Caloric Restriction Stimulates Revascularization in Response to Ischemia via Adiponectin-mediated Activation of Endothelial Nitric-oxide Synthase (original) (raw)
Abstract
Caloric restriction (CR) can extend longevity and modulate the features of obesity-related metabolic and vascular diseases. However, the functional roles of CR in regulation of revascularization in response to ischemia have not been examined. Here we investigated whether CR modulates vascular response by employing a murine hindlimb ischemia model. Wild-type (WT) mice were randomly divided into two groups that were fed either ad libitum (AL) or CR (65% of the diet consumption of AL). Four weeks later, mice were subjected to unilateral hindlimb ischemic surgery. Body weight of WT mice fed CR (CR-WT) was decreased by 26% compared with WT mice fed AL (AL-WT). Revascularization of ischemic hindlimb relative to the contralateral limb was accelerated in CR-WT compared with AL-WT as evaluated by laser Doppler blood flow and capillary density analyses. CR-WT mice had significantly higher plasma levels of the fat-derived hormone adiponectin compared with AL-WT mice. In contrast to WT mice, CR did not affect the revascularization of ischemic limbs of adiponectin-deficient (APN-KO) mice. CR stimulated the phosphorylation of endothelial nitric-oxide synthase (eNOS) in the ischemic limbs of WT mice. CR increased plasma adiponectin levels in eNOS-KO mice but did not stimulate limb perfusion in this strain. CR-WT mice showed enhanced phosphorylation of AMP-activated protein kinase (AMPK) in ischemic muscle, and administration of AMPK inhibitor compound C abolished CR-induced increase in limb perfusion and eNOS phosphorylation in WT mice. Our observations indicate that CR can promote revascularization in response to tissue ischemia via an AMPK-eNOS-dependent mechanism that is mediated by adiponectin.
Obesity is closely associated with the development of metabolic syndrome and type 2 diabetes (1), which contribute to microvascular rarefaction and impaired collateral vessel growth under ischemic conditions (2–4). These conditions lead to increased vulnerability to ischemic injury and impaired wound healing and promote the progression of cardiovascular diseases. Thus, therapeutic approaches that enhance revascularization could be beneficial for ischemic vascular disease.
Caloric restriction (CR)4 has been shown to extend the life span of multiple species by retarding the aging process (5). In obese subjects CR has been shown to reduce visceral fat accumulation and also decrease body weight (6). CR have also been reported to lead to a reduction of hyperglycemia and hyperlipidemia that are major risk factors for ischemic heart diseases (7–12). A number of experimental studies have shown that CR attenuates atherosclerotic lesion formation (11), pathological cardiac hypertrophy (13), and ischemia-induced myocardial damage (14). These findings suggest that CR counteracts the unfavorable features of obese complications. However, the consequences of CR on vascular responses to tissue ischemia have not been examined.
Adipose tissue secretes a variety of bioactive molecules, referred to as adipokines, that directly affect obesity-linked disorders in remote organs (15). Adiponectin is an adipokine that is down-regulated in obesity-linked diseases, including ischemic heart disease and peripheral arterial disease (16,17). Adiponectin exerts protective actions on a variety of metabolic and cardiovascular disorders, including insulin resistance, atherosclerosis, vascular dysfunction, and cardiac injury (18). CR has been shown to markedly increase circulating levels of adiponectin (14,19). Thus, it is plausible that adiponectin mediates the salutary actions of CR in the setting of obesity-linked vascular complications. Here we investigated whether CR modulates the process of ischemia-induced revascularization in vivo. We first examined the effect of CR on revascularization using a mouse model of vascular insufficiency. We also examined the potential contribution of adiponectin to CR-mediated revascularization process in ischemic muscles. Our observations indicate that CR promotes revascularization in response to tissue ischemia via modulation of adiponectin production.
EXPERIMENTAL PROCEDURES
_Materials_—Endothelial nitric-oxide synthase (eNOS) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-eNOS (Ser-1177), phospho-AMPK (Thr-172), and pan-α-AMPK antibody were purchased from Cell Signaling Technology (Beverly, MA). Tubulin antibody was from Oncogene (Cambridge, MA). Adenovirus vectors containing the gene for β-galactosidase (Ad-βgal) and full-length mouse adiponectin (Ad-APN) were described previously (20). Compound C was purchased from Calbiochem.
_CR Protocols_—Studies using wild-type (WT), eNOS-deficient (eNOS-KO), and adiponectin-deficient (APN-KO) mice in a C57/BL6 background were approved by the Institutional Animal Care and Use Committee at Nagoya University. Male mice at the ages of 6 weeks were housed in individual cages and fed ad libitum (AL) on a normal chow for 2 weeks. Food was provided at the same time (3:00 p.m.), and food intake of individual mice was measured daily. The average value of caloric intake was calculated from daily food intake for 2 weeks. After that, mice were randomly divided into two groups. The AL group was fed ad libitum for an additional 4 weeks. CR mice were fed with 65% of the average caloric intake of control AL diet for the next 4 weeks. At 4 weeks after the CR or control AL diet, mice were subjected to unilateral hindlimb surgery. All mice were weighed at weekly intervals. Heart rate (HR) and systolic blood pressure were determined using a tail-cuff pressure analysis system in the conscious state.
_Mouse Model of Revascularization_—Mice were subjected to unilateral hindlimb surgery under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally). In this model, the entire left femoral artery and vein were removed surgically as described previously (21). In some experiments, 2 × 108 plaque-forming units (pfu) of Ad-APN or Ad-βgal were systemically injected into the jugular vein of mice 3 days before the ischemic hindlimb surgery (22). In some experiments, we intraperitoneally injected NOS inhibitor l-NAME (20 mg/kg/day) dissolved in phosphate-buffered saline or vehicle (phosphate-buffered saline) into WT mice 1 day prior to surgery and daily until sacrifice. In some experiments, AMPK inhibitor compound C (20 mg/kg/3 times per week) dissolved in dimethyl sulfoxide (DMSO) or vehicle (DMSO) was intraperitoneally injected into the abdomen of WT mice 1 day before the operation until sacrifice (23,24).
_Analysis of Hindlimb Blood Flow_—Hindlimb blood flow was determined using a laser Doppler blood flow (LDBF) analyzer (Moor LDI; Moor Instruments, Devon, UK). LDBF analyses were performed on legs and feet before surgery and on postoperative days 3, 7, and 14. Blood flow was shown as changes in the laser frequency using different color pixels. Quantitative analysis of blood flow was expressed as the ratio of left (ischemic) to right (nonischemic) LDBF to avoid data variations because of ambient light and temperature (22).
_Measurement of Capillary Density_—Capillary density in adductor muscle was assessed by immunohistochemical analysis. Muscle samples were embedded in OCT compound (Miles Scientific, Elkhart, IN) and snap-frozen in liquid nitrogen. Tissue slices (7 μm in thickness) were prepared and stained with CD31 (PECAM-1, BD Biosciences) followed by treatment with fluorescein isothiocyanate-conjugated secondary antibody to detect CD31. The signals were detected and analyzed by fluorescence microscopy. Capillary endothelial cells were quantified by measuring the number of CD31-positive cells per high power field (×400), and the number of capillaries per muscle fiber in 15 randomly chosen microscopic fields from three different sections in each tissue block (22).
_Western Blot Analysis_—Tissue samples obtained on postoperative day 7 were homogenized in lysis buffer containing 20 mm Tris-HCl (pH 8.0), 1% Nonidet P-40, 150 mm NaCl, 0.5% deoxycholic acid, 1 mm sodium orthovanadate, and protease inhibitor mixture (Sigma). Protein content was determined by the Bradford method. The same amounts of protein (50 μg) were separated with denaturing SDS-10% polyacrylamide gels. The membranes were immunoblotted with the primary antibodies at a 1:1000 dilution followed by the secondary antibody conjugated with horseradish peroxidase at a 1:5000 dilution. Bands were visualized using ECL Western blotting detection kit (Amersham Biosciences).
_Measurement of Plasma Parameters_—Total cholesterol, high density lipoprotein cholesterol, and glucose levels were measured with enzymatic kits (Wako Chemicals, Richmond, VA). Insulin levels were measured with electroimmunoassay kit (Wako Chemicals). Adiponectin levels were determined using enzyme-linked immunosorbent assay kits (Otsuka Pharmaceutical Co Ltd., Tokyo, Japan). Blood was collected by heart puncture from mice on 4 weeks of CR.
_Statistical Analysis_—Data are presented as mean ± S.E. Statistical analysis was performed by analysis of variance followed by Scheffe's F test. A value of p < 0.05 was accepted as statistically significant.
RESULTS
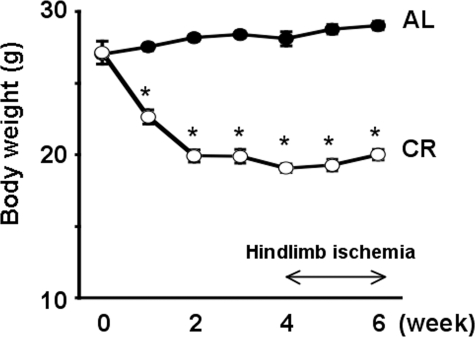
_Effect of CR on Body Weight in WT Mice_—A linear reduction in body weight occurred during the first 2 weeks of CR, and the reduction in body weight was maintained between 2 and 6 weeks (Fig. 1). The WT mice fed AL (AL-WT mice) showed a moderate increase in body weight of 7.3% over this time course. At the end of the 6-week time course, the difference in body weight between AL-WT mice and WT mice fed CR diet (CR-WT mice) was 26% (Fig. 1). HR and systolic blood pressure did not differ between the two groups (Table 1). Consistent with previous reports (12,25), there was a significant reduction in total cholesterol, triglyceride, and plasma glucose levels in the CR-WT mice compared with AL-WT mice.
FIGURE 1.
Effect of CR on body weight of WT mice. The CR was 65% of the AL diet fed to control. Values presented are the mean ± S.E. of six mice per group. *, p < 0.01 versus AL-WT mice.
TABLE 1.
Characteristics of AL-WT and CR-WT mice Blood samples were obtained from WT mice fed AL or CR diet for 4 weeks (n = 6). Each value is mean ± S.E. HR indicates heart rate (beats/min); sBP, systolic blood pressure (mm Hg); TC, total cholesterol (mg/dl); HDL-C, high density lipoprotein cholesterol (mg/dl); PG, plasma glucose (mg/dl); TG, triglyceride.
| Diet | HR | sBP | TC | HDL-C | TG | PG |
|---|---|---|---|---|---|---|
| AL | 640 ± 22 | 104 ± 6 | 77.6 ± 1.5 | 54.9 ± 1.9 | 112.4 ± 7.0 | 95.3 ± 10.1 |
| CR | 605 ± 12 | 101 ± 7 | 69.3 ± 3.0a | 52.6 ± 1.3 | 93.4 ± 2.4a | 70.6 ± 4.4a |
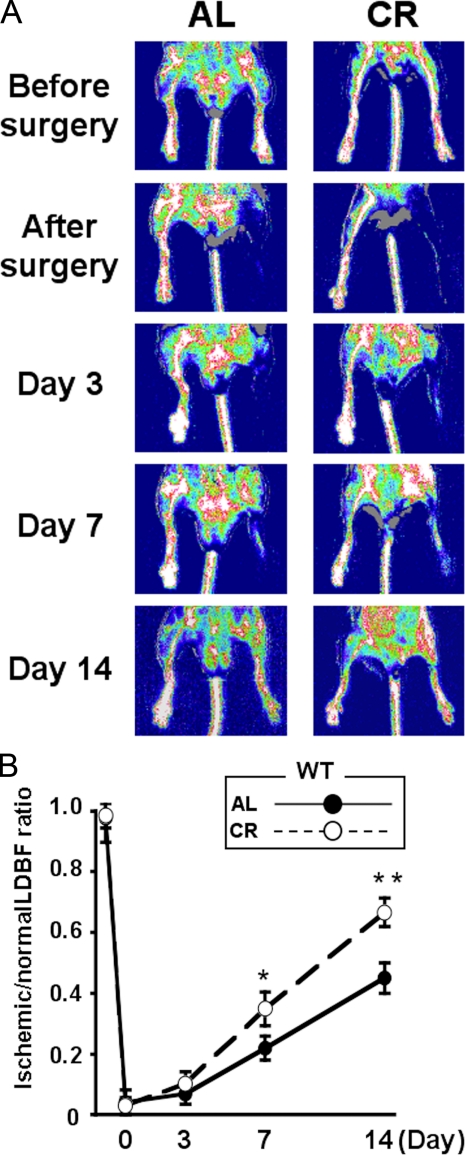
_CR Promotes Revascularization in Response to Tissue Ischemia in WT Mice_—AL-WT and CR-WT mice underwent surgical induction of unilateral hindlimb ischemia 4 weeks after the initiation of diets. All mice survived and appeared healthy during the follow-up period.Fig. 2_A_ shows representative LDBF images of hindlimb blood flow before surgery and at different time points after surgery in the AL- and CR-WT mice. In AL-WT mice, hindlimb perfusion fell precipitously after surgery, increased to 20–30% of the nonischemic limb by day 7, and ultimately returned to 50% of the nonischemic limb by day 14 (Fig. 2_B_). CR-WT mice showed a significant increase in limb flow at 7 and 14 days after hindlimb surgery compared with AL-WT mice (Fig. 2_B_) (p < 0.05; n = 6).
FIGURE 2.
Caloric restriction improves perfusion of ischemic limbs in WT mice. A, representative LDBF images showing improved perfusion in ischemic limb of WT-fed AL or CR. A low perfusion signal (dark blue) was observed in the ischemic hindlimb of AL-WT mice, whereas a high perfusion signal (white to red) was detected in CR-WT mice on post-operative day 7 and 14. B, quantitative analysis of ischemic/nonischemic LDBF ratio in AL-WT and CR-WT mice on postoperative day 3, 7, and 14. *, p < 0.05; **, p < 0.01 versus AL-WT mice; n = 6.
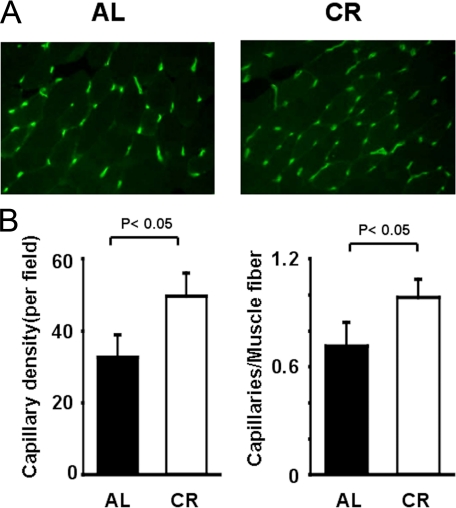
To investigate the extent of revascularization at the microcirculatory level, capillary density was measured in histological sections harvested from the ischemic tissues. Representative photomicrographs of CD31-stained ischemic muscles are shown in Fig. 3_A_. Quantitative analysis revealed that the capillary density was significantly increased in CR-WT mice compared with AL-WT mice at 14 days after surgery (Fig. 3_B_).
FIGURE 3.
Increased capillary density in ischemic CR-WT mice. A, fluorescence staining of ischemic tissues with anti-CD31 monoclonal antibody (green) on post-operative day 14. B, quantitative analysis of capillary density in AL-WT and CR-WT mice on post-operative day 14 (n = 6 in each group). Capillary density was expressed as the number of capillaries per high power field (×400, left) and capillaries per muscle fiber (right).
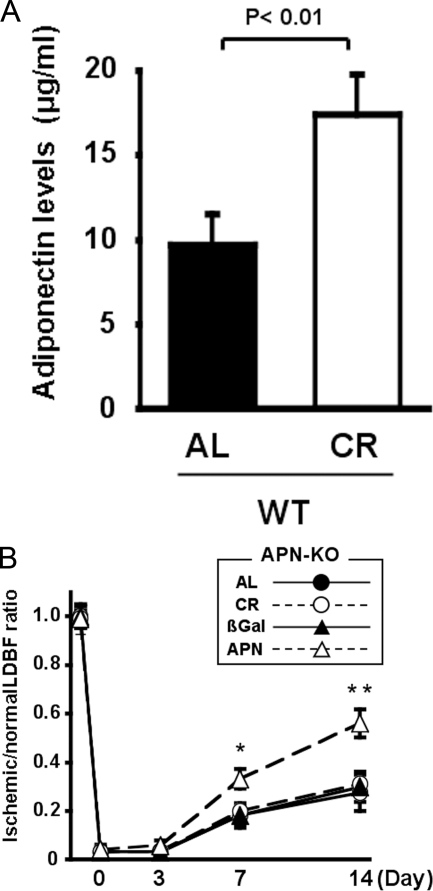
_Role of Adiponectin in CR-mediated Revascularization_—CR has been shown to increase serum levels of adiponectin (14,19), and we have shown that adiponectin promotes blood flow recovery in response to ischemia (22). These findings led us to hypothesize that the increase in circulating adiponectin levels contributes to the improved vascular response in the CR-treated animals. We assessed plasma adiponectin levels in WT treated with AL or CR. At the time of surgery, CR increased plasma adiponectin levels to a level 1.8 times higher compared with AL-WT mice (9.7 ± 1.8 in AL-WT mice and 17.4 ± 2.4 μg/ml in CR-WT mice, p < 0.05) (Fig. 4_A_). To examine the involvement of adiponectin in ischemia-induced revascularization caused by CR, we investigated the effect of CR on blood flow recovery of ischemic muscles of APN-KO mice on postoperative day 0, 3, 7, and 14. AL-APN-KO mice had a reduced blood flow of ischemic limbs in agreement with our previous work (22). Importantly, CR did not affect ischemic limb perfusion of APN-KO mice after surgery as compared with AL-APN-KO (Fig. 4_B_). In contrast, the treatment of AL-APN-KO mice with an adenovirus expressing adiponectin (Ad-APN) led to a significant increase in plasma adiponectin levels. In this experiment Ad-APN is delivered systemically 3 days prior to surgery. This procedure leads to transduction of the liver, and vector-encoded adiponectin can be detected in serum (26). Whereas circulating levels of adiponectin are not detectable in AL-APN-KO mice and Ad-βgal-treated AL-APN-KO mice, Ad-APN treatment increased circulating levels to 9.1 ± 2.2 μg/ml in AL-APN-KO mice at day 7 after surgery that is similar to AL-WT mice. This restoration of circulating adiponectin led to an enhancement of limb perfusion after hindlimb surgery (Fig. 4_B_). Collectively, these data suggest that the stimulant effect of CR on ischemia-induced revascularization is mediated by the up-regulation of adiponectin.
FIGURE 4.
Effects of CR on ischemia-induced revascularization in APN-KO mice. A, plasma adiponectin levels in AL-WT and CR-WT mice. Blood samples were obtained from WT mice fed AL or CR for 4 weeks (n = 5).B, quantitative analysis of ischemic/nonischemic LDBF ratio in AL or CR-APN-KO mice and AL-APN-KO mice treated with Ad-APN or Ad-βgal on post-operative day 0, 3, 7, and 14 (n = 5). The adenoviral vector expressing adiponectin (Ad-APN) or Ad-βgal at 2 × 108 pfu total was delivered intravenously to AL-APN-KO mice via the jugular vein 3 days before ischemic surgery (*, p < 0.05; **, p < 0.01 versus Ad-βgal).
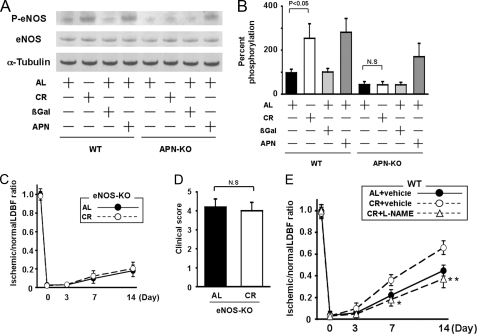
_eNOS Activation Is Essential for CR-induced Revascularization_— eNOS plays an important role in revascularization following hindlimb ischemia (21). To analyze the potential involvement of eNOS in CR-induced revascularization, the expression and phosphorylation of eNOS in ischemic adductor muscle were assessed by Western blot analysis. The expression of total eNOS protein in ischemic muscles did not differ between AL and CR-mice. However, phosphorylation of eNOS at Ser-1177 in ischemic muscle was significantly greater in CR-WT mice than in AL-WT mice (Fig. 5, A and_B_). To test the possible contribution of adiponectin to regulation of eNOS activation by CR, we assessed the phosphorylation of eNOS in ischemic tissue of APN-KO mice treated with AL or CR. In contrast to the stimulatory effects of CR on eNOS phosphorylation in WT mice, CR did not influence eNOS phosphorylation in APN-KO mice (Fig. 5, A and_B_).
FIGURE 5.
CR promotes eNOS phosphorylation in response to tissue ischemia. A, representative immunoblots for phosphorylated eNOS (P-eNOS) and eNOS in WT and APN-KO mice fed AL or CR, and AL-WT or AL-APN-KO mice treated with Ad-APN or Ad-βgal. Western immunoblots with the indicated antibodies were performed on the ischemic adductor muscle at 7 days after surgery. Ad-APN or Ad-βgal at 2 × 108 pfu total was intravenously injected into AL-WT and AL-APN-KO mice 3 days before the induction of hindlimb ischemia. B, quantitative analysis of relative changes in phosphorylation of eNOS in WT and APN-KO mice fed AL or CR, and AL-WT or AL-APN-KO mice treated with Ad-APN or Ad-βgal. Phosphorylation of eNOS was normalized to the tubulin signal and expressed as percentage of the signal intensity of AL-WT mice (n = 4). C, quantitative analysis of ischemic/nonischemic LDBF ratio in AL-eNOS-KO or CR-eNOS-KO mice on post-operative day 0, 3, 7, and 14 (n = 5).D, clinical score in AL-eNOS-KO or CR-eNOS-KO mice as determined by an index of severity of limb ischemia. (0 = normal, 1 = pale foot or gait abnormalities, 2 = less than half of foot is necrotic,3 = more than half of foot is necrotic without lower leg necrosis,4 = more than half of foot is necrotic with some lower leg necrosis,5 = necrosis or auto amputation of entire lower limb.) E, quantitative analysis of ischemic/nonischemic LDBF ratio in AL or CR-WT mice in the presence of l-NAME or vehicle on post-operative day 0, 3, 7, and 14 (*, p < 0.05; **, p < 0.01 versus CR+vehicle; n = 5).
To test whether increased expression of adiponectin affects phosphorylation of eNOS in ischemic muscle, Ad-βgal or Ad-APN was intravenously delivered to APN-KO and WT mice. Circulating adiponectin levels were 9.9 ± 2.1 μg/ml in wild-type/Ad-βgal, 18.1 ± 3.6 μg/ml in wild-type/Ad-APN, <0.05 μg/ml in APN-KO/Ad-βgal, and 9.1 ± 2.2 μg/ml in APN-KO/Ad-APN on postoperative day 7. Treatment with Ad-APN significantly increased eNOS phosphorylation in ischemic muscle of WT and APN-KO mice at day 7 after surgery (Fig. 5,A and B).
To further analyze the involvement of eNOS signaling in revascularization by CR, we examined the impact of CR on blood flow of ischemic muscles in eNOS-KO mice. CR-eNOS-KO mice had increased plasma adiponectin levels compared with AL-eNOS-KO mice (8.1 ± 1.4 in AL-eNOS-KO mice and 15.1 ± 2.3 μg/ml in CR-eNOS-KO mice, respectively, p < 0.05). LDBF analysis revealed that no significant differences in limb perfusion were observed between AL-eNOS-KO and CR-eNOS-KO mice on postoperative day 0, 3, 7, and 14 (Fig. 5_C_). Because eNOS-KO mice exhibit severe ischemia-induced vascular insufficiency, which is accompanied by amputation, we assessed lower limb function and tissue salvage post-surgery using a clinical scoring system (27). Similarly, the index of severity of tissue ischemia after hindlimb surgery did not differ between AL-eNOS-KO and CR-eNOS-KO mice (Fig. 5_D_). We also analyzed the effect of CR on blood flow recovery of ischemic muscles in WT mice receiving NOS inhibitorl-NAME or vehicle. Although vehicle-treated CR-WT mice showed increased recovery of blood flow compared with vehicle-treated AL-WT mice, treatment with l-NAME abrogated the increase in limb perfusion recovery in CR-WT mice (Fig. 5_E_). Collectively, these data suggest that CR-stimulated revascularization is attributed to eNOS activation that is involved in adiponectin production.
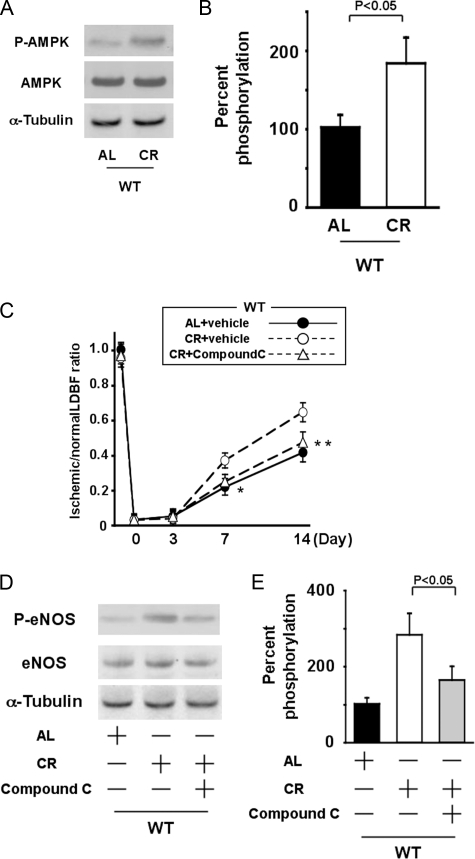
_Role of AMPK in CR-induced Revascularization_—To examine the possible participation of AMPK in CR-induced revascularization, the expression and phosphorylation of AMPK in ischemic adductor muscle were assessed by Western blot analysis. The expression of total AMPK protein in ischemic muscles did not differ between AL and CR mice. However, phosphorylation of AMPK in ischemic muscle was significantly greater in CR-WT mice than in AL-WT mice at day 7 after the operation (Fig. 6,A and B). To further analyze the involvement of AMPK in CR-induced revascularization, we examined the effect of CR on blood flow recovery of ischemic muscles in WT mice receiving AMPK inhibitor compound C or vehicle. CR-WT mice receiving compound C or vehicle had increased plasma adiponectin levels compared with AL-WT mice receiving vehicle (8.3 ± 1.6 in vehicle-treated AL-WT mice, 16.1 ± 2.4 in vehicle-treated CR-WT mice, and 15.4 ± 2.4 μg/ml in compound C-treated CR-WT mice, respectively). Treatment of CR-WT mice with compound C blocked increased limb perfusion caused by CR compared with vehicle-treated CR-WT mice (Fig. 6_C_). Furthermore, treatment with compound C significantly diminished CR-induced increase in eNOS phosphorylation in ischemic muscle tissue (Fig. 6, D and_E_). These data suggest that AMPK is involved in CR-induced eNOS activation and revascularization.
FIGURE 6.
AMPK signaling is required for CR-induced revascularization and eNOS phosphorylation in response to tissue ischemia. A, representative immunoblots for phosphorylated AMPK (P-AMPK) and AMPK in AL-WT and CR-WT mice on day 7 after induction of limb ischemia. Western immunoblots with the indicated antibodies were performed on the ischemic adductor muscle.B, quantitative analysis of relative changes in phosphorylation of AMPK in AL-WT and CR-WT mice on day 7 after induction of limb ischemia. Phosphorylation of AMPK was normalized to the tubulin signal and expressed as percentage of the signal intensity of AL-WT mice (n = 4). C, quantitative analysis of ischemic/nonischemic LDBF ratio in AL or CR-WT mice in the presence of compound C or vehicle on post-operative day 0, 3, 7, and 14 (*, p < 0.05; **, p < 0.01_versus CR_+vehicle; n = 5). D, representative immunoblots for phosphorylated eNOS (P-eNOS) and eNOS in AL-WT or CR-WT mice in the presence or absence of compound C on day 7 after induction of limb ischemia. E, quantitative analysis of relative changes in phosphorylation of eNOS in AL-WT or CR-WT mice in the presence or absence of compound C. Phosphorylation of eNOS was normalized to the tubulin signal and expressed as percentage of the signal intensity of AL-WT mice (n = 4).
DISCUSSION
This study provides evidence that CR promotes ischemia-induced revascularization in a well established hindlimb model. CR enhanced blood flow recovery and capillary formation in ischemic muscle of wild-type mice, which was accompanied by increased levels of eNOS phosphorylation and plasma adiponectin. The stimulatory actions of CR on reperfusion and eNOS phosphorylation of ischemic limbs were abolished in APN-KO mice. Furthermore, CR did not improve flow recovery in ischemic limbs of eNOS-KO mice.
The ability of CR to increase adiponectin levels is likely to contribute to the stimulation of revascularization under our experimental conditions. We have shown that adiponectin overexpression will accelerate revascularization of ischemic limbs in wild-type mice (22). Our observations here show that CR increased plasma adiponectin levels 1.8-fold in wild-type mice, in agreement with previous reports (14,19). CR promoted ischemia-induced revascularization in wild-type mice but not APN-KO mice. Because adiponectin promotes vascular cell function and survival under conditions of stress (22,28), we propose that adiponectin functions as an important mediator of CR-stimulated vascular responses. Recently, it was reported that CR confers resistance to myocardial ischemia-reperfusion injury by increasing adiponectin levels (14). CR also improves the survival and myocardial damage in obese mice with viral myocarditis, which is accompanied by increased adiponectin levels in plasma and myocardium (29). Thus, the up-regulation of adiponectin by CR could represent a common mechanism in the protection of cardiovascular tissues to stress.
It is well established that eNOS is beneficial for various types of vascular and metabolic diseases (21,30,31). In this study, CR increased the activating phosphorylation of eNOS in ischemic muscles, and the ability of CR to enhance revascularization was abrogated in eNOS-KO mice or WT mice receiving NOS inhibitor. Thus, CR appears to promote revascularization in ischemic states through its ability to activate eNOS. It is reported that CR induces mitochondrial biogenesis in various tissues of mice, and these effects are diminished in eNOS-KO mice (32). Collectively, these observations suggest that eNOS acts as a key mediator of the protective actions of CR.
It has been shown that adiponectin exerts vascular protection through modulation of eNOS signaling. We have shown that adiponectin stimulates phosphorylation of eNOS, which is associated with enhanced endothelial cell migration and differentiation into capillary-like structures (28,33,34). Adiponectin stimulates nitric oxide production by endothelial cells via the activating phosphorylation of eNOS (33,35,36). Recently, we have shown that APN-KO mice develop increased cerebral ischemia-reperfusion injury compared with WT mice, which is accompanied by reduced eNOS activation in ischemic cerebral tissue (37). Conversely, administration of adiponectin reduces cerebral infarct size and stimulates eNOS phosphorylation in ischemic brain. Of importance, the cerebroprotective actions of adiponectin were diminished in eNOS-KO mice, suggesting that eNOS is required for salutary vascular responses to adiponectin in ischemic brain. Consistent with these observations, this study shows that APN-KO mice have reduced phosphorylation of eNOS in ischemic muscles. The CR-induced increase in eNOS phosphorylation during ischemia was abolished in APN-KO mice. Furthermore, despite the increased plasma adiponectin in eNOS-KO mice following CR, CR had no effects on perfusion recovery of ischemic limbs in eNOS-KO mice. Taken together, these data provide evidence that the vasculo-protective actions of CR are medicated, at least in part, through the adiponectin-eNOS regulatory axis.
Our data show that AMPK is required for CR-stimulated revascularization in ischemic tissue. CR stimulated the phosphorylation of AMPK in the ischemic tissue, and administration of an AMPK inhibitor abrogated CR-induced increase in ischemia-induced revascularization and eNOS phosphorylation. AMPK is reported to directly phosphorylate eNOS at Ser-1177 (38). It has also been shown that adiponectin stimulates phosphorylation of eNOS in endothelial cells through its ability to activate AMPK (33,36). AMPK activation has also been shown to stimulate vascular endothelial growth factor production in ischemic muscle (39). However, vascular endothelial growth factor levels in ischemic muscles did not differ between AL and CR-WT mice (data not shown), suggesting that the beneficial effect of CR on revascularization is not mediated by the angiogenic properties of this cytokine. Although other proangiogenic cytokines may be involved in CR-induced revascularization, we favor the hypothesis that CR-mediated increase in adiponectin has a salutary effect on the vasculature under conditions of ischemic stress by promoting AMPK-eNOS signaling and thereby promotes the revascularization response.
Obesity-linked diseases, including type 2 diabetes, cause increased mortality and morbidity of coronary and peripheral artery diseases because of microvascular rarefaction and impaired collateral vessel growth under ischemic conditions (2,40,41). The findings reported here suggest that CR could favor revascularization in response to tissue ischemia. Thus, nutritional approaches aimed at limiting caloric intake could be useful for treatment of ischemic heart and limb diseases.
*
This work was supported, in whole or in part, by National Institutes of Health Grants HL86785, HL91949, and HL81587 (to K. W.). This work was also supported by a grant-in-aid for young scientists A, The Nakashima Foundation, and The Aichi D.R.G. Foundation (to R. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
4
The abbreviations used are: CR, caloric restriction; AL, ad libitum; WT, wild-type; KO, knockout; eNOS, endothelial nitric-oxide synthase; HR, heart rate; LDBF, laser Doppler blood flow; AMO PK, AMP-activated protein kinase; pfu, plaque-forming unit; NOS, nitric-oxide synthase; Ad-APN, adenovirus expressing adiponectin.
References
- 1.Reilly, M. P., and Rader, D. J. (2003) Circulation 108 1546-1551 [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz, M. B., Biyikoglu, S. F., Akin, Y., Guray, U., Kisacik, H. L., and Korkmaz, S. (2003) Int. J. Obes. Relat. Metab. Disord. 27 1541-1545 [DOI] [PubMed] [Google Scholar]
- 3.Schiekofer, S., Galasso, G., Sato, K., Kraus, B. J., and Walsh, K. (2005) Arterioscler. Thromb. Vasc. Biol. 25 1603-1609 [DOI] [PubMed] [Google Scholar]
- 4.Tirziu, D., Moodie, K. L., Zhuang, Z. W., Singer, K., Helisch, A., Dunn, J. F., Li, W., Singh, J., and Simons, M. (2005) Circulation 112 2501-2509 [DOI] [PubMed] [Google Scholar]
- 5.Ingram, D. K., Anson, R. M., de Cabo, R., Mamczarz, J., Zhu, M., Mattison, J., Lane, M. A., and Roth, G. S. (2004) Ann. N. Y. Acad. Sci. 1019 412-423 [DOI] [PubMed] [Google Scholar]
- 6.Fontana, L., and Klein, S. (2007) J. Am. Med. Assoc. 297 986-994 [Google Scholar]
- 7.Zimmerman, J., Kaufmann, N. A., Fainaru, M., Eisenberg, S., Oschry, Y., Friedlander, Y., and Stein, Y. (1984) Arteriosclerosis 4 115-123 [DOI] [PubMed] [Google Scholar]
- 8.Verdery, R. B., and Walford, R. L. (1998) Arch. Intern. Med. 158 900-906 [DOI] [PubMed] [Google Scholar]
- 9.Verdery, R. B., Ingram, D. K., Roth, G. S., and Lane, M. A. (1997) Am. J. Physiol. 273 E714-E719 [DOI] [PubMed] [Google Scholar]
- 10.Lane, M. A., Tilmont, E. M., De Angelis, H., Handy, A., Ingram, D. K., Kemnitz, J. W., and Roth, G. S. (2000) Mech. Ageing Dev. 112 185-196 [DOI] [PubMed] [Google Scholar]
- 11.Guo, Z., Mitchell-Raymundo, F., Yang, H., Ikeno, Y., Nelson, J., Diaz, V., Richardson, A., and Reddick, R. (2002) Mech. Ageing Dev. 123 1121-1131 [DOI] [PubMed] [Google Scholar]
- 12.Mahoney, L. B., Denny, C. A., and Seyfried, T. N. (2006) Lipids Health Dis. 5 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour, E. M., Parikh, R. V., Singer, A. A., and Bolling, S. F. (2006) J. Mol. Cell. Cardiol. 41 661-668 [DOI] [PubMed] [Google Scholar]
- 14.Shinmura, K., Tamaki, K., Saito, K., Nakano, Y., Tobe, T., and Bolli, R. (2007) Circulation 116 2809-2817 [DOI] [PubMed] [Google Scholar]
- 15.Lago, F., Dieguez, C., Gomez-Reino, J., and Gualillo, O. (2007) Nat. Clin. Pract. Rheumatol. 3 716-724 [DOI] [PubMed] [Google Scholar]
- 16.Ouchi, N., Kihara, S., Funahashi, T., Matsuzawa, Y., and Walsh, K. (2003) Curr. Opin. Lipidol. 14 561-566 [DOI] [PubMed] [Google Scholar]
- 17.Iwashima, Y., Horio, T., Suzuki, Y., Kihara, S., Rakugi, H., Kangawa, K., Funahashi, T., Ogihara, T., and Kawano, Y. (2006) Atherosclerosis 188 384-390 [DOI] [PubMed] [Google Scholar]
- 18.Ouchi, N., and Walsh, K. (2007) Clin. Chim. Acta 380 24-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu, M., Miura, J., Lu, L. X., Bernier, M., DeCabo, R., Lane, M. A., Roth, G. S., and Ingram, D. K. (2004) Exp. Gerontol. 39 1049-1059 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda, M., Shimomura, I., Sata, M., Arita, Y., Nishida, M., Maeda, N., Kumada, M., Okamoto, Y., Nagaretani, H., Nishizawa, H., Kishida, K., Komuro, R., Ouchi, N., Kihara, S., Nagai, R., Funahashi, T., and Matsuzawa, Y. (2002) J. Biol. Chem. 277 37487-37491 [DOI] [PubMed] [Google Scholar]
- 21.Murohara, T., Asahara, T., Silver, M., Bauters, C., Masuda, H., Kalka, C., Kearney, M., Chen, D., Symes, J. F., Fishman, M. C., Huang, P. L., and Isner, J. M. (1998) J. Clin. Investig. 101 2567-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata, R., Ouchi, N., Kihara, S., Sato, K., Funahashi, T., and Walsh, K. (2004) J. Biol. Chem. 279 28670-28674 [DOI] [PubMed] [Google Scholar]
- 23.Li, P., Kondo, T., Numaguchi, Y., Kobayashi, K., Aoki, M., Inoue, N., Okumura, K., and Murohara, T. (2008) Hypertension 51 252-258 [DOI] [PubMed] [Google Scholar]
- 24.McCullough, L. D., Zeng, Z., Li, H., Landree, L. E., McFadden, J., and Ronnett, G. V. (2005) J. Biol. Chem. 280 20493-20502 [DOI] [PubMed] [Google Scholar]
- 25.Stein, O., Dabach, Y., Halperin, G., Ben-Naim, M., and Stein, Y. (2003) Biochem. Biophys. Res. Commun. 308 29-34 [DOI] [PubMed] [Google Scholar]
- 26.Shibata, R., Sato, K., Kumada, M., Izumiya, Y., Sonoda, M., Kihara, S., Ouchi, N., and Walsh, K. (2007) Cardiovasc. Res. 74 471-479 [DOI] [PubMed] [Google Scholar]
- 27.Yu, J., deMuinck, E. D., Zhuang, Z., Drinane, M., Kauser, K., Rubanyi, G. M., Qian, H. S., Murata, T., Escalante, B., and Sessa, W. C. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 10999-11004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi, H., Ouchi, N., Kihara, S., Walsh, K., Kumada, M., Abe, Y., Funahashi, T., and Matsuzawa, Y. (2004) Circ. Res. 94 e27-e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanda, T., Saegusa, S., Takahashi, T., Sumino, H., Morimoto, S., Nakahashi, T., Iwai, K., and Matsumoto, M. (2007) Int. J. Cardiol. 119 310-318 [DOI] [PubMed] [Google Scholar]
- 30.Williams, I. L., Wheatcroft, S. B., Shah, A. M., and Kearney, M. T. (2002) Int. J. Obes. Relat. Metab. Disord. 26 754-764 [DOI] [PubMed] [Google Scholar]
- 31.Li, H., Wallerath, T., Munzel, T., and Forstermann, U. (2002) Nitric Oxide 7 149-164 [DOI] [PubMed] [Google Scholar]
- 32.Nisoli, E., Tonello, C., Cardile, A., Cozzi, V., Bracale, R., Tedesco, L., Falcone, S., Valerio, A., Cantoni, O., Clementi, E., Moncada, S., and Carruba, M. O. (2005) Science 310 314-317 [DOI] [PubMed] [Google Scholar]
- 33.Ouchi, N., Kobayashi, H., Kihara, S., Kumada, M., Sato, K., Inoue, T., Funahashi, T., and Walsh, K. (2004) J. Biol. Chem. 279 1304-1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata, R., Skurk, C., Ouchi, N., Galasso, G., Kondo, K., Ohashi, T., Shimano, M., Kihara, S., Murohara, T., and Walsh, K. (2008) FEBS Lett. 582 1607-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng, K. K., Lam, K. S., Wang, Y., Huang, Y., Carling, D., Wu, D., Wong, C., and Xu, A. (2007) Diabetes 56 1387-1394 [DOI] [PubMed] [Google Scholar]
- 36.Chen, H., Montagnani, M., Funahashi, T., Shimomura, I., and Quon, M. J. (2003) J. Biol. Chem. 278 45021-45026 [DOI] [PubMed] [Google Scholar]
- 37.Nishimura, M., Izumiya, Y., Higuchi, A., Shibata, R., Qiu, J., Kudo, C., Shin, H. K., Moskowitz, M. A., and Ouchi, N. (2008) Circulation 117 216-223 [DOI] [PubMed] [Google Scholar]
- 38.Chen, Z. P., Mitchelhill, K. I., Michell, B. J., Stapleton, D., Rodriguez-Crespo, I., Witters, L. A., Power, D. A., Ortiz de Montellano, P. R., and Kemp, B. E. (1999) FEBS Lett. 443 285-289 [DOI] [PubMed] [Google Scholar]
- 39.Ouchi, N., Shibata, R., and Walsh, K. (2005) Circ. Res. 96 838-846 [DOI] [PubMed] [Google Scholar]
- 40.Abaci, A., Oguzhan, A., Kahraman, S., Eryol, N. K., Unal, S., Arinc, H., and Ergin, A. (1999) Circulation 99 2239-2242 [DOI] [PubMed] [Google Scholar]
- 41.Yarom, R., Zirkin, H., Stammler, G., and Rose, A. G. (1992) J. Pathol. 166 265-270 [DOI] [PubMed] [Google Scholar]