Pyk2- and Src-Dependent Tyrosine Phosphorylation of PDK1 Regulates Focal Adhesions (original) (raw)
Abstract
3-Phosphoinositide-dependent protein kinase 1 (PDK1) is a signal integrator that activates the AGC superfamily of serine/threonine kinases. PDK1 is phosphorylated on tyrosine by oxidants, although its regulation by agonists that stimulate G-protein-coupled receptor signaling pathways and the physiological consequences of tyrosine phosphorylation in this setting have not been fully identified. We found that angiotensin II stimulates the tyrosine phosphorylation of PDK1 in vascular smooth muscle in a calcium- and c-Src-dependent manner. The calcium-activated tyrosine kinase Pyk2 acts as a scaffold for Src-dependent phosphorylation of PDK1 on Tyr9, which permits phosphorylation of Tyr373 and -376 by Src. This critical function of Pyk2 is further supported by the observation that Pyk2 and tyrosine-phosphorylated PDK1 colocalize in focal adhesions after angiotensin II stimulation. Importantly, infection of smooth muscle cells with a Tyr9 mutant of PDK1 inhibits angiotensin II-induced tyrosine phosphorylation of paxillin and focal adhesion formation. These observations identify a novel interaction between PDK1 and Pyk2 that regulates the integrity of focal adhesions, which are major compartments for integrating signals for cell growth, apoptosis, and migration.
Focal adhesions (FAs) are specialized sites of cell attachment to the extracellular matrix where transmembrane integrins link the extracellular matrix to the actin cytoskeleton (17, 44). Recent work has shown that they also represent key centers of signal integration for receptor tyrosine kinases and G-protein-coupled receptors (GPCR), whose assembly and disassembly can regulate various signal transduction pathways. Among the proteins localized to FAs are the multifunctional Src family kinases, focal adhesion kinase, and the serine/threonine kinase p21-activated kinase (PAK), as well as the scaffolding proteins paxillin and Shc and the actin binding proteins vinculin and talin (17). Dynamic turnover of FAs is critically important in the modulation of growth, survival, and gene expression as well as in responses involving cell motility, such as migration and mitosis (17, 44). Gaining insight into the mechanisms regulating the assembly and disassembly of FAs is thus central to understanding the control of many cellular functions.
3-Phosphoinositide-dependent protein kinase 1 (PDK1) was originally identified as the activator of the cell survival kinase Akt/PKB (2, 47). Subsequently, PDK1 has been shown to be a more versatile kinase and is involved in a variety of cell functions, including protein synthesis, cell survival, glucose metabolism, and cell adhesion and migration. PDK1 represents a pivotal point of divergence, leading to the activation of multiple members of the AGC superfamily of serine/threonine kinases, including Akt/PKB, protein kinase C (PKC), PKC-related protein kinases (PRK and PKN), serum- and glucocorticoid-induced protein kinase, p70 ribosomal protein S6 kinase (p70S6K), p90 ribosomal protein S6 kinase (RSK), and PAK (50, 54). Of these proteins, PAK and Akt/PKB have been shown to be involved in actin cytoskeletal reorganization (10, 35), an important mechanism of FA regulation.
The regulation of PDK1 activity, its subcellular localization, and its interactions with other proteins have been intense areas of investigation. Although it was originally thought to be constitutively active, recent studies have suggested that it can be stimulated by extracellular agonists such as the growth factor insulin (9) and the oxidants hydrogen peroxide (39) and peroxovanadate (38, 39). In cells transfected with tagged PDK1 constructs, PDK1 is found in the cytosol and translocates to the membrane upon activation with platelet-derived growth factor (3) and insulin (16), where it interacts with its downstream targets such as Akt (3, 16). However, the basal and agonist-stimulated subcellular localization of endogenous PDK1 is unknown. Casamayor et al. (8) showed that constitutive phosphorylation of PDK1 on Ser241 is critical for kinase activity, but recent results suggest that PDK1 can also be phosphorylated on tyrosine (Tyr) residues, leading to an increase in activity (38, 39). In response to peroxovanadate, three tyrosine residues, Tyr9, Tyr373, and Tyr376, are phosphorylated (24, 38). Surprisingly, there have been no reports showing a regulation of PDK1 by activation of GPCRs.
There are several similarities between the functional activity of PDK1 and the response of vascular smooth muscle cells (VSMCs) to angiotensin II (Ang II), a peptide hormone that exerts its effects through the GPCR AT1 receptor. Ang II has numerous effects on VSMCs, such as contraction, hypertrophy, extracellular matrix deposition, and migration (19). Importantly, Ang II activates many of the substrates of PDK1, including Akt/PKB (51), PKC (23, 33), p70S6K (14), RSK (48), and PAK (46). Furthermore, Ang II activates c-Src as well as several other tyrosine kinases (5). One of these, the calcium-dependent tyrosine kinase Pyk2 (also designated CADTK/RAFTK/CAKbeta), is of particular interest because of its demonstrated involvement in many of the same physiological responses as PDK1, including regulation of cytoskeleton-associated proteins. These observations suggest that Ang II may activate PDK1 and that VSMCs may provide an ideal system in which to investigate the physiological importance of potential mechanisms of PDK1 activation.
In this study, we demonstrate that in VSMCs, Ang II induces tyrosine phosphorylation of PDK1 in a calcium- and Src-dependent fashion. We identify the calcium-dependent tyrosine kinase Pyk2 as a critical mediator of PDK1 phosphorylation by Ang II and show that Pyk2 physically interacts with PDK1, leading to tyrosine phosphorylation via activation of c-Src. Both Pyk2 and tyrosine-phosphorylated PDK1 localize in FAs after Ang II stimulation. The phosphotyrosine-dependent activation of PDK1 by Pyk2 appears to be involved in the formation of FAs, possibly in part through regulation of paxillin phosphorylation, suggesting that this interaction may be centrally important for controlling signaling events involved in cell growth, apoptosis, and migration.
MATERIALS AND METHODS
Materials.
Sheep anti-PDK1 antibody, antiphosphotyrosine antibody (clone 4G10), the PDK1 immunoprecipitation kinase assay kit PDKtide, and expression vectors for constitutively active c-Src and dominant-negative c-Src were obtained from Upstate Biotechnology (Lake Placid, N.Y.). Anti-PKB kinase/PDK1 and paxillin monoclonal antibodies were from Transduction Laboratories (Lexington, Ky.). Antiphosphopaxillin antibody was from Cell Signaling (Beverly, Mass.). Protein A/G Plus-agarose and anti-Myc-tag antibody (clone 9E10) were from Santa Cruz Biotechnology (Santa Cruz, Calif.). BAPTA/AM [1,2-bis(_o_-aminophenoxy)ethane-N,N,_N_′,_N_′-tetraacetic acid tetra(acetoxymethyl) ester] was from Calbiochem Corporation (San Diego, Calif.). PentaHis antibody and Effectene and Polyfect transfection reagents were from QIAGEN (Valencia, Calif.). Lipofectin was purchased from Life Technologies, Inc. (Gaithersburg, Md.). Rhodamine Red X-conjugated goat anti-mouse immunoglobulin G and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G were from Jackson ImmunoResearch (West Grove, Pa.). [γ-32P]ATP (3,000 Ci/mmol) was from NEN Life Science Products (Wilmington, Del.). All other chemicals and reagents, including antivinculin mouse monoclonal antibody and Dulbecco's modified Eagle's medium (DMEM) with 25 mM HEPES and 4.5 g of glucose per liter, were from Sigma (St. Louis, Mo.).
The expression vector for wild-type (WT) Myc-tagged human PDK1 (Myc-PDK1) was a kind gift of D. R. Alessi (University of Dundee, Dundee, United Kingdom) and was described previously (1). Expression vectors for WT Pyk2, a kinase-inactive (KI) Pyk2 mutant (lysine 457 to alanine [K457A]), and an autophosphorylation-site mutant (tyrosine 402 to phenylalanine [Y402F]) were kindly provided by W. Xiong (University of Alabama at Birmingham) (56). Phospho-PDK1-specific antibodies and Y9F mutant PDK1 expression vector were described previously (38). Pyk2 antisense oligonucleotides and scrambled control oligonucleotides were custom-made by Biognostik (Goetingen, Germany) as described previously (41) and were a kind gift of P. Lucchesi (University of Alabama at Birmingham).
Cell culture.
VSMCs were isolated from male Harlan Sprague-Dawley rat thoracic aortas by enzymatic digestion as described previously (22). Cells were grown in DMEM supplemented with 10% calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. For experiments, cells between passages 6 and 17 were used at confluence.
Chinese hamster ovary cells (CHO/AT1) were permanently transfected with the rat angiotensin AT1 receptor cDNA (36) in pCEP4 by use of Lipofectin. Transfected cells were selected based on hygromycin B resistance, and AT1 receptor binding was measured as described previously (28). These cells express 2,747 fmol of AT1 receptor binding sites per mg of protein. Cells were maintained in DMEM supplemented with 20 μg of l-proline per ml, 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 600 μg of hygromycin B per ml.
HEK293 cells were purchased from Clontech (Palo Alto, Calif.) and cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Oligonucleotide transfection of VSMCs.
Scrambled or antisense Pyk2 oligonucleotides were transfected into VSMCs as described previously (41).
Detection of PDK1 tyrosine phosphorylation.
Cells at 80 to 90% confluence in 100-mm-diameter dishes were made quiescent by incubation with DMEM containing 0.1% calf serum for 24 h. Cells were stimulated with agonist at 37°C in serum-free DMEM for the specified times. Some cells were preincubated with various inhibitors as indicated. After treatment, cells were lysed as described previously (51), and solubilized proteins were isolated by centrifugation and quantified by the Bradford assay. For immunoprecipitation, cell lysates were incubated with either sheep anti-PDK1 antibody or mouse anti-Myc antibody overnight, and the immunocomplex was collected with protein A/G-agarose beads for 2 h at 4°C with gentle rocking. The beads were washed with lysis buffer and boiled in 1× sodium dodecyl sulfate sample buffer. Proteins were separated by sodium dodecyl sulfate-9% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked at room temperature with phosphate-buffered saline (PBS) containing 5% nonfat dry milk and 0.1% Tween 20 for 1 h. Blots were incubated with antiphosphotyrosine antibody 4G10, and phosphorylated forms of proteins were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, N.J.). Band intensity was quantified by densitometry of immunoblots using NIH Image, version 1.61.
PDK1 kinase activity assay.
PDK1 activity was measured with the PDK1 immunoprecipitation kinase assay kit. Briefly, VSMC lysates were prepared and subjected to immunoprecipitation with anti-PDK1 antibody as described above. The kinase reaction was carried out by incubating the beads for 30 min at 30°C in kinase buffer containing [γ-32P]ATP mixed with a magnesium-ATP cocktail and 200 μM PDKtide (a specific substrate peptide for PDK1). After incubation, the reaction mixture was transferred onto P81 phosphocellulose paper and washed three times in 0.75% phosphoric acid and once in acetone, and radioactivity was measured by liquid scintillation counting.
Construction of expression vectors.
cDNAs for WT Pyk2, kinase-inactivated Pyk2, and autophosphorylation-site mutant Pyk2 were subcloned into the expression vector pcDNA4/HisMax (Clontech). Pyk2 proteins were thus expressed with a polyhistidine (His) tag. Y373F mutant PDK1 expression vector was made by using the Quick Change site-directed mutagenesis kit (Stratagene) with primers as follows: sense, 5′-CGA CGA GGA CTG CTT TGG CAA TTA TGA CAA TCT CC-3′; and antisense, 5′-GGA GAT TGT CAT AAT TGC CAA AGC AGT CCT CGT CG-3′ (underlining indicates mutated nucleotides). Introduction of the designed mutation was confirmed by sequencing.
Transient transfection of HEK293 cells.
HEK293 cells at 80% confluency in 6-well plates were transiently transfected with PDK1, c-Src, and Pyk2 expression vectors by use of Effectene transfection reagent. Cells were incubated for 6 h with the DNA-Effectine mixture, and then the medium was changed to serum-free DMEM. Thirty hours after transfection, cells were lysed and used for immunoprecipitation experiments.
Transient transfection of CHO/AT1 cells.
CHO/AT1 cells at 80% confluency in 60-mm-diameter dishes were transiently transfected with PDK1, c-Src, and Pyk2 expression vectors by use of Polyfect transfection reagent. Cells were incubated overnight with the Polyfect-DNA mixture, and then the medium was changed to serum-free DMEM. Thirty-six hours after transfection, cells were lysed as described above and lysates were subjected to immunoprecipitation as described above.
Preparation of recombinant adenoviruses and infection of VSMCs.
Recombinant adenoviruses to express Y9F PDK1 or WT PDK1 were prepared with the pAdEasy system as described previously (25, 29). VSMCs were infected with recombinant adenovirus at a multiplicity of infection of 100 in serum-free culture medium for 1 h with occasional shaking, washed twice with medium with 0.1% calf serum, and incubated for another 72 h.
Immunocytochemistry.
VSMCs plated onto 22-mm-diameter round glass coverslips were washed with ice-cold PBS and fixed in 4% paraformaldehyde. After washing, cells were permeabilized with 0.1% Triton X-100 and rinsed sequentially in PBS, 50 mM NH4Cl, and PBS. After incubating for 1 h in blocking buffer (3% bovine serum albumin in PBS), the cells were incubated with primary antibodies for 1 h, washed, and incubated for 1 h with corresponding secondary antibodies conjugated to either rhodamine Red X or fluorescein isothiocyanate. Cells on coverslips were mounted onto glass slides in a Vectashield apparatus and examined with a laser scanning confocal microscope (Bio-Rad; MRC 1024 system). Confocal images of VSMCs following vinculin staining were used to quantify the area of FA formation. NIH Image was used to measure the total area of a green fluorescent protein (GFP)-expressing cell, after which the area of vinculin expression in that same cell was determined. When comparing cells from different treatment groups, all image threshold settings of NIH Image remained constant. Data were expressed as percentages of vinculin staining relative to the total cell area.
Statistical analysis.
Results are expressed as means ± standard errors (SE). Statistical significance was assessed by analysis of variance. A P value of <0.05 was considered statistically significant.
RESULTS
Ang II-induced PDK1 tyrosine phosphorylation and upstream mediators.
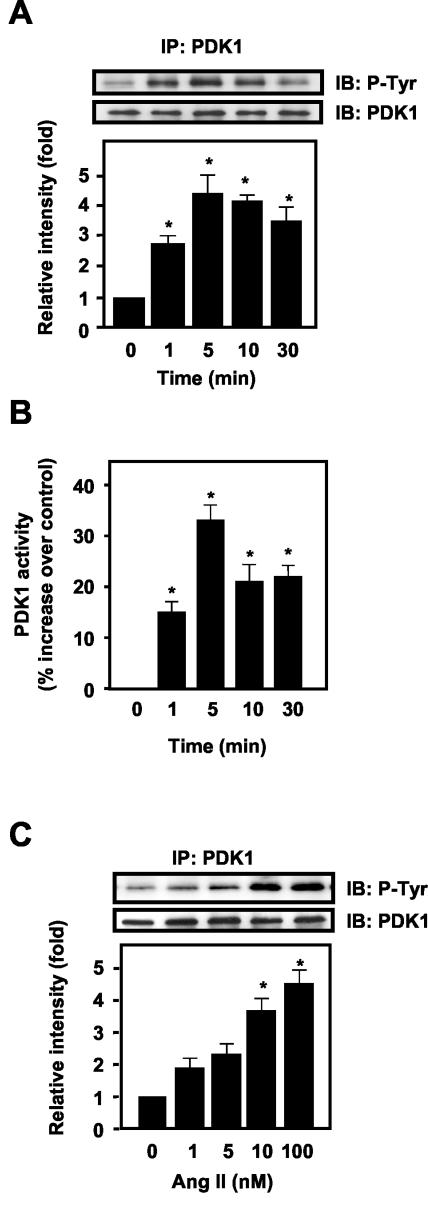
In VSMCs, Ang II induced a rapid, robust tyrosine phosphorylation of PDK1 (Fig. 1A) which peaked at 5 min (4.3 ± 0.9-fold) and then gradually decreased, remaining above the baseline for at least 30 min. This increase in tyrosine phosphorylation was accompanied by an increase in PDK1 activity, which was significantly elevated above control for 30 min (Fig. 1B). Ang II-induced tyrosine phosphorylation of PDK1 was dose dependent between 1 and 100 nM (Fig. 1C).
FIG.1.
Effect of Ang II on tyrosine phosphorylation of PDK1 in VSMCs. VSMCs were stimulated with Ang II for the indicated times and concentrations and prepared for immunoprecipitation analysis or PDK1 activity assay as described in Materials and Methods. (A) Cells were stimulated with 100 nM Ang II for the indicated times, immunoprecipitated (IP) with anti-PDK1 antibody, and analyzed by Western blot analysis (IB). (B) Immunoprecipitates prepared as for panel A were assayed for activity against the PDK1 substrate PDKtide. (C) VSMCs were stimulated with various concentrations of Ang II (1 to 100 nM) for 5 min. In panels A and C, the top panels are representative immunoblots of Ang II-induced tyrosine phosphorylation of PDK1. The bottom panels represent averaged data quantified by densitometry of immunoblots, expressed as fold increases in phosphorylation. In all panels, values are means ± SE for four independent experiments. *, P < 0.05 versus control.
In VSMCs, Ang II initiates calcium-dependent signal generation (21), transactivation of the epidermal growth factor receptor (EGF-R) (15), and activation of phosphatidylinositol 3-kinase (PI3-K)-dependent pathways (45). To define which of these signaling events mediates Ang II-induced tyrosine phosphorylation of PDK1, we examined the effects of specific inhibitors of the EGF-R tyrosine kinase or PI3-K, as well as of calcium chelation, on the response to Ang II. Neither the EGF-R kinase inhibitor AG1478 nor the PI3-K inhibitors LY294002 and wortmannin had an effect on PDK1 tyrosine phosphorylation by Ang II at concentrations previously shown to be effective in VSMCs (data not shown). In contrast, BAPTA/AM (20 μM for 30 min) markedly inhibited the ability of Ang II to induce phosphorylation of PDK1 on tyrosine (Fig. 2).
FIG. 2.
Role of intracellular calcium in Ang II-induced tyrosine phosphorylation in VSMCs. VSMCs were preincubated with BAPTA/AM (20 μM) for 30 min before exposure to Ang II (100 nM for 5 min). Top panels, representative immunoblots; bottom panels, averaged data quantified by densitometry of immunoblots, expressed as fold increases in phosphorylation. Values are means ± SE for three independent experiments.
Pyk2 is upstream of PDK1.
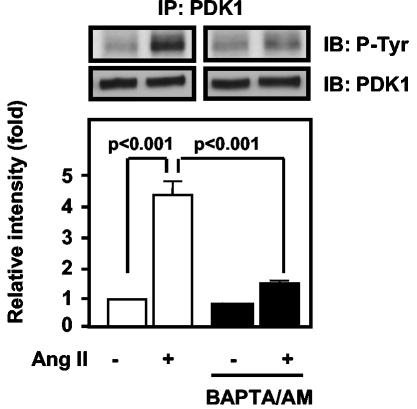
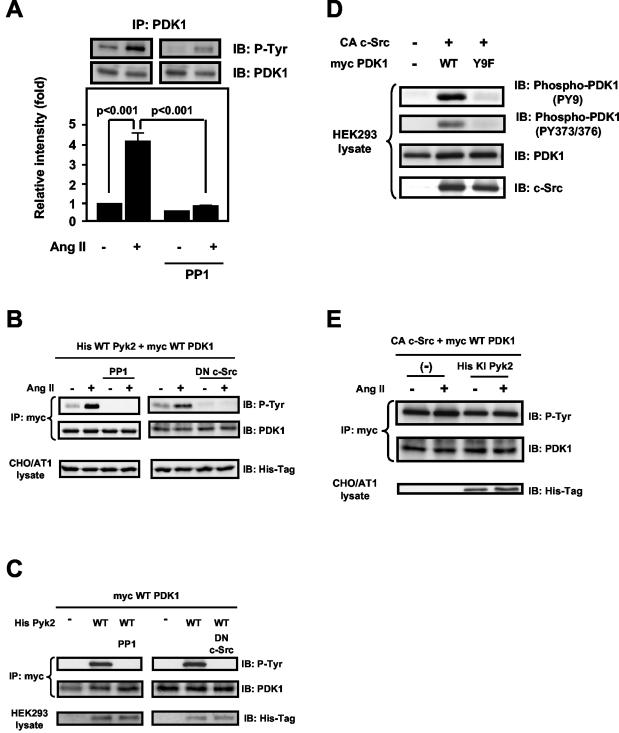
The inhibition of PDK1 tyrosine phosphorylation by BAPTA/AM suggests the possibility that a calcium-sensitive tyrosine kinase is an upstream mediator of PDK1 signaling. In VSMCs, the calcium-dependent tyrosine kinase Pyk2 is rapidly activated by Ang II and acts as a scaffold, coordinating downstream signaling events (13, 40, 42). We therefore hypothesized that Pyk2 is involved in PDK1 phosphorylation in response to Ang II. To investigate the role of Pyk2 in Ang II-induced PDK1 tyrosine phosphorylation, we downregulated Pyk2 protein expression by using antisense oligonucleotides. As shown in Fig. 3A, Pyk2 antisense oligonucleotides completely blocked PDK1 tyrosine phosphorylation in response to Ang II in VSMCs.
FIG. 3.
Role of Pyk2 in PDK1 tyrosine phosphorylation. (A) VSMCs were transfected with either no oligonucleotide (no oligo), scrambled oligonucleotide for Pyk2 (scrambled Pyk2), or antisense oligonucleotide for Pyk2 (AS Pyk2) and were stimulated with Ang II (100 nM for 5 min). Lysates were immunoprecipitated (IP) with anti-PDK1 antibody, followed by immunoblotting (IB) with antiphosphotyrosine antibody (P-Tyr) (top) or anti-PDK1 antibody (middle). Blots are representative of three independent experiments. (B) CHO/AT1 cells were cotransfected with the indicated expression vectors and treated with either 100 nM Ang II (+) or vehicle (−) for 5 min. Lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with antiphosphotyrosine antibody (P-Tyr) (top) or anit-PDK1 antibody (middle). Immunoblotting of lysates with anti-His antibody shows the expression of His-tagged Pyk2 proteins (bottom). (C) HEK293 cells were cotransfected with the indicated expression vectors, and lysates were subjected to immunoprecipitation with anti-Myc antibody, followed by immunoblotting with antiphosphotyrosine antibody (P-Tyr) (top) or anti-PDK1 antibody (middle). Immunoblotting of lysates with anti-His antibody shows the expression of His-tagged Pyk2 proteins (bottom). For all panels, immunoblots are representative of three independent experiments.
To determine if Pyk2 activation is necessary and sufficient for PDK1 tyrosine phosphorylation, we transfected CHO cells that stably express AT1 receptors (CHO/AT1) (36) and have very little endogenous Pyk2 (data not shown) with WT PDK1 and either WT Pyk2 or KI Pyk2 and examined the ability of Ang II to induce phosphorylation of PDK1. As previously demonstrated in HEK293 cells (11), overexpression of Pyk2 was sufficient to activate its intrinsic kinase activity (data not shown). Tyrosine phosphorylation of PDK1 could be observed only when WT, but not KI, Pyk2, was coexpressed, indicating that the kinase activity of Pyk2 was required for PDK1 phosphorylation (Fig. 3B). Treatment of cells with Ang II further enhanced the tyrosine phosphorylation of PDK1 in cells expressing WT Pyk2, suggesting that receptor activation recruits additional signaling components that can enhance PDK1 phosphorylation.
Pyk2 has both kinase and scaffolding functions (4). A major consequence of Pyk2 kinase activity is autophosphorylation at Tyr402, which then serves as a binding site for the SH2 domain of Src family kinases (11). To further confirm the requirement for Pyk2 kinase activity and to determine if autophosphorylation on Tyr402 is necessary for PDK1 tyrosine phosphorylation, we transfected HEK293 cells with WT PDK1 and either WT Pyk2, KI Pyk2, or the autophosphorylation-site mutant Pyk2 Y402F. Similar to our results in CHO/AT1 cells, WT, but not KI, Pyk2 phosphorylated PDK1 (Fig. 3C). Importantly, the Y402F Pyk2 mutant was unable to support PDK1 tyrosine phosphorylation, indicating that the scaffolding function of Pyk2 is required for PDK1 phosphorylation.
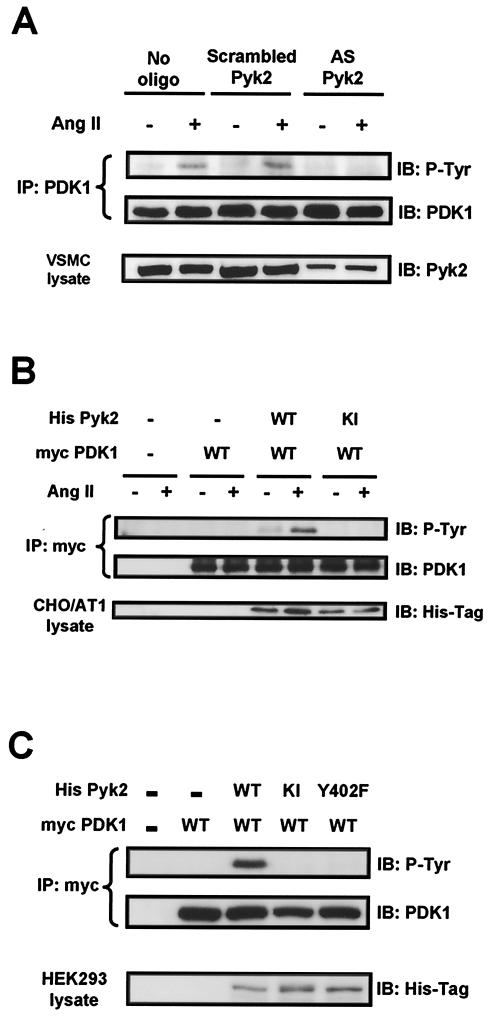
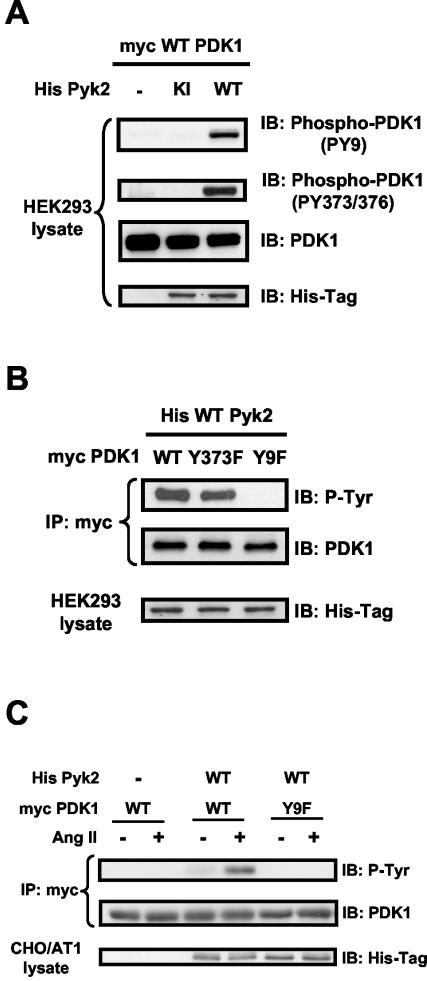
Pyk2 mediates PDK1 phosphorylation at tyrosines 9, 373, and 376.
PDK1 has been shown to be phosphorylated on Tyr9, Tyr373, and Tyr376 (38). To investigate whether Pyk2 is specifically required for phosphorylation of any of these tyrosines, we cotransfected HEK293 cells with Myc-tagged PDK1 and either WT or KI Pyk2. Using phosphorylation-site-specific antibodies, we found that overexpression of WT Pyk2 dramatically induced phosphorylation on both Tyr9 and Tyr373-Tyr376 of PDK1 (Fig. 4A). It has been shown previously that in vanadate-stimulated HEK293 cells, phosphorylation on Tyr9 of PDK1 is necessary for subsequent phosphorylation on Tyr373-Tyr376 (38). To determine if this is also true for Pyk2-dependent PDK1 phosphorylation, we expressed in HEK293 cells site-specific PDK1 mutants in which individual tyrosines were replaced with phenylalanines (Y373F PDK1 and Y9F PDK1). As shown in Fig. 4B, overexpression of Pyk2 failed to phosphorylate the Y9F PDK1 mutant, but the phosphorylation of Y373F PDK1 remained intact. Furthermore, mutation of Tyr9 eliminated the ability of Ang II to enhance Pyk2-dependent PDK1 phosphorylation (Fig. 4C), suggesting that activation of Pyk2 leads to the sequential phosphorylation of PDK1 on Tyr9 and Tyr373-Tyr376.
FIG.4.
Pyk2-dependent phosphorylation sites on PDK1. (A) HEK293 cells were cotransfected with the indicated expression vectors, and lysates were subjected to Western blot analysis (IB) with phosphorylation-site-specific anti-PDK1 antibodies (PY9 and PY373/376), total PDK-1 antibody, or anti-His antibody. (B) HEK293 cells were cotransfected with the indicated expression vectors, and lysates were subjected to immunoprecipitation (IP) with anti-Myc antibody, followed by immunoblotting with antiphosphotyrosine antibody (P-Tyr) or anti-PDK1 antibody. Separate aliquots of the lysates were immunoblotted with anti-His antibody to confirm equal expression of Pyk2. (C) CHO/AT1 cells were cotransfected with the indicated expression vectors and treated with either vehicle (−) or Ang II (+) (100 nM for 5 min). Lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with antiphosphotyrosine antibody (P-Tyr) (top) oranti-PDK1 antibody (middle). Immunoblotting of lysates with anti-His antibody demonstrates the expression of His-tagged Pyk2 proteins (bottom). For all panels, immunoblots are representative of three independent experiments.
Involvement of Src in Pyk2-dependent PDK1 phosphorylation.
It has been previously shown that the tyrosine phosphorylation of PDK1 is induced by expression of v-Src, which is a constitutively active form of Src (24, 38), and by c-Src in vitro (38, 39). In many cell systems, there is a close association between Src and Pyk2 (4). These observations led us to investigate whether Src mediates Pyk2-dependent PDK1 phosphorylation. In VSMCs stimulated with Ang II, the Src family kinase inhibitor PP1 (20 μM for 30 min) completely abolished PDK1 tyrosine phosphorylation (Fig. 5A). Similarly, in CHO/AT1 cells expressing WT Pyk2 and WT PDK1, both PP1 (10 μM for 30 min) and coexpression of dominant-negative c-Src eliminated basal and Ang II-stimulated PDK1 tyrosine phosphorylation (Fig. 5B). Identical results were obtained with HEK293 cells (Fig. 5C). Importantly, coexpression of constitutively active c-Src (CA-Src) and WT PDK1 led to tyrosine phosphorylation of PDK1 on both Tyr9 and Tyr373-Tyr376 (Fig. 5D). Mutation of Tyr9 abolished the ability of CA-Src to phosphorylate PDK1 at either site. These results are similar to those in Fig. 4 showing that Pyk2-dependent phosphorylation of Tyr373-Tyr376 is dependent upon prior Tyr9 phosphorylation. Furthermore, expression of CA-Src is sufficient to induce PDK1 tyrosine phosphorylation in the absence of Pyk2 and abolishes the need for Ang II (Fig. 5D and E). Consistent with these findings, expression of KI Pyk2 did not affect the ability of CA-Src to tyrosine phosphorylate PDK1 when coexpressed in CHO/AT1 cells (Fig. 5E). Taken together, these results clearly show that Src mediates Pyk2-dependent PDK1 phosphorylation and is likely the immediate upstream kinase responsible for PDK1 phosphorylation.
FIG.5.
Role of c-Src in Pyk2-dependent tyrosine phosphorylation of PDK1. (A) VSMCs were preincubated with PP1 (20 μM) for 30 min before exposure to Ang II (100 nM for 5 min). The lysates were immunoprecipitated (IP) with anti-PDK1 antibody, followed by immunoblotting (IB) with antiphosphotyrosine antibody (P-Tyr) or anti-PDK1 antibody. Top, representative immunoblots; bottom, averaged data quantified by densitometry of immunoblots, expressed as fold increases in phosphorylation. Values are means ± SE for three independent experiments. (B) CHO/AT1 cells were cotransfected with the indicated expression vectors and treated with either vehicle (−) or Ang II (+) (100 nM for 5 min). Left panels, some cells were pretreated with PP1 (10 μM for 30 min); right panels, some cells were transfected with dominant-negative (DN) c-Src together with WT His-Pyk2 and WT Myc-PDK1. Lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with antiphosphotyrosine antibody (P-Tyr) (top) or anti-PDK1 antibody (middle). Immunoblotting of lysates with anti-His antibody demonstrates the expression of His-tagged Pyk2 proteins (bottom). (C) HEK293 cells were cotransfected with the indicated expression vectors. Left panels, some cells were pretreated with PP1 (10 μM for 30 min); right panels, some cells were transfected with dominant-negative c-Src together with WT His-Pyk2 and WT Myc-PDK1. Cells were lysed and subjected to immunoprecipitation and immunoblotting. Immunoblotting of lysates with anti-His antibody demonstrates the expression of His-tagged Pyk2 proteins (bottom). (D) HEK293 cells were cotransfected with the indicated expression vectors and lysed, and the lysates were subjected to Western blot analysis. (E) CHO/AT1 cells were cotransfected with the indicated expression vectors and treated with either vehicle (−) or Ang II (+) (100 nM for 5 min). Lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with the indicated antibodies. All panels show representative immunoblots of three independent experiments.
PDK1 physically associates with Pyk2.
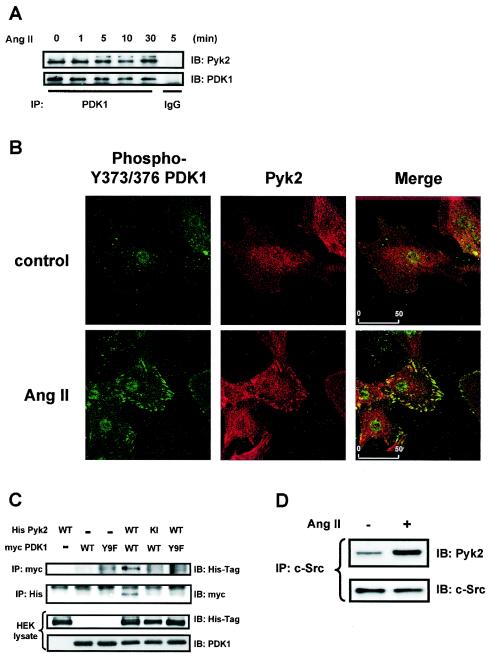
The requirement for both Src and Pyk2 in PDK1 phosphorylation suggests that these proteins might be physically associated. Using a coimmunoprecipitation approach, we found that the PDK1 immunoprecipitate contained Pyk2 in the basal state as well as after Ang II stimulation, indicating that the two proteins constitutively associate in VSMCs (Fig. 6A). To gain insight into the subcellular site at which this interaction occurs, we used confocal microscopy. In agreement with Western blot analysis (Fig. 1A), tyrosine-phosphorylated PDK1 was almost undetectable in quiescent VSMCs (Fig. 6B, top left panel). Upon Ang II stimulation, PDK1 phosphorylated on Tyr373-Tyr376 was detected in an FA-like distribution (Fig. 6B, bottom left panel) and colocalized with the FA protein paxillin (data not shown). Similar to results with other cell types (18, 31, 34), Pyk2 was also detected in FAs after agonist stimulation (Fig. 6B, middle panels). Importantly, analysis of the merged image clearly shows colocalization of Pyk2 and tyrosine-phosphorylated PDK1 in FAs in Ang II-stimulated VSMCs (Fig. 6B, right panels).
FIG. 6.
Pyk2 association with PDK1. (A) VSMCs were stimulated with 100 nM Ang II for the indicated times, and lysates were immunoprecipitated (IP) with anti-PDK1 antibody, followed by immunoblotting (IB) with Pyk2 antibody. (B) VSMCs grown on coverslips were treated with either vehicle (control) or Ang II (100 nM for 5 min) and fluorescently stained for PDK1 phosphorylated on Y373-Y376 (green) (left panels) or Pyk2 (red) (middle panels). Right panels, merged images showing colocalization (yellow). Scale bar = 50 μm. (C) HEK293 cells were cotransfected with the indicated expression vectors, and lysates were subjected to immunoprecipitation and immunoblotting. Separate aliquots of the lysates were immunoblotted with anti-His antibody or anti-PDK1 antibody to confirm equal expression of Pyk2 or PDK1. (D) VSMCs were stimulated with Ang II (100 nM for 5 min), and lysates were immunoprecipitated with anti-c-Src antibody, followed by immunoblotting with Pyk2 antibody (top). Equal amounts of c-Src protein in the immunoprecipitates were confirmed by immunoblotting with anti-c-Src antibody (bottom). For panels A, C, and D, the data are representative immunoblots of three independent experiments.
To further characterize the protein-protein binding between Pyk2 and PDK1, we expressed a combination of His-tagged Pyk2 proteins (WT or KI) and Myc-tagged PDK1 proteins (WT and Y9F) in HEK293 cells. As shown in Fig. 6C, His-tagged WT Pyk2 protein was present when WT PDK1 was immunoprecipitated with anti-Myc antibody (top panel). This was confirmed by the reverse experiment of immunoprecipitation with anti-His antibody followed by immunoblotting with anti-Myc antibody (second panel from the top). In contrast, no band was detected when KI His-Pyk2 and WT Myc-PDK1 or WT His-Pyk2 and Y9F Myc-PDK1 were coexpressed. These observations suggest that Pyk2-PDK1 interaction requires Pyk2 kinase activity and the protein structure surrounding Tyr9 in PDK1.
Because c-Src mediates Pyk2-dependent tyrosine phosphorylation of PDK1 (Fig. 5), we also investigated potential c-Src-Pyk2 interactions in the presence and absence of Ang II in VSMCs. As shown in Fig. 6D, Pyk2 was detected in c-Src immunoprecipitates from untreated VSMCs, indicating an association between c-Src and Pyk2. Moreover, Ang II treatment increased the association between these proteins. Taken together, these results suggest that PDK1 and Pyk2 exist basally in a protein complex and that c-Src is recruited to this complex upon activation, leading to tyrosine phosphorylation of PDK1.
Role in FA formation.
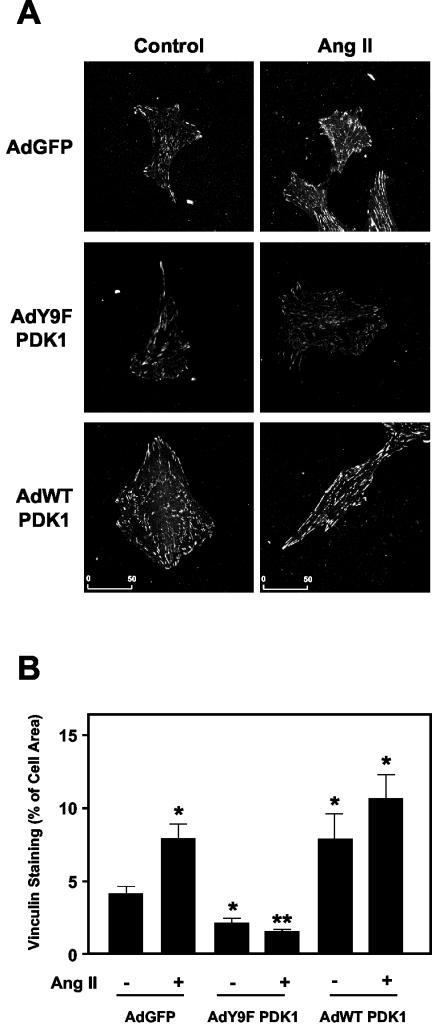
Because of the striking colocalization of Pyk2 and tyrosine-phosphorylated PDK1 in FAs and the fact that both Pyk2 and PDK1 have been shown to regulate the actin cytoskeleton (12, 49, 55), we investigated the consequences of the loss of PDK1 Tyr9 phosphorylation on FA formation by Ang II in VSMCs. We visualized FAs by immunostaining of vinculin, a well-known integral FA protein (17, 44). As shown in Fig. 7, treatment with Ang II (100 nM for 5 min) significantly increased the number, size, and area of distribution of FAs in VSMCs infected with a control adenovirus expressing GFP. Overexpression of WT PDK1 also increased FA area, and exposure to Ang II had little additional effect. In contrast, adenovirus-mediated expression of Y9F PDK1 together with GFP dramatically impaired FA formation by Ang II. Mean data are shown in Fig. 7B. These results support a novel function of Pyk2-induced PDK1 tyrosine phosphorylation, namely the regulation of FA formation.
FIG. 7.
Role of PDK1 tyrosine phosphorylation in Ang II-induced FA formation in VSMCs. (A) VSMCs were infected with either control adenovirus expressing GFP (AdGFP), virus expressing WT PDK1 (AdWT PDK1), or virus expressing Y9F PDK1 (AdY9F PDK1). Cells were treated with either vehicle (control) or Ang II (100 nM for 5 min). The results are representative of two independent experiments in which 30 cells in 10 fields were observed. Scale bar = 50 μm. (B) Quantification of vinculin staining in the cells described for panel A. A total of 7 to 10 cells from each group were analyzed as described in Materials and Methods, and the area of vinculin staining was expressed as a percentage of the total cell area. *, significant difference from GFP alone (P < 0.05); **, significant difference from Ang II alone in GFP-infected cells (P < 0.01).
Mechanism of PDK1-mediated FA formation.
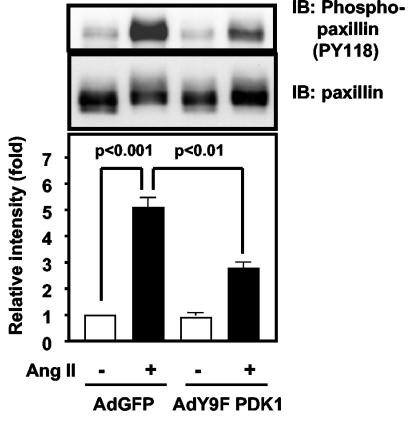
FA formation is mediated by multiple proteins, including small G proteins (17), PAK (6), and paxillin (37). The last protein is of particular interest because it is phosphorylated by Ang II (52), it is downstream of Pyk2 (32, 43), and phosphorylation of it at Tyr31 and Tyr118 is required for agonist-induced formation of FAs in epithelial cells (37). To determine if tyrosine phosphorylation of paxillin by Ang II is mediated by PDK1, we stimulated VSMCs with Ang II in the presence and absence of Y9F PDK1. As shown in Fig. 8, Ang II-induced phosphorylation of paxillin on Tyr118 is significantly inhibited by Y9F PDK1. These data suggest that regulation of tyrosine phosphorylation of paxillin is one mechanism by which PDK1 may modulate FA formation.
FIG. 8.
Role of PDK1 tyrosine phosphorylation in Ang II-induced paxillin phosphorylation. VSMCs were infected with the indicated adenoviruses, treated with Ang II (100 nM for 5 min), lysed, and immunoblotted (IB) with phospho (pY118)-paxillin antibody. Top panels, representative immunoblots; bottom panel, averaged data quantified by densitometry, expressed as fold increases in phosphorylation. Values are means ± SE for four independent experiments.
DISCUSSION
In the present study, we demonstrate for the first time that PDK1 can be tyrosine phosphorylated by a GPCR agonist such as Ang II. We describe a novel role for Pyk2 and its interaction with Src in phosphorylation of specific tyrosine residues Tyr9, Tyr373, and Tyr376 of PDK1. Tyrosine-phosphorylated PDK1 is colocalized with Pyk2 in FAs after Ang II stimulation and appears to play a role in FA function. Mutation of Tyr9 completely abolishes the ability of Ang II to assemble FAs, possibly in part by inhibiting tyrosine phosphorylation of paxillin. These results suggest a key role for PDK1 in the molecular mechanisms responsible for the dynamic regulation of these important signaling domains.
The finding that Pyk2 plays a critical role in the mechanism leading to phosphorylation of PDK1 represents a new function for this important enzyme. Pyk2 was originally identified as a calcium-dependent protein kinase expressed in the central nervous system that played a role in ion channel function (30). Subsequent work showed that Pyk2 can act as an important scaffolding molecule, coordinating signaling through c-Src, p130Cas, and PI3-K (40, 42). In the present study, Pyk2 is indeed shown to act as a scaffold, binding to both PDK1 and c-Src, thereby allowing c-Src to phosphorylate Tyr9, Tyr373, and Tyr376 on PDK1. Pyk2-dependent PDK1 activation may explain in part its wide-reaching cellular functions. Pyk2 has been shown to play a central role in cytoskeletal reorganization, inhibition of apoptosis, glucose metabolism, and protein synthesis (4). Interestingly, these functions of Pyk2 seem to be common to those of PDK1. Our data provide evidence that Pyk2 and PDK1 are in the same axis of signal transduction and, in fact, the same subcellular location. The current data thus identify an important, previously unknown consequence of Pyk2-PDK1 interaction—the regulation of FA formation, an event critical to many of these phenotypic changes.
Pyk2- and c-Src-dependent tyrosine phosphorylation of PDK1 appears to play an integral role in the response of VSMCs to Ang II. The early signaling events initiated by Ang II have been extensively studied. Immediately after binding to the AT1 receptor, Ang II increases intracellular calcium, resulting in activation of the tyrosine kinase Pyk2 (7, 42), and increases NAD(P)H oxidase-dependent production of reactive oxygen species (20), leading to the activation of the tyrosine kinase c-Src (26, 42). Pyk2 (13) and c-Src in turn converge to transactivate the EGF-R (52), which is essential for the activation of mitogen-activated protein kinases and possibly PI3-K (15). In the present study, we demonstrate that Ang II-induced PDK1 tyrosine phosphorylation is abolished by calcium chelation, transfection of Pyk2 antisense oligonucleotides, and a Src-family kinase inhibitor (Fig. 2, 3A, and 5A). On the other hand, neither EGF-R transactivation nor PI3-K activity is required for the tyrosine phosphorylation of PDK1 (data not shown). This indicates that PDK1 is downstream of the calcium-sensitive Pyk2 and redox-sensitive activation of c-Src but that it is independent of the EGF-R transactivation pathway in Ang II-stimulated VSMCs.
The localization of tyrosine-phosphorylated PDK1 in FAs and the fact that PDK1 tyrosine phosphorylation is downstream of Pyk2 and c-Src led us to hypothesize that PDK1 may play a role in the dynamic regulation of FAs. FAs are not only involved in cell motility but also integrate signaling pathways initiated by growth factors and GPCR agonists (17). Activation of both Pyk2 and c-Src has been shown to be critical for the formation of FAs (27, 49). This suggests that tyrosine-phosphorylated PDK1 may play a role in FA assembly and disassembly as well. It has been reported previously that PDK1 is important for reorganization of the actin cytoskeleton (12). In this study, we found that tyrosine phosphorylation of PDK1 is critical for this function. A replication-deficient adenovirus overexpressing Y9F PDK1 that is not phosphorylated by overexpression of Pyk2 or Ang II (Fig. 4) completely prevented the ability of Ang II to increase the number, size, and distribution of FAs (Fig. 7). This result clearly indicates that PDK1 tyrosine phosphorylation is crucial for the regulation of FAs by Ang II and thus may profoundly influence the integration of downstream signaling pathways.
We have previously shown that FAs are important signaling domains required for the spatial and temporal organization of Ang II signaling in VSMCs (53). Disruption of FAs inhibits subsequent EGF-R-dependent activation of Akt, which is required for Ang II-induced hypertrophy and cell survival. PDK1 is known to phosphorylate Akt on Thr308, thus permitting phosphorylation on Ser473 and activation of Akt (50). The present data suggest an additional novel mechanism of Akt regulation by PDK1. PDK1, by virtue of its ability to regulate FA formation, may facilitate formation of signaling complexes in this domain, enhancing Akt activation and activation of other downstream signaling molecules.
As noted above, there are several known proteins involved in FA formation, including small G proteins (17) and PAK (6). Very recently, phosphorylation of paxillin at Tyr31 and Tyr118 was shown to be required for agonist-induced formation of FAs in epithelial cells (37). Because paxillin is phosphorylated by Ang II in VSMCs (52) and has been shown to be mediated by Pyk2 in other cell types (32, 43), paxillin represents a potentially important downstream effector of PDK1. The data in Fig. 8 indicate that this is indeed the case, as expression of Y9F PDK1 substantially inhibited Ang II-induced paxillin phosphorylation on Tyr118. Thus, regulation of paxillin phosphorylation is one mechanism by which PDK1 may mediate FA formation. It is likely that other pathways are also involved, because several other PDK1 targets, such as PRKs and PAKs, have been implicated in organization of the actin cytoskeleton (50, 54). These kinases thus may also serve as downstream mediators of PDK1-dependent FA formation. Further investigation is required to define the mechanisms of PDK1-dependent FA formation, including the intermediate steps by which the serine/threonine kinase PDK1 regulates tyrosine phosphorylation of paxillin.
In summary, Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 occurs in FAs in response to the physiological GPCR agonist Ang II and participates in FA formation. The role of tyrosine-phosphorylated PDK1 in the regulation of FA assembly may underlie its ability to modify multiple downstream targets in a coordinated fashion. The localized interaction of these key signaling molecules may thus represent a novel pathway that plays a critical role in such diverse processes as cell growth, apoptosis, and cell migration and may provide insight into understanding the broader function of FAs in cell biology.
Acknowledgments
This work was supported by NIH grants HL38206, HL58000, and HL60728 and by a grant to Y.T. from the Japan Heart Foundation and Bayer Yakuhin Research Grant Abroad.
REFERENCES
- 1.Alessi, D. R., M. Deak, A. Casamayor, F. B. Caudwell, N. Morrice, D. G. Norman, P. Gaffney, C. B. Reese, C. N. MacDougall, D. Harbison, A. Ashworth, and M. Bownes. 1997. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7**:**776-789. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr. Biol. 7**:**261-269. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8**:**684-691. [DOI] [PubMed] [Google Scholar]
- 4.Avraham, H., S. Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12**:**123-133. [DOI] [PubMed] [Google Scholar]
- 5.Berk, B. C. 1999. Angiotensin II signal transduction in vascular smooth muscle: pathways activated by specific tyrosine kinases. J. Am. Soc. Nephrol. 10(Suppl. 11)**:**S62-S68. [PubMed] [Google Scholar]
- 6.Bokoch, G. M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72**:**743-781. [DOI] [PubMed] [Google Scholar]
- 7.Brinson, A. E., T. Harding, P. A. Diliberto, Y. He, X. Li, D. Hunter, B. Herman, H. S. Earp, and L. M. Graves. 1998. Regulation of a calcium-dependent tyrosine kinase in vascular smooth muscle cells by angiotensin II and platelet-derived growth factor. Dependence on calcium and the actin cytoskeleton. J. Biol. Chem. 273**:**1711-1718. [DOI] [PubMed] [Google Scholar]
- 8.Casamayor, A., N. A. Morrice, and D. R. Alessi. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342**:**287-292. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, H., F. H. Nystrom, L. Q. Dong, Y. Li, S. Song, F. Liu, and M. J. Quon. 2001. Insulin stimulates increased catalytic activity of phosphoinositide-dependent kinase-1 by a phosphorylation-dependent mechanism. Biochemistry 40**:**11851-11859. [DOI] [PubMed] [Google Scholar]
- 10.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24**:**350-355. [DOI] [PubMed] [Google Scholar]
- 11.Dikic, I., G. Tokiwa, S. Lev, S. A. Courtneidge, and J. Schlessinger. 1996. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383**:**547-550. [DOI] [PubMed] [Google Scholar]
- 12.Dong, L. Q., L. R. Landa, M. J. Wick, L. Zhu, H. Mukai, Y. Ono, and F. Liu. 2000. Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97**:**5089-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi, S., H. Iwasaki, T. Inagami, K. Numaguchi, T. Yamakawa, E. D. Motley, K. M. Owada, F. Marumo, and Y. Hirata. 1999. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension 33**:**201-206. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi, S., H. Iwasaki, H. Ueno, G. D. Frank, E. D. Motley, K. Eguchi, F. Marumo, Y. Hirata, and T. Inagami. 1999. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J. Biol. Chem. 274**:**36843-36851. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi, S., K. Numaguchi, H. Iwasaki, T. Matsumoto, T. Yamakawa, H. Utsunomiya, E. D. Motley, H. Kawakatsu, K. M. Owada, Y. Hirata, F. Marumo, and T. Inagami. 1998. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 273**:**8890-8896. [DOI] [PubMed] [Google Scholar]
- 16.Filippa, N., C. L. Sable, B. A. Hemmings, and E. Van Obberghen. 2000. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol. Cell. Biol. 20**:**5712-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger, B., A. Bershadsky, R. Pankov, and K. M. Yamada. 2001. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell. Biol. 2**:**793-805. [DOI] [PubMed] [Google Scholar]
- 18.Gismondi, A., L. Bisogno, F. Mainiero, G. Palmieri, M. Piccoli, L. Frati, and A. Santoni. 1997. Proline-rich tyrosine kinase-2 activation by beta 1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J. Immunol. 159**:**4729-4736. [PubMed] [Google Scholar]
- 19.Griendling, K. K., B. Lassègue, and R. W. Alexander. 1996. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 36**:**281-306. [DOI] [PubMed] [Google Scholar]
- 20.Griendling, K. K., C. A. Minieri, J. D. Ollerenshaw, and R. W. Alexander. 1994. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 74**:**1141-1148. [DOI] [PubMed] [Google Scholar]
- 21.Griendling, K. K., S. E. Rittenhouse, T. A. Brock, L. S. Ekstein, M. A. Gimbrone, Jr., and R. W. Alexander. 1986. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J. Biol. Chem. 261**:**5901-5906. [PubMed] [Google Scholar]
- 22.Griendling, K. K., M. B. Taubman, M. Akers, M. Mendlowitz, and R. W. Alexander. 1991. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J. Biol. Chem. 266**:**15498-15504. [PubMed] [Google Scholar]
- 23.Griendling, K. K., T. Tsuda, B. C. Berk, and R. W. Alexander. 1989. Angiotensin II stimulation of vascular smooth muscle cells. Secondary signalling mechanisms. Am. J. Hypertens. 2**:**659-665. [DOI] [PubMed] [Google Scholar]
- 24.Grillo, S., T. Gremeaux, A. Casamayor, D. R. Alessi, Y. Le Marchand-Brustel, and J. F. Tanti. 2000. Peroxovanadate induces tyrosine phosphorylation of phosphoinositide-dependent protein kinase-1 potential involvement of src kinase. Eur. J. Biochem. 267**:**6642-6649. [DOI] [PubMed] [Google Scholar]
- 25.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95**:**2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida, M., M. B. Marrero, B. Schieffer, T. Ishida, K. E. Bernstein, and B. C. Berk. 1995. Angiotensin II activates pp60c-src in vascular smooth muscle cells. Circ. Res. 77**:**1053-1059. [DOI] [PubMed] [Google Scholar]
- 27.Ishida, T., M. Ishida, J. Suero, M. Takahashi, and B. C. Berk. 1999. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J. Clin. Investig. 103**:**789-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lassègue, B., R. W. Alexander, G. Nickenig, M. Clark, T. J. Murphy, and K. K. Griendling. 1995. Angiotensin II down-regulates the vascular smooth muscle AT1 receptor by transcriptional and post-transcriptional mechanisms: evidence for homologous and heterologous regulation. Mol. Pharmacol. 48**:**601-609. [PubMed] [Google Scholar]
- 29.Lassègue, B., D. Sorescu, K. Szocs, Q. Yin, M. Akers, Y. Zhang, S. L. Grant, J. D. Lambeth, and K. K. Griendling. 2001. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 88**:**888-894. [DOI] [PubMed] [Google Scholar]
- 30.Lev, S., H. Moreno, R. Martinez, P. Canoll, E. Peles, J. M. Musacchio, G. D. Plowman, B. Rudy, and J. Schlessinger. 1995. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376**:**737-745. [DOI] [PubMed] [Google Scholar]
- 31.Li, J., H. Avraham, R. A. Rogers, S. Raja, and S. Avraham. 1996. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood 88**:**417-428. [PubMed] [Google Scholar]
- 32.Li, X., and H. S. Earp. 1997. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J. Biol. Chem. 272**:**14341-14348. [DOI] [PubMed] [Google Scholar]
- 33.Liao, D. F., B. Monia, N. Dean, and B. C. Berk. 1997. Protein kinase C-zeta mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J. Biol. Chem. 272**:**6146-6150. [DOI] [PubMed] [Google Scholar]
- 34.Litvak, V., D. Tian, Y. D. Shaul, and S. Lev. 2000. Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J. Biol. Chem. 275**:**32736-32746. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Ruiz, M., D. Fulton, G. Sowa, L. R. Languino, Y. Fujio, K. Walsh, and W. C. Sessa. 2000. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 86**:**892-896. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, T. J., R. W. Alexander, K. K. Griendling, M. S. Runge, and K. E. Bernstein. 1991. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351**:**233-236. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura, K., H. Yano, H. Uchida, S. Hashimoto, E. Schaefer, and H. Sabe. 2000. Tyrosine phosphorylation of paxillin alpha is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J. Biol. Chem. 275**:**27155-27164. [DOI] [PubMed] [Google Scholar]
- 38.Park, J., M. M. Hill, D. Hess, D. P. Brazil, J. Hofsteenge, and B. A. Hemmings. 2001. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J. Biol. Chem. 276**:**37459-37471. [DOI] [PubMed] [Google Scholar]
- 39.Prasad, N., R. S. Topping, D. Zhou, and S. J. Decker. 2000. Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1). Biochemistry 39**:**6929-6935. [DOI] [PubMed] [Google Scholar]
- 40.Rocic, P., G. Govindarajan, A. Sabri, and P. A. Lucchesi. 2001. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am. J. Physiol. Cell Physiol. 280**:**C90-C99. [DOI] [PubMed] [Google Scholar]
- 41.Rocic, P., and P. A. Lucchesi. 2001. Down-regulation by antisense oligonucleotides establishes a role for the proline-rich tyrosine kinase PYK2 in angiotensin II-induced signaling in vascular smooth muscle. J. Biol. Chem. 276**:**21902-21906. [DOI] [PubMed] [Google Scholar]
- 42.Sabri, A., G. Govindarajan, T. M. Griffin, K. L. Byron, A. M. Samarel, and P. A. Lucchesi. 1998. Calcium- and protein kinase C-dependent activation of the tyrosine kinase PYK2 by angiotensin II in vascular smooth muscle. Circ. Res. 83**:**841-851. [DOI] [PubMed] [Google Scholar]
- 43.Salgia, R., S. Avraham, E. Pisick, J. L. Li, S. Raja, E. A. Greenfield, M. Sattler, H. Avraham, and J. D. Griffin. 1996. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J. Biol. Chem. 271**:**31222-31226. [DOI] [PubMed] [Google Scholar]
- 44.Sastry, S. K., and K. Burridge. 2000. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261**:**25-36. [DOI] [PubMed] [Google Scholar]
- 45.Saward, I., and P. Zahradka. 1997. Angiotensin II activates phosphatidylinositol 3-kinase in vascular smooth muscle cells. Circ. Res. 81**:**249-257. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz, U., T. Ishida, M. Ishida, J. Surapisitchat, M. I. Hasham, S. Pelech, and B. C. Berk. 1998. Angiotensin II stimulates p21-activated kinase in vascular smooth muscle cells: role in activation of JNK. Circ. Res. 82**:**1272-1278. [DOI] [PubMed] [Google Scholar]
- 47.Stephens, L., K. Anderson, D. Stokoe, H. Erdjument-Bromage, G. F. Painter, A. B. Holmes, P. R. Gaffney, C. B. Reese, F. McCormick, P. Tempst, J. Coadwell, and P. T. Hawkins. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279**:**710-714. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi, E., J. Abe, and B. C. Berk. 1997. Angiotensin II stimulates p90rsk in vascular smooth muscle cells. A potential Na(+)-H+ exchanger kinase. Circ. Res. 81**:**268-273. [DOI] [PubMed] [Google Scholar]
- 49.Tang, H., Q. Hao, T. Fitzgerald, T. Sasaki, E. J. Landon, and T. Inagami. 2002. Pyk2/CAKbeta tyrosine kinase activity-mediated angiogenesis of pulmonary vascular endothelial cells. J. Biol. Chem. 277**:**5441-5447. [DOI] [PubMed] [Google Scholar]
- 50.Toker, A., and A. C. Newton. 2000. Cellular signaling: pivoting around PDK-1. Cell 103**:**185-188. [DOI] [PubMed] [Google Scholar]
- 51.Ushio-Fukai, M., R. W. Alexander, M. Akers, Q. Yin, Y. Fujio, K. Walsh, and K. K. Griendling. 1999. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 274**:**22699-22704. [DOI] [PubMed] [Google Scholar]
- 52.Ushio-Fukai, M., K. K. Griendling, P. L. Becker, L. Hilenski, S. Halleran, and R. W. Alexander. 2001. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 21**:**489-495. [DOI] [PubMed] [Google Scholar]
- 53.Ushio-Fukai, M., L. Hilenski, N. Santanam, P. L. Becker, Y. Ma, K. K. Griendling, and R. W. Alexander. 2001. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J. Biol. Chem. 276**:**48269-48275. [DOI] [PubMed] [Google Scholar]
- 54.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346**:**561-576. [PMC free article] [PubMed] [Google Scholar]
- 55.Watson, J. M., T. W. Harding, V. Golubovskaya, J. S. Morris, D. Hunter, X. Li, J. S. Haskill, and H. S. Earp. 2001. Inhibition of the calcium-dependent tyrosine kinase (CADTK) blocks monocyte spreading and motility. J. Biol. Chem. 276**:**3536-3542. [DOI] [PubMed] [Google Scholar]
- 56.Xiong, W., and J. T. Parsons. 1997. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J. Cell Biol. 139**:**529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]