Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy (original) (raw)
Abstract
Background Recent studies showed that angiotensin II type 1 receptor blocker (ARB) slows progression of chronic renal disease in patients with type 2 diabetes, regardless of changes in blood pressure. We showed that the imbalance of nitric oxide (NO) and reactive oxygen species (ROS) due to endothelial NO synthase (eNOS) uncoupling contributed to renal dysfunction in the diabetic nephropathy. The aim of this study was to determine the effects of ARB on uncoupled eNOS in rat diabetic nephropathy.
Methods. Diabetes was induced in Sprague-Dawley rats with streptozotocin (65 mg/ kg body weight). After 6 weeks, rats were divided into saline (DM; n = 11) and ARB, losartan groups (DM+Los; n = 11). After 2-week treatment, glomerular ROS production was assessed by 2′,7′-dichlorofluorescin diacetate (DCFH-DA)-derived chemiluminescence. Renal NO and ROS production were imaged by confocal laser microscopy after renal perfusion with DCFH-DA and diaminorhodamine-4M acetoxymethyl ester with l-arginine. The dimeric form of eNOS was measured by low-temperature sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Serum tetrahydrobiopterin (BH4) concentrations were determined by high-performance liquid chromatography. Protein and mRNA expression of GTP cyclohydrolase 1 (GTPCH1), key enzyme of BH4 synthesis, were examined.
Results Losartan attenuated glomerular ROS production in DM. Accelerated ROS production and diminished bioavailable NO caused by NOS uncoupling were noted in DM glomeruli. Losartan reversed the decreased GTPCH1 and decreased dimeric form of eNOS and glomerular NO production by increased BH4 bioavailability.
Conclusions. ARB improved the NOS uncoupling in diabetic nephropathy by increasing BH4 bioavailability.
Keywords: angiotensin II type 1 receptor blocker, endothelial dysfunction, eNOS uncoupling, GTP cyclohydrolase 1, tetrahydrobiopterin
Introduction
There is growing evidence that endothelial dysfunction, which is often defined as a decrease in the bioavailability of endothelial-derived nitric oxide (NO), is a critical pathological state that could lead to vascular disease states such as hypertension, atherosclerosis and diabetes. Because superoxide rapidly scavenges NO within the endothelium, a reduction in bioactive NO might occur despite the increase in NO generation. Among many enzymatic systems that are capable of producing superoxide, the NAD(P)H oxidase and the uncoupled endothelial NO synthase (eNOS) are the main sources of superoxide in vascular wall [1] in diabetic patients. We also reported that in rat diabetic kidney, NAD(P)H oxidase and uncoupled eNOS were major sources of glomerular superoxide [2]. Accordingly, these mechanisms are potential key targets for therapeutic interventions in endothelial dysfunction.
It was demonstrated that administration of angiotensin II (Ang II) receptor blockers (ARBs) slows the progression of diabetic nephropathy [3] and the development of proteinuria [4] in patients with type 2 diabetes. This effect is specific and independent of their blood pressure lowering effect. Inhibition of the Ang II type 1 (AT1) receptor in diabetic patients reverses endothelial dysfunction [5]. Furthermore, long-term treatment with the ARB normalizes the structure of subcutaneous small arteries of hypertensive patients with non-insulin-dependent diabetes [6]. Thus, the control of Ang II signalling by ARB could be potentially effective in improving endothelial function in diabetes. However, it is not clear whether ARB could improve eNOS uncoupling in hyperglycaemia-induced diabetic nephropathy. Accordingly, it was decided to examine whether short-term blockade of the AT1 receptor using losartan would have favourable effects on uncoupled eNOS in the diabetic glomeruli of rats with streptozotocin (STZ)-induced diabetes by lowering oxidative stress.
Subjects and methods
Experimental protocol and tissue preparation
The experimental protocol (No. 06-029) was approved in advance by the Ethics Review Committee for Animal Experimentation of the Kawasaki Medical School (Kurashiki, Japan). Male Sprague-Dawley rats (6–7 weeks old) weighing 190 to 220 g were purchased from Charles River Japan Inc. (Kanagawa, Japan). Diabetes was induced by a single tail vein injection of STZ (65 mg/kg body weight; Sigma-Aldrich Japan, Tokyo, Japan) diluted in a citrate buffer, pH 4.5 [diabetes mellitus (DM); n = 22]. Age-matched non-diabetic control rats (Cont; n = 22) were each injected with an equal volume of citrate buffer. Six weeks after induction of DM, 11 rats from each group were provided over a period of 2 weeks with drinking water containing losartan (Banyu Pharmaceutical, Japan; 30 mg/kg/day, Cont+Los and DM+Los group). During the experimental period, body weight and blood glucose were measured weekly in each rat. At 8 weeks after induction of DM, the mean blood pressure was measured by the tail-cuff method, and 24-h urine samples were obtained by using metabolic cages. Albumin concentrations in 24-h urine samples were measured by an ELISA kit (Exocell, Philadelphia, PA, USA). Eight weeks after induction of DM, the rats were anaesthetized with pentobarbital (50 mg/kg body weight) and serum was isolated and assayed for creatinine. From six rats in each group, the cortical tissue of the left kidney was cut into small pieces and the glomeruli were isolated by the mechanical graded sieving technique. The suspension of the glomeruli was then used for reactive oxygen species (ROS) production assay and protein isolation. The right kidney was used for immunohistochemistry. Five rats in each group were used for the in situ detection of NO and ROS.
Fluorescence spectrometric assay for production of ROS in isolated glomeruli
Production of peroxides, including H2O2 and peroxynitrite, was measured in isolated glomeruli using the 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) method, as previously described [7]. Isolated glomeruli from each group were incubated in RPMI-1640 containing 0.5 mM DCFH-DA (excitation 495 nm, emission 530 nm, Invitrogen, Tokyo, Japan) for 1 h, and immediately fluorescent compound 2′,7′-dichlorofluorescein (DCF) was detected by confocal laser microscopy (Leica-Microsystems, Tokyo, Japan). In some experiments, the glomeruli were pre-exposed RPMI-1640 with diphenylene iodinium (DPI, 100 μM), allopurinol (Allo, 1 mM), N(G)-nitro-l-arginine methyl ester (L-NAME, 1 mM) or 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS, 100 μM) before the experiment. The mean fluorescence intensity of the isolated glomeruli (total 600 glomeruli from six rats in each group) was analysed by Leica TCS-NT system software (Leica-Microsystems).
Lucigenin chemiluminescence assay for production of ROS in isolated glomeruli
The superoxide production in glomeruli was measured by using lucigenin chemiluminescence as described [8]. Lucigenin chemiluminescence was expressed as units per minute per milligram of weight (unit/min/mg).
In situ detection of NO and ROS
The production levels of NO and ROS resulting from NOS coupling were imaged by confocal laser microscopy after renal perfusion with DCFH-DA and diaminorhodamine-4M acetoxymethyl ester (DAR-4M AM; excitation 560 nm, emission 575 nm, Sekisui Medical, Tokyo, Japan) with l-arginine as previously described [2]. The mean NO and ROS fluorescence intensity of glomeruli (total 100 glomeruli from five rats in each group) was analysed by Leica TCS-NT system software (Leica-Microsystems).
Western immunoblotting
Portions of the isolated glomeruli (100 μg/lane) were subjected to SDS–PAGE. For immunoblot analysis of the dimeric form of eNOS, samples were not heated and the temperature of the gel was maintained below 10°C during electrophoresis (low-temperature SDS–PAGE). Anti-eNOS antibody (Santa Cruz biotechnology, Santa Cruz, CA, USA), anti-phospho eNOS antibody (Santa Cruz), anti-actin antibody (Sigma), anti-3-nitrotyrosine antibody (Abcam, Tokyo, Japan) and anti-GTP cyclohydrolase 1 (GTPCH1) antibody (Santa Cruz) were used as the primary antibodies. Signals were detected using the ECL system (Amersham Biosciences, Piscataway, NJ, USA).
Immunohistochemistry of nitrotyrosine
Cryostat sections (6 μm) fixed in 4% buffered paraformaldehyde were stained with an antibody against nitrotyrosine (Upstate, Lake Placid, NY, USA). Detection was carried out using the DAKO EnVision+TM system and diaminobenzidine (Dako Japan, Tokyo, Japan).
Determination of serum hydrobiopterin concentrations
Tetrahydrobiopterin (BH4) and dihydrobiopterin (BH2) concentrations were determined by high-performance liquid chromatography (HPLC) as previously described [2].
Quantitative real-time polymerase chain reaction for GTPCH1
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR) were performed as previously described [2]. Primers and probes for GTPCH1 (NM_024356) are listed below: GTPCH1 forward probe, 5′-CAGATGTCCTGAACGATGCT-3′; reverse probe, 5′-ATATGGACCCTTCCCACAAA-3′; TaqMan probe, 5′-FAM-TCCATGTGTGAGCATCACCTGGTC-BHQ-3′. The change in the expression was expressed by standardizing RNA levels corrected for glyceraldehyde-3-phosphate dehydrogenase expression in the sample.
Statistical analysis
Values were expressed as mean ± SD. All parameters were evaluated with the two-tailed unpaired Student's _t_-test, Welch's _t_-test or Mann-Whitney's _U-_test. A _P_-value <0.05 denoted the presence of a statistically significant difference.
Results
Effects of losartan on renal function in diabetic rats
Table 1 summarizes the characteristics of Cont, Cont+Los, DM and DM+Los rats. There was no difference in physiological data between Cont and Cont+Los groups. At 8 weeks after STZ injection, the body weight of diabetic rats was significantly lower and glucose levels higher compared with the control rats (P < 0.05). Administration of losartan resulted in a slight but not significant decrease in MAP. There were no differences in serum creatinine levels between the groups. Urinary albumin excretion was increased significantly in DM rat (0.06 ± 0.02 versus 0.89 ± 0.19 mg/day, respectively, P < 0.05) and losartan treatment significantly decreased the level of albuminuria (0.48 ± 0.25 mg/day, P < 0.05 versus DM).
Table 1.
Physiological data
| Control | DM | |||
|---|---|---|---|---|
| Group | No treatment | Losartan | No treatment | Losartan |
| Number | 11 | 11 | 11 | 11 |
| Body weight (g) | 401 ± 12 | 421 ± 18 | 355 ± 23a | 338 ± 12b |
| Blood glucose (mg/dl) | 127 ± 7 | 121 ± 9 | 499 ± 50a | 448 ± 76b |
| Mean blood pressure (mmHg) | 94 ± 5 | 92 ± 5 | 104 ± 7 | 98 ± 5 |
| Urinary albumin excretion (mg/day) | 0.06 ± 0.02 | 0.08 ± 0.03 | 0.89 ± 0.19a | 0.48 ± 0.25b,c |
| Serum creatinine (mg/dl) | 0.30 ± 0.02 | 0.31 ± 0.03 | 0.28 ± 0.05 | 0.31 ± 0.04 |
Effects of losartan on ROS generation in diabetic nephropathy
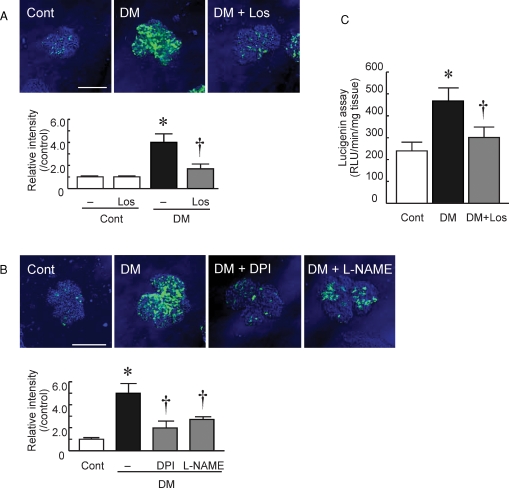
ROS production in isolated glomeruli was evaluated by DCFH conversion to DCF and imaged by confocal laser-scanning microscopy. DCF fluorescence was strong, indicating superoxide production, in the isolated glomeruli of diabetic rats compared with those of control rats (Figure 1A; 4.0 ± 0.6 fold, P < 0.05 versus Cont). The DCF fluorescence was blocked when the glomeruli were incubated in the presence of the superoxide scavenger Tiron, confirming that the signal originated from superoxide (data not shown). The fluorescence intensity in the isolated glomeruli from losartan-treated DM rats was significantly reduced (Figure 1A; 1.8 ± 0.5 fold, P < 0.05 versus DM).
Fig. 1.
Superoxide production and pathway in diabetic glomeruli. (A) Isolated glomeruli of control with no treatment rats (Cont), diabetic rats (DM) and losartan-treated diabetic rats (DM+Los) were incubated with 2′,7′-dichlorofluorescein diacetate (DCFH) and superoxide production was measured (Bar = 100 μm). Relative intensity was quantified. Data are shown relative to the Cont. Data are mean ± SD of 100 glomeruli from six rats in each group (total 600 glomeruli). Los; losartan treatment. *P < 0.05 versus Cont, †P < 0.05 versus DM + no treatment. (B) Glomeruli of DM were separately incubated with substrates of various enzymes and superoxide production was measured by DCFH staining (Bar = 100 μm). Relative intensity was quantified. Data are shown relative to the Cont. Data are mean ± SD of 100 glomeruli from six Cont or DM rats in each group (total 600 glomeruli). DPI, diphenylene iodonium; L-NAME, N(G)-nitro-l-arginine methyl ester. *P < 0.05 versus Cont, †P < 0.05 versus DM (−). (C) The superoxide production in glomeruli was measured by lucigenin chemiluminescence. Extracted protein from Cont, Cont+Los, DM and DM+Los glomeruli were used. Data are mean ± SD of six rats in each group. *P < 0.05 versus Cont, †P < 0.05 versus DM + no treatment.
Mechanism of ROS production in diabetic nephropathy
Glomerular ROS generation in DM was blocked by NAD(P)H oxidase inhibitor, DPI and NOS inhibitor and L-NAME (Figure 1B; 2.0 ± 0.6 fold and 2.6 ± 0.4 fold, respectively, P < 0.05 versus DM). However, pretreatment with mitochondrial anion channel inhibitor, DIDS and xanthine oxidase inhibitor, Allo had no effects (data are not shown). These results suggest that ROS production in the isolated glomeruli of DM rats was mediated via NAD(P)H oxidase and NOS activation, but not via a xanthine oxidase or mitochondrial pathway.
Effects of losartan NAD(P)H oxidase activity in diabetic nephropathy
ROS production by NAD(P)H oxidase in isolated glomeruli was determined by lucigenin chemiluminescence assay (Figure 1C). ROS production by NAD(P)H oxidase was significantly increased in DM rats compared with Cont rats (411 ± 80 versus 205 ± 33 RLU/min/mg tissue, respectively, P < 0.05) and losartan treatment attenuated ROS production (297 ± 52 RLU/min/mg tissue, P <0.05 versus DM).
Effect of losartan on NO and ROS production in diabetic nephropathy
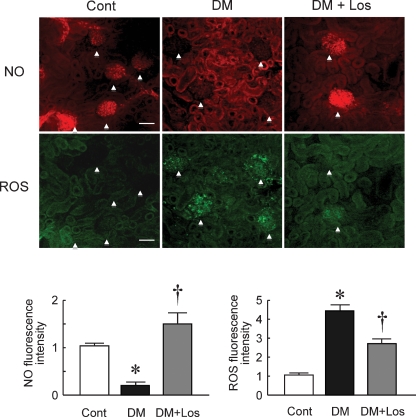
Figure 2 shows ROS and NO imaging in control and diabetic glomeruli treated with or without losartan. Generation of ROS and NO in the glomeruli were evaluated by the fluorescent intensity of DCF and DAR-4M, respectively. NO, but not ROS, was detected in the glomeruli of the Cont rats. However, bioavailable NO was decreased and ROS production was increased in the glomeruli of DM rats compared with the Cont rats (1.5 ± 0.3 fold and 4.4 ± 0.3 fold, respectively, P < 0.05 versus Cont). Losartan treatment significantly increased NO production and decreased ROS production in the glomeruli of DM (0.2 ± 0.1 fold and 2.7 ± 0.3 fold, respectively, P < 0.05 versus DM). These results suggest that treatment of diabetic nephropathy with losartan normalized the imbalance of NO/ROS in the glomeruli.
Fig. 2.
In situ detection of nitrate oxide (NO) and reactive oxygen species (ROS). Representative images of NO (top) and ROS (bottom) in renal cortex of control rats (Cont), diabetic rats (DM) and DM rats treated with losartan (DM+Los). Upper NO and lower ROS images are the same region of renal cortex. Arrowhead indicates glomerulus. Generation of ROS and NO was evaluated by the fluorescent intensity of DCF and DAR-4M, respectively (Bar = 100 μm). Data are mean ± SD of 20 glomeruli from five rats in each group (total 100 glomeruli). *P < 0.05 versus Cont, †P < 0.05 versus DM + no treatment.
Effects of losartan on eNOS dimerization in diabetic nephropathy
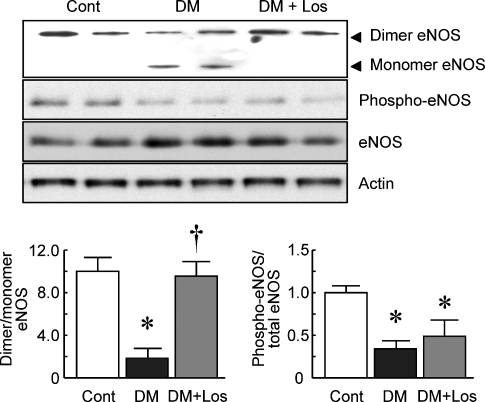
The eNOS protein dimer/monomer ratio was decreased in the glomeruli of DM rats compared with those of Cont rats (Figure 3; 2.0 ± 1.0 and 9.7 ± 1.1, respectively, P < 0.05 versus Cont). However, losartan treatment prevented the decrease of the ratio in the diabetic glomeruli (9.4 ± 1.1, P < 0.05 versus DM). Losartan treatment did not influence the eNOS phosphorylation in the glomeruli of DM.
Fig. 3.
Western blot analysis for eNOS in the glomeruli. Representative blots of eNOS after low-temperature sodium dodecyl sulfate–polyacrylamide gel electrophoresis and phosphorylated (phospho)-eNOS. The intensity of dimers, monomers eNOS, phospho-eNOS and total eNOS was determined by NIH Image software, and the results are expressed as the dimer/monomer ratio and phospho-eNOS/total eNOS ratio. Data are mean ± SD of six rats in each group. *P < 0.05 versus Cont, †P < 0.05 versus DM.
Effects of losartan on protein nitrosification in diabetic nephropathy
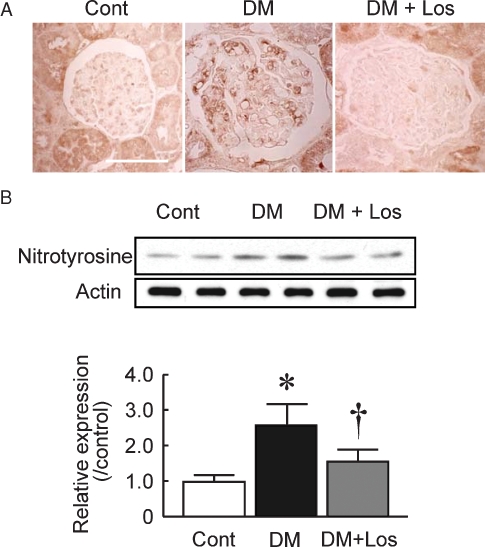
Bioavailable NO is decreased by reacting with superoxide to form peroxynitrite. Generation of peroxynitrite in diabetic glomeruli, especially in endothelial cells, was confirmed by immunohistochemistry using the nitrotyrosine antibody (Figure 4A). Losartan treatment decreased nitrotyrosine staining in glomeruli. The elevated level of nitrotyrosine in isolated glomeruli from DM rats was also confirmed (Figure 4B; 2.5 ± 0.6 fold, P < 0.05 versus Cont), and the elevation was completely inhibited by losartan treatment (1.6 ± 0.3 fold, P < 0.05 versus DM).
Fig. 4.
Nitrotyrosine formation in the glomeruli. (A) Immunohistochemistry of nitrotyrosine in control rats (Cont), diabetic rats (DM) and DM rats treated with losartan (DM+Los) (Bar = 100 μm). (B) Representative blots of nitrotyrosine. The intensity of the 55 kDa band was determined by NIH Image software, and the results are expressed as relative to the Cont. Data are mean ± SD of six rats in each group. *P < 0.05 versus Cont, †P < 0.05 versus DM.
Serum tetrahydrobiopterin levels in diabetic rats
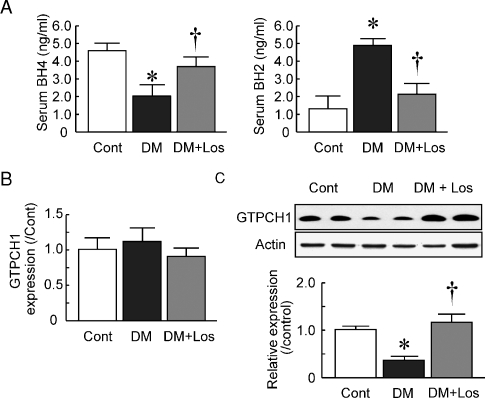
BH4 is an essential cofactor of NOS. ROS such as peroxynitrite or nitrogen dioxide convert BH4 into BH2, and BH4 deficiency decreases NO bioactivity. Serum BH4 and BH2 levels were measured by HPLC. The BH4 levels were decreased in DM rats compared with Cont rats (Figure 5A; 2.0 ± 0.7 and 4.5 ± 0.6 ng/ml, respectively, P < 0.05 versus Cont), and losartan treatment increased the levels in rats with diabetic nephropathy (3.6 ± 0.6 ng/ml, P < 0.05 versus DM). In contrast, the BH2 levels were increased in DM rats compared with Cont rats (Figure 5A; 4.8 ± 0.5 and 1.2 ± 0.9 ng/ml, respectively, P < 0.05 versus Cont) and losartan treatment decreased the levels in rats with diabetic nephropathy (2.2 ± 0.6 ng/ml, P < 0.05 versus DM).
Fig. 5.
Serum biopterin concentration and glomerular GTPCH1 expression. (A) Serum levels of tetrahydrobiopterin (BH4) and dihydrobiopterin (BH2) in control rats (Cont), diabetic rats (DM) and DM rats treated with losartan (DM+Los) were determined using HPLC. Data are mean ± SD of six rats in each group. *P < 0.05 versus Cont, †P < 0.05 versus DM. (B) Glomerular mRNA expression of GTPCH1 in Cont, DM and DM+Los. (C) Glomerular protein expression of GTPCH1 in Cont, DM and DM+Los. Representative blots of GTPCH1 are shown. Data are mean ± SD of six rats in each group. *P < 0.05 versus Cont, †P < 0.05 versus DM.
The key enzyme involved in de novo BH4 synthesis is GTPCH1. The GTPCH1 mRNA expression in glomeruli was not changed in the DM group compared to the Cont group (Figure 5B; 1.15 ± 0.15 fold). Losartan treatment also showed no changes in GTPCH1 expression in DM glomeruli (0.88 ± 0.14 fold, P < 0.05 versus DM). However, the protein expression was significantly decreased in DM and losartan restored protein expression (Figure 5C; 0.4 ± 0.1 fold and 1.2 ± 0.2 fold, respectively). These results suggest that GTPCH1 was decreased in the protein level in the isolated glomeruli of DM rats and losartan restored GTPCH1 protein levels and kept serum BH4.
Discussion
In the present study, we explored whether losartan improves NO production in the glomeruli of rats with diabetic nephropathy independent of the blood pressure lowering effects. Our data demonstrated that blockade of Ang II signalling in diabetic glomeruli reduced ROS production and improved the eNOS coupling statement, and indeed restored NO bioavailability in glomeruli.
In the present study, we used an STZ-induced type 1 diabetes model to demonstrate the therapeutic efficacy of losartan on glomerular eNOS uncoupling in early diabetic nephropathy. But some of the characteristic early alterations in human diabetic nephropathy such as increased albuminuria, the development of glomerular hyperfiltration and some of the characteristic histopathological changes can be observed in the STZ-induced diabetic model [9]. Moreover, studies in the STZ-induced diabetic rat model have shown increased renal oxidative stress induced by hyperglycaemia [10,11]. So we have used this model for the study of glomerular endothelial dysfunction induced by hyperglycaemia [2,12]. In the present study, we also used this STZ model for the investigation of hyperglycaemia-induced eNOS uncoupling and the effects of losartan.
It has been reported that ARB have anti-oxidative effects in human essential hypertension [13], hypertensive diabetic nephropathy [14] and mouse type 2 diabetic nephropathy [15]. Data of the present study also indicated that ARB treatment suppressed ROS production in diabetic glomeruli. It is known that Ang II mediates ROS generation through activation of NAD(P)H oxidase [16]. As we reported previously [2], activation of NAD(P)H oxidase is also involved in ROS production in the diabetic glomeruli. We confirmed by direct observation that ARB reduced ROS production through the reduction of NAD(P)H oxidase activity. These finding are consistent with the previous reports that ARB attenuated NAD(P)H oxidase-dependent ROS production [17].
In addition, using two different methods, we confirmed uncoupled eNOS in diabetic glomeruli. ARB improved the eNOS coupling state in diabetic glomeruli and recovered NO production to normal levels. The uncoupled NOS in diabetic glomeruli was confirmed by the decrease of eNOS dimer form and by in situ NO imaging. The in situ NO imaging method is useful for detecting NOS dysfunction. We used a kidney perfusate containing l-arginine and Ca2+ to enhance NO production. Under normal conditions, coupled NOS converts l-arginine into l-citrulline and produces NO. However, when NOS cofactor BH4 is at suboptimal concentrations, l-arginine produces superoxide rather than NO through uncoupled NOS [18]. This ROS production could be detected by the ROS fluorescent indicator, DCFH, in our in situ imaging method. Administration of ARB improved NOS function, as determined by increased glomerular NO production and decreased glomerular ROS production. These results also suggest that ARB can improve uncoupled NOS in the diabetic glomeruli.
BH4 serves as a critical co-factor for eNOS and deficiency of BH4 results in eNOS uncoupling, which is associated with increased superoxide and decreased NO production. We found that serum levels of BH4 in the diabetic rats were lower than those in control rats, and ARB restored BH4 bioavailability in diabetic nephropathy by reduced catabolism for oxidation to BH2. The reduced BH4 oxidation by ARB leads to eNOS recoupling and results in increased NO and reduced ROS production by the enzyme. BH4 bioavailability is potently influenced by oxidative stress, by the downregulation of GTPCH1 [19] and by oxidation to inactive BH2 [20]. Recently, Chalupsky et al. [21] demonstrated the role of dihydrofolate reductase in the regulation of BH4 and NO bioavailability in the endothelium. Endothelial NAD(P)H oxidase-derived hydrogen peroxide downregulated dihydrofolate reductase expression in response to angiotensin II, resulting in BH4 deficiency and uncoupling of eNOS. Moreover, Xu et al. [22] demonstrated that diabetic hyperglycaemia activates the 26S proteasome via peroxynitrite and results in the ubiquitination and degradation of GTPCH1, which is the rate-limiting enzyme for BH4 de novo synthesis. We confirmed that nitrotyrosine formation, peroxynitrite indicator, was elevated in diabetic glomeruli and attenuated by losartan treatment. Moreover, we confirmed that GTPCH1 protein expression levels were decreased in diabetic glomeruli and attenuated by losartan treatment without affecting mRNA expression, so it may influence the GTPCH1 activity. As a result, losartan may improve eNOS uncoupling in STZ-induced diabetic glomeruli.
On the other hand, statins are reported to potentiate GTPCH1 gene expression and BH4 synthesis, thereby improving eNOS function [23]. Administration of the calcium channel blocker, benidipine, is also reported to be effective in preventing BH4 deficiency by activating GTPCH1 in type II diabetic rats [24]. GTPCH1 is also subject to posttranslational modification by phosphorylation. In rat mesangial cells, Ang II and platelet-derived growth factor are reported to increase GTPCH1 activity by phosphorylation via a protein kinase C-dependent mechanism [25]. However, the role of GTPCH1 phosphorylation in ECs has not been determined.
The 2-week ARB treatment did not cause significant pathological changes in the kidneys of STZ rats. However, ARB treatment resulted in a significant reduction of albuminuria. It is possible that this effect is the result of improvement of endothelial function. Improvement of eNOS uncoupling including BH4 by ARB treatment leads to increased NO viability and maintenance of endothelial function; then ARB would reduce microalbuminuria in STZ rat. Recently, the usefulness of microalbuminuria in predicting increased risk of cardiovascular and renal diseases is well established in diabetic patients, as well as in essential hypertensive patients and in the general population [26]. Thus, the reduction of microalbuminuria could then be a relevant therapeutic strategy for reducing or preventing cardiovascular events in patients with diabetes and essential hypertension.
Changes of NO production have been associated with glomerular hyperfiltration, vascular permeability [27]. ARB including losartan can induce glomerular haemodynamics improvement by reducing efferent artery resistance [28]. So losartan may improve the eNOS–BH4 coupling by improving glomerular hyperfiltration and/or endothelial function. In renal diseases, glomerular haemodynamic improvement reduces oxidative stress. We have demonstrated that, in a subtotal nephrectomized rats model that shows increased glomerular hyperfiltration, glomerular superoxide production was increased and ARB ameliorated superoxide production by attenuating NAD(P)H oxidase activity [29]. On the other hand, in aorta of the rat STZ model, ARB has been reported to improve eNOS uncoupling by inhibiting NAD(P)H oxidase activity, so losartan may improve eNOS uncoupling by direct action to the glomerular endothelium. So, losartan may demonstrate its anti-oxidative effects by both reducing glomerular hypertension and direct endothelial protection.
In conclusion, we have demonstrated in the present study that the imbalance between NO and ROS contributes to renal dysfunction in diabetic nephropathy. ARB improved eNOS uncoupling by increasing serum BH4 level, which contributed to the reduction in diabetic glomerular ROS production. These mechanisms add a new dimension to the renoprotective effects of ARB in diabetic nephropathy.
Acknowledgments
We thank Mrs Sawako Tsujita for the animal care. We also thank Prof. Katsuhiko Tsujioka (Department of Physiology, Kawasaki Medical School, Kurashiki, Japan) for his technical assistance with the in situ detection of ROS and NO. We also thank Dr F.G. Issa (Department of Medicine, University of Sydney, Sydney, Australia) for careful reading and editing of this manuscript.
Conflict of interest statement. None declared.
References
- 1.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 2.Satoh M, Fujimoto S, Haruna Y, et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F1144–F1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 5.Malik RA, Schofield IJ, Izzard A, et al. Effects of angiotensin type-1 receptor antagonism on small artery function in patients with type 2 diabetes mellitus. Hypertension. 2005;45:264–269. doi: 10.1161/01.HYP.0000153305.50128.a1. [DOI] [PubMed] [Google Scholar]
- 6.Rizzoni D, Porteri E, De Ciuceis C, et al. Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with noninsulin-dependent diabetes mellitus. Hypertension. 2005;45:659–665. doi: 10.1161/01.HYP.0000153308.91043.97. [DOI] [PubMed] [Google Scholar]
- 7.Asaba K, Tojo A, Onozato ML, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Satoh M, Ogita H, Takeshita K, et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breyer MD, Bottinger E, Brosius FC, III, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 10.Onozato ML, Tojo A, Goto A, et al. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 11.Palm F, Cederberg J, Hansell P, et al. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Satoh M, Namikoshi T, et al. Blockade of serotonin 2A receptor improves glomerular endothelial function in rats with streptozotocin-induced diabetic nephropathy. Clin Exp Nephrol. 2008;12:119–125. doi: 10.1007/s10157-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 13.Dohi Y, Ohashi M, Sugiyama M, et al. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens Res. 2003;26:691–697. doi: 10.1291/hypres.26.691. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa S, Mori T, Nako K, et al. Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension. 2006;47:699–705. doi: 10.1161/01.HYP.0000203826.15076.4b. [DOI] [PubMed] [Google Scholar]
- 15.Fan Q, Liao J, Kobayashi M, et al. Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dial Transplant. 2004;19:3012–3020. doi: 10.1093/ndt/gfh499. [DOI] [PubMed] [Google Scholar]
- 16.Seshiah PN, Weber DS, Rocic P, et al. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama H, Kobayashi M, Wang DH, et al. Telmisartan inhibits both oxidative stress and renal fibrosis after unilateral ureteral obstruction in acatalasemic mice. Nephrol Dial Transplant. 2005;20:2670–2680. doi: 10.1093/ndt/gfi045. [DOI] [PubMed] [Google Scholar]
- 18.Pou S, Pou WS, Bredt DS, et al. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 19.Zheng JS, Yang XQ, Lookingland KJ, et al. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation. 2003;108:1238–1245. doi: 10.1161/01.CIR.0000089082.40285.C3. [DOI] [PubMed] [Google Scholar]
- 20.Kawashima S. The two faces of endothelial nitric oxide synthase in the pathophysiology of atherosclerosis. Endothelium. 2004;11:99–107. doi: 10.1080/10623320490482637. [DOI] [PubMed] [Google Scholar]
- 21.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Wu Y, Song P, et al. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 23.Hattori Y, Nakanishi N, Akimoto K, et al. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:176–182. doi: 10.1161/01.atv.0000054659.72231.a1. [DOI] [PubMed] [Google Scholar]
- 24.Okumura M, Masada M, Yoshida Y, et al. Decrease in tetrahydrobiopterin as a possible cause of nephropathy in type II diabetic rats. Kidney Int. 2006;70:471–476. doi: 10.1038/sj.ki.5000431. [DOI] [PubMed] [Google Scholar]
- 25.Lapize C, Pluss C, Werner ER, et al. Protein kinase C phosphorylates and activates GTP cyclohydrolase I in rat renal mesangial cells. Biochem Biophys Res Commun. 1998;251:802–805. doi: 10.1006/bbrc.1998.9552. [DOI] [PubMed] [Google Scholar]
- 26.Segura J, Ruilope LM, Rodicio JL. Microalbuminuria. Clin Exp Hypertens. 2004;26:701–707. doi: 10.1081/ceh-200031985. [DOI] [PubMed] [Google Scholar]
- 27.Chiarelli F, Cipollone F, Romano F, et al. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes. 2000;49:1258–1263. doi: 10.2337/diabetes.49.7.1258. [DOI] [PubMed] [Google Scholar]
- 28.Arima S, Ito S. The mechanisms underlying altered vascular resistance of glomerular afferent and efferent arterioles in diabetic nephropathy. Nephrol Dial Transplant. 2003;18:1966–1969. doi: 10.1093/ndt/gfg263. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto S, Satoh M, Horike H, et al. Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. Hypertens Res. 2008;31:305–313. doi: 10.1291/hypres.31.305. [DOI] [PubMed] [Google Scholar]