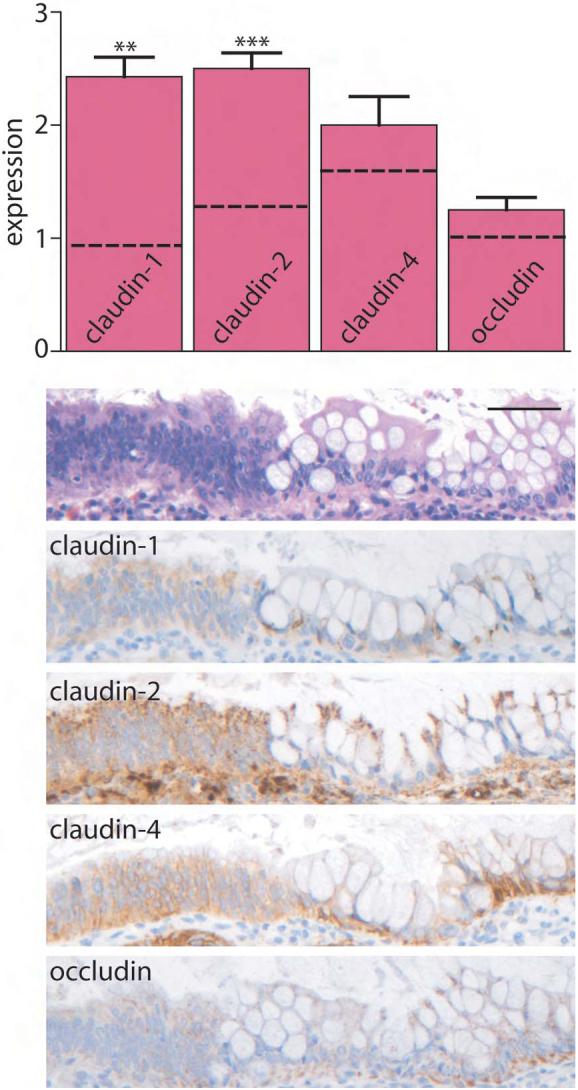
Immunohistochemical staining in IBD dysplasia was scored semi-quantitatively from 0-3. Claudin-1 and claudin-2 expression is increased relative to control tissues (dashed lines). **, P≤0.01; ***, P≤0.001. Scale bar =60 μm.
Nuclear β-catenin localization is increased in IBD
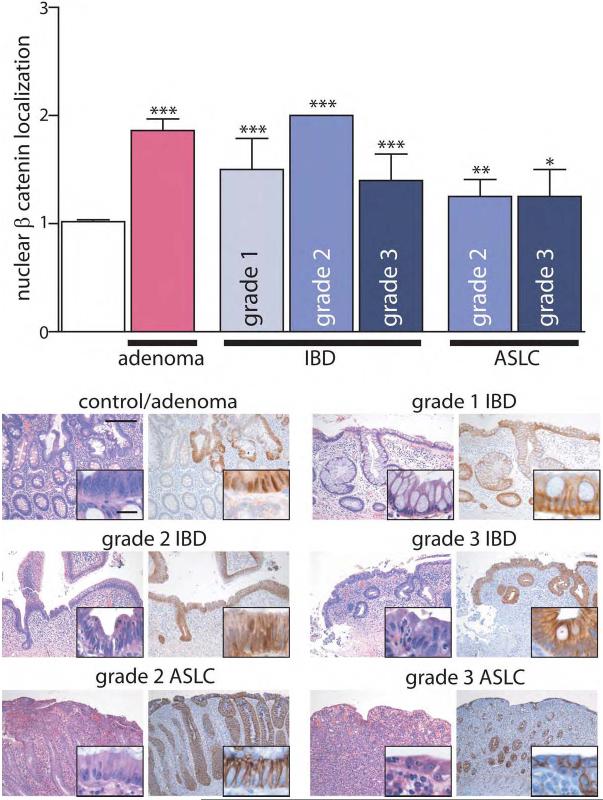
Claudin-1 and claudin-2 expression have been reported to be modulated by β-catenin/Tcf/lef signaling and, conversely, claudin-1 may regulate β-catenin.28 We therefore asked if the observed increases in claudin-1 and claudin-2 expression in IBD, IBD-associated dysplasia, and sporadic adenomas are associated with increased β-catenin nuclear localization, a correlate of β-catenin activation. β-catenin showed weak nuclear localization in control tissues, most prominently in crypts. This was increased in sporadic adenomas (Fig 5a, P<0.001). Nuclear β-catenin localization was also elevated in IBD compared to control tissue (P<0.001); 11 of 16 IBD specimens had increased nuclear β-catenin (9 of 13 UC and 3 of 3 CD). Overall, there was no correlation between nuclear β-catenin localization and disease activity in IBD (r = −0.12, P>0.6), but nuclear localization did increase from Grade 1 to Grade 2 IBD (P<0.05). Nuclear β-catenin in IBD-associated dysplasia was greater than that in non-dysplastic IBD (P<0.005), and was quantitatively similar to staining in sporadic adenomas. In contrast, nuclear β-catenin was only increased in 3 of 12 cases of ASLC and did not correlate with disease activity (Fig 5b). Thus, like claudin-1 and claudin-2, β-catenin staining is increased in IBD and dysplasia (sporadic and IBD-associated), but is not increased in most cases of ASLC. Thus, it may be that activation of β-catenin explains increases in claudin-1 and claudin-2 expression in IBD and IBD-associated dysplasia.
Figure 5. Nuclear β-Catenin localization is enhanced in dysplasia and IBD.
(a) Nuclear β-catenin localization was assessed semi-quantitatively and compared to control tissue (white bar). β-catenin within the nucleus was increased in sporadic adenomas, IBD, and, to a lesser extent in ASLC. *, P≤0.05; **, P≤0.01; ***, P≤0.001. Scale bar =200μm, inset scale bar = 20 μm.
DISCUSSION
Patients with IBD have an increased propensity to develop colon carcinoma.30-34, 41 The mechanisms underlying this susceptibility are not well-understood, and the molecular events that contribute to this phenotype are ill-defined. On the basis of previous data suggesting that expression of claudin family members is altered in IBD and invasive colon cancer,13, 15-23, 25-27 we hypothesized that modified expression of tight junction proteins could be associated with the increased risk of colon carcinoma in IBD. However, claudin family member distribution and expression has not been characterized in either sporadic or colitis-associated premalignant colonic dysplasia.
Claudin-1 protein has been reported to be slightly decreased in IBD on the basis of SDS-PAGE and immunoblot analysis of whole mucosal homogenates.4, 8 However, claudin-1 expression and localization within colonocytes of IBD patients have not been assessed in the context of inflammation grade. We observed increased claudin-1 expression in colonocytes of IBD patients. This was linked to active inflammation as claudin-1 expression was not increased in inactive IBD. Although the number of CD cases with dysplasia was small, it is interesting to note that overall results were similar in UC and CD. In agreement with previous immunohistochemical and SDS-PAGE immunoblot analyses,3, 4, 8 we found that claudin-2 expression is increased in colonocytes of patients with active IBD. This is consistent with the reported in vitro effects of IL13 and TNF on claudin-2 expression,3, 8. As these cytokines are also elevated in active UC8, 42 and CD9, respectively, they may explain the similar elevations of claudin-2 expression in these diseases. Importantly, the mere presence of active inflammation was not sufficient to increase expression of either claudin-1 or claudin-2, as expression of claudin-1 was unaffected and claudin-2 was only slightly increased in ASLC. This also implies that increases in mitotic activity alone cannot explain our results.
In contrast to claudin-1 and claudin-2, claudin-4 expression was not correlated with disease activity. The observation is consistent with a previous study that used the same antibody used here to detect claudin-4.3 Moreover, since claudin-4 expression was increased in ASLC, the data suggest that the observed effects may be simply due to inflammatory activity and not a consequence of the chronic disease process.
To assess the association between altered claudin expression in IBD and neoplastic risk, we compared expression in non-neoplastic IBD to that in IBD-associated dysplasia and sporadic adenomas. Similar increases in expression of both claudin-1 and claudin-2 was present in IBD-associated dysplasia and sporadic adenomas. Given the emerging association of neoplastic risk in IBD with inflammatory activity,33, 34 these data suggest that increased expression of claudin-1 and claudin-2 in this setting may contribute to neoplastic progression. While these changes in claudin-1 and claudin-2 expression are expected to impact barrier function, it is not clear that barrier modulation is the mechanism by which claudin protein expression influences neoplasia. It is possible that claudin proteins interact with signaling pathways, including TGFβ/SMAD and β-catenin,13, 43 in a manner that is separate from their effects on barrier function.
While the relationship we have identified between claudin-1 and claudin-2 expression and active inflammation in IBD is correlative, the previous observation that these tight junction proteins can either regulate or be regulated by β-catenin/Tcf/lef signaling13, 28, 29 further suggests that the increased claudin-1 and claudin-2 expression observed may be due to β-catenin activation. We therefore assessed β-catenin activation as nuclear localization. As expected, increased translocation of β-catenin from the lateral membranes to the nucleus was present in sporadic adenomas. Strikingly, this enhanced nuclear localization was also present in IBD and IBD-associated dysplasia. These observations, together with previous in vitro analyses, point to β-catenin as an upstream regulator of claudin-1 and claudin-2 in IBD.
Some discrepancies exist among studies of claudin family member expression in neoplasia, but this may, in part, be due to differences in tumor stage. For example, although we observed increases in claudin-1 and claudin-2 expression in adenomas and IBD-associated dysplasia, we did note decreased expression of these same proteins at sites of invasion. Similar findings of reduced claudin expression have been reported in high grade colorectal carcinomas.25 This may reflect the loss of proteins associated with epithelial differentiation during epithelial to mesenchymal transformation. Therefore, although claudin-1 and claudin-2 are elevated in preinvasive IBD-associated and sporadic neoplasia, expression may not remain increased in invasive lesions.
In summary, these data suggest that active inflammation in the setting of chronic IBD results in an increase in β-catenin transcriptional activity that may contribute to increased claudin-1 and claudin-2 expression. The data support the hypothesis that elevated claudin-1 and claudin-2 expression contribute to neoplastic transformation and provide human data that corroborates previous work using cell lines and tumor xenografts.13 Thus, increased claudin-1 and claudin-2 expression may contribute to carcinogenesis in IBD.
ACKNOWLEDGEMENTS
We thank Can Gong and Leslie Martin for excellent technical assistance, Cami McBride for statistical consultation, and Alana Bunnag for chart review. This work was supported by The National Institutes of Health (grants R01DK061931, R01DK068271, and P30CA14599).
Support: The National Institutes of Health (grants R01DK061931, R01DK068271, and P30CA14599).
Glossary
Abbreviations used
Acute
self-limited colitis
ASLC
Crohn's disease
CD
inflammatory bowel disease
IBD
ulcerative colitis, UC
REFERENCES
- 1.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss H-D, Bentzel CJ, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 2.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 3.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 4.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 8.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 Is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald TT, Hutchings P, Choy M-Y, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clinical & Experimental Immunology. 1990;81:301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, et al. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113(Pt 11):2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kominsky SL. Claudins: emerging targets for cancer therapy. Expert Rev Mol Med. 2006;8:1–11. doi: 10.1017/S1462399406000056. [DOI] [PubMed] [Google Scholar]
- 17.Soini Y. Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumours. Histopathology. 2005;46:551–560. doi: 10.1111/j.1365-2559.2005.02127.x. [DOI] [PubMed] [Google Scholar]
- 18.Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57:919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 20.Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–6271. [PubMed] [Google Scholar]
- 21.Gyorffy H, Holczbauer A, Nagy P, Szabo Z, Kupcsulik P, Paska C, et al. Claudin expression in Barrett's esophagus and adenocarcinoma. Virchows Arch. 2005;447:961–968. doi: 10.1007/s00428-005-0045-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee SK, Moon J, Park SW, Song SY, Chung JB, Kang JK. Loss of the tight junction protein claudin 4 correlates with histological growth-pattern and differentiation in advanced gastric adenocarcinoma. Oncol Rep. 2005;13:193–199. [PubMed] [Google Scholar]
- 23.Grone J, Weber B, Staub E, Heinze M, Klaman I, Pilarsky C, et al. Differential expression of genes encoding tight junction proteins in colorectal cancer: frequent dysregulation of claudin-1, -8 and -12. Int J Colorectal Dis. 2007;22:651–659. doi: 10.1007/s00384-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 24.Kinugasa T, Huo Q, Higashi D, Shibaguchi H, Kuroki M, Tanaka T, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27:3729–3734. [PubMed] [Google Scholar]
- 25.Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2005;18:511–518. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- 26.Aung PP, Mitani Y, Sanada Y, Nakayama H, Matsusaki K, Yasui W. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch. 2006;448:428–434. doi: 10.1007/s00428-005-0120-2. [DOI] [PubMed] [Google Scholar]
- 27.Hahn-Stromberg V, Edvardsson H, Bodin L, Franzen L. Disturbed expression of E-cadherin, beta-catenin and tight junction proteins in colon carcinoma is unrelated to growth pattern and genetic polymorphisms. APMIS. 2008;116:253–262. doi: 10.1111/j.1600-0463.2008.00894.x. [DOI] [PubMed] [Google Scholar]
- 28.Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469–476. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 29.Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke J-D. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Comm. 2004;314:1001–1007. doi: 10.1016/j.bbrc.2003.12.185. [DOI] [PubMed] [Google Scholar]
- 30.Eaden J. Review article: colorectal carcinoma and inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl 4):24–30. doi: 10.1111/j.1365-2036.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 31.von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007;50:839–855. doi: 10.1007/s10350-006-0848-z. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 34.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Kumar NB, Nostrant TT, Appelman HD. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis) Am J Surg Pathol. 1982;6:523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Surawicz CM. The role of rectal biopsy in infectious colitis. Am J Surg Pathol. 1988;12(Suppl 1):82–88. [PubMed] [Google Scholar]
- 37.Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterol. 1984;86:104–113. [PubMed] [Google Scholar]
- 38.Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Mucosal biopsy diagnosis of colitis: acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterol. 1994;107:755–763. doi: 10.1016/0016-5085(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 39.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 41.Vagefi PA, Longo WE. Colorectal cancer in patients with inflammatory bowel disease. Clin Colorectal Cancer. 2005;4:313–319. doi: 10.3816/ccc.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 42.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiou SR, Singh AB, Moorthy K, Datta PK, Washington MK, Beauchamp RD, et al. Smad4 regulates claudin-1 expression in a transforming growth factor-betaindependent manner in colon cancer cells. Cancer Res. 2007;67:1571–1579. doi: 10.1158/0008-5472.CAN-06-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]