Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents (original) (raw)
. Author manuscript; available in PMC: 2009 Dec 1.
Summary
We describe herein that a pyrazine derivative, T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide), is protective for a lethal West Nile virus infection in rodents. Oral T-705 at 200 mg/kg administered twice daily beginning 4 hours after subcutaneous (s.c.) viral challenge protected mice and hamsters against WNV-induced mortality, and reduced viral protein expression and viral RNA in brains. The minimal effective oral dose was between 20 and 65 mg/kg when administered twice a day beginning 1 day after viral s.c. challenge of mice. Treatment could be delayed out to 2 days after viral challenge to still achieve efficacy in mice. Further development of this compound should be considered for treatment of WNV.
Keywords: T-705, West Nile virus, hamsters, mice, 6-fluoro-3-hydroxy-2-pyrazinecarboxamide
A pyrazine analog, T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide), is orally active against influenza viruses and other RNA viruses in cell culture and animal models (Furuta et al., 2005; Gowen et al., 2007; Takahashi et al., 2003). T-705 was first shown to have potent inhibitory activity against influenza A, B, and C viruses with 50% effective concentrations (EC50) of 0.013 to 0.48 μg/mL (Furuta et al., 2002; Takahashi et al., 2003), whereas, it had no cytotoxicity up to 1,000 μg/mL in various cell lines with a selectivity index >2,000. Animal studies have further verified the antiviral activity of T-705. Orally administered T-705 (>50 mg/kg four times daily) reduced viral load and mortality of influenza A virus pulmonary infection in mice (Furuta et al., 2002). In a follow-up publication (Sidwell et al., 2007), T-705 inhibited influenza A/Duck/MN/1525/81 (H5N1) virus and disease in a mouse model when treatment was initiated at various times after virus, including 4 days after intranasal virus installation. Preclinical development for influenza has progressed to the submission of an IND and the initiation of clinical trials in Japan and the U.S. (2007). T-705 is also efficacious in treating a bunyavirus (Punta Toro virus) in mouse and hamster infection models, as well as an arenavirus (Pichinde virus) infection in hamsters (Gowen et al., 2007). Based on the preclinical broad-spectrum activity of T-705 and its evaluation in clinical trials, we evaluated T-705 in cell culture and rodent models against West Nile virus.
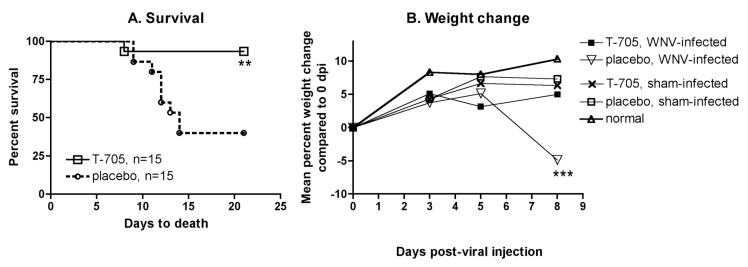
In this report, animal studies were initiated based on the finding that the EC50 of T-705 in Vero cells was 53 ± 4 μg/mL from 6 different replicates (data not shown) (Morrey et al., 2002). T-705 (Toyama Chemical Co. Ltd, Shinjuku-ku, Tokyo, Japan) was suspended in 0.4% carboxymethyl cellulose (CMC) and stored at 4°C during the experiment. T-705 at a dose of 200 mg/kg administered orally twice daily beginning 4 hours after subcutaneous (s.c.) injection of WNV until day 13 statistically improved survival of mice (P ≤ 0.01) (Figure 1A). Additionally, the same animals treated with T-705 did not lose whole body weight in contrast to the placebo-treated, infected animals (P ≤ 0.001) (Figure 1B). WNV RNA was also reduced in the brains (P ≤ 0.05) and spleens (P ≤ 0.001) of C57BL/6 mice treated with T-705 beginning within 4 hours after viral challenge and assayed 6 days later, as compared to data from vehicle-treated mice (Figure 2). Ampligen, an interferon inducer (Morrey et al., 2004), at the treatment schedule used, was less effective than the T-705 at reducing viral RNA in these tissues.
Figure 1.
Effect of T-705 at 200 mg/kg administered orally twice daily beginning 4 hours until day 13 after s.c. injection of WNV (8.5 X 104 plaque-forming units, pfu, prototypic New York 1999 isolate designated CDC 996625, Robert Lanciotti, CDC) in C57BL/6 female mice with 15 animals per group. Animal use was in compliance with the Utah State University Institutional Animal Care and Use Committee in an AAALAC-accredited facility. A.) Survival of mice, **P ≤ 0.01 using the log rank test, B.) Weight change of mice, ***P ≤ 0.001 compared to all other groups using one-way analysis of variance Kruskal-Wallis test.
Figure 2.
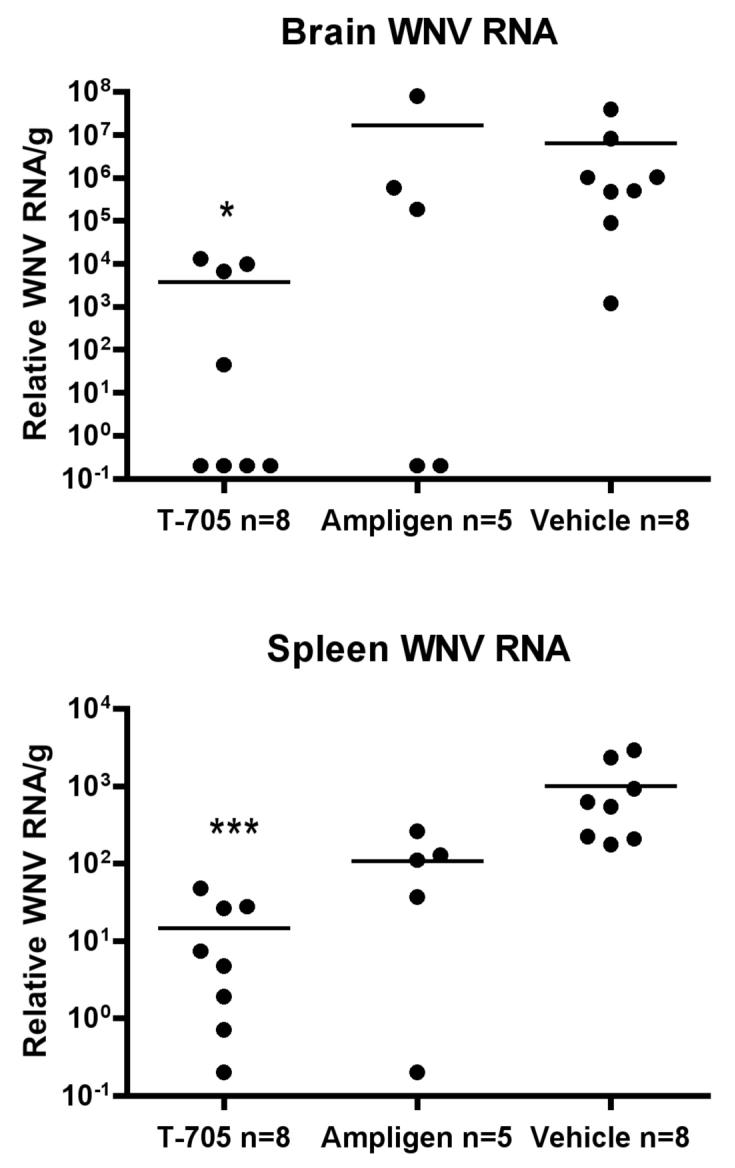
Effect of T-705 at 200 mg/kg twice daily on WNV RNA (Morrey et al., 2006) at day 6 when administered orally twice daily beginning 4 hours after s.c. injection of WNV in C57BL/6 female mice as described in Figure 1. (*P ≤ 0.05, ***P ≤ 0.001 using one-way analysis of variance)
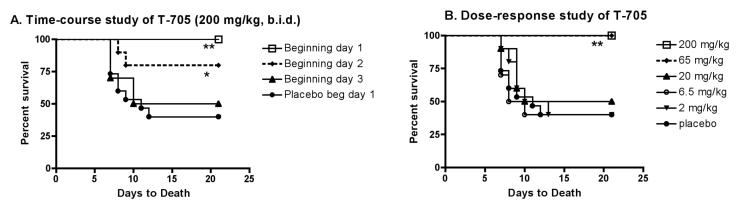
Experiments were conducted to determine the limits of efficacy for reduced doses of T-705 or in delaying treatment. T-705 at a dose of 200 mg/kg administered orally, twice daily beginning 4 hours, or 1 day after s.c. injection of WNV statistically improved survival of mice (P ≤ 0.01, P ≤ 0.05, respectively), but T-705 was not effective if treatment was delayed until day 4 (data not shown). The experiment was repeated, except that treatments were delayed until days 1, 2, or 3 (Figure 3A). The treatment statistically improved survival if delayed for 2 days, but not at 3 days after viral challenge.
Figure 3.
Effect of T-705 (200 mg/kg, b.i.d.) on survival A.) when administered orally twice daily beginning on day 1, 2 or 3 days for 13 day after s.c. injection of WNV in C57BL/6 female mice; or B.) when administered serial doses of T-705 beginning on day 1 as described in Figure 1. Ten animals were used per group. (*P ≤ 0.05, **P ≤ 0.01).
To determine a minimal effective dose, ½ log dilutions of T-705 administered orally twice a day beginning 1 day after viral challenge were evaluated in s.c. challenged of C57BL/6 mice. Doses at 200 and 65 mg/kg administered twice daily protected all mice from mortality, whereas, the survivals of animals treated with lower doses of 20, 6.5, or 2.0 mg/kg administered orally twice daily were not statistically different from placebo control mice (Figure 3B). Therefore, the minimal effective dose was between 65 and 20 mg/kg administered twice daily in mice.
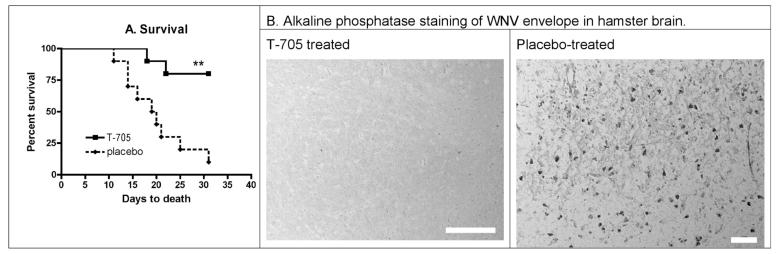
To verify T-705 activity in a second species, hamsters were orally treated with 200 mg/kg twice daily beginning at 4 hours after s.c. viral challenge (Figure 4). As with mice, the T-705 statistically improved survival (P ≤ 0.01) compared to placebo-treated hamsters (Figure 4A). Likewise, WNV envelope protein was detected immunohistochemically in the brains of three placebo-treated hamsters, but not in brain sections of three T-705-treated hamsters when assayed 7 days after viral challenge (Figure 4B).
Figure 4.
Effect of T-705 (200 mg/kg, b.i.d.) on A.) survival, B.) alkaline phosphatase detection of WNV envelope protein (Morrey et al., 2006) in brain at day 7 when administered orally twice daily beginning on day 0 for 14 days after s.c. injection with WNV in female Syrian golden hamsters (Charles River Laboratory). Fifteen animals were included in each group, wherein five animals were randomly removed on day 7 and their brains were processed for immunocytochemistry. The sections were stained with 7H2 MAb, a monoclonal antibody specific for WNV envelope, and counter-stained with hematoxylin (Morrey et al., 2006). Scale bar = 50 μm. (**P ≤ 0.01)
In this report we describe the first compound, to date, converted to a nucleoside analog that is active against WNV in rodent models. Other nucleoside analogs are active against WNV in cell culture systems, such as 6-azauridine, cyclopententylcytosine, and ribavirin (Morrey et al., 2002), but have not been demonstrated to be active in animal models (Morrey et al., 2004) (unpublished data).
The mechanism of action of T-705 against influenza A was investigated elsewhere (Furuta et al., 2005) to show that T-705 is converted to T-705 ribomonophosphate (T-705RMP) and to T-705 ribotriphosphate (T-705RTP). Moreover, T-705RTP dose-dependently inhibits influenza virus RNA-dependent RNA-polymerase (RdRp) in a GTP-competitive manner, but T-705RMP is a poor inhibitor of cellular inosine monophosphate dehydrogenase (IMPDH). Based on this, T-705 should be investigated in the future for its inhibitory effect on WNV RNA polymerase.
In this study, orally administered T-705 at 200 mg/kg twice daily was effective when initiated 2 days after s.c. injection of WNV in mice, but not at day 3 or 4. Day 2 after viral challenge may be just before the virus infects the brain (Morrey et al., 2006; Morrey et al., 2007). The lack of activity past day 2, however, might be due to the absence of an optimized treatment regimen, insufficient bioavailability in the brain, or the lack of appropriate metabolism in neuronal cells for conversion to active T-705RTP.
T-705 should be investigated for treatment of WNV, because it is the first compound converted to a nucleoside analog to have WNV activity in animal models, it is broadly active against other RNA viruses, namely influenza A, B and C, arenaviruses, bunyaviruses, and flaviviruses, and it is currently in clinical trials.
Acknowledgements
Funding: NIH NO1-AI-15435, NIH U54 AI-065357. Potential conflict of interest: Y. Furuta is an employee of Toyama Chemical, the producer of T-705.
References
- Toyama starts U.S. trials of polymerase inhibitor. FDAnews Drug Pipeline Alert. 2007;4 [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–81. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–6. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW. In Vitro and In Vivo Activities of T-705 against Arenavirus and Bunyavirus Infections. Antimicrob Agents Chemother. 2007;51:3168–76. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster models. Antiviral Chemistry and Chemotherapy. 2004;15:101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang HC, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized monoclonal antibody against West Nile virus E protein administered after neuronal infection protects against lethal encephalitis in hamsters. Journal of Infectious Disease. 2006;194:1300–08. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining the limit of effective treatment for West Nile virus neurological infection with a humanized neutralizing monoclonal antibody. Antimicrob Agents Chemother. 2007;51:2396–402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Smee DF, Sidwell RW, Tseng CK. Identification of active compounds against a New York isolate of West Nile virus. Antiviral Research. 2002;55:107–116. doi: 10.1016/s0166-3542(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wang M-H, Morrey JD, Furuta Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007;51:845–51. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Furuta Y, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Shiraki K. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir Chem Chemother. 2003;14:235–41. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]