A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice (original) (raw)
Abstract
Interleukin 21 (IL-21) is a pleiotropic cytokine produced by CD4 T cells that affects the differentiation and function of T, B, and NK cells by binding to a receptor consisting of the common cytokine receptor γ chain and the IL-21 receptor (IL-21R). IL-21, a product associated with IL-17-producing CD4 T cells (TH17) and follicular CD4 T helper cells (TFH), has been implicated in autoimmune disorders including the severe systemic lupus erythematosus (SLE)-like disease characteristic of BXSB-Yaa mice. To determine whether IL-21 plays a significant role in this disease, we compared IL-21R-deficient and -competent BXSB-Yaa mice for multiple parameters of SLE. The deficient mice showed none of the abnormalities characteristic of SLE in IL-21R-competent Yaa mice, including hypergammaglobulinemia, autoantibody production, reduced frequencies of marginal zone B cells and monocytosis, renal disease, and premature morbidity. IL-21 production associated with this autoimmune disease was not a product of TH17 cells and was not limited to conventional CXCR5+ TFH but instead was produced broadly by ICOS+ CD4+ splenic T cells. IL-21 arising from an abnormal population of CD4 T cells is thus central to the development of this lethal disease, and, more generally, could play an important role in human SLE and related autoimmune disorders.
Keywords: autoimmune disease, autoantibodies, B cells, T cells
Systemic lupus erythematosus (SLE) in humans is a chronic, multigenic autoimmune disease characterized by a wide spectrum of clinical abnormalities, the production of multiple autoantibodies, and the generation of immune complexes that often lead to severe renal disease. The production of autoantibodies is indicative of a profound breakdown in humoral tolerance mechanisms and B cell hyperactivity caused either by B cell-intrinsic abnormalities or immunoregulatory defects of other cell types. Mouse models genetically programmed to develop characteristics of SLE have proven useful for characterizing this disease process and for identifying potential therapeutic targets. Among the most interesting models of SLE are the BXSB mice bearing the Y chromosome-linked autoimmune acceleration (Yaa) mutation (1). Affected animals develop a remarkably severe disease characterized by lymphoid hyperplasia, monocytosis, hypergammaglobulinemia, and severe immune complex-mediated glomerulonephritis. In contrast to the female prevalence of SLE in humans, this disease is male-biased because of the epistatic effects of BXSB-background autosomal alleles in combination with Yaa. This mutant locus is the result of the duplication of at least 17 genes in the X chromosome, including Toll-like receptor 7 (Tlr7), and their placement in the Y chromosome (2, 3). Remarkably, severe autoimmune disease results from the presence of an extra copy of these X-chromosome genes in Yaa mice in combination with BXSB-background autosomal alleles.
IL-21 is a pleiotropic member of the γ-chain family of cytokines, which engages the IL-21 receptor (IL-21R) and the common cytokine receptor γ-chain expressed on cells of both lymphoid and myeloid lineages (ref. 4, reviewed in ref. 5). This cytokine is expressed by antigen-stimulated peripheral T cells (4) and by T follicular cells (TFH) cells (6, 7), and may act in an autocrine and/or paracrine manner to support the development and maintenance of TH17 cells (8–10). IL-21 is a potent inducer of primary CD8+ T cell and NK cell activity, and acts in synergy with IL-15 in regulating CD8+ T cell expansion and memory function (11). In B cells, the effect of IL-21 is dependent on their stage of differentiation, such that engagement with naïve B cells confers an apoptotic signal while it promotes T cell-dependent maturation of antigen-experienced B cells to plasma cells (12, 13) and is essential for germinal center (GC) formation (6). Recent studies in humans suggest that allelic variation in the IL-21 gene is a risk factor for SLE (14). Previous work showed that BXSB-Yaa mice have elevated IL-21 at the transcriptional and serum protein levels (12). Here we show that IL-21 signaling is essential for the SLE-like autoimmune disease of BXSB-Yaa mice and describe the mechanisms responsible.
Results
Elevation of Il21 Expression by Noncirculating T Cells from Aging BXSB-Yaa Mice.
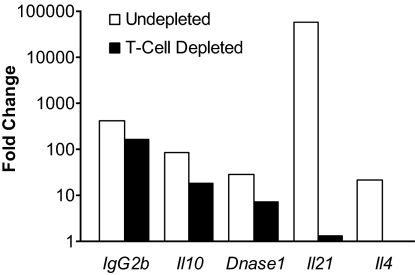
Our previous studies showed that Il21 transcripts and serum levels of IL-21 protein are elevated in BXSB-Yaa but not BXSB-wild type (wt) mice at 16 weeks of age (12). To determine if this elevation is age-related, we used real-time quantitative RT-PCR (qPCR) arrays (15) to monitor transcript levels of Il21 and other genes in spleen cells of mice at 8 weeks of age, before overt disease, and at 16 weeks of age, when the mice are uniformly ill. The results showed that levels of transcripts for Il21, as expected, as well as Il4, Il10, and Dnase1, were significantly increased in 2 cohorts of 16-week-old but not in 8-week-old BXSB-Yaa mice (Table 1). The marked increases in IgG2b transcripts provide an independent marker for the striking B cell activation and maturation associated with this disease. Parallel studies of peripheral blood leukocytes from mice of the same ages showed that none of these genes were expressed at increased levels (data not shown). To determine the cellular origins of these transcripts, we performed the same studies on spleens acutely depleted of T cells (Fig. 1). This treatment resulted in striking reductions in transcripts for Il21 and Il4 and substantial but less profound decreases in expression of Il10 and Dnase1. Levels of IgG2b transcripts were essentially unchanged. These studies showed that expression of Il21 is T cell-dependent and increases in a time-dependent manner in association with the development of disease.
Table 1.
Expression of Il21 by splenic T cells increases as BXSB-Yaa mice age
| Gene | Gene expression, fold change | ||
|---|---|---|---|
| 8-week spleen | 16-week group A spleen | 16-week group B spleen | |
| IgG2b | N.S. | 40.7 | 51.4 |
| Il10 | N.S. | 17.4 | 8.4 |
| Il21 | N.S. | 16.6 | 24 |
| Il4 | N.S. | N.S. | 6 |
| Dnase1 | N.S. | 6.4 | 10.6 |
Fig. 1.
IL-21 is the product of T cells. Fold change computations were based on the mean CT (cycle threshold) values normalized to 18S RNA from spleen cells pooled from 2 21-week-old BXSB-Yaa and 2 BXSB-wt mice, depleted of both CD4+ and CD8+ T cells or left intact.
IL-21R Deficiency Prevents the Humoral Components of the BXSB-Yaa Autoimmune Disease.
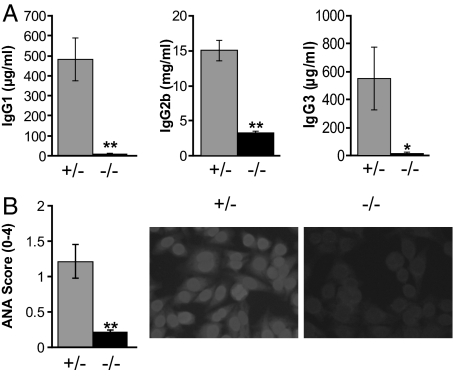
The humoral manifestations of disease in Yaa mice include marked elevation in serum levels of class-switched Ig isotypes and autoantibodies. To examine whether IL-21 contributes to these phenotypes, we studied BXSB-Yaa mice homozygous for a null allele of IL-21R (_Yaa/Il21_−/−) at backcross generation 5–6 and used littermates heterozygous for IL-21R (Yaa/Il21r+/−) as controls. We first compared 16-week-old mice of both genotypes for serum levels of IgG1, IgG2b, and IgG3 (Fig. 2A). The levels of all 3 isotypes were markedly lower in sera of _Yaa/Il21_−/− mice. We then tested sera from the same mice for the presence of serum anti-nuclear autoantibodies (ANA) as determined by immunostaining of Hep-2 cell nuclei (Fig. 2B). These data showed that ANA were greatly decreased in mice unable to signal through the IL-21R. The overall results demonstrated that IL-21 signaling is required for the humoral manifestations of BXSB-Yaa autoimmunity.
Fig. 2.
IL-21R deficiency prevents humoral components of the BXSB-Yaa autoimmune disease. (A) ELISA analysis of Ig subclass levels in plasma from 10 Yaa/Il21r+/− (gray bars) and 10 _Yaa/Il21_−/− (black bars) 16-week-old mice. (B) Anti-nuclear antibodies (ANA) from Yaa/Il21r+/− mice compared with _Yaa/Il21_−/− mice detected by staining of Hep-2 cell slides (Right) with quantitation by ImageJ (Left). *, P < 0.05; **, P < 0.002.
IL-21R Deficiency Prevents the Abnormal Leukocyte Populations Characteristic of BXSB-Yaa Mice.
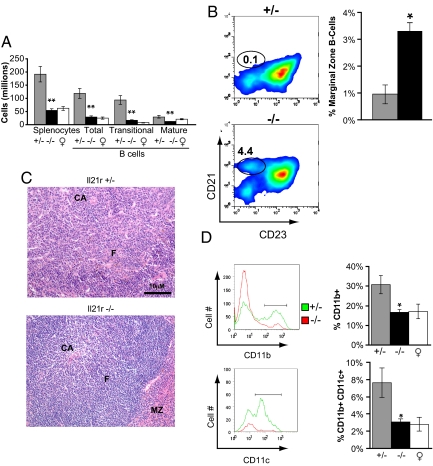
Yaa mice develop a number of poorly understood abnormal leukocyte phenotypes coincident with autoimmune disease. These include lymphoid hyperplasia manifested by splenomegaly and lymphadenopathy, marked reductions in the size of the splenic marginal zone (MZ) and the frequencies of MZ B cells, and a striking increase in the frequency of monocytes. To evaluate the potential contribution of heightened IL-21 signaling to these phenotypes, we examined the spleens of the above-described Yaa/Il21r+/− and _Yaa/Il21_−/− mice (Fig. 3). In contrast to the enlarged, hypercellular spleens from Yaa/Il21r+/−mice, spleens from _Yaa/Il21_−/− mice did not differ significantly from control non-Yaa female BXSB mice in total weight (data not shown) or cellularity as assessed by flow cytometry (Fig. 3A). In addition, spleens of _Yaa/Il21_−/− mice had significantly fewer total B cells as well as transitional and mature B cell subsets as determined by flow cytometry (Fig. 3A). A similar dissociation was observed for newly formed and postswitch splenic B cells, which are typically expanded in BXSB-Yaa mice but were present in normal numbers in IL-21R-deficient animals (Fig. S1). In contrast to the low numbers of MZ B cells, poorly developed MZs and disorganized splenic architecture typical of Yaa/Il21r+/− mice, the frequencies of MZ B cells were substantially elevated (Fig. 3B) and the MZs and follicular organization of _Yaa/Il21_−/− mice appeared normal in structure (Fig. 3C). In addition, spontaneous atypical GC formation, a characteristic of Yaa/Il21r+/− mice, was not observed in mice deficient in Il21r expression (data not shown). As expected, a high proportion of T cells and B cells in 16-week-old Yaa/Il21r+/− mice expressed the activation markers Ly6a/e and CD69, whereas the expression of these activation markers was reduced in T and B cells of _Yaa/Il21_−/− mice (Table S1). Yaa mice are also characterized by a progressive monocytosis marked by the presence of increasing numbers of CD11b+ monocytes and CD11b+ CD11c+ dendritic cells in the peripheral blood and spleen (16, 17). Studies of spleen cells showed that the frequencies of cells with this phenotype were profoundly decreased in IL-21R-deficient mice (Fig. 3D). We conclude that heightened IL-21 signaling is responsible for each of the _Yaa_-dependent histological, numeric, and cellular abnormalities that characterize BXSB-Yaa mice.
Fig. 3.
IL-21R deficiency prevents the abnormal leukocyte populations characteristic of BXSB-Yaa mice. (A) Spleens of _Yaa/Il21_−/− mice exhibit greatly reduced cellularity compared with spleens of Yaa/Il21r+/− mice. Yaa/Il21r+/− mice have high numbers of total B cells, and immature and mature B cells; this phenotype was abolished in _Yaa/Il21_−/− mice. (B) The frequencies of splenic MZ B cells (CD19+ CD21hi CD23lo) typically depleted in Yaa/Il21r+/− mice are restored in _Yaa/Il21_−/− mice. (C) Yaa/Il21r+/− but not _Yaa/Il21_−/− spleens lack an obvious MZ and show expansion in red pulp with monocytosis and accumulations of plasma cells resulting in compression of the white pulp. Central arteriole (CA), follicle (F) of H&E-stained sections. (D) _Yaa/Il21_−/− mice do not develop spleen cell monocytosis as measured by CD11b expression and have fewer CD11c-positive dendritic cells compared with Yaa/Il21r+/− littermate controls. Data are representative of spleens from 16-week-old mice. Data from BXSB female mice thus lacking Yaa of the same age are included for comparison.
IL-21R Deficiency Prevents Renal Disease and Mortality Characteristic of BXSB-Yaa Mice.
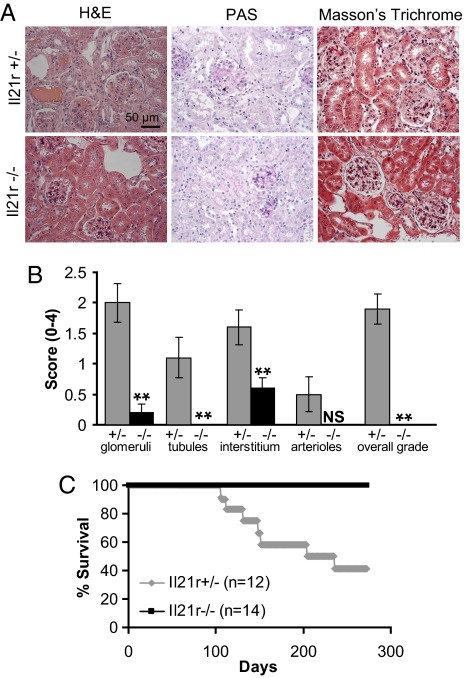
Accelerated mortality in Yaa mice is due to the progressive development of immune-complex-mediated glomerulonephritis. The observations that _Yaa/Il21_−/− mice are not hypergammaglobulinemic and have greatly reduced levels of autoantibodies suggested that these mice might be protected from the development of lethal renal disease. To examine this issue, we first compared the histologic features of kidneys from 16-week-old _Yaa/Il21_−/− and Yaa/Il21r+/− mice. Kidneys of Yaa/Il21r+/− mice exhibited multiple pathological features, including a thickening of the glomerular basement membrane, perivascular inflammation associated with neutrophilic infiltrates, and focal adhesions with marked glomerular fibrosis, sclerosis, and Ig deposits (Fig. 4 A and B). In contrast, kidneys from _Yaa/Il21_−/− mice were essentially free of all these abnormalities. To determine if abrogation of renal disease would be predictive of prolonged survival, we followed a cohort of _Yaa/Il21_−/− and Yaa/Il21r+/− mice for 275 days. As expected, nearly 50% of the Yaa/Il21r+/− mice had become moribund by ≈180 days. In contrast, all Yaa/Il21r+/− mice remained healthy for the duration of the experiment (Fig. 4C). These data indicate that a deficiency in IL-21R prevented the development of key immunopathological processes responsible for the shortened lifespans of BXSB-Yaa mice.
Fig. 4.
IL-21R deficiency prevents renal disease and mortality characteristic of BXSB-Yaa mice. (A and B) Kidney sections from 16-week-old Yaa/Il21r+/− and _Yaa/Il21_−/− mice were stained with H&E, periodic acid/Schiff reagent (PAS), or Masson's trichrome. (A) Representative sections. (B) Kidneys were graded for severity of changes. N.S., not significant at P ≤ 0.05. (C) Kaplan–Meier lifespan analysis indicated a significant Wilcoxon P value of 0.0017 for survival differences.
Analysis of Involvement of TH17 and TFH.
We then addressed the potential involvement of TH17 and TFH as the source of IL-21 needed to promote this autoimmune disease. In addition to strikingly reduced expression of 2 switched Ig isotypes, there were marked reductions in transcripts for Il10, and, predictably, Il21r, with splenic cDNAs of _Yaa/Il21_−/− mice compared with those of Yaa/Il21r+/− mice (Fig. S2_A_). The reduced levels of Il10 transcripts are in keeping with earlier studies showing that IL-10 is produced by human B cells and mouse NK cells stimulated with IL-21 (18, 19). However, we observed only variable and statistically insignificant differences in Il17 expression in studies of cDNAs prepared from either total spleen cells or semipurified splenic CD4 T cells from 16-week-old Yaa/Il21r+ and _Yaa/Il21_−/− mice (Fig. S2 A and B). By using flow cytometry, we also failed to detect any significant difference in intracellular IL-17 protein staining of splenic CD4 T cells from mice similarly aged before and after culture conditions that promote TH17 cell polarization (Fig. S2_C_). In addition, we failed to detect changes in Il17 gene expression or intracellular IL-17 protein expression of either freshly isolated CD4 T cells or purified naïve CD4 T cells after polarization as well as in CD4 T cells that were expanded with IL-23 in vitro (data not shown). The results showed that the transcript levels of Il17 and frequencies of TH17 cells did not correlate with autoimmune manifestations that distinguish IL-21R-deficient and -competent BXSB-Yaa mice and that TH17-cell frequencies ex vivo and after culture did not appreciably differ between mice of these 2 genotypes.
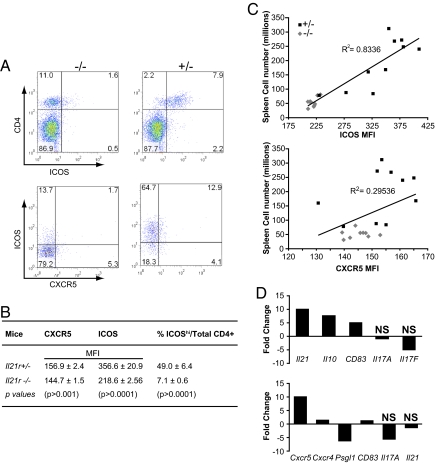
Because IL-21 is also considered to be a product of the TFH subset of CD4 T cells, we then addressed whether a deficiency in IL-21R would influence the frequency of splenic cells that are characterized by the coexpression of the TFH markers CXCR5 and ICOS (20, 21). The proportions of CXCR5 and ICOS-positive CD4+ cells were abnormally high in spleens of Yaa/Il21r+/− mice, with ICOS+ CD4 T cells comprising 49.0 ± 6.4% of CD4 T cells. In contrast, the frequencies of ICOS+ CD4+ cells in spleens of _Yaa/Il21_−/− mice were substantially reduced to 7.1 ± 0.6% of CD4 T cells (Fig. 5 A and B). Moreover, strong correlation (_R_2 = 0.83) was observed comparing ICOS expression of CD4 T cells with total spleen cell numbers of the Yaa/Il21r+/− and _Yaa/Il21_−/− cohorts (Fig. 5C). However, although the frequencies of CD4+ T cells expressing CXCR5 were also elevated in Yaa/Il21r+/− mice (Fig. 5 A and B), the correlation with total spleen cell numbers was weak (_R_2 = 0.29) (Fig. 5C). Gene expression analysis of flow-sorted CD4 T cells was then carried out to address the relationships of ICOS and CXCR5 to Il21 expression. Expression of Il21 was enriched ≈10-fold in ICOShi as compared with ICOSlo CD4 T cells that were isolated from 5-month-old BXSB-Yaa mice (Fig. 5D). To determine whether the level of CXCR5 affected Il21 expression, in a separate experiment we compared flow cytometry-sorted splenic ICOShi CXCR5hi and ICOShi CXCR5lo CD4 T cells pooled from 5-month-old BXSB-Yaa mice. The levels of Il21 transcripts did not differ between CXCR5hi and CXCR5lo T cells, whereas Cxcr5 transcripts were significantly increased, confirming the enrichment procedure. In addition, sorted CXCR5lo cells exhibited lower expression of the P-selectin glycoprotein ligand-1 (Psgl1), a finding in keeping with recent studies of SLE-predisposed MRL/lpr mice showing that low expression of this marker is characteristic of a novel non-TFH population that was a major source of IL-21 (22). Finally, although the expression of Cxcr4 was decreased and Cd83 was elevated in the sorted CXCR5hi T cells, these makers did not correlate with IL-21 expression. Taken together, these results suggest that the increased IL-21 that is critically required for the BXSB-Yaa autoimmune disease does not necessarily derive from conventional TFH cells but instead appears to arise more generally from ICOS+ CD4 T cells.
Fig. 5.
IL-21R deficiency decreases numbers of TFH cells. Splenic CD4 T cells from 16-week-old _Yaa/Il21_−/−, Yaa, and Yaa/Il21r+/− mice were analyzed for ICOS and CXCR5 expression by flow cytometry analysis. (A) Representative data. (B) Mean fluorescence intensity (MFI) and percent ICOShi splenic CD4 T cells; there were 10 mice per group. (C) Correlation of total mononuclear spleen cell numbers with MFI of ICOS (Upper) or CXCR5 (Lower) on CD4 T cells of individual 16-week-old Yaa/Il21r+/− and _Yaa/Il21_−/− mice. (D) Gene expression comparison of flow cytometry-sorted ICOShi and ICOSlo splenic CD4 T cells (Upper) and ICOShi CXCR5hi and CXCR5lo splenic CD4 T cells (Lower) from BXSB-Yaa mice. See Fig. S4 for flow cytometric gates.
Discussion
The results described here demonstrate that development of the severe SLE-like disease characteristic of BXSB-Yaa mice is critically dependent on IL-21 signaling. BXSB-Yaa mice deficient in IL-21R exhibited none of the prominently abnormal phenotypes typical of this autoimmune syndrome, resulting in healthy mice with a greatly extended, if not normal, lifespan. It is very unlikely that the effects observed in mice homozygous for the _Il21r_-null allele at backcross generation 5–6 can be attributed to residual genetic variation or passenger alleles linked to the Il21r gene. We base this conclusion first on the demonstration that Il21r+/− BXSB-Yaa mice tested after 11 backcross generations developed similarly severe autoimmune disease, which is abrogated in _Il21r_−/− littermate controls (Fig. S3). Second, the major susceptibility loci associated with autoimmune disease in this model have been rigorously mapped and none are located near Il21r (23, 24). IL-21 signaling is therefore fundamental to one or more critical steps in the pathogenesis of SLE in BXSB-Yaa mice. In a previous study, we sought to block IL-21 signaling by repeated treatment of BXSB-Yaa mice with a soluble IL-21R-Fc fusion protein, but documented only minimal and variable changes in SLE biomarkers and overall survival (25). The basis for this apparent discrepancy with the results from the current study of IL-21R-deficient mice is not known. Most likely, however, it can be ascribed to a complete absence of IL-21/IL-21R signaling in IL-21R-deficient mice as compared with only partial effectiveness of IL-21R-Fc treatment in blocking these interactions. A more appreciable but still only partial benefit with the same IL-21R-Fc compound was observed with the MRL-lpr SLE model (26) and in models for experimentally-induced diseases of inflammatory cell etiology, including collagen-induced arthritis (27). Although it remains to be shown definitely that IL-21 contributes to human SLE, the observation that allelic variation in the IL-21 gene is a risk factor for SLE (14) is consistent with such a possibility. Taken together, these results suggest that interruption of the IL-21 signaling pathway merits intensive investigation as a therapeutic option for treating SLE and potentially other autoimmune diseases. The very striking effects of IL-21R deficiency on the severe autoimmune disease of BXSB-Yaa mice and the lesser effects seen with mice treated with the soluble IL-21R-Fc fusion proteins suggest that the latter approach to treatment may require modification or supplementary interventions.
IL-21 is a product of CD4 T cells, with evidence for both inflammatory TH17 cells and TFH as potential sources (7). Studies have reported that mice with defective IL-21 signaling have reduced numbers of TH17 cells (8) and a role for IL-21-producing TH17 cells has been proposed for humoral aspects of autoimmune disease developed by BXD2 mice (28). Although initial studies suggested a role for IL-21 acting through TH17 in experimental allergic encephalomyelitis (9), recent studies analyzing both IL-21R-deficient and IL-21-deficient mice have questioned this conclusion (29–32). Our studies argue strongly that TH17 cells are not responsible for the IL-21-dependent signaling that is required for the BXSB-Yaa disease.
In contrast, there is considerable evidence that TFH cells, characterized by the coexpression of ICOS and CXCR5, are a robust source for IL-21 (6, 7). Studies in humans have shown that by producing IL-21, TFH cells play a key role in promoting the differentiation of B cells to Ig-secreting plasma cells in the GC (33). This is consistent with studies showing that IL-21 is critical for Ig production and plasma cell differentiation in both mice and humans, presumably through its induction of Blimp-1 (12, 34). BXSB-Yaa mice exhibited a marked expansion of ICOS+ CD4 T cells that were markedly reduced in mice deficient in IL-21R. The elevations of ICOS expression on CD4 T cells, which correlated nicely with overall splenic cellularity as a measure of disease, were greatly diminished by an IL-21R deficiency. In addition, Il21 mRNA was enriched 10-fold in ICOShi vs. ICOSlo CD4 T cells from BXSB-Yaa mice. In contrast, there was no correlation between splenic cellularity and CXCR5 expression and Il21 mRNA was not enriched in CXCR5hi vs. CXCR5lo ICOS+ CD4 T cells. These results suggest that the cellular origin of IL-21 critically required to cause autoimmune disease in the BXSB-Yaa model is not limited to conventional TFH. Similar conclusions supporting a non-TFH origin of IL-21 have been very recently described in the MRL/lpr SLE mouse model with IL-21 being produced by extrafollicular CXCR5− ICOS+ T cells that express low levels of Psgl1 (22). Expression of IL-21 by noncanonical TFH that are not restricted to B cell follicles may thus lead to the disorganized follicular structure, ectopic GC formation, and the excessive autoantibody production characteristic of severe SLE.
Our study also helps to clarify the role of Yaa in the pathogenesis of SLE-like autoimmunity. The Yaa mutation required for this severe autoimmune syndrome is the result of a 4-Mb X → Y chromosome duplication of genes including Tlr7 (2, 3). This duplication causes B cells and dendritic cells to be more readily activated by endogenous TLR7 stimuli, such as single-stranded RNA, through an NFκB pathway (2). The Yaa phenotype is B cell, but not T cell, intrinsic (35–38), and B cells are absolutely required for development of this disease (unpublished results). This suggests that the atypical B cells of BXSB-Yaa mice may directly or indirectly promote the differentiation/expansion of IL-21-secreting CD4+ T cells in response to TLR7 ligation.
An expansion of ICOShi CD4 T cells that overexpress IL-21 similar to that which is found in BXSB-Yaa mice is observed in SLE-prone Sanroque mice that carry a hypomorphic mutation in the RING-type ubiquitin ligase gene, Rc3h1 (20, 39). The product of the wild-type allele of a gene destabilizes ICOS mRNA, repressing the differentiation of ICOS+ CD4 T cells (21, 39). Elevated TLR7 signaling in B cells may cause T cells to override this natural repressor mechanism, resulting in ICOS+ T cells that generically produce the IL-21. Thus, endogenous innate immune stimuli may act through B cells to disrupt normal T cell differentiation and homing. The IL-21 produced outside of the normal constraints of the lymphoid follicles may then drive the differentiation of autoreactive Yaa B cells into autoantibody-secreting plasma cells while also inducing additional manifestations of this severe SLE-like disease, a scenario that may also extend to other antibody-mediated autoimmune syndromes.
Materials and Methods
Mice.
BXSB/MpJ-Yaa mice were bred and maintained in a specific pathogen-free mouse colony at the Jackson Laboratory. BXSB.B6-Yaa+/J were used as BXSB-wt mice for data in Fig. 1. We generated IL-21R-deficient STOCK-Il21rtm1Wjl mice (13) serially backcrossed 5–6 generations to BXSB/MpJ-Yaa to create BXSB.129 Il21rtm1Wjl mice. For some experiments, _Il21r_−/− and control littermate Il21r+/− or Il21r+/+ BXSB-Yaa male mice from backcross generations 10–11 were used. Oligonucleotide primer sequences for mutation genotyping detecting the wild-type band were mIL-21R F-5′-CATTTCCAAAGAGCTCCAGTAAACAG-3′; R- 5′-CTTGGCCTGCAGTTCTGACG-3′ used in combination with standard neo primers.
ELISA and ANA.
ELISAs for Ig subclasses were performed by using unlabeled goat anti-mouse Ig (IgG1, IgG2b, and IgG3) as described in ref. 40. Data are expressed as concentration of Ig/ml based on interpolation by using a standard curve based on titration of purified mouse Igs. Anti-double-stranded DNA antibodies were determined by ELISA as described in ref. 41. For ANA, serum was diluted 1:40 in PBS and 15 μl was applied to each spot on Hep-2 slides (Antibodies, Inc.) following the supplier's recommendations. ANA intensity was quantitated by using ImageJ software (National Institutes of Health) containing the RGB Measure plug-in. Negative and positive serum control samples provided with the slides were used to set the 0 and 4 values, respectively.
Flow Cytometry.
Flow cytometric analysis was performed by using conventional multiparameter procedures. Analysis was carried out on a FACScan (Becton Dickinson) with CELLQuest software for acquisition and FlowJo software (Tree Star) for analysis. Viable cells were gated by propidium iodide exclusion. Immature (transitional) B cells were assayed as being B220+, AA4.1high. Mature B cells were considered to be those that were B220+, AA4.1lo. MZ B cells were identified as CD19+, CD21hi, and CD23lo. The activation status of T cells and B cells was monitored by using CD69 or Ly6a/e (SCA-1) mAbs in combination with CD3 (T cell) and CD19 (B cell) markers, and mAb to CXCR5 and ICOS on CD4+ T cells were used to identify TFH cells. Monocytosis was evaluated by staining with mAb to CD11b and CD11c.
Histology.
Tissues obtained at necropsy were fixed, embedded in formalin, and stained. Histological sections were graded in a blinded manner. Four major structures of the kidney were graded on a scale of 0 (normal) to 4 (severe) pathology. At least 10 glomeruli were examined for each animal. An overall grade was applied that reflects all of the changes and takes into account the proportion of the appropriate structures affected in the entire section (42, 43).
Gene Expression.
Gene expression was performed by using ImmunoQuantArrays and analyzed by the GPR algorithm as described (15) or by conventional ΔCT normalization procedures. For a gene list of the 192 and 384 gene ImmunoQuantArrays used in Fig. 1, write to D.C.R. For genes and primer sequences used for data in Fig. 5 and Fig. S2, see Table S2. Validated primers for Il17b,- c,- d, and -f were obtained from RealTimePrimers.com. For T cell depletion studies, T cells were negatively depleted by anti-CD4 + anti-CD8 MAC beads, and depletion was ≈95% complete as assessed by flow cytometry using an anti-CD3 mAb. For TFH enrichment studies, we sort-purified ICOShi CD4+ and ICOSlo CD4+ populations. For CXCR5 subset studies, we sort-purified CD4+ ICOS+ CXCR5hi and CD4+ ICOS+ CXCR5lo populations by using a FACSVantageSE, and RNA was prepared by using Qiagen MicroRNeasy kits.
Supplementary Material
Supporting Information
Acknowledgments.
This work was supported in part by a postdoctoral fellowship from the Arthritis Foundation to J.A.B.; an Arthritis Foundation Innovative Research Grant and National Institutes of Health Grants DK56597 and R21 DK074463 to D.C.R.; and the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Heart, Lung, and Blood Institute.
Footnotes
Conflict of interest statement: The authors are listed as co-inventors on applications for and/or issued patents related to IL-21.
This article is a PNAS Direct Submission.
References
- 1.Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979;22:1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 2.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 5.Spolski R, Leonard WJ. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 6.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 11.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki K, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 14.Sawalha AH, et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:458–461. doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- 15.Akilesh S, Shaffer DJ, Roopenian D. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 2003;13:1719–1727. doi: 10.1101/gr.533003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wofsy D, Kerger CE, Seaman WE. Monocytosis in the BXSB model for systemic lupus erythematosus. J Exp Med. 1984;159:629–634. doi: 10.1084/jem.159.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amano H, et al. Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum. 2005;52:2790–2798. doi: 10.1002/art.21365. [DOI] [PubMed] [Google Scholar]
- 18.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 19.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 20.Vinuesa CG, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 21.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 22.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maibaum MA, Haywood ME, Walport MJ, Morley BJ. Lupus susceptibility loci map within regions of BXSB derived from the SB/Le parental strain. Immunogenetics. 2000;51:370–372. doi: 10.1007/s002510050632. [DOI] [PubMed] [Google Scholar]
- 24.Rogers NJ, et al. Monocytosis in BXSB mice is due to epistasis between Yaa and the telomeric region of chromosome 1 but does not drive the disease process. Genes Immun. 2007;8:619–627. doi: 10.1038/sj.gene.6364424. [DOI] [PubMed] [Google Scholar]
- 25.Bubier JA, et al. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1110:590–601. doi: 10.1196/annals.1423.063. [DOI] [PubMed] [Google Scholar]
- 26.Herber D, et al. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R. Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 27.Young DA, et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007;56:1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]
- 28.Hsu HC, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 29.Suto A, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 31.Holmdahl R. IL-21 and autoimmune disease - hypothesis and reality? Eur J Immunol. 2008;38:1800–1802. doi: 10.1002/eji.200838529. [DOI] [PubMed] [Google Scholar]
- 32.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 33.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: Predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 34.Ettinger R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 35.Fossati L, et al. The Yaa gene-mediated acceleration of murine lupus: Yaa− T cells from non-autoimmune mice collaborate with Yaa+ B cells to produce lupus autoantibodies in vivo. Eur J Immunol. 1995;25:3412–3417. doi: 10.1002/eji.1830251231. [DOI] [PubMed] [Google Scholar]
- 36.Merino R, Fossati L, Lacour M, Izui S. Selective autoantibody production by Yaa+ B cells in autoimmune Yaa(+)-Yaa− bone marrow chimeric mice. J Exp Med. 1991;174:1023–1029. doi: 10.1084/jem.174.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DesJardin LE, Butfiloski EJ, Sobel ES, Schiffenbauer J. Hyperproliferation of BXSB B cells is linked to the Yaa allele. Clin Immunol Immunopathol. 1996;81:145–152. doi: 10.1006/clin.1996.0170. [DOI] [PubMed] [Google Scholar]
- 38.Lawson BR, et al. The role of alpha beta+ T cells and homeostatic T cell proliferation in Y-chromosome-associated murine lupus. J Immunol. 2001;167:2354–2360. doi: 10.4049/jimmunol.167.4.2354. [DOI] [PubMed] [Google Scholar]
- 39.Yu D, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 40.Roopenian DC, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 41.Christianson GJ, et al. Beta2-microglobulin dependence of the lupus-like autoimmune syndrome of MRL-lpr mice. J Immunol. 1996;156:4932–4939. [PubMed] [Google Scholar]
- 42.Pirani CL, Pollak VE, Schwartz FD. The reproducibility of semiquantitative analyses of renal histology. Nephron. 1964;29:230–237. doi: 10.1159/000179336. [DOI] [PubMed] [Google Scholar]
- 43.Appel GB, Pirani CL, D'Agati V. Renal vascular complications of systemic lupus erythematosus. J Am Soc Nephrol. 1994;4:1499–1515. doi: 10.1681/ASN.V481499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information