Interleukin-6 and Neural Stem Cells: More Than Gliogenesis (original) (raw)
Abstract
Besides its wide range of action as a proinflammatory cytokine in the immune system, interleukin-6 (IL-6) has also attracted much attention due to its influence on the nervous system. In the present study we show that the designer fusion protein H-IL-6, consisting of IL-6 and its specific receptor IL-6R-α, but not IL-6 alone, mediates both neuro- as well as gliogenesis. Using immunocytochemistry, Western blot, and patch-clamp recording, we demonstrate that H-IL-6 induces the differentiation of neural stem cells (NSCs) specifically into glutamate-responsive neurons and two morphological distinctive astroglia cell types. H-IL-6–activated neurogenesis seems to be induced by the MAPK/CREB (mitogen-activated protein kinase/cAMP response element-binding protein) cascade, whereas gliogenesis is mediated via the STAT-3 (signal transducers and activators of transcription protein-3) signaling pathway. Our finding that IL-6 mediates both processes depending on its specific soluble receptor sIL-6R-α has implications for the potential treatment of neurodegenerative diseases.
INTRODUCTION
In recent years it has been noted that the adult brain has “self-repair-capacity” to replace lost neurons in several selected regions of the CNS such as the olfactory bulb, hippocampus, adult human subependymal zone, and the cortex. Active neurogenesis occurs in the subgranular zone (SGZ) of the hippocampal dentate gyrus, and in the subventricular zone (SVZ) of the lateral ventricles (Kempermann and Gage, 1999; Gage, 2000; Okano, 2002). Neural stem cells (NSCs) within these neurogenic regions can self-renew, proliferate, and differentiate into neurons or glia, providing a reservoir for replacement of cells lost during normal cell turnover and after brain injury. Newborn neurons and glia then migrate to appropriate regions in the brain and integrate into neuronal circuits (Brazel and Rao, 2004; Campos, 2004; Ming and Song, 2005; Reynolds and Rietze, 2005). Recent findings show that impairment of neurogenesis is sufficient to deteriorate learning and memory, hinting that abnormalities in the proliferation and differentiation of NSCs could play a role in the pathogenesis of cognitive disorders such as Alzheimer's disease (Shors, 2004). The question facing modern medicine is how best to use NSCs to produce functional recovery in neurodegenerative disorders in the aging brain (Arvidsson et al., 2002; Sugaya, 2005; Tanne, 2005).
The interleukin-6 (IL-6) receptor family is comprised of multisubunit receptors associated with a common receptor subunit, the transmembrane protein gp130 (Taga and Kishimoto, 1997; Heinrich et al., 2003). Natural soluble forms of those integral-membrane receptors have been described for numerous cytokines (Jones and Rose-John, 2002). Although most of them act as antagonists by competing for their ligands with the membrane bound receptors, the soluble IL-6R (sIL-6R), which is generated by limited proteolysis (shedding) or alternative splicing, behaves as an agonist (Jones and Rose-John, 2002; Rose-John and Neurath, 2004). Thus, the complex of sIL-6R bound to IL-6 is able to activate target cells that express gp130 on their cell surface but lack membrane-bound IL-6R (gp80)—a process called trans-signaling (Rose-John and Neurath, 2004; Jones et al., 2005). It is worth noting that all cells in the body express gp130, whereas only few cells express IL-6R. Cells responding to IL-6 during inflammatory states do not express IL-6R. Interestingly, the gp130 receptor subunit also occurs as a soluble protein, which is believed to have antagonistic activity (Rose-John et al., 2006). These findings designate the IL-6R system as an interesting target for the treatment of various inflammatory and cancer diseases (Jones et al., 2001; Scheller and Rose-John, 2006; Rose-John et al., 2007).
Although IL-6 has been found originally to serve as a major inducer of immune and inflammatory responses (Rose-John et al., 2006), accumulating evidence point to a crucial role of IL-6 within the CNS. Thus, increasing attention has been focused on the functional role of the hematopoietic cytokines belonging to the IL-6R family in the CNS. For instance, IL-6 promotes the differentiation of cortical precursor cells into oligodendrocytes and astrocytes (Kahn and De Vellis, 1994; Wagner, 1996; Bonni et al., 1997; Gruol and Nelson, 1997; Rajan and McKay 1998; Nakanishi et al., 2007), activates adult astrocytes (Campbell et al., 1993), and also functions as a neurotrophic and differentiation factor for neurons of the central and peripheral nervous system (Satoh et al., 1988; Gadient and Otten, 1997; März et al., 1997, 1998; Schäfer et al., 1999; Thier et al., 1999).
To further clarify the specific role of IL-6 and its specific IL-6R on NSCs' phenotype change and differentiation, we used a highly active fusion protein of IL-6 and sIL-6R designated as Hyper-IL-6 (H-IL-6) and compared its activity to that of IL-6 alone (Jones and Rose-John, 2002). We found that H-IL-6 induces NSCs to differentiate specifically into glutamate-responsive neurons, oligodendrocytes as well as into phenotypically different glia types and that this effect is strongly depending on the specific sIL-6R. Our results may have implications for the combined use of IL-6 and its specific soluble receptor sIL-6R-α for the treatment of neurodegenerative diseases.
MATERIALS AND METHODS
Reagents
Unless indicated, all reagents used for biochemical methods were purchased from Sigma-Aldrich (Sigma-Aldrich, Milwaukee, WI). Glutamate (Glu, glutamic acid), NMDA (_N_-methyl-d-aspartate), (RS)-AMPA (α-amino-3- hydroxy-5-methyl-4-isoxazole propionic acid, AMPA-receptor agonist), and the selective noncompetitive AMPA-receptor inhibitor GYKI-52466 hydrochloride were from Tocris Bioscience (Bristol, United Kingdom), STAT-3 (Signal Transducers and Activators of Transcription) inhibitors (cucurbitacin-I, Cucumis sativus L., a dual inhibitor of phosphorylated JAK-2 and phosphorylated STAT-3 (Calbiochem, San Diego, CA), and a selective inhibitor of STAT-3 (a cell-permeable inhibitor peptide, a STAT-3–SH2-domain–binding phospho-peptide (p-iP-STAT-3) that acts as a selective inhibitor of STAT-3 signaling. It lowers the DNA-binding activity of STAT-3 by forming an inactive STAT-3–peptide complex and reduces the levels of STAT-3–STAT-3 dimers that can bind DNA with no effects on STAT-3–independent Ras/MAPK signaling (Calbiochem).
Antibodies
For Western blotting: anti-ERK (extracellular-regulated kinase, 1:500, rabbit polyclonal; Chemicon, Temecula, CA), anti-pERK (phosphorylated ERK, 1:2000, rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA), donkey/sheep polyclonal anti-goat/rabbit/mouse IgG (horseradish peroxidase [HRP]-coupled, 1:5000; Amersham, Piscataway, NJ), anti-α-tubulin (1:1000, mouse monoclonal; Santa Cruz), anti-nestin (1:2000, mouse monoclonal; Chemicon), anti-GFAP (glial fibrillary acidic protein, 1:2000, mouse monoclonal; Chemicon), anti-TRKA (tropomyosin-related kinase A receptor, 1:1000, rabbit polyclonal; Cell Signaling Technology, Danvers, MA; and rabbit polyclonal, kindly provided by Dr. L. F. Reichardt, UCSF/HHMI, CA), anti-phospho-TRKA (1:1000, rabbit polyclonal; Cell Signaling), anti-β-III-tubulin (anti-TUJ-1, neuron-specific β-III-tubulin antibody, 1:2500, mouse monoclonal IgG; BabCO, Richmond, CA), anti-gp130 (1:1000, rabbit polyclonal; Santa Cruz), anti-IL-6R-α (1:1000, rabbit polyclonal; Santa Cruz), anti-LIFR (leukemia inhibitory factor receptor, 1:1000, rabbit polyclonal; Santa Cruz), anti-GAD-65/67 (glutamic-acid-decarboxylase, 1:1000, mouse monoclonal; Stressgen Bioreagents, Victoria, BC, Canada), anti-STMN2 (stathmin-like 2; also: SCG10), neuronal GAP (silencer element), 1:2000, rabbit, polyclonal; Proteintech Group, Chicago, IL), anti-MBP (myelin basic protein, 1:1000, mouse monoclonal, Chemicon), anti-STAT-3 protein (1:500, rabbit polyclonal; Santa Cruz), anti-phospho-STAT-3 (1:1000, mouse monoclonal; Santa Cruz), anti-p53 (1:2000, rabbit polyclonal; Cell Signaling), anti-phospho-p53 (1:500, rabbit polyclonal; Cell Signaling), anti-AKT (protein kinase B, 1:500, rabbit polyclonal; Santa Cruz), anti-phospho-AKT (1:1000, rabbit polyclonal; Santa Cruz), anti-MAP2 (microtubule-associated protein, 1:1000, mouse monoclonal, Chemicon), anti-GLU-V (VGLUT1; vesicular glutamate transporter), 1:2000, mouse monoclonal; Chemicon), anti-CREB (cAMP response-element binding, 1:1000, mouse monoclonal; Upstate Biotechnology, Lake Placid, NY), anti-phospho-CREB (1:1000, mouse monoclonal; Upstate), anti-TH antibody (tyrosine hydroxylase, 1:2000, mouse monoclonal; Santa Cruz), anti-PKAα-cat (catalytic subunit of protein kinase A α, 1:1000, rabbit polyclonal; Santa Cruz).
For immunocytochemistry: anti-GFAP (1:500; rabbit polyclonal; Sigma), anti-β-III-tubulin (anti-TUJ-1, 1:500, mouse monoclonal IgG; BabCO), goat serum (Vector Laboratories, Burlingame, CA) and anti-MAP2 (1:200, mouse monoclonal; Chemicon), anti-STMN2 (1:200, rabbit polyclonal; Proteintech Group, Chicago, IL), anti-GLU-V (1:500, mouse monoclonal; Chemicon), anti-GAD-67 (1:500, mouse monoclonal; Chemicon), anti-MBP (1:200, rabbit polyclonal; Chemicon), and anti-nestin (1:500, mouse-monoclonal; Chemicon).
Growth Factors
Recombinant epidermal growth factor (EGF), nerve growth factor (NGF), brain-derived growth factor (BDNF), and neurotrophin-3 (NT-3) were purchased from PeproTech (Rocky Hill, NJ). Recombinant IL-6 and Hyper-IL-6 were kindly provided by Professor Dr. S. Rose-John (Department of Biochemistry, Christian-Albrechts University, D-24098 Kiel, Germany). The designer cytokine H-IL-6, a stable fusion protein of human IL-6 and human IL-6R linked by a flexible peptide chain, was expressed and purified as described previously (Fischer et al., 1997). This construct stimulated gp130-expressing cells at 100- to 1000-fold lower concentrations than the combination of unlinked human IL-6 and human sIL-6R (Fischer et al., 1997).
Animal Material
Methods of experimentation, including the sacrificing of animals, were in accordance with the International Guiding Principles for Animal Research (WHO) and were approved by the local Institutional Animal Care and Use Committee (NTU-IACUC). Mouse tissue (C57BL/6J) was isolated after humanely killing the animals using approved anesthetic methods. For the isolation of NSCs all efforts were made to minimize the animals' suffering and to reduce the number of animals used.
NSC Culture
The advanced pregnant female (C57BL/6J) was anesthetized, and embryonic day 14 (E14) fetuses were removed one at a time. Fetusus were killed by rapid decapitation, followed by immediate removal of the brain and its surrounding membranes. Primary cultures were established from the forebrains (SVZ) of the fetuses. Dissociated embryonic tissue was digested with 0.5% trypsin (Invitrogen, Singapore) for 10 min, dissociated mechanically, and then passed through a 70-μm nylon mesh (cell strainer, BD Falcon, BD Biosciences, Bedford, MA). After two washing steps with DMEM-F12 (1:1; Invitrogen, Invitrogen), cells were exposed to the mitogen EGF in serum-free conditions. Obtained neurospheres of NSCs were grown in DMEM/F-12 (1:1) culture media with 15 mM HEPES-buffer solution (Hyclone Laboratories, Logan, UT) and antibiotics supplemented with B27-supplement (1:50; Invitrogen) in the presence of EGF (20 ng/ml) for three passages (3P, 1P ∼4 d). Cells were cultured in noncoated 75-cm2 Nunc (Thermo Fisher Scientific, Roskilde, Denmark) flasks at clonal density (2 × 105 cells/ml), and the media was changed every 3 to 4 d. H-IL6 or IL-6 was applied as described in the text. In some cases NSCs were also grown with EGF, EGF and BDNF (100 ng/ml), EGF and NGF (100 ng/ml), or EGF and NT-3 (100 ng/ml) for 3P as indicated in the text.
NSC Differentiation
EGF-derived neurospheres, from E14 cortex tissue, were cultured for 3P and thereafter were differentiated by seeding mechanically dissociated (single cell suspension) NSCs onto poly-l-lysine (PLL)-coated glass coverslips in four-well plates at a density of 5 × 103 cells/ml in EGF-free neurobasal medium (NB; Invitrogen, Invitrogen) containing 2 mM glutamax, 2 mM glutamine, and B-27 supplements, and H-IL-6 (100 ng/ml) or IL-6 (100 ng/ml) where applicable. Cells were incubated in a 37°C incubator for 5 d to observe the differentiation property.
Cell Proliferation Assays
The innovative CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI; Heese et al., 2004; Yokota et al., 2006) was used for the cell proliferation applications. The assay generates a “glow” luminescent signal based on quantitation of the ATP present from viable cells, which signals the presence of metabolically active cells. The homogeneous “add-mix-measure” format results in cell lysis and generation of a luminescent signal (that relies on the properties of a proprietary thermostable luciferase) proportional to the amount of ATP present. The amount of ATP is directly proportional to the number of cells present in culture. Briefly, according to the manufacturer's protocol, NSCs were cultured for 3P and thereafter were dissociated and plated at a density of 20,000 cells/well on an opaque-walled 96-well plate with 100 μl complete media (as mentioned above), and H-IL-6 or IL-6 were added at different concentrations (0, 60, 120, 240, and 480 ng/ml) for 72 h. On stimulation, one volume of CellTiter-Glo reagent was added, and 20 min later luminescence was recorded using a plate reader luminometer (Fluoroskan Ascent FL; Thermo Fisher Scientific, Waltham, MA). In addition, to quantify the numbers of proliferating NSCs, the FITC (fluorescein isothiocyanate) 5-bromo-2′-deoxyuridine (BrdU) Flow Kit (BD Biosciences, Piscataway, NJ) was used. NSCs (4 × 106 cells obtained after 3P in EGF, 20 ng/ml) were labeled with 3 mM BrdU for 72 h. Controls were grown with EGF (20 ng/ml) only or with EGF (20 ng/ml) plus H-IL-6 (100 ng/ml), and then the BrdU-labeled NSCs were stained with FITC-conjugated anti-BrdU antibody (1:50) with the reagents provided in accordance to the manufacturer's protocol, and finally 10,000 cells were analyzed with BD FACSCalibur (BD Biosciences) using FL1 detector for BrdU-positive cells and FL3 detector for 7-amino-actionmycin D (7-AAD)-positive cells to determine the percentage of proliferating cells (BrdU and 7-AAD-positive) among the total cell population analyzed. Results shown as relative proliferation represent experiments done four times, each performed in triplicates.
Immunocytochemistry
NSCs were differentiated with neurobasal (NB) medium for 5 d and thereafter fixed with 4% paraformaldehyde for 20 min at room temperature (RT). Cells were rinsed three times with PBS (phosphate-buffered saline) and blocked for 1 h in PBS containing 0.01% Triton X-100 (USB, Cleveland, OH) and 10% normal goat serum (Vector Laboratories, Burlingame, CA) before incubation with the different primary antibodies overnight at 4°C. After washing three times these primary antibodies were followed by secondary antibodies labeled with FITC Alexa Fluor 568 (goat polyclonal anti-rabbit 1:400, or Alex Fluor 488 goat monoclonal anti-mouse 1:400, Molecular Probes, Rochester, MN) for 1 h. After washing three times the coverslips were mounted onto glass slides using DAPI/Antifade glue (Chemicon) and analyzed with a Carl Zeiss Live imaging microscope (Axiovert 200; Carl Zeiss, Göttingen, Germany).
H-IL-6/IL-6 Stimulation for H-IL-6/IL-6–mediated Signaling Pathway Analysis
EGF-grown NSCs (for 3P) were washed with fresh DMEM/F-12 media without supplements to remove all traces of EGF and cultured in three different 25-cm2 flasks of EGF-free NSCs media (5 ml) supplemented with B27 for 5 h. H-IL-6 (100 ng/ml) or IL-6 (100 ng/ml) was then added to the respective flask (nothing was added into the control flask) and incubated for 10 min at 37°C, before the reaction was stopped with ice-cold PBS (15 ml), and washed twice with ice-cold PBS. H-IL-6 and IL-6–stimulated as well as control cells were then lysed and used for protein expression analysis by SDS-PAGE and Western blot with respective antibodies.
Cell Lysis and Protein Extraction
For washing, adherent cells were resuspended using a disposable cell scraper (Greiner Bio-One GmbH, Frickenhausen, Germany) and collected into 15-ml centrifuge tubes (BD Falcon, BD Biosciences), while floating neurospheres were aspirated and collected into 15-ml centrifuge tubes. Cells were washed twice with Ca2+/Mg2+-free PBS (−/−) via centrifugation, and the supernatant was removed after the last wash. Lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 40 mM NaF, 5 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 1% (vol/vol) Nonidet P-40, 0.1% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, 1 mM phenylmethylsulfonyl fluoride, and 10 ng/ml aprotinin) was added to each respective centrifuge tube followed by 5 min of incubation on ice. Lysed cells were centrifuged at 10,000 × g at 4°C for 10 min. The supernatant containing the protein extract was either immediately used for further analysis or stored at −80°C.
For the subcellular protein isolation, the cell lysates were prepared according to the manufacturer's protocol using Qproteome Cell Compartment kit (Qiagen, Hilden, Germany) and analyzed by Western blot. Briefly, NSCs were collected and washed three times with ice-cold PBS followed by protein extraction using the CE1 buffer to obtain the cytosolic proteins. Thereafter, the pellets were extracted stepwise with CE2 buffer and CE3 buffer, which gives membrane proteins and nuclear proteins, respectively. Finally, the pellets were suspended in CE4 buffer to get the cytoskeletal proteins. The various fractions were applied for SDS-PAGE analysis.
SDS-PAGE and Western Blot Analysis
Twenty micrograms of cell lysates were separated on 8–12% resolving SDS-PAGE at 0.02 Ampere (A) constant current and transferred to a polyvinylidine fluoride membrane (0.22 μm; Amersham) by applying the “semidry” transfer method (Bio-Rad Laboratories, Singapore) for 60 min at 0.12 A in buffer containing 25 mM Tris, 192 mM glycine, 20% methanol, and 0.01% (wt/vol) SDS. The membrane was blocked with 5% bovine serum albumin (BSA, Bio-Rad) in Tris-buffered saline (TBS) solution plus 0.1% Tween-20 (TBS-T) or PBS-T for 2 h at RT, washed three times in PBS-T for 10 min each, and incubated with primary antibody (diluted in 2% BSA in PBS-T) for 1 h at RT. Membranes were then washed as above and incubated with HRP-conjugated secondary antibody for 1 h at RT. The membranes were developed using ECL plus Western blot Detection Reagent (Amersham) and x-ray films (Konica Minolta, Osaka, Japan) were exposed to the membranes before film development in a Kodak X-OMAT 2000 processor (Eastman Kodak, Toronto, ON, Canada). For equal sample loading, protein quantification was done with a 2D Quant kit (Amersham) with at least two independent replicates. BSA was used as standard for protein quantification. For reprobing the same membrane with another primary antibody, Pierce's (Pierce Biotechnology, Rockford, IL) stripping solution was used to strip the membranes. In addition, equal sample loading was checked using α-tubulin as reference protein. Western blot experiments were done at least four times for statistical quantification and analysis (n = 4) and representative blots are shown. Values (= relative protein expression) are the ratio of densitometric scores (GS-800 Calibrated Densitometer and Quantity One quantification analysis software version 4.5.2; Bio-Rad) for the respective Western blot products (mean ± SD) using the α-tubulin bands as reference.
Electrophysiology
Patch-clamp recordings were made using the whole-cell recording configuration of the patch-clamp technique. The extracellular solution contained 140 mM NaCl, 10 mM HEPES, 1 mM NaHCO3, 0.5 mM Na2HPO4, 5 mM KCl, 0.5 mM KH2PO4, 2 mM CaCl2, and 10 mM glucose; the intracellular solution (pipette) contained 130 mM CsCl, 10 mM HEPES, 10 mM tetraethylammonium chloride (TEA-Cl), 1 mM CaCl2, 2 mM MgCl2, and 2 mM EGTA. The extracellular solution was adjusted to pH 7.4 with 1 N NaOH, and the intracellular solution was adjusted to pH 7.2 with 1 N CsCl. Unless stated otherwise, all chemicals were from Sigma-Aldrich. Membrane potential was held at −70 mV. The current-voltage (I-V) relationships were constructed using depolarizing voltage-ramp protocols. Briefly, currents activated by NMDA or AMPA were elicited with voltage-ramp protocol from −100 to 60 mV within 3000 ms. Current amplitude was at the beginning (20 ms) and end (480 ms). The 20-ms time point was selected to ensure that the cell membrane capacitive current did not contribute to the current measured. Currents were filtered at 1 kHz using an eight-pole Bessel filter (Frequency Devices, Ottawa, IL), amplified using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA), digitized at 5 kHz using a DigiData 1322A interface and analyzed using pCLAMP 9.2 (Axon Instruments).
Statistical Evaluation
The data obtained in this investigation are illustrated as mean ± SD. Differences between the groups were established using an unpaired Student's t test, whereas within-group comparisons were performed using the paired Student's t test. Differences in relative cell proliferation were also assessed by a two-way ANOVA followed by a post hoc t test with Bonferroni correction. To be considered statistically significant, we required a probability value to be at least < 0.05 (95% confidence limit).
RESULTS
IL-6 Mediates Neural Stem Cell Differentiation Depending on Its Soluble Receptor sIL-6R
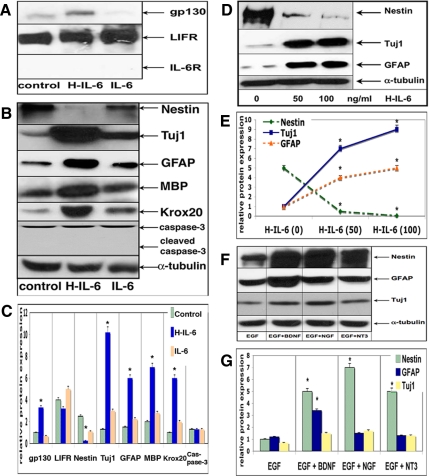
To demonstrate that IL-6 mediates neural stem cell differentiation depending on its soluble receptor sIL-6R, we initially determined the expression of the components of the entire IL-6R system on neurospheres. Spheres were cultured for 3P with EGF only or with EGF plus H-IL-6 or with IL-6. Although the gp130 receptor subunit was up-regulated in the presence of H-IL-6 only, the specific IL-6R could not be detected (though NSCs may express very low levels), thereby explaining why NSCs do not respond to IL-6 but do react upon activation by H-IL-6 (Figure 1A). Accordingly, the stem cell marker nestin was significantly reduced upon H-IL-6 stimulation but only slightly down-regulated when NSCs were grown with EGF plus IL-6 (Figure 1, B and C). Surprisingly, we observed a very strong induction of the neural marker TUJ-1 (neuron-specific β-III-tubulin) by H-IL-6. We also tested the glia/progenitor-marker GFAP as well as the oligodendrocyte-markers MBP and KROX20 and found that H-IL-6 mediated the differentiation of NSCs into all the different glia cells as indicated by an increased expression of GFAP, MBP, and KROX20. To test whether the reduction in nestin was due to differentiation or rather due to cell death, we also investigated the potential induction of apoptosis. However, neither the total caspase-3 expression nor its cleaved product (activated caspase-3) was altered. In addition, an annexin V-FITC Apoptosis Detection Kit (BD Biosciences) was used to assess apoptosis within the NSC cultures, but no significant apoptotic signs could be detected (data not shown), thus reemphasizing the fact that H-IL-6 is mediating the differentiation of NSCs independent of apoptotic mechanisms (Figure 1B). As shown in the Figure 1, D and E, the effect of H-IL-6 on the down-regulation of nestin was dose-dependent.
Figure 1.
IL-6 mediates neural stem cell differentiation depending on its soluble receptor sIL-6R. (A) H-IL-6 enhances gp130 receptor expression in EGF-responsive neural stem cells in vitro. NSCs were grown in EGF (20 ng/ml), EGF (20 ng/ml) plus H-IL-6 (100 ng/ml), or EGF (20 ng/ml) plus IL-6 (100 ng/ml) for three passages (3P) as described in Materials and Methods. (B) Thereafter, lysates were prepared and Western blot was performed using the respective antibodies as indicated. NSCs were treated as in A. The presence of H-IL-6 (100 ng/ml) enhances differentiation of NSCs, thus mediating neurogliogenesis. H-IL-6 down-regulates the stem cell marker nestin while up-regulating the neural marker TUJ-1 as well as the glia markers GFAP (astrocytes) and MBP and KROX20 (oligodendrocytes). No change in caspase-3 or activated caspase-3 (due to apoptosis) expression could be detected; α-tubulin was used as control for equal sample loading and as reference protein for the quantitative analysis. (C) Quantitative analysis of the Western blots shown in A and B (*p < 0.05, compared with control). (D) Dose-dependent effect of H-IL-6 on NSCs. NSCs were grown as in A and tended to differentiate (decrease in nestin expression) if exposed to higher concentrations of H-IL-6 (0, 50, or 100 ng/ml H-IL-6). (E) Quantitative analysis of the Western blots shown in D (*p < 0.05, compared with control). (F) The classical neurotrophic factors NGF, BDNF, and NT-3 (100 ng/ml each) do not mediate NSCs differentiation in the presence of EGF as the neural stem cell marker nestin, and the differentiation markers TUJ-1 and GFAP are almost unchanged after culturing for 3P. (G) Quantitative analysis of the Western blots shown in F (*p < 0.05, compared with control).
It has also to be noted that H-IL-6 is able to override the action of the strong mitogen EGF, thereby allowing the NSCs to enter the differentiation pathway. In contrast, the classical neurotrophins (NGF, BDNF, or NT-3) are unable to trigger differentiation of multipotent NSCs as long as EGF is present—almost no significant changes could be observed for any of the differentiation markers tested (TUJ-1, GFAP; Figure 1, F and G).
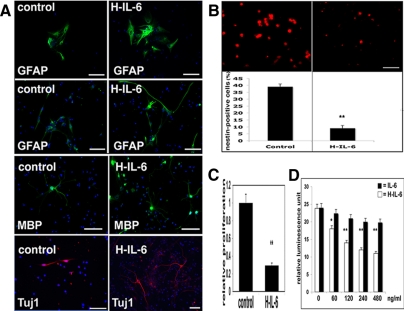
The differentiation effect of H-IL-6 was confirmed by immunocytochemistry. For this purpose NSCs were grown for 3P with (only) EGF before differentiation was induced in EGF-free NB-media (with B27 supplement) for 5 d with or without H-IL-6. NSCs showed significant more neuronal (TUJ-1–positive) and glia (GFAP- and MBP-positive) cells if differentiated in the presence of H-IL-6 (Figure 2A).
Figure 2.
IL-6 mediates neural stem cell differentiation by decreasing the nestin-positive population. (A) Immunocytochemical analysis of H-IL-6–treated NSCs. NSCs were first grown (3P) in EGF (20 ng/ml) before inducing differentiation in neurobasal medium (NB, plus B27 supplement) with H-IL-6 (100 ng/ml) or without (control) for 5 d. H-IL-6 enhances neurogliogenesis by up-regulating the neural marker TUJ-1 as well as the glia markers GFAP (astrocytes) and MBP (oligodendrocytes). GFAP staining indicates two morphological different types of glia cells (see also Figure 3A). Scale bar, 100 μm. (B) H-IL-6 differentiates nestin-positive NSCs. H-IL-6 mediates NSCs differentiation by reducing the number of nestin-positive cells. EGF (20 ng/ml) (control) and EGF plus H-IL-6 (100 ng/ml)-treated NSCs (grown for 3P) were made single by mechanical trituration. Immunocytochemistry was performed with a nestin antibody as described in Materials and Methods, and the nestin-positive cells were counted with a fluorescence microscope (Axiovert 200, Zeiss). Data are shown as mean ± SD of three independent experiments, each done in duplicate (**p < 0.01, compared with control). Scale bar, 20 μm. (C) Proliferation of NSCs is greatly reduced by H-IL-6. NSCs were first grown in EGF (20 ng/ml) for 3P before the proliferation assay (BrdU incorporation) was done. Quantification of BrdU incorporation is shown as mean ± SD of four independent determinations (**p < 0.01; H-IL-6,100 ng/ml plus EGF, compared with control, EGF only). (D) H-IL-6 inhibits NSCs proliferation in a dose-dependent manner as the neurospheres differentiate into neurons and glia cells. NSCs were grown as in C before the proliferation assay (ATP consumption) was done with various doses of IL-6 or H-IL-6 as described in Materials and Methods. Values represent the mean (±SD) from experiments done four times, each performed in triplicate (*p < 0.05, **p < 0.01; H-IL-6 compared with IL-6).
H-IL-6 Differentiates Nestin-positive NSCs
To check whether H-IL-6 was responsible for the survival of neurons and glia or rather initiated the differentiation of nestin-positive cells, we counted the number of nestin-positive cells and found that those were significantly reduced if NSCs were grown in the presence of H-IL-6 for 3P, thus confirming the TUJ-1/GFAP-immunocytochemical and Western blot results obtained in Figures 1 and 2A (Figure 2B).
Additional proliferation analyses revealed that H-IL-6 indeed induced the differentiation of NSCs, thereby explaining why the nestin-positive cells were down-regulated. Although the effect of IL-6 on NSCs proliferation was marginal, H-IL-6 significantly inhibited NSCs proliferation in a dose-dependent manner (Figure 2, C and D).
H-IL-6 Differentiates NSCs into Neurons and Two Morphological Distinctive Astroglia Cell Types
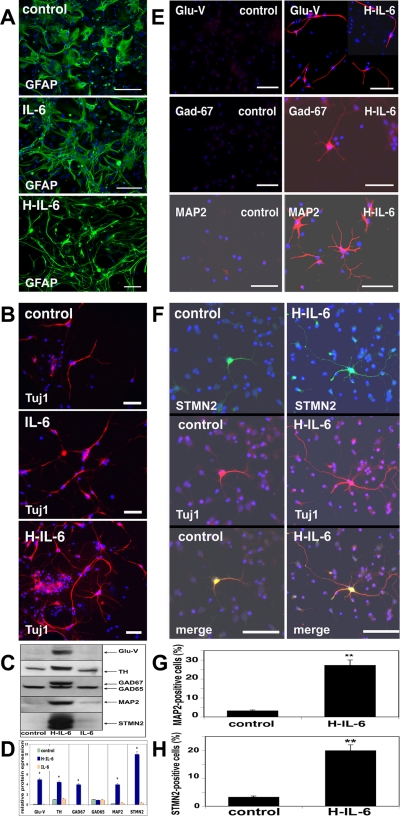
We noted that H-IL-6 not only induced the differentiation of NSCs into GFAP-positive astrocytes but was also responsible for the formation of at least two morphological distinctive astroglia types as shown in Figure 2A.
To analyze this phenomenon we cultured NSCs in the presence of EGF and H-IL-6 for 3P before differentiating them into glia cells for 5 d in NB media with either IL-6 alone or H-IL-6. Immunocytochemistry analysis showed that the presence of H-IL-6 in the NSCs' culture (maintained with EGF plus H-IL-6 for 3P) allowed us to obtain clearly more differentiated GFAP-positive glia cells upon differentiation than without H-IL-6 (compare Figure 3A, control, with Figure 2A, control). Moreover, differentiation of the EGF/H-IL-6–grown NSCs either in the presence of IL-6 or H-IL-6 again showed that IL-6 alone did not have a significant effect on the morphological appearance of the astrocytes because NSCs still do not express the specific IL-6R (Figure 3A, control, vs. IL-6). In contrast, if differentiated in the presence of H-IL-6, astroglia cells show a significant different morphology—presenting less flat cytoplasm and having much more thin outgrowths (Figure 3A, control, and IL-6 vs. H-IL6).
Figure 3.
H-IL-6 mediates neurogliogenesis. (A) H-IL-6 mediates gliogenesis. NSCs were grown with EGF (20 ng/ml) in the presence of H-IL-6 (100 ng/ml) for 3P in vitro and then were differentiated for 5 d in NB (plus B27 supplement) media only (control) or in the presence of either H-IL-6 or IL-6 (each 100 ng/ml), and immunocytochemistry was performed using an anti-GFAP antibody as described in Materials and Methods. Differentiation of NSCs results in two morphological distinctive glia cell types. Scale-bar, 100 μm. (B) H-IL-6 mediates neurogenesis. NSCs were grown with EGF (20 ng/ml) in the presence of H-IL-6 (100 ng/ml) for 3P in vitro and then were differentiated as in A, and immunocytochemistry was performed using an anti-TUJ-1 antibody as described in Materials and Methods. H-IL-6–treated cells showed more neuronal branches than control or IL-6–stimulated cells. Scale-bar, 100 μm. (C) NSCs were grown only in EGF (control) or EGF plus H-IL-6 or plus IL-6 for 3P and were differentiated for 5 d, as indicated, in NB media (control group) or in the presence of H-IL-6 or IL-6. Western blot analysis shows the up-regulation of different neuronal subtypes upon differentiation with H-IL-6: TH (dopaminergic neurons), GLU-V (glutamatergic neurons), and GAD-65/67 (GABAergic neurons). (D) Quantitative analysis of the Western blots shown in C (*p < 0.05, compared with control). (E) NSCs were grown and differentiated as in C (control and H-IL-6 groups). Also with the immunocytochemistry method different neuronal subtypes could be detected if H-IL-6 was present during the differentiation process but were absent in control-differentiated cells. Scale-bar, 50 μm. (F) NSCs were grown and differentiated as in E. STMN2-specific immunocytochemistry (costaining with TUJ-1) revealed that H-IL-6–differentiated neurons display more branches than control neurons (for appropriate comparison, representative neurons were selected). Scale-bar, 50 μm. (G) NSCs were grown and differentiated as in E. Immunocytochemistry was done as in E with an anti-MAP2 antibody. MAP2-positive cells were counted in control and H-IL-6–treated cells. Data are shown as mean ± SD of four independent experiments, each done in duplicates (**p < 0.01, compared with control). (H) STMN2-positive cells from F were counted in control and H-IL-6–differentiated cells. Data are shown as mean ± SD of four independent experiments, each done in duplicates (**p < 0.01, compared with control).
Of utmost interest was our finding of the up-regulated expression of the neural marker TUJ-1. This finding prompted us to investigate the effect of H-IL-6 on neuronal differentiation in detail. NSCs were cultured in the presence of EGF and H-IL-6 for 3P before differentiating them for 5 d in NB media (control) or with either IL-6 alone or H-IL-6. This time, the common neural maker TUJ-1 was only slightly up-regulated in our immunohistochemical analysis when differentiated with NB medium or with IL-6. However, if neurospheres were differentiated with H-IL-6, we obtained not only more neurons but those neurons also showed significant more branches that might be neuronal dendrites or axons (Figure 3B).
H-IL-6 Differentiates NSCs into Different Neuronal Subtypes
To examine whether H-IL-6 can differentiate NSCs into different specific neuronal subtypes, a Western blot analysis was performed of those cells grown either in EGF (control) or in EGF plus H-IL-6 or plus IL-6 for 3P and differentiated for 5 d in NB media with H-IL-6 or IL-6 as described in Figure 3, C and D. Interestingly, when NSCs were grown and differentiated in the presence of H-IL-6, H-IL-6 (but not IL-6 alone) induced the protein expression of several neuronal subtype-specific markers such as TH (dopaminergic neurons), GLU-V (glutamatergic neurons), GAD-67 (GABAergic neurons), MAP2 (a major MAP of brain tissue known to promote microtubule assembly and to form side-arms on microtubules, which also interacts with neurofilaments, actin, and other elements of the cytoskeleton) and STMN2, a neuron-specific stathmin protein with a potent microtubule-destabilizing factor that is enriched in the growth cones of developing neurons with high expression levels in the hippocampus. In addition, it has been suggested that STMN2 serves as an important regulatory factor of growth cone motility by enhancing microtubule dynamics, possibly through increasing the catastrophe frequency (Himi et al., 1994; Mori and Morii, 2002, 2006; Figure 3, C and D).
On the basis of these Western blot results, we evaluated the differentiated cells again with neuronal type-specific antibodies by immunocytochemistry (Figure 3E). For this purpose, NSCs were again grown with EGF or EGF plus H-IL-6 for 3P before differentiating them for 5 d in NB media (plus B27 supplement) with or without H-IL-6. The specific staining for MAP2, GLU-V, and GAD-67 clearly showed that H-IL-6 enhanced neurogenesis. In addition, the STMN2 staining (representative neurons were selected) was used to show that the H-IL-6–differentiated neurons showed obviously more and longer branches than the neurons in the control-differentiated group (Figure 3F). MAP2- and STMN2-positive cells obtained by immunocytochemistry were then counted in control and H-IL-6–treated cells (Figure 3, G and H).
H-IL-6 Mediates Neuronal Differentiation of NSCs via the MAPK/CREB Pathway
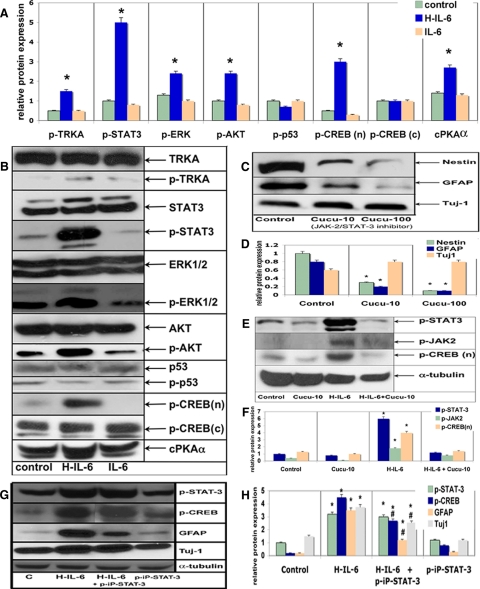
Previous reports have pointed out that the STAT-3 pathway is responsible for gliogenesis and suppression of STAT-3 enhances neurogenesis (Bonni et al., 1997; Gu et al., 2005). Thus, we analyzed additional signaling molecules that might be responsible for the H-IL-6–mediated neurogenesis. NSCs were grown in EGF (20 ng/ml) for 3P and then stimulated with H-IL-6 (100 ng/ml) or IL-6 (100 ng/ml) for 10 min as described in Materials and Methods before being subjected to Western blot analysis. Nonsurprisingly, we found a strong activation of STAT-3 as indicated by its phosphorylation. However, additionally, H-IL-6 activates via gp130 other known signaling pathways such as the RAS-MAPK ERK1/2 cascade (Heinrich et al., 2003). The key activator of the transcription factor CREB is the PKA that is upstream to the MAPK pathway and is responsible for the activation (phosphorylation) of CREB initiated in our study by H-IL-6 (Figure 4, A and B). On activation (phosphorylation), CREB translocates to the nucleus to activate gene transcription (Figure 4, A and B; Waltereit and Weller, 2003; Sands and Palmer, 2008). The finding that IL-6 cooperates with the NGF/TRKA signaling to promote neuronal differentiation inspired us to look also for the phosphorylation status of the NGF receptor kinase TRKA (Sterneck et al., 1996). Indeed, upon stimulation of the NSCs with H-IL-6 the TRKA receptor became significantly phosphorylated (Figure 4, A and B). Another pivotal signaling molecule in controlling neuronal survival and differentiation is protein kinase B (AKT; Huang and Reichardt, 2003). Besides, very recently it has been shown that IL-6 can activate STAT-3 via AKT, which by itself is activated by the Ras/phosphatidylinositol 3-kinase (PI3K) pathway and responsible for neuronal survival (Ohbayashi et al., 2007). Therefore, we were also interested in AKT and found its activation through H-IL-6 as indicated by a significant elevated phosphorylation status. Another finding being worth to mention here was that the phosphorylation status of the crucial cell cycle control protein p53 was slightly reduced (Figure 4, A and B).
Figure 4.
Signaling pathway analysis of H-IL-6–activated NSCs neuronal-differentiation. (A and B) NSCs were grown in EGF (20 ng/ml) for 3P and then stimulated with H-IL-6 (100 ng/ml) or IL-6 (100 ng/ml) for 10 min as described in Materials and Methods before being subjected to Western blot. Results clearly show the activation of the JAK/STAT-3 and PKA/MAPK pathways. In addition, nuclear phosphorylated CREB (n) (CREB (c), cytoplasmic fraction) is significantly increased compared with control and IL-6–treated cells. cPKAα, catalytic subunit of protein kinase A α. (A) represents the quantitative analysis of the Western blots shown in B (*p < 0.05, compared with control). (C) Western blot is shown where NSCs were grown in the presence of the STAT-3 inhibitor cucurbitacin-I (Cucu-10/-100; 10 and 100 ng/ml) for 3P with EGF (20 ng/ml) and H-IL-6 (100 ng/ml). Almost all nestin- and GFAP-positive NSCs/progenitors seem to die (very low signal for either of them in the presence of Cucu-I), whereas TUJ-1–positive neuronal cells survive allowing neural precursors to differentiate upon H-IL-6 stimulation into neurons via activation/phosphorylation of MAPK/CREB (nuclear p-CREB (n)). (D) Quantitative analysis of the Western blots shown in C (*pp < 0.05, compared with control). (E) NSCs were grown in EGF for 3P and then stimulated with H-IL-6 as in A and B. The less specific STAT-3 inhibitor cucurbitacin-I (Cucu-10, 10 ng/ml) inhibits H-IL-6 (100 ng/ml)-activated CREB (phosphorylated fraction of nuclear CREB (p-CREB (n)) and STAT-3 phosphorylation. (F) Quantitative analysis of the Western blots shown in E (*p < 0.05, compared with control). (G) NSCs were grown in EGF and then stimulated with H-IL-6 as in A and B. Applying the specific STAT-3 signaling inhibitor (p-iP-STAT-3, 30 μM) still allows H-IL-6 (100 ng/ml) to stimulate the phosphorylation of STAT-3 but inhibits its action and thus GFAP activation. The presence of the inhibitor also permits CREB activation (phosphorylation, p-CREB) and by this neuronal (TUJ-1 up-regulation) differentiation and survival. (H) Quantitative analysis of the Western blots shown in G (*p < 0.05, compared with control; # p < 0.05, compared with H-IL-6–stimulated cells).
STAT-3 Inhibition Leads to Neuronal Survival
Other scientists have indicated (at mRNA level) that the STAT-3 pathway is responsible for gliogenesis and that inhibition of STAT-3 leads to neurogenesis (Gu et al., 2005). Thus, to support our data that H-IL-6 indeed supports neuronal differentiation and survival in a STAT-3–independent manner, we applied the JAK/STAT-3 inhibitor cucurbitacin-I as well as a more specific STAT-3-inhibitory peptide. When cells were grown for 3P with EGF and H-IL6 in the presence of the JAK/STAT-3 pathway inhibitor (cucurbitacin-I), mainly TUJ-1-positive neuronal progenitors could be detected and nestin- and GFAP-positive NSCs/progenitors were almost absent (Figure 4, C and D). When applying inhibitors of the MAPK pathways (for instance MEK1/2- or ERK1/2 inhibitors), NSCs could not be maintained for 3P—even in the presence of H-IL-6 (due to the inhibition of the EGF and IL-6 signaling cascades, data not shown). Additionally, in the presence of cucurbitacin-I H-IL-6–induced STAT-3 and CREB phosphorylation were inhibited—confirming that cucurbitacin-I acts more upstream of STAT-3 in the IL-6 signaling pathways and also indicating that other (CREB-independent) pathways might exist in H-IL-6-mediated neuronal differentiation (Figure 4, E and F). Therefore, we applied the more specific STAT-3 inhibitor (p-iP-STAT-3, a cell permeable peptide), which inhibits STAT-3 signaling (it does not inhibit STAT-3 phosphorylation but it only lowers the DNA-binding activity of STAT-3 and reduces the levels of active STAT-3–STAT-3 dimers) but neither H-IL-6–activated MAPK nor the phosphorylation of CREB. Consequently, GFAP was also down-regulated while TUJ-1 still got up-regulated (Figure 4G, 4H). These data confirm that H-IL-6 mediates neuronal differentiation and survival via a STAT-3–independent pathway such as the MAPK/CREB cascade.
H-IL-6–differentiated Neurons Respond Specifically to the Ionic Glutamate/AMPA Receptor
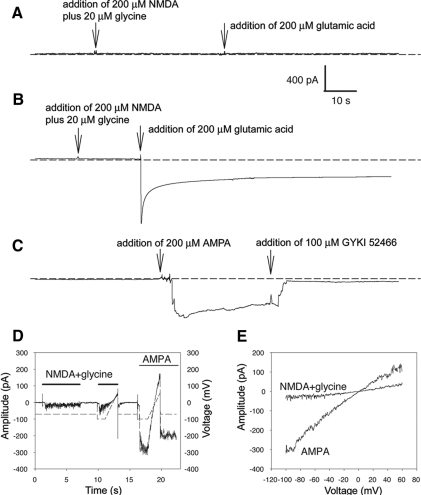
To test the functionality of the H-IL-6–differentiated neurons, we applied various neurotransmitters and recorded the neuronal response. NSCs were grown in EGF for 3P. Control cells were differentiated in NB media while the H-IL-6 cells were grown in EGF plus H-IL-6 and also kept in H-IL-6 during the 5 d differentiation period. For ionic current measurement using a whole-cell patch clamp technique, 130 mM CsCl and 10 mM TEA-Cl were used in the internal solution to block potassium channels. Effects in detecting the NMDA- (NMDA-specific glutamate receptors) and glutamic acid–activated (ionotropic glutamate receptors) currents had not met with success in neurons of control-differentiated NSCs (n = 8, Figure 5A). However, when the H-IL-6–differentiated NSCs were studied, rapid inward currents of a few hundreds up to 3000 pA could be recorded in those neurons when 200 μM glutamic acid was applied into the extra-cellular solution. Similar to the control cells, NMDA did not induce a response in H-IL-6–differentiated neurons. As shown, the activated currents were not due to the opening of NMDA receptors, as there was no current activation when 200 μM NMDA plus 20 μM glycine were applied to the H-IL-6–differentiated cells (n = 8, Figure 5B). To test whether the currents activated by glutamic acid were via AMPA receptors, AMPA (the selective AMPA receptor agonist) and the selective noncompetitive AMPA antagonist GYKI-52466 were used to detect AMPA receptor protein expression in these H-IL-6–differentiated neural stem cells. Figure 5C reveals that H-IL-6–differentiated neurons contain glutamate receptors with an activated current displaying the characteristics of the AMPA receptor subtype (n = 7, Figure 5C). To study the current voltage (I-V) relationship of the H-IL-6–differentiated neural stem cells, we applied a ramp protocol. The I-V relationship was linear for both NMDA and AMPA activating currents, though the current activated by NMDA was very small, ∼10 pA only (Figure 5, D and E). Concluding, H-IL-6–differentiated neurons respond specifically to the ionic glutamate/AMPA receptor.
Figure 5.
Ionic glutamate receptors in differentiated neural stem cells. (A) NSCs were grown only with EGF (20 ng/ml) for 3P in vitro and then differentiated in NB media plus B27 supplement for 5 d (without H-IL-6) as described in Materials and Methods. No ionic glutamate receptors were detected in these control-differentiated NSCs (n = 8). (B) NSCs were grown with EGF (20 ng/ml) in the presence of H-IL-6 (100 ng/ml) for 3P in vitro and then differentiated in the presence of H-IL-6 for 5 d as described in Materials and Methods. In H-IL-6–differentiated neural stem cells, glutamic acid-activated ionic receptors (non-NMDA receptors) were detected (n = 8). (C) H-IL-6–differentiated cells from B were further analyzed. The activation current was with AMPA characteristics as the selective noncompetitive AMPA antagonist GYKI-52466 could completely inhibit the AMPA-receptor-agonist–mediated effect. The membrane potential was held at −70 mV. The short dashed line indicates the baseline of zero current (n = 7). (D) Currents activated by NMDA in H-IL-6–differentiated cells from B were very small, 10 pA only. The short dashed line is the membrane potential. The bold lines indicate the addition of 200 μM NMDA plus 20 μM glycine or the addition of 200 μM AMPA. (E) The current voltage (I-V) relationships of NMDA and AMPA receptor channels of H-IL-6–differentiated cells from B.
DISCUSSION
The present study aimed at gaining more insight into the functional role of the IL-6R system during NSCs differentiation because increasing evidence supports an essential role for the IL-6 receptor family during development, differentiation, as well as de- and regeneration of neurons in the CNS. Ciliary neurotrophic factor (CNTF), for instance, mediates the maintenance of forebrain NSCs and enhances astrocyte differentiation (of those cells already committed to the glial lineage) but not astrocyte commitment through its specific receptor complex consisting of CNTFα, LIFR (leukemia inhibitory factor) and gp130 (Bonni et al., 1997; Shimazaki et al., 2001). Similarly, LIF does not mediate astrocytic differentiation (Molne et al., 2000). Although the JAK-STAT–signaling pathway has been described as being activated by the IL-6R family members and being responsible for gliogenesis, its action is opposed by the RAS-MAPK pathway that is rather responsible for neuronal differentiation (Bonni et al., 1997; Shimazaki et al., 2001; Gu et al., 2005; Taga and Fukuda, 2005).
In the CNS both IL-6 and its specific receptor (IL-6R) are expressed on neurons and glial cells including astrocytes (Gadient and Otten, 1997). Additional data indicate that IL-6—in conjunction with sIL-6R—regulates specific neurotrophin release from astrocytes in a brain-region–dependent manner (März et al., 1999). Moreover, IL-6 does not only promote neurite outgrowth and neuronal survival in cultured enteric and sensory neurons (Schäfer et al., 1999; Thier et al., 1999) but also supports neuro-regeneration of hippocampal neurons in the CNS (Hakkoum et al., 2007).
Here we demonstrate that NSCs do not express a functional IL-6R and thus do not properly respond to IL-6. We also did not observe any release of IL-6 nor NGF by NSCs in NSC-conditional medium (using ELISA, data not shown). However, if stimulated with the IL-6–sIL6R fusion protein H-IL-6, NSCs differentiate into glia and neurons. Besides the observation that H-IL-6 induces gliogenesis with morphological distinctive glia cells, the most interesting point in the present study is that we could detect an up-regulation of a wide range of different neuronal subtypes (glutamatergic, GABAergic, dopaminergic) that were responding specifically to glutamate via AMPA receptors and thus characterizing H-IL-6 as a more general differentiation factor for NSCs mediating neurogliogenesis.
Several years ago it has already been shown in the CNS that IL-6 can significantly alter neuronal development and response to the neurotransmitter glutamate by interfering with the expression and function of the glutamate receptor system (Qiu et al., 1995, 1998). Moreover, it has been described that different metabotropic glutamate receptors (mGluRs) support self-renewal of NSCs and that functional NMDA-receptors may be expressed constitutively in neural progenitor cells to play a crucial role in commitment and differentiation (probably together with other mGluR subtypes) into neurons in the hippocampus (Melchiorri et al., 2007; Kitayama et al., 2004). Although our H-IL-6–differentiated neurons responded to AMPA only, we cannot rule out that NMDA-responsive neurons were among those differentiated cells. Taken together, future studies have to disclose the importance of the interplay between the IL-6 and Glu systems during NSCs differentiation.
Our analysis also revealed that H-IL-6 triggered gliogenesis via STAT-3 activation and neurogenesis through the activation of the MAPK pathway respectively resulting in an accumulation of nuclear phosphorylated CREB (p-CREB). Recent data provided evidence that CREB regulates differentiation and survival of newborn neurons thus supporting our observation (Giachino et al., 2005; Josselyn and Nguyen, 2005; Dworkin et al., 2007; Peltier et al., 2007). Although Gu et al. (2005) have shown that inhibition of STAT-3 enhances neurogenesis, more precisely, we could show that nestin-/GFAP-positive NSCs/progenitors died during complete STAT-3 inhibition while—in the presence of H-IL-6—TUJ-1–positive neuronal progenitors and NSCs could survive and differentiate into different neuronal subtypes via CREB and AKT activation.
Previously, it has been shown in Stat-3–deficient mice that the activity of the ERK-MAPK pathway remained intact as STAT-3 likely plays only a critical role in the commitment to cell cycle progression and gliogenesis (Gu et al., 2005; Chan et al., 2004). This is in line with our observation where the less specific JAK/p-STAT-3 inhibitor (cucurbitacin-I) leads to a down-regulation of nestin- and GFAP-positive NSCs/progenitors, whereas neuronal cells (TUJ-1-positive) remained alive—and, if applying the more specific p-STAT-3 inhibitor (p-iP-STAT-3), H-IL-6 is still capable of activating the RAS/MAPK/AKT cascade and the transcription factor CREB, thereby (whereas STAT-3 signaling and gliogenesis is inhibited) mediating neuronal differentiation and survival.
Cucurbitacin-I is effective at suppressing the levels of p-STAT3 and p-JAK2, but unable to directly inhibit Src, JAK1, or JAK2 kinase activities. The Ras/Raf/MEK/ERK and the JNK signaling pathways are not inhibited by cucurbitacin-I. Because the phosphotyrosine JAK2 levels were also reduced by cucurbitacin-I, it has been suggested that JAK2 is likely not the target. Moreover, it may even slightly interfere with the EGFR signaling pathways (Blaskovich et al., 2003). This may explain our observation that higher doses of cucurbitacin-I led to NSCs death. As the gp130 subunit and the EGFR seem to form a receptor complex, it is likely that cucurbitacin-I inhibits the common gp130/EGFR-GAB1/SHP2/JAK-Ras/MAPK-pathway which explains our observation of reduced p-CREB levels in the presence of cucurbitacin-I (Badache and Hynes, 2001). Our preliminary efforts could not clarify the detailed mechanism of H-IL-6–activated neuronal differentiation. For future experiments, any interference with the STATs and MAPK pathways has to take into account the complex interplay between growth and differentiation factors acting on the same signaling pathways which may lead to proliferation or differentiation depending only on different dynamics of MAPK/ERK activation (Kholodenko, 2007).
In addition, a recent report has shown that STAT-3 is not only acting as a transcription factor in the nucleus but might be also essential for neuronal differentiation because cytoplasmic STAT-3 modulates the microtubules network by binding to the COOH-terminal tubulin-interacting domain of stathmin and thereby antagonizing its microtubules destabilization activity (Ng et al., 2006). Thus, a complete down-regulation of STAT-3 expression (for instance by siRNA) my also affect neurogenesis. Because the cytoplasmic and nuclear functions of STAT-3 appear to be independent of one another, inhibition of (nuclear) p-STAT-3 may prevent gliogenesis while cytoplasmic STAT-3 is still able to support IL-6–mediated neurogenesis.
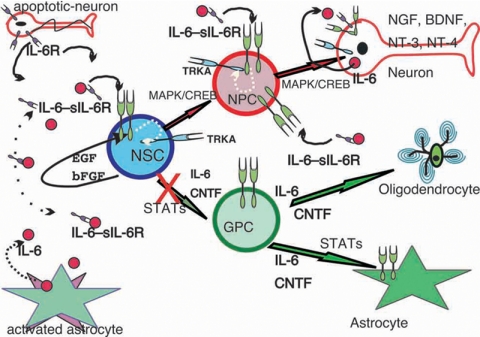
Early reports have described highest expression of both IL-6 and IL-6R within the hippocampus/dentate gyrus area of the adult mouse brain nearby zones of active continuous neurogenesis (Gadient and Otten, 1995; Otten et al., 2001; Kempermann and Gage, 1999; Brazel and Rao, 2004; Ming and Song, 2005). Potential cellular sources have been described as astrocytes for IL-6 and neurons for IL-6 and IL-6R (Gadient and Otten, 1995, 1997). Moreover, in the CNS IL-6 has the capability to act as proinflammatory cytokine or neurotrophic factor (Gadient and Otten, 1997; Otten et al., 2001). Thus, it is tempting to speculate that, during early stages of neurodegenerative diseases, apoptotic neurons my release the soluble IL-6R via an active shedding mechanism (similar as recently described for apoptotic neutrophils in the immune system; Chalaris et al., 2007; DeLeo, 2007), which in turn acts via _trans-_signaling with IL-6, released by activated astrocytes, on NSCs to induce neurogenesis (Figure 6). It would be of interest to see whether in vivo an intrinsic mechanism exists that could inhibit gliogenesis but enhances neurogenesis if activated by this trans-signaling pathway. The up-regulated gp130 receptor may sensitize NSCs as part of an amplification loop in this _trans-_signaling cascade (Klouche et al., 1999).
Figure 6.
IL-6—and depending on the presence of its soluble IL-6R—induces neural stem cells (NSCs) differentiation into astrocytes [via glia progenitor cells (GPC), pathway indicated by green arrows] and neurons [via neuronal progenitor cells (NPC), pathway indicated by red arrows]. During neurodegenerative processes apoptotic neurons release sIL-6R (purple receptor symbols) by shedding, whereas activated astrocytes release IL-6 (red circle). Via _trans-_signaling they can act on NSCs, which express (as GPCs and NPCs) very low or no IL-6 receptors but the common signaling receptor subunit gp130 (shown as green receptor-dimer symbols) that is used by all members of the IL-6-receptor family (Taga and Kishimoto, 1997; Heinrich et al., 2003). Although EGF and bFGF maintain NSCs' self-renewal, the IL-6–sIL-6R complex overrides those activities mediating NSCs differentiation. In contrast, classical neurotrophic factors such as NGF, BDNF, or NT-3 are unable to overcome EGF signaling. Increased gp130 expression on NSCs/NPCs and IL-6R-NGFR/TRKA (blue receptor symbols) transactivation by IL-6R/gp130 (white dotted line) enhances neuronal differentiation. Differentiated neurons express then various neurotrophic factors for their survival and proper functioning. Inhibiting the glia-differentiation pathway by, for instance, p-STAT-inhibitors (inhibition of nuclear transcription factor activity of p-STATs but not complete down-regulation of their expression) may turn H-IL-6 into an interesting potential therapeutic factor, acting on the MAPK/CREB signaling pathway, for the treatment of neurodegenerative diseases.
Moreover, the associative function between the IL-6R complex and the NGF receptor kinase TRKA has been described previously as “transactivation” (without its ligand NGF) of TRKA (Sterneck et al., 1996; Chao, 2003; Sorkin, 2005). Thus, IL-6–induced TRKA transactivation, as described in our study, might be one additional mechanism through which neuronal differentiation of NSCs is initiated.
Concluding, similar to the wide range of activities the classical neurotrophins show in the nervous and immune systems (Ayyadhury and Heese, 2007), IL-6 may also play a crucial role in the CNS (Balschun et al., 2004; Chiaretti et al., 2008) with sIL-6R as a pivotal regulator of regenerative processes and thus displaying H-IL-6 as potential neurotrophic survival and differentiation factor that might be useful, for instance in combination with inhibitors of the p-STAT–signaling pathways, for therapeutic approaches in various clinical applications.
ACKNOWLEDGMENTS
We thank Dr. Reto Gadient (AstraZeneca Pharmaceuticals, Wilmington, DE) for helpful suggestions and critical reading of the manuscript. This study was supported by A*STAR Grant BMRC/04/1/22/19/360 to K.H.
Footnotes
REFERENCES
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Ayyadhury S., Heese K. Neurotrophins—more than neurotrophic. Curr. Immunol. Rev. 2007;3:189–215. [Google Scholar]
- Badache A., Hynes N. E. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 2001;61:383–391. [PubMed] [Google Scholar]
- Balschun D., Wetzel W., Del Rey A., Pitossi F., Schneider H., Zuschratter W., Besedovsky H. O. Interleukin-6, a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Blaskovich M. A., Sun J., Cantor A., Turkson J., Jove R., Sebti S. M. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signalling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Bonni A., Sun Y., Nadal-Vicens M., Bhatt A., Frank D. A., Rozovsky I., Stahl N., Yancopoulos G. D., Greenberg M. E. Regulation of gliogenesis in the central nervous system by the JAK-STAT signalling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Brazel C. Y., Rao M. S. Aging and neuronal replacement. Ageing Res. Rev. 2004;3:465–483. doi: 10.1016/j.arr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Abraham C. R., Masliah F., Kemper P., Inglis J. D., Oldstone M.B.A., Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin-6. Proc. Natl. Acad. Sci. USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos L. S. Neurospheres: insights into neural stem cell biology. J. Neurosci. Res. 2004;78:761–769. doi: 10.1002/jnr.20333. [DOI] [PubMed] [Google Scholar]
- Chalaris A., Rabe B., Paliga K., Lange H., Laskay T., Fielding C. A., Jones S. A., Rose-John S., Scheller J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signalling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- Chan K. S., Sano S., Kiguchi K., Anders J., Komazawa N., Takeda J., DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J. Clin. Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chiaretti A., Antonelli A., Mastrangelo A., Pezzotti P., Tortorolo L., Tosi F., Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J. Neurotrauma. 2008;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- DeLeo F. R. Attractive shedding. Blood. 2007;110:1711–1712. doi: 10.1182/blood-2007-06-096677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin S., Heath J. K., DeJong-Curtain T. A., Hogan B. M., Lieschke G. J., Malaterre J., Ramsay R. G., Mantamadiotis T. CREB activity modulates neural cell proliferation, midbrain-hindbrain organization and patterning in zebrafish. Dev. Biol. 2007;307:127–141. doi: 10.1016/j.ydbio.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Fischer M., Goldschmitt J., Peschel C., Kallen K. J., Brakenhoff J.P.J., Wollmer A., Grötzinger J., Rose-John S. A designer cytokine with activity on human hematopoietic progenitor cells. Nat. Biotech. 1997;15:142–145. doi: 10.1038/nbt0297-142. [DOI] [PubMed] [Google Scholar]
- Gadient R. A., Otten U. Interleukin-6 and interleukin-6 receptor mRNA expression in rat central nervous system. Ann. NY Acad. Sci. 1995;762:403–406. doi: 10.1111/j.1749-6632.1995.tb32348.x. [DOI] [PubMed] [Google Scholar]
- Gadient R. A., Otten U. Interleukin-6 (IL-6): a molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997;521:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Gage F. H. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Giachino C., De Marchis S., Giampietro C., Parlato R., Perroteau I., Schutz G., Fasolo A., Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D. L., Nelson T. E. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Gu F., Hata R., Ma Y. J., Tanaka J., Mitsuda N., Kumon Y., Hanakawa Y., Hashimoto K., Nakajima K., Sakanaka M. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J. Neurosci. Res. 2005;81:163–171. doi: 10.1002/jnr.20561. [DOI] [PubMed] [Google Scholar]
- Hakkoum D., Stoppini L., Muller D. Interleukin-6 promotes sprouting and functional recovery in lesioned organotypic hippocampal slice cultures. J. Neurochem. 2007;100:747–757. doi: 10.1111/j.1471-4159.2006.04257.x. [DOI] [PubMed] [Google Scholar]
- Heese K., Yamada T., Akatsu H., Yamamoto T., Kosaka K., Nagai Y., Sawada T. Characterization of the new transcription-regulator-protein p60TRP. J. Cell. Biochem. 2004;91:1030–1042. doi: 10.1002/jcb.20010. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Behrmann I., Haan S., Hermann H. M., Muller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himi T., Okazaki T., Mori N. SCG10 mRNA localization in the hippocampus: comparison with other mRNAs encoding neuronal growth-associated proteins (nGAPs) Brain Res. 1994;655:177–185. doi: 10.1016/0006-8993(94)91612-8. [DOI] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Horiuchi S., Topley N., Yamamoto N., Fuller G. M. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Richards P. J., Scheller J., Rose-John S. IL-6 transsignalling: the in vivo consequences. J. Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim. Biophys. Acta. 2002;1592:251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- Josselyn S. A., Nguyen P. V. CREB, synapses and memory disorders: past progress and future challenges. Curr. Drug Targets CNS Neurol. Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- Kahn M. A., De Vellis J. Regulation of an oligodendrocyte progenitor cell line by the interleukin-6 family of cytokines. Glia. 1994;12:87–98. doi: 10.1002/glia.440120202. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F. H. New nerve cells for the adult brain. Sci. Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kholodenko B. N. Untangling the signalling wires. Nat. Cell Biol. 2007;9:247–249. doi: 10.1038/ncb0307-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama T., Yoneyama M., Tamaki K., Yoneda Y. Regulation of neuronal differentiation by N-methyl-D-aspartate receptors expressed in neural progenitor cells isolated from adult mouse hippocampus. J. Neurosci. Res. 2004;76:599–612. doi: 10.1002/jnr.20095. [DOI] [PubMed] [Google Scholar]
- Klouche M., Bhakdi S., Hemmes M., Rose-John S. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J. Immunol. 1999;163:4583–4589. [PubMed] [Google Scholar]
- März P., Cheng J. G., Gadient R. A., Patterson P. H., Stoyan T., Otten U., Rose-John S. Sympathetic neurons can produce and respond to interleukin 6. Proc. Natl. Acad. Sci. USA. 1998;95:3251–3256. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- März P., Heese K., Dimitriades-Schmutz B., Rose-John S., Otten U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26:191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- März P., Herget T., Lang E., Otten U., Rose-John S. Activation of gp130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur. J. Neurosci. 1997;9:2765–2773. doi: 10.1111/j.1460-9568.1997.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Ming G. L., Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Melchiorri D., Cappuccio I., Ciceroni C., Spinsanti P., Mosillo P., Sarichelou I., Sale P., Nicoletti F. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology. 2007;53:473–480. doi: 10.1016/j.neuropharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Molne M., Studer L., Tabar V., Ting Y. T., Eiden M. V., McKay R. D. Early cortical precursors do not undergo LIF-mediated astrocytic differentiation. J. Neurosci. Res. 2000;59:301–311. doi: 10.1002/(sici)1097-4547(20000201)59:3<301::aid-jnr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mori N., Morii H. SCG10-related neuronal growth-associated proteins in neural development, plasticity, degeneration, and aging. J. Neurosci. Res. 2002;70:264–273. doi: 10.1002/jnr.10353. [DOI] [PubMed] [Google Scholar]
- Morii H., Shiraishi-Yamaguchi Y., Mori N. SCG10, a microtubule destabilizing factor, stimulates the neurite outgrowth by modulating microtubule dynamics in rat hippocampal primary cultured neurons. J. Neurobiol. 2006;66:1101–1114. doi: 10.1002/neu.20295. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Niidome T., Matsuda S., Akaike A., Kihara T., Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur. J. Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Ng D. C., Lin B. H., Lim C. P., Huang G., Zhang T., Poli V., Cao X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J. Cell Biol. 2006;172:245–257. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi N., Ikeda O., Taira N., Yamamoto Y., Muromoto R., Sekine Y., Sugiyama K., Honjoh T., Matsuda T. LIF- and IL-6-induced acetylation of STAT3 at Lys-685 through PI3K/Akt activation. Biol. Pharm. Bull. 2007;30:1860–1864. doi: 10.1248/bpb.30.1860. [DOI] [PubMed] [Google Scholar]
- Okano H. Stem cell biology of the central nervous system. J. Neurosci. Res. 2002;69:698–707. doi: 10.1002/jnr.10343. [DOI] [PubMed] [Google Scholar]
- Otten U., März P., Heese K., Hock C., Kunz D., Rose-John S. Signals regulating neurotrophin expression in glial cells. Prog. Brain Res. 2001;132:545–554. doi: 10.1016/S0079-6123(01)32102-7. [DOI] [PubMed] [Google Scholar]
- Peltier J., O'Neill A., Schaffer D. V. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev. Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- Qiu Z., Parsons K. L., Gruol D. L. Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. J. Neurosci. 1995;15:6688–6699. doi: 10.1523/JNEUROSCI.15-10-06688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z., Sweeney D. D., Netzeband J. G., Gruol D. L. Chronic interleukin-6 alters NMDA receptor-mediated membrane responses and enhances neurotoxicity in developing CNS neurons. J. Neurosci. 1998;18:10445–10456. doi: 10.1523/JNEUROSCI.18-24-10445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan P., McKay R.D.G. Multiple routes to astrocytic differentiation in the CNS. J. Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A., Rietze R. L. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Neurath M. F. IL-6 trans-signalling: the heat is on. Immunity. 2004;20:2–4. doi: 10.1016/s1074-7613(04)00003-2. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Scheller J., Elson G., Jones S. A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Rose-John S., Waetzig G. H., Scheller J., Grotzinger J., Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- Sands W. A., Palmer T. M. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Taga T., Matsuda T., Hirano T., Kishimoto T., Kaziro Y. Induction of neural differentiation in PC12 cells by B cell stimulatory factor 2/interleukin 6. Mol. Cell. Biol. 1988;8:3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer K. H., Mestres P., März P., Rose-John S. The IL-6/sIL-6R fusion protein hyper-IL-6 promotes neurite outgrowth and neuron survival in cultured enteric neurons. J. Interferon Cytokine Res. 1999;19:527–532. doi: 10.1089/107999099313974. [DOI] [PubMed] [Google Scholar]
- Scheller J., Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med. Microbiol. Immunol. 2006;195:173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- Shimazaki T., Shingo T., Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J. Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T. J. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A. TRKing signals through the Golgi. Sci. STKE. 2005;267:1–3. doi: 10.1126/stke.2672005pe1. [DOI] [PubMed] [Google Scholar]
- Sterneck E., Kaplan D. R., Johnson P. F. Interleukin-6 induces expression of peripherin and cooperates with Trk receptor signalling to promote neuronal differentiation in PC12 cells. J. Neurochem. 1996;67:1365–1374. doi: 10.1046/j.1471-4159.1996.67041365.x. [DOI] [PubMed] [Google Scholar]
- Sugaya K. Possible use of autologous stem cell therapies for Alzheimer's disease. Curr. Alzheimer Res. 2005;2:367–376. doi: 10.2174/1567205054367919. [DOI] [PubMed] [Google Scholar]
- Taga T., Fukuda S. Role of IL-6 in the neural stem cell differentiation. Clin. Rev. Allergy Immunol. 2005;28:249–256. doi: 10.1385/CRIAI:28:3:249. [DOI] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Tanne J. H. Activating stem cells may treat Alzheimer's. Br. Med. J. 2005;330:622. doi: 10.1136/bmj.330.7492.622-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier M., März P., Otten U., Weis J., Rose-John S. Interleukin-6 (IL-6) and its soluble receptor support survival of sensory neurons. J. Neurosci. Res. 1999;55:411–422. doi: 10.1002/(SICI)1097-4547(19990215)55:4<411::AID-JNR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wagner J. A. Is IL-6 both a cytokine and a neurotrophic factor? J. Exp. Med. 1996;183:2417–2419. doi: 10.1084/jem.183.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R., Weller M. Signalling from cAMP/PKA to MAPK and synaptic plasticity. Mol. Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- Yokota T., Mishra M., Akatsu H., Tani Y., Miyauchi T., Yamamoto T., Kosaka K., Nagai Y., Sawada T., Heese K. Brain site-specific gene expression analysis in Alzheimer's disease patients. Eur. J. Clin. Invest. 2006;36:820–830. doi: 10.1111/j.1365-2362.2006.01722.x. [DOI] [PubMed] [Google Scholar]