Infection with Multiple Human Immunodeficiency Virus Type 1 Variants Is Associated with Faster Disease Progression (original) (raw)
Abstract
Human immunodeficiency virus type 1 (HIV-1)-infected individuals develop a genetically diverse virus population over time, but often only a limited number of viral variants are transmitted from a chronic carrier to a newly infected person. Interestingly, many women but few men are infected by multiple HIV-1 variants from a single partner. To determine whether the complexity of the infecting virus population influences clinical outcome, we examined viral diversity in the HIV-1 envelope sequences present at primary infection in 156 women from Kenya for whom we had follow-up data on viral RNA levels and CD4 T-cell counts. Eighty-nine women had multiple viral genotypes, while 67 women had a single genotype at primary infection. Women who acquired multiple viral genotypes had a significantly higher viral load (median, 4.84 versus 4.64 log10 copies/ml, P = 0.04) and a significantly lower CD4+-T-cell count (median, 416 versus 617 cells/mm3, P = 0.01) 4 to 24 months after infection compared to women who were infected with a single viral genotype. These studies suggest that early HIV-1 genetic diversity is linked to faster disease progression.
The human immunodeficiency virus type 1 (HIV-1) genome evolves during the course of an infection, especially the envelope gene, which encodes the antigenic surface glycoprotein used for viral entry (25). The envelope gene may vary in sequence by as much as 10% among the viral quasispecies present within a single individual (4). Interestingly, newly infected subjects typically acquire only a limited number of these viral genomes from the chronically infected index case. In fact, men and infants have generally been found to have a genetically homogeneous envelope gene at or near the time of their infection regardless of the route of transmission (33, 34, 38, 39). Our group and others showed that women from Africa were often infected with heterogeneous envelope genotypes early in infection (12, 15, 28). In the first 32 women examined in our previous studies, 20 had multiple variants at primary infection. These variants often differed by more than 5% in the envelope gene and constituted more than 10% of the HIV-1 population. Phylogenetic studies showed that the variants within each woman clustered together, and therefore they were highly likely to have been acquired from a single source. This finding strongly suggests that diversity at this early stage of infection was not due to superinfection by viruses from a second sex partner (15, 28). In addition, it was quite unlikely that the observed viral diversity was due to rapid mutation of a single virus after transmission because the same variants could be readily detected both before and after seroconversion (15).
It is unknown whether the complexity of the infecting HIV-1 population affects the level of virus replication in the host and the subsequent course of disease. The steady-state level of viral replication, often called the viral set point, is generally established about 4 months postinfection, after the host has responded to the primary infection (13, 31). This viral set point and the decline in CD4+-T-lymphocyte counts over time provide surrogate markers for HIV-1 disease progression (10, 23). While there have been several studies that have examined the difference in viral evolution in fast and slow progressors (5, 8, 9, 17-19, 21, 22, 30, 35), there are no published studies that have prospectively examined the relationship between the genetic diversity of the infecting virus population and subsequent disease progression. Thus, the role that the genetic complexity of the transmitted virus plays in determining the steady-state levels of HIV-1 replication and disease outcome is not known.
We studied antiretroviral drug-naive female sex workers in Kenya who were followed prospectively before and after HIV-1 seroconversion. This cohort has been described previously in detail (20). Briefly, HIV-1-seronegative women were monitored approximately every month for HIV-1 seroconversion. Stored plasma samples from the two visits prior to seroconversion were then tested for HIV-1 RNA with the Gen-Probe quantitative HIV-1 assay, which is sensitive to the subtypes found in Kenya (7). A time of infection was assigned to each subject on the basis of results of both HIV-1 serologic and RNA tests. For subjects who tested positive for HIV-1 RNA prior to seroconversion, the date of infection was estimated to be 17 days earlier than the visit at which HIV-1 RNA was detected (2). For women who were negative for HIV-1 RNA prior to seroconversion, the day of infection was estimated to be the midpoint between the seroconversion visit and the previous visit. Women positive for HIV-1 RNA prior to seroconversion or with less than 1 year between the last HIV-1 negative serology result and the first HIV-1 antibody-positive test result were selected for inclusion in the study. Women from whom no peripheral blood mononuclear cell (PBMC) sample was available within 1 year after the estimated date of infection were excluded.
To examine viral diversity, proviral DNA was isolated from PBMC samples by QIAamp DNA Blood Midi Kit (Qiagen, Valencia, Calif.). To ensure that all of the major infecting HIV-1 variants were present in the subsequent PCRs, the number of HIV-1 proviral copies in the PBMC sample was first determined by real-time quantitative PCR. Briefly, _Pol_-specific primers Pol 15 (5′-TACAGTGCAGGGGAAAGAATA-3′; corresponding to nucleotide [nt] positions 4809 to 4829 in the HXB2 genome) and Pol 4 (5′-GGAAAGGTGAAGGGGCAG-3′; nt 4957 to 4974) and a Pol probe (6FAM-TTTCGGGTTTATTACAGGGACAGCAG-TAMRA [nt 4896 to 4921]) (C. M. Rousseau and J. Overbaugh et al., unpublished data) were used in quantitative real-time PCR. If no product was detected by this method, envelope-specific primers and probes were used (Env 77 [5′-GTCTGGGCCACACATGCTTGT-3′; nt 6426 to 6446] and Env 78 [5′-TGCTCTACCATGTTATTTTTCCACA-3′; nt 6508 to 6532] and Env Probe 6FAM-CCCACAGACCCCAACCCACAAGAAATA-TAMRA [nt 6508 to 6532]). The mean and standard deviation of the cycle count at which the detection threshold was achieved was calculated for each quantitative standard from at least six independent experiments. Each subsequent real-time quantitative PCR run was verified by ensuring that the cycle counts for the standards were within the estimated range and by showing that 1, 10, and 100 HIV-1 proviral copies of the ACH-2 cell line (3), a T-cell clone latently infected with HIV-1, were accurately quantified every time. All subject samples were tested in duplicate, and the average of the two results was used to estimate the proviral copy number. If the two results were not within threefold of each other, the samples were retested. If no signal was detected by either real-time method, then limiting dilution was used to estimate the number of viral genomes. After determining the number of HIV-1 copies, the envelope gene fragment encompassing variable regions V1 through V5 or V3 was amplified through nested PCR from at least 10 proviral genomes each in two independent PCRs (26, 28). The amplified product from the two independent PCRs was analyzed individually and in combination by the heteroduplex mobility assay (HMA), an electrophoresis-based method that discriminates between heteroduplexes and homoduplexes (6). The virus population was classified as heterogeneous if a heteroduplex was observed in the HMA of the mixture of the two PCRs.
In earlier studies of this cohort, diversity in the envelope gene of 32 women was evaluated by using multiple independent PCRs either by analyzing cloned sequences (28) or by HMA (15). In order to examine a larger sample of women, we tested whether viral diversity could be accurately defined by HMA with fewer PCRs. In 19 subjects examined, we found that analyzing the envelope gene from a minimum of 20 HIV-1 genomes, amplified in two independent PCRs, yielded the same results as those obtained from earlier methods. Repeated analysis of the same sample yielded reproducible results (data not shown). In nine samples where multiple envelope genotypes were previously documented, heteroduplexes were observed in the HMA of the combination of the products from the two PCRs. In fact, heteroduplexes were observed in the HMA of the products from each individual PCR in eight of these nine samples.
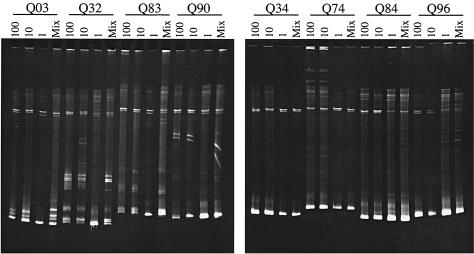
To further validate that HMA of the products of PCRs from 10 to 100 input templates provides an accurate assessment of viral diversity, we analyzed the PCR product from a range of input templates and a mixture of 10 independent PCR products from one input proviral copy (Fig. 1). Four samples previously classified as heterogeneous (Q03, Q32, Q83, and Q90) displayed heteroduplexes in all cases except in those products from PCRs of one input template copy, as would be expected. For the four individuals previously determined to have a homogeneous virus population (Q34, Q74, Q84, and Q96), no heteroduplexes were detected in the HMA of products from PCRs that had 10 and 100 proviral copies (Fig. 1). Similar results were obtained with products from PCRs starting with one proviral template, although in some cases, such as Q84, a smear was observed above the homoduplex. This tended to be detected in cases in which there was more PCR product (e.g., Q84, Q96, and Q90 HMA of the one-copy PCRs). These cases with smearing above the homoduplex were classified as homogeneous if there was no discernible distinct heteroduplex band above them in the gel. Consistent with this interpretation, only a single genotype was detected in the sample from Q84 when the sequences from 18 clones from six independent PCRs were analyzed (28). This contrasts with Q03, where we observed distinct heteroduplex bands in the gel and therefore categorized her virus as heterogeneous; for Q03, the results of the HMA were also consistent with the results of previous sequence analysis (28). The qualitative assignment of viral diversity with HMA and detailed sequence analysis produced consistent results in our previous studies (15, 16, 28), and this was further confirmed in five cases with HMA results from the products of two PCRs starting with 10 to 100 template copies and sequence analysis of multiple envelope clones from independent PCRs. While the HMA is not meant to be a perfect replacement for detailed sequence analyses, in part because insertions and deletions may cause heteroduplexes even if the sequences are otherwise identical (15), these pilot studies on a fraction of the cohort suggest that it provides a reliable high-throughput screen to analyze diversity in a larger cohort. Importantly, the investigator interpreting the HMA did not have access to clinical data and the investigator analyzing the data did not have input into the interpretation of the HMA.
FIG. 1.
Representative HMAs of a 1.2-kb envelope fragment amplified in PCRs starting with different amounts of proviral templates from PBMCs from eight subjects. The designations represent the different subjects; Q03, Q32, Q83, and Q90 were classified as heterogeneous, and Q34, Q74, Q84, and Q96 were classified as homogeneous virus populations with a combination of two PCRs starting with approximately 10 proviral copies. For each subject, the first three lanes of product are from PCRs with 100, 10, and 1 proviral copies as determined by real-time quantitative PCR, respectively. The number of proviral copies added to the PCR is indicated above each lane. Twenty-four to 36 independent PCRs from one input template were performed for each subject, and the last lane (Mix) contains a mixture of 10 of these PCRs that yielded a positive result.
We have qualitatively described viral diversity in an additional 124 women by this protocol and found that 69 (56%) had viral heterogeneity and 55 (44%) had viral homogeneity at primary infection. The number of input proviral copies for each PCR analyzed on the HMA was not significantly different between the subjects with a heterogeneous virus (median, 30; range, 10 to 100) compared to the women with a homogeneous virus (median, 20; range, 10 to 100, P = 0.6, Mann-Whitney U test). This, further, suggests that the observed differences in diversity are not due to sampling differences. Combining the women analyzed here and the results from our previous studies (15, 28), 89 (57%) of 156 women had a heterogeneous virus population while 67 (43%) of the 156 had viral homogeneity at or near the time of their infection. Of the 156 women in this cohort, 62 tested positive for HIV-1 RNA prior to seroconversion. In the other 94 women, HIV-1 seroconversion was documented within a median of 89 days (range, 28 to 348) from their last seronegative visit. The proportion of women in whom HIV-1 RNA was detected prior to seroconversion and those that were HIV-1 RNA negative prior to seroconversion was not significantly different between the two groups (P = 0.6). For the entire cohort, the median interval from the estimated infection date to the day of collection of the PBMC sample assayed for viral diversity was 71 days (range, 14 to 352). For women who lacked preseroconversion HIV-1 RNA, the median interval of time from the last HIV-1 seronegativity date to the day on which the PBMC sample used for the diversity analysis was collected was 124 days (range, 28 to 356).
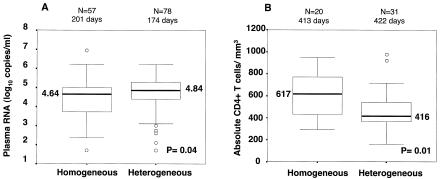
After HIV-1 infection, the HIV-1 RNA level in plasma was measured a median of six different times (range, 1 to 26) over a median of 907 days (range, 14 to 2,933) of follow-up. Women with a heterogeneous virus had significantly higher viral RNA levels at the time that viral diversity was assessed during acute infection than did women with a homogeneous envelope genotype (median, 5.11 versus 4.63 log10 copies/ml, P = 0.003; Mann-Whitney U test). To examine whether this difference persisted after primary infection, when the viral set point was established, we examined viral diversity in relation to HIV-1 RNA levels in plasma at 4 to 24 months postinfection. The median of the first HIV-1 RNA level in plasma, measured 4 to 24 months after infection, was significantly higher in women infected with multiple envelope genotypes than in women infected with a single genotype (median, 4.84 versus 4.64 log10 copies/ml, P = 0.04; Fig. 2A). The interval from the estimated infection date to the viral load measurement day was not significantly different in the two groups (median, 174 versus 201 days; P = 0.2, Mann-Whitney U test).
FIG. 2.
Box plots of the first viral load (A) and absolute CD4+-T-cell count (B) measured 4 to 24 months after infection comparing women infected with a heterogeneous virus population to those with a homogeneous virus population. The number of subjects contributing to the data and the median interval from the estimated date of infection to the day of viral RNA or CD4+-T-cell measurement are above each box plot. The thick black line in each box represents the median values for each group; the numbered values are presented beside the lines. Boxes show the interquartile range, the 25th and 75th percentiles of the data, while the whiskers are values 1.5 times the interquartile range above and below the median. Οpen circles represent any data outside the whiskers. Comparisons between groups were done with the Mann-Whitney U test.
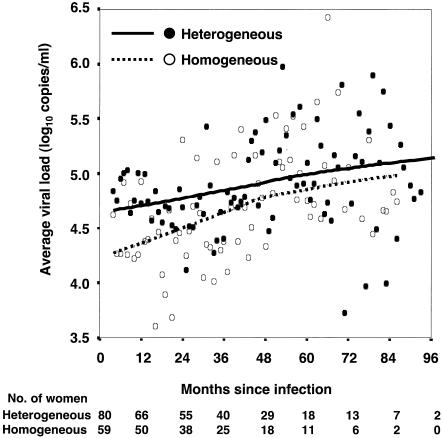
To examine whether the difference in viral load persisted over the course of the infection, mean monthly viral levels after 4 months of infection were compared for each group over time. This longitudinal analysis revealed that women infected with a heterogeneous HIV-1 population consistently maintained a higher viral load over the course of follow up than those women infected with a homogeneous HIV-1 population (Fig. 3). The HIV-1 level in plasma was 0.27 log10 copies/ml (95% confidence interval [CI], 0.019 to 0.526, P = 0.04) higher in women with a heterogeneous than in those with a homogeneous virus population over time when a linear mixed-effects model was used. Thus, women with heterogeneous genotypes have a higher viral load during primary infection and maintain this difference over the course of the infection.
FIG. 3.
Mean viral loads of women infected with a heterogeneous virus population versus those of women with a homogeneous virus population over the course of follow-up. Each symbol represents the mean of the available individual log-transformed viral loads for the women in each group for each month, starting 4 months after infection. In cases in which a woman contributed more than one viral load measurement for a month, an average was used for her results for that month. Loess-smoothed curves were generated with 99% of the mean monthly viral loads with 20 iterations. The number of women in each group with active follow-up over time is shown at the bottom.
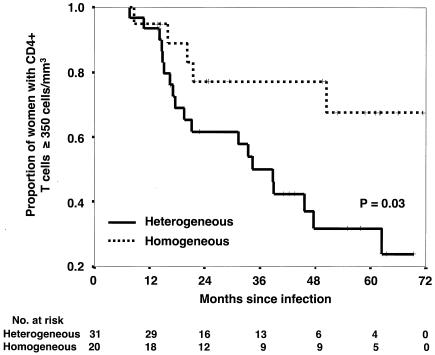
CD4+-T-lymphocyte counts were also available for these women, although there were fewer of these measurements than viral RNA level measurements. Women infected with multiple HIV-1 genotypes had a lower absolute CD4+-T-cell count, measured within 4 to 24 months after infection, than did women infected with a single HIV-1 genotype (median, 416 versus 617 cells/mm3; P = 0.01; Fig. 2B). Again, the interval from the infection date to the CD4+-T-cell measurement day was not significantly different in the two groups (median, 422 versus 413 days; P = 0.3, Mann-Whitney U test). Longitudinal analysis revealed a significant difference between the two groups in the time to absolute CD4+-T-cell count of less than 350 cells/mm3, which is often used as a threshold for initiation of antiretroviral therapy (Fig. 4) (36). The mean time to a CD4+-T-cell count of less than 350 cells/mm3 was 39.2 months (95% CI, 30.8 to 47.6) in the women infected with multiple variants compared to 56.8 months (95% CI, 46.0 to 67.7) for the women infected with a single variant.
FIG. 4.
Kaplan-Meier analysis of time to a CD4+-T-cell count of less than 350 cells/mm3. Survival analysis was performed on a subset of women who had at least one CD4+-T-cell measurement 4 to 24 months after infection. The event, a CD4+-T-cell count of less than 350/mm3, was assumed to have occurred on the date of the clinic visit at which the CD4+-T-cell count was done. Kaplan-Meier curves and log-rank tests were used for the survival analyses. The numbers of women at risk in both groups are shown at the bottom.
The interval of time from the date of estimated infection to the day on which the PBMC sample analyzed for viral diversity was collected was not significantly different between the women infected with multiple genotypes (median, 75.5 days; range, 14 to 352) and women with a single genotype (median, 67.0 days; range, 16 to 330; P = 0.1, Mann-Whitney U test). Thus, early viral diversity observed in the women examined in this study was not associated with the amount of time the virus had to evolve and adapt in the host. Given that women with genetically diverse viruses had higher levels of viral RNA even during acute infection, we cannot rule out the possibility that greater viral turnover partially contributed to the diversity found. Previous estimates of the rate of HIV-1 envelope gene diversification of approximately 1% per year (32), however, argues against a significant contribution of de novo diversification in this early window. In addition, studies with the simian immunodeficiency virus model, in which the viral loads are often greater than the HIV-1 infection in humans, suggest that only minor envelope changes accrue in this early interval (11, 27, 29). Thus, because viral diversity was examined early after infection, rapid diversification is not likely to explain the presence of multiple envelope genotypes, especially since these variants can be detected prior to seroconversion (15).
In this cohort, we have examined viral diversity early in infection, when there has been limited opportunity for reinfection. These women reported a range of zero to five sexual contacts per week in the interval of time from infection to when we analyzed viral diversity (a median of 71 days), and there was a similar frequency of reported sexual activity and condom use in women in whom a heterogeneous virus was detected versus those in whom we detected only a single variant (data not shown). Thus, in the majority of women in whom a heterogeneous virus was detected near seroconversion, the number of HIV-1 exposures in the time between when they first became infected and when we analyzed viral diversity was considerably less than the approximate 1 in 1,000 episodes of penile-vaginal sexual intercourse that result in HIV-1 acquisition (1). Together, these data support the findings from our previous phylogenetic analyses of sequences in a subset of women, which demonstrated that the HIV-1 variants detected in women early in infection were transmitted from a single partner (15, 28).
This study demonstrates a strong association between the genetic diversity of the infecting virus population and clinical outcome. Women infected with multiple genotypes had a higher steady-state level of HIV-1 viremia and faster CD4+-T-cell decline, which are two key surrogate markers of HIV-1 disease progression. The magnitude of these differences is clinically significant, with the mean time to CD4+-T-cell depletion below 350 cells/mm3 occurring about 18 months earlier in the group with viral heterogeneity. This cohort has not had a sufficient length of follow-up for evaluation of other end points such as death, but previous studies have demonstrated that the level of viral replication predicts the times to AIDS and death (10, 23).
Several previous studies have retrospectively examined the rate of viral evolution over the course of infection in relation to the rate of disease progression. These studies have not produced a consistent model perhaps, in part, because only small numbers of selected subjects were evaluated in any given study. Some of these studies have suggested that greater viral replication and faster progression to AIDS are correlated with greater viral diversity (18, 19, 22), while others have proposed that a stronger immune response and slower disease progression lead to greater genetic complexity (5, 8, 9, 17, 21, 30, 35). In aggregate, these studies have focused on virus-host factors after primary infection that may influence the generation of viral diversity over time. In essence, they are using the development of diversity either as a measure of immune selection or as a marker of viral replication within the infected individual. Our study addressed a distinct question that focuses on the effect of the genetic diversity of the infecting HIV-1 population in shaping the subsequent virus-host dynamics and the rate of disease progression. This prospective examination of a large number of subjects early after infection indicates that greater genetic complexity in the infecting HIV-1 population leads to a decreased ability to control viral replication that can be detected early in acute infection and over the course of the disease.
The nature of the selective bottleneck that determines the genetic diversity of the transmitted HIV-1 strain is unknown, but it is likely that this bottleneck prevents the majority of the highly adapted viral variants in a chronic carrier from establishing an infection within the new host. These selective bottlenecks represent fitness losses, and therefore, this may decrease the replication capacity of the infecting virus population (24, 37). Studies with the simian immunodeficiency virus model have shown that both the level of virus replication and disease progression are dependent on the properties of the infecting virus (14). Therefore, the less restrictive selective barrier in the women with early viral heterogeneity may increase the likelihood of acquiring more pathogenic HIV-1 variants. These studies indicate that the genetic complexity of the transmitted virus population is important in determining the subsequent course of HIV-1 disease.
Acknowledgments
We thank Dana Panteleeff for providing HIV-1 RNA data; H. Martin, E. M. Long, M. Poss, and the Mombasa field staff for their contributions to this study; C. M. Rousseau for assistance with quantitative PCR; and the Kenya HIV/STD Research Project for continued collaborations. We are indebted to all of the women in the Mombasa cohort.
This study was supported by grants AI38518, D43-TW00007, and A1-33873, subcontract N01-A1-36173-119, from the National Institutes of Health.
REFERENCES
- 1.Baeten, J. M., and J. Overbaugh. 2003. Measuring the infectiousness of persons with HIV-1: opportunities for preventing sexual HIV-1 transmission. Curr. HIV Res. 1**:**69-86. [DOI] [PubMed]
- 2.Busch, M. P., L. L. L. Lee, D. R. Satten, D. R. Henrard, K. E. Farzadegan, K. E. Nelson, S. Read, R. Y. Dodd, and L. R. Petersen. 1995. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion 35**:**91-97. [DOI] [PubMed] [Google Scholar]
- 3.Clouse, K. A., D. Powell, I. Washington, G. Poli, K. Strebel, W. Farrar, P. Barstad, J. Kovacs, A. S. Fauci, and T. M. Folks. 1989. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J. Immunol. 142**:**431-438. [PubMed] [Google Scholar]
- 4.Delwart, E. L., H. W. Sheppard, B. D. Walker, J. Goudsmit, and J. I. Mullins. 1994. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J. Virol. 68**:**6672-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delwart, E. L., H. Pan, H. W. Sheppard, D. Wolpert, A. U. Neumann, B. Korber, and J. I. Mullins. 1997. Slower evolution of human immunodeficiency virus type 1 quasispecies during progression to AIDS. J. Virol. 71**:**7498-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rubsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science 262**:**1257-1261. [DOI] [PubMed] [Google Scholar]
- 7.Emery, S., S. Bodrug, B. A. Richardson, C. Giachetti, M. A. Bott, D. Panteleeff, G. L. Jagodzinski, N. L. Michael, R. Nduati, J. Bwayo, J. K. Kreiss, and J. Overbaugh. 2000. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J. Clin. Microbiol. 38**:**2688-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganeshan, S., R. E. Dickover, B. T. Korber, Y. J. Bryson, and S. M. Wolinsky. 1997. Human immunodeficiency virus type 1 genetic evolution in children with different rates of development of disease. J. Virol. 71**:**663-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halapi, E., T. Leitner, M. Jansson, G. Scarlatti, P. Orlandi, A. Plebani, L. Romiti, J. Albert, H. Wigzell, and P. Rossi. 1997. Correlation between HIV sequence evolution, specific immune response and clinical outcome in vertically infected infants. AIDS 11**:**1709-1717. [DOI] [PubMed] [Google Scholar]
- 10.Henrard, D. R., J. F. Phillips, L. R. Muenz, W. A. Blattner, D. Wiesner, M. E. Eyster, and J. J. Goedert. 1995. Natural history of HIV-1 cell-free viremia. JAMA 274**:**554-558. [PubMed] [Google Scholar]
- 11.Johnson, P. R., T. E. Hamm, S. Goldstein, S. Kitov, and V. M. Hirsch. 1991. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology 185**:**217-228. [DOI] [PubMed] [Google Scholar]
- 12.Kampinga, G. A., A. Simonon, P. Van de Perre, E. Karita, P. Msellati, and J. Goudsmit. 1997. Primary infections with HIV-1 of women and their offspring in Rwanda: findings of heterogeneity at seroconversion, coinfection, and recombinants of HIV-1 subtypes A and C. Virology 227**:**63-76. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann, G. R., P. Cunningham, A. D. Kelleher, J. Zaunders, A. Carr, J. Vizzard, M. Law, and D. A. Cooper. 1998. Patterns of viral dynamics during primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 178**:**1812-1815. [DOI] [PubMed] [Google Scholar]
- 14.Kimata, J. T., L. Kuller, D. B. Anderson, P. Dailey, and J. Overbaugh. 1999. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat. Med. 5**:**535-541. [DOI] [PubMed] [Google Scholar]
- 15.Long, E. M., H. L. Martin, Jr., J. K. Kreiss, et al. 2000. Gender differences in HIV-1 diversity at time of infection. Nat. Med. 6**:**71-75. [DOI] [PubMed] [Google Scholar]
- 16.Long, E. M., S. M. J. Rainwater, L. Lavreys, K. Mandaliya, and J. Overbaugh. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retrovir. 18**:**567-576. [DOI] [PubMed] [Google Scholar]
- 17.Lukashov, V. V., C. L. Kuiken, and J. Goudsmit. 1995. Intrahost human immunodeficiency virus type 1 evolution is related to length of the immunocompetent period. J. Virol. 69**:**6911-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani, I., P. Gilbert, J.-L. Sankalé, G. Eisen, S. Mboup, and P. J. Kanki. 2002. Intrapatient diversity and its correlation with viral setpoint in human immunodeficiency virus type 1 CRF02_A/G-IbNG infection. J. Virol. 76**:**10745-10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markham, R. B., W. C. Wang, A. E. Weisstein, Z. Wang, A. Munoz, A. Templeton, J. Margolick, D. Vlahov, T. Quinn, H. Farzadegan, and X. F. Yu. 1998. Patterns of HIV-1 evolution in individuals with differing rates of CD4 T cell decline. Proc. Natl. Acad. Sci. USA 95**:**12568-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, H. L., P. M. Nyange, B. A. Richardson, L. Lavreys, K. Mandaliya, D. J. Jackson, J. O. Ndinya-Achola, and J. K. Kreiss. 1998. Hormonal contraception, sexually transmitted diseases, and the risk of heterosexual transmission of HIV-1. J. Infect. Dis. 178**:**1053-1059. [DOI] [PubMed] [Google Scholar]
- 21.McDonald, R. A., D. L. Mayers, R. C. Chung, K. F. Wagner, S. Ratto-Kim, D. L. Brix, and N. L. Michael. 1997. Evolution of human immunodeficiency virus type 1 env sequence variation in patients with diverse rates of disease progression and T-cell function. J. Virol. 71**:**1871-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNearney, T., Z. Hornickova, R. Markham, A. Birdwell, M. Arens, A. Saah, and L. Ratner. 1992. Relationship of human immunodeficiency virus type 1 sequence heterogeneity to stage of disease. Proc. Natl. Acad. Sci. USA 89**:**10247-10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellors, J. W., L. A. Kingsley, C. R. Rinaldo, Jr., J. A. Todd, B. S. Hoo, R. P. Kokka, and P. Gupta. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann. Intern. Med. 122**:**573-579. [DOI] [PubMed] [Google Scholar]
- 24.Novella, I. S., J. Quer, E. Domingo, and J. J. Holland. 1999. Exponential fitness gains of RNA virus populations are limited by bottleneck effects. J. Virol. 73**:**1668-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overbaugh, J., and C. R. Bangham. 2001. Selection forces and constraints on retroviral sequence variation. Science 292**:**1106-1109. [DOI] [PubMed] [Google Scholar]
- 26.Overbaugh, J., R. J. Anderson, J. O. Ndinya-Achola, and J. K. Kreiss. 1996. Distinct but related human immunodeficiency virus type 1 variant populations in genital secretions and blood. AIDS Res. Hum. Retrovir. 12**:**107-115. [DOI] [PubMed] [Google Scholar]
- 27.Overbaugh, J., L. M. Rudensey, M. D. Papenhausen, R. E. Benveniste, and W. R. Morton. 1991. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J. Virol. 65**:**7025-7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poss, M., H. L. Martin, J. K. Kreiss, L. Granville, B. Chohan, P. Nyange, K. Mandaliya, and J. Overbaugh. 1995. Diversity in virus populations from genital secretions and peripheral blood from women recently infected with human immunodeficiency virus type 1. J. Virol. 69**:**8118-8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudensey, L. M., J. T. Kimata, E. M. Long, B. Chackerian, and J. Overbaugh. 1998. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVMne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72**:**209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvatori, F., S. Masiero, C. Giaquinto, C. M. Wade, A. J. L. Brown, L. Chieco-Bianchi, and A. De Rossi. 1997. Evolution of human immunodeficiency virus type 1 in perinatally infected infants with rapid and slow progression to disease. J. Virol. 71**:**4694-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schacker, T. W., J. P. Hughes, T. Shea, R. W. Coombs, and L. Corey. 1998. Biological and virologic characteristics of primary HIV infection. Ann. Intern. Med. 128**:**613-620. [DOI] [PubMed] [Google Scholar]
- 32.Shankarappa, R., J. B. Margolick, S. J. Gange, A. G. Rodrigo, D. Upchurch, J. Farzadegan, P. Gupta, C. R. Rinaldo, G. H. Learn, X. He, X.-L. Huang, and J. I. Mullins. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73**:**10489-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfs, T. F., G. Zwart, M. Bakker, and J. Goudsmit. 1992. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology 189**:**103-110. [DOI] [PubMed] [Google Scholar]
- 34.Wolinsky, S. M., C. M. Wike, B. T. Korber, C. Hutto, W. P. Parks, L. L. Rosenblum, K. J. Kunstman, M. R. Furtado, and J. L. Munoz. 1992. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science 255**:**1134-1137. [DOI] [PubMed] [Google Scholar]
- 35.Wolinsky, S. M., B. T. Korber, A. U. Neumann, M. Daniels, K. J. Kunstman, A. J. Whetsell, M. R. Furtado, Y. Cao, D. D. Ho, J. T. Safrit, and R. A. Koup. 1996. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science 272**:**537-542. [DOI] [PubMed] [Google Scholar]
- 36.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society—USA Panel. JAMA 288**:**222-235. [DOI] [PubMed] [Google Scholar]
- 37.Yuste, E., S. Sanchez-Palomino, C. Casado, E. Domingo, and C. Lopez-Galindez. 1999. Drastic fitness loss in human immunodeficiency virus type 1 upon serial bottleneck events. J. Virol. 73**:**2745-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, L. Q., P. MacKenzie, A. Cleland, E. C. Holmes, A. J. Brown, and P. Simmonds. 1993. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 67**:**3345-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science 261**:**1179-1181. [DOI] [PubMed] [Google Scholar]