A meta-analysis of randomized controlled trials in pulmonary arterial hypertension (original) (raw)
Abstract
Aims
There is no cure for pulmonary arterial hypertension, but current approved treatment options include prostanoids, endothelin-receptor antagonists, and phosphodiesterase type-5 inhibitors. The effect on survival of these compounds has not been appropriately assessed in individual trials because of small sample size and short duration. We performed a meta-analysis of all randomized controlled trials with drugs published in this condition.
Methods and results
Trials were searched in the Medline database from January 1990 to October 2008. The primary analysis included only studies with a placebo comparator arm, the sensitivity analysis also included studies comparing two active treatment arms. The main outcome measure was all-cause mortality. Twenty-one trials were included in the primary analysis (3140 patients) and two additional studies (59 patients) were included in the sensitivity analysis. Average duration of the trials was 14.3 weeks. All-cause mortality rate in the control group was 3.8%. Active treatments were associated with a reduction in mortality of 43% (RR 0.57; 95% CI 0.35–0.92; P = 0.023); the sensitivity analysis confirmed a reduction in mortality of 38% (RR 0.62; 95% CI 0.39–1.00; P = 0.048).
Conclusion
The results of this meta-analysis suggest an improvement of survival in the patients treated with the targeted therapies approved for pulmonary arterial hypertension.
Keywords: Pulmonary hypertension, Meta-analysis, Randomized controlled trials, Endothelin receptor antagonists, Phosphodiesterase type-5 inhibitors, Prostanoids
Introduction
Pulmonary arterial hypertension is a devastating, progressive disease with increasingly debilitating symptoms.1 Increased pulmonary vascular resistance owing to obstructive proliferative changes in the lung microcirculation results in extensive heart structural changes, limits patients exercise capacity, and eventually leads to right heart failure and premature death.1
The pathogenesis of pulmonary arterial hypertension is poorly understood, but an imbalance between vasoconstrictor/proliferative agents (e.g. endothelin) and vasodilator/antiproliferative substances (e.g. prostacyclin and nitric oxide) have been identified in the lung vasculature.2,3
There is no cure for pulmonary arterial hypertension, but current approved treatment options include prostanoids, endothelin-receptor antagonists, and the phosphodiesterase type-5 inhibitors.4 These therapies improve symptoms, exercise capacity, haemodynamics, and outcome but the clinical relevance of these effects have been recently challenged.5–7 The main criticisms include the limited improvements observed on the exercise capacity and the short duration and the small sample size of the individual studies which have precluded any insight on the prognostic relevance of the treatments.
A meta-analysis on 16 randomized controlled trials (RCTs) performed in pulmonary arterial hypertension8 concluded that the treatments ‘produced limited benefits in clinical endpoints and failed to support a significant survival advantage’. However, the meta-analysis did not consider six RCTs9–14 published before its submission, included both acute15,16 and long-term studies and included one study on patients with lung fibrosis.16
We present the data of a meta-analysis on 23 RCTs9–14,17–33 with drugs performed exclusively in pulmonary arterial hypertension patients (only in one study a minority of patients with inoperable chronic thrombo-embolic pulmonary hypertension was included23) published as of October 2008. We excluded acute studies assessing only haemodynamic variables.
Methods
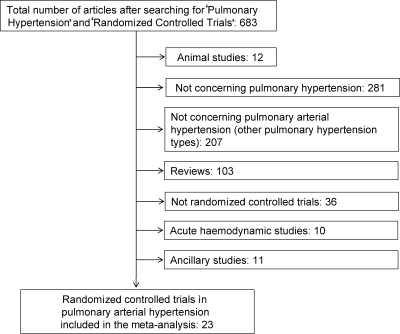
RCTs in patients with pulmonary arterial hypertension (Group 1 according to the Venice clinical classification34 of pulmonary hypertension) published in English from January 1990 to October 2008 were identified by the commonly adopted approach of computer-based literature search on the MEDLINE database (Figure 1). As we were interested in the analysis of the effects on mortality, acute studies assessing only haemodynamic variables were excluded. Twenty-three RCTs with drugs (Tables 1 and 2) with these characteristics were identified. Each study was used as a unit for statistical analysis. The data were analysed by intention-to-treat including all randomized patients.
Figure 1.
Flow chart of the search strategy and selection of the trials.
Table 1.
Randomized controlled trial characteristics
| First author/year | Official acronym | Number of patients | Active drug | Comparator | Study period (weeks) | Etiology (%) |
|---|---|---|---|---|---|---|
| Rubin et al.17 | – | 23 | Epoprostenol | Randomized controls* | 8 | IPAH (100) |
| Barst et al.18 | – | 81 | Epoprostenol | Randomized controls* | 12 | IPAH (100) |
| Badesch et al.19 | – | 111 | Epoprostenol | Randomized controls* | 12 | APAH (100) |
| Channick et al.20 | – | 32 | Bosentan | Placebo | 12 | IPAH (84), APAH (16) |
| Langleben et al.9 | – | 71 | Terbogrel | Placebo | 12 | IPAH (100) |
| Simmoneau et al.21 | – | 470 | Treprostinil | Placebo | 12 | IPAH (58), APAH (42) |
| Galiè et al.22 | ALPHABET | 130 | Beraprost | Placebo | 12 | IPAH (48), APAH (52) |
| Olschewski et al.23 | AIR | 203 | Iloprost | Placebo | 12 | IPAH (50), APAH (22), CTEPH (28) |
| Rubin et al.24 | BREATHE-1 | 213 | Bosentan | Placebo | 16 | IPAH (70), APAH (30) |
| Barst et al.25 | – | 116 | Beraprost | Placebo | 36 | IPAH (74), APAH (26) |
| Sastry et al.26 | – | 22 | Sildenafil | Placebo | 12 | IPAH (100) |
| Humbert et al.27 | BREATHE-2 | 33 | Epoprostenol + Bosentan | Epoprostenol + placebo | 16 | IPAH (82), APAH (18) |
| Barst et al.28 | STRIDE-1 | 178 | Sitaxsentan | Placebo | 12 | IPAH (53), APAH (47) |
| Galiè et al.29 | SUPER-1 | 278 | Sildenafil | Placebo | 12 | IPAH (64), APAH (30), Other (6) |
| Wilkins et al.30 | SERAPH | 26 | Bosentan | Sildenafil | 16 | IPAH (88), APAH (12) |
| Singh et al.10 | – | 20 | Sildenafil | Placebo | 8 | IPAH (50), PAH-ES (50) |
| Galiè et al.11 | BREATHE-5 | 54 | Bosentan | Placebo | 16 | PAH-ES (100) |
| Barst et al.13 | STRIDE-2 | 185 | Sitaxsentan | Placebo | 18 | IPAH (59), APAH (30), Other (11) |
| McLaughlin et al.12 | STEP | 67 | Inhaled iloprost† | Placebo† | 12 | IPAH (55), APAH (45) |
| Hoeper et al.14 | COMBI | 40 | Inhaled iloprost† | Placebo† | 12 | IPAH (100) |
| Galiè et al.31 | ARIES | 394 | Ambrisentan | Placebo | 12 | IPAH (64), APAH (32), Other (4) |
| Galiè et al.32 | EARLY | 185 | Bosentan‡ | Placebo‡ | 24 | IPAH (61),APAH (35), Other(4) |
| Simonneau et al.33 | PACES | 267 | Sildenafil§ | Placebo§ | 16 | IPAH (79), APAH (21) |
Table 2.
Randomized controlled trial characteristics
| First author/year | Official acronym | NYHA/WHO functional class (%) | Primary endpoint | Invasive haemodynamic evaluation | Explicitly reported hospitalizations | Reported survival | |||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||
| Rubin et al.17 | – | – | 9 | 65 | 26 | 6MWD | Yes | No | Yes |
| Barst et al.18 | – | – | – | 74 | 26 | 6MWD | Yes | No | Yes |
| Badesch et al.19 | – | – | 5 | 78 | 17 | 6MWD | Yes | No | Yes |
| Channick et al.20 | – | – | – | 100 | – | 6MWD | Yes | No | Yes |
| Langleben et al.9 | – | – | 49 | 51 | – | 6MWD | Yes | No | Yes |
| Simmoneau et al.21 | – | – | 11 | 81 | 8 | 6MWD | Yes | No | Yes |
| Galiè et al.22 | ALPHABET | – | 49 | 51 | – | 6MWD | Yes | No | Yes |
| Olschewski et al.23 | AIR | – | – | 59 | 41 | 6MWD&FC | Yes | No | Yes |
| Rubin et al.24 | BREATHE-1 | – | – | 91 | 9 | 6MWD | No | Yes | Yes |
| Barst et al.25 | – | – | 52 | 48 | – | VO2 max | Yes | No | Yes |
| Sastry et al.26 | – | – | 82 | 18 | – | TT | No | No | Yes |
| Humbert et al.27 | BREATHE-2 | – | – | 75 | 25 | PVR | Yes | No | Yes |
| Barst et al.28 | STRIDE-1 | – | 33 | 66 | 1 | VO2 max | Yes | No | Yes |
| Galiè et al.29 | SUPER-1 | – | 39 | 58 | 3 | 6MWD | Yes | Yes | Yes |
| Wilkins et al.30 | SERAPH | 1 | – | 100 | – | RV mass | No | No | Yes |
| Singh et al.10 | – | – | 40 | 55 | 5 | 6MWD | No | No | Yes |
| Galiè et al.11 | BREATHE-5 | – | – | 100 | – | SaO2 and PVR | Yes | No | Yes |
| Barst et al.13 | STRIDE-2 | – | 37 | 59 | 4 | 6MWD | No | Yes | Yes |
| McLaughlin et al.12 | STEP | – | 1 | 94 | 5 | 6MWD | Yes | Yes | Yes |
| Hoeper et al.14 | COMBI | – | – | 100 | – | 6MWD | No | Yes | Yes |
| Galiè et al.31 | ARIES | 2 | 38 | 55 | 5 | 6MWD | No | Yes | Yes |
| Galiè et al.32 | EARLY | – | 100 | – | – | PVR and 6MWD | Yes | Yes | Yes |
| Simonneau et al.33 | PACES | 1 | 25 | 68 | 6 | 6MWD | Yes | Yes | Yes |
Main outcome measure for the present analysis was all-cause mortality, which was reported in all RCTs. The following additional secondary parameters which were reported explicitly and clearly in the text and/or tables of only part of the RCTs were also assessed: hospitalizations owing to pulmonary arterial hypertension, exercise capacity as assessed by the 6-min walk distance (6MWD),35 NYHA/WHO functional class improvement,36 right atrial pressure, mean pulmonary arterial pressure, cardiac index, and pulmonary vascular resistance.
The primary analysis was performed in 21 RCTs in which a clear identification of a placebo comparator arm was possible. Two additional RCTs27,30 assessed two different, concurrently initiated treatment regimens in naïve patients and the decision on which arm is considered the reference ‘placebo’ comparator arm may be arbitrary. RCTs with patients on background treatment with approved drugs for pulmonary arterial hypertension in which the addition of a new active compound (combination therapy) was tested as compared with placebo were included.12,14,32,33 Studies with compounds that were eventually not approved owing to lack of efficacy9,22,25 and doses of approved drugs, which were not endorsed because less effective or for increased side effects13,24,28,31 were also included. In the three RCTs with epoprostenol,17–19 a randomized control group was included but it was not blinded because for ethical reasons tunnelled central venous catheters and portable pumps for placebo infusion were not utilized.
A secondary, sensitivity analysis on total mortality was performed including all 23 studies. In this case the reference ‘placebo’ comparator arm in the two studies assessing two different, concurrently initiated treatment regimens in naïve patients was arbitrarily identified: in the BREATHE-2 study27 the group treated with epoprostenol alone (as compared with the association of epoprostenol and bosentan) and in the SERAPH study30 the bosentan arm (as compared with the sildenafil arm) were considered as control arms, respectively. These regimens could be considered as ‘standard of care’ when the studies were conceived and performed.
Statistical methods
Treatment effects for total mortality were evaluated as relative risks (RR) according to the inverse variance fixed-effect method.37 In order to identify biases owing to the exclusion of trials from the analysis, the continuity correction method was also used by adding 0.5 in each cell with null events. To confirm the robustness of the data in case of statistically significant results (P < 0.05) of the primary analysis,38 the Mantel–Haenszel and the Peto fixed-effect methods were also tested.
Treatment effects for explicitly reported hospitalizations and NYHA/WHO improvement were evaluated as RR according to the inverse variance fixed-effect method.
Number needed to benefit (NNT) and number of avoided events per 1000 treated patients were calculated applying the RR to the control group event rate.
For exercise capacity (as assessed by 6MWD), right atrial pressure, pulmonary arterial pressure, cardiac index, and pulmonary vascular resistance (as assessed by right heart catheterization), we computed the effect size of tested drugs by using the weighted mean difference, which was calculated after subtracting from baseline the end-study values in treated and control groups. When studies did not directly supply the standard error of the mean (SEM) for the calculation of effect size, it was estimated from the published data.39 When either the values at the end of follow-up or the SEM were not reported in the article, they were manually calculated from figures (if available).
Multi-arm studies13,24,28,29,31 were assessed combining all active arms in one and comparing it with the control group. The arms testing doses of drugs, which were eventually not approved because less effective or for increased side effects13,24,28,31 were included. The Cochran Q test and I-squared were used to assess the magnitude of effect size heterogeneity. When the heterogeneity test reached the formal level for statistical significance to assess heterogeneity (P < 0.10), the null hypothesis of homogeneity of the treatment effects across the studies was rejected and the analysis was repeated by calculating a random-effect model.40
Additional analyses were performed according to the pharmacological category of tested drugs and disease severity (estimated using the median value of the 6MWD at baseline).
All analyses were performed using Stata 9.0 (Stata Statistical Software: Release 9.0, 2005. StataCorp LP, College Station, TX, USA).
Results
Characteristics of the studies
Tables 1 and 2 show the 23 RCTs characteristics recruiting 3199 patients with pulmonary arterial hypertension that have been published over a 18-year period (January 1990–October 2008, Figure 1). In the 21 studies included in the primary analysis, 3140 patients were enrolled. Only in one study23 57 patients with non-operable chronic thrombo-embolic pulmonary hypertension (Group 434) were recruited. Eight RCTs assessed the effects of prostanoids (intravenous epoprostenol, subcutaneous treprostinil, inhaled iloprost, and oral beraprost), eight RCTs assessed the effects of endothelin receptor antagonists (oral bosentan, sitxsentan, and ambrisentan), four RCTs assessed the effects of the phosphodiesterase type-5 inhibitor sildenafil, and one study the effects of the thromboxane synthase inhibitor terbogrel. Two studies27,30 compared two different, concurrently initiated treatment regimens in naïve patients: in the BREATHE-2 study, the groups were treated with epoprostenol alone or with a combination of epoprostenol and bosentan, respectively, and in the SERAPH study, the groups were treated with bosentan or sildenafil, respectively. In four studies background therapy with bosentan,12,14 sildenafil,32 or epoprostenol,33 respectively, was allowed.
The average length of the study periods was 14.3 ± 5.9 weeks (range 8–36 weeks).
In 17 studies, the exclusive or predominant aetiology was idiopathic pulmonary arterial hypertension, two studies included exclusively patients with the scleroderma spectrum of diseases19 or patients with Eisenmenger's syndrome.11
The majority of the patients included in the RCTs were in NYHA/WHO functional class III, only one study included exclusively NYHA/WHO functional class II patients.32
The 6MWD alone or in combination was the primary endpoint in 17 studies; additional primary endpoints included maximal oxygen consumption, treadmill exercise test duration, pulmonary vascular resistance, right ventricular muscle mass, and systemic blood oxygen saturation.
All-cause mortality
Overall mortality (Table 3, Figure 2) in the 21 studies included in the primary analysis was 2.48% (78 of the 3140 patients). Mortality in the actively treated group was 1.54% (28 of the 1825patients) and in the placebo group was 3.80% (50 of the 1315 patients). These cumulative data do not consider the different randomization fractions and the different durations of the RCTs and should be intended as descriptive.
Table 3.
Number of patients who died or were hospitalized in the randomized phase of the clinical trials (intent-to-treat)
| First author/year | Active drug | Comparator | Active drug | Comparator | ||||
|---|---|---|---|---|---|---|---|---|
| Death | Death | Hospitalization* | Hospitalization* | |||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Rubin et al.17 | 1 | 10 | 3 | 9 | NA | NA | NA | NA |
| Barst et al.18 | 0 | 41 | 8 | 32 | NA | NA | NA | NA |
| Badesch et al.19 | 4 | 52 | 5 | 50 | NA | NA | NA | NA |
| Channick et al.20 | 0 | 21 | 0 | 11 | NA | NA | NA | NA |
| Langleben et al.9 | 1 | 45 | 0 | 25 | NA | NA | NA | NA |
| Simmoneau et al.21 | 9 | 224 | 10 | 227 | NA | NA | NA | NA |
| Galiè et al.22 | 1 | 64 | 1 | 64 | NA | NA | NA | NA |
| Olschewski et al.23 | 1 | 100 | 4 | 98 | NA | NA | NA | NA |
| Rubin et al.24 | 1 | 143 | 2 | 67 | 6 | 138 | 9 | 60 |
| Barst et al.25 | 1 | 59 | 2 | 54 | NA | NA | NA | NA |
| Sastry et al.26 | 0 | 10 | 1 | 11 | NA | NA | NA | NA |
| Humbert et al.27 | 3 | 19 | 0 | 11 | NA | NA | NA | NA |
| Barst et al.28 | 1 | 117 | 0 | 60 | NA | NA | NA | NA |
| Galiè et al.29 | 3 | 205 | 1 | 69 | 6 | 201 | 7 | 63 |
| Wilkins et al.30 | 1 | 13 | 0 | 12 | NA | NA | NA | NA |
| Singh et al.10 | 0 | 10 | 0 | 10 | NA | NA | NA | NA |
| Galiè et al.11 | 0 | 37 | 0 | 17 | NA | NA | NA | NA |
| Barst et al.13 | 0 | 123 | 0 | 62 | 2 | 121 | 4 | 58 |
| McLaughlin et al.12 | 0 | 34 | 0 | 33 | 0 | 34 | 4 | 29 |
| Hoeper et al.14 | 0 | 19 | 0 | 21 | 0 | 19 | 0 | 21 |
| Galiè et al.31 | 4 | 257 | 5 | 128 | 9 | 252 | 11 | 121 |
| Galiè et al.32 | 1 | 92 | 1 | 91 | 1 | 92 | 3 | 89 |
| Simonneau et al.33 | 0 | 134 | 7 | 126 | 8 | 126 | 11 | 120 |
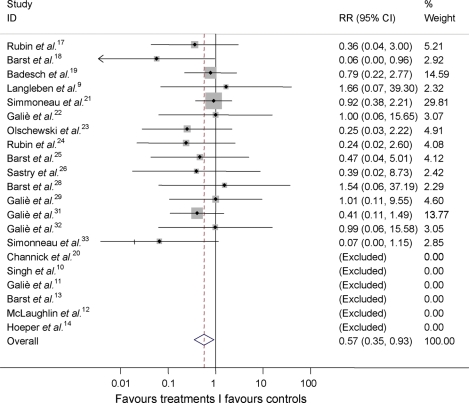
Figure 2.
Cumulative RR estimate of death in active treatment groups when compared with control groups (RR [95% CI]). P = 0.023 for the overall estimate of the primary analysis by inverse variance method. Studies with no events in both groups (Table 3) were excluded.
The cumulative RR estimate of death was a reduction of 43% (RR 0.57; 95% CI 0.35, 0.92; P = 0.023) with the inverse variance method (Figure 2), no heterogeneity (I-squared = 0.0%; P = 0.830) was detected among studies. The analysis with the continuity correction (P < 0.022), the Mantel–Haenszel and the Peto methods (both P < 0.001) confirmed the statistical significance.
Number of patients to be treated (NNT) to prevent one death was 61.6 and 16.2 (95% CI 2.7–24.0) deaths were prevented in each 1000 patients treated; these data were based on a RR = 0.573 applied to the control group event rate.
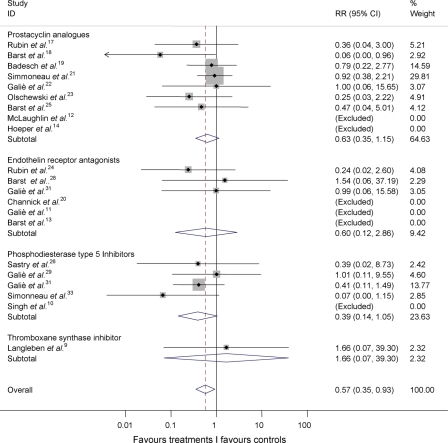
With respect to the effects of the different classes of drugs (prostanoids, thromboxane synthase inhibitors, endothelin receptor antagonists, and phosphodiesterase type-5 inhibitors), no statistically significant between-group heterogeneity (I-squared = 0.0%; P = 0.771) emerged in subgroup analyses in total mortality (Figure 3) or between the subgroups testing each of the treatments (I-squared = 0.0%; P = 0.830).
Figure 3.
Cumulative RR estimate of death in active treatment groups when compared with control groups stratified according to treatment class (inverse variance method). Heterogeneity between groups: P = 0.771. Studies with no events in both groups (Table 3) were excluded. RR, relative risk.
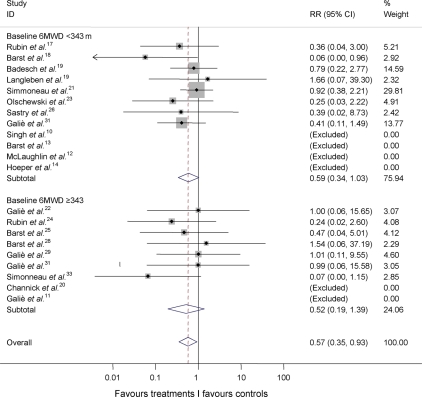
Cumulative RR estimate of death in active treatment groups when compared with control groups stratified by baseline exercise capacity according to the median value of the 6MWD of 343 m (Figure 4) did not show between-group heterogeneity (I-squared = 0.0%; P = 0.825).
Figure 4.
Cumulative RR estimate of death in active treatment groups when compared with control groups stratified by the median of baseline exercise capacity of the studies (inverse variance method). Studies with no events in both groups (Table 3) were excluded. Heterogeneity between groups: P = 0.825. 6MWD, six-minute walk distance; RR, relative risk.
In the sensitivity analysis including all 23 studies, overall mortality (Table 3) was 2.56% (82 of the 3199 patients). Mortality in the actively treated group was 1.72% (32 of the 1861 patients) and in the placebo group was 3.74% (50 of the 1338 patients).
The cumulative RR estimate of death was a reduction of 38% (RR 0.62; 95% CI 0.39, 1.00; P = 0.048) with the inverse variance method, no heterogeneity was apparent among studies (I-squared = 0.0%; P = 0.784). Analysis with the continuity correction (P < 0.044), the Mantel–Haenszel and Peto methods (P < 0.004 and P < 0.003, respectively) confirmed the statistical significance.
Explicitly reported hospitalizations for pulmonary arterial hypertension
Overall hospitalization rate in the eight RCTs (35%) reporting this information (Tables 2 and 3) was 4.98% (81 of the 1625). Hospitalization rate in the actively treated group was 3.2% (32 of the 1015) and in the placebo group was 8.03% (49 of the 610). These cumulative data do not consider the different randomization fraction and the different durations of the RCTs and should be intended as descriptive.
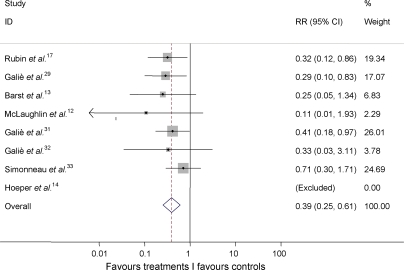
The cumulative RR estimate of hospitalizations (Figure 5) was a reduction of 61% (RR 0.39; 95% CI 0.25, 0.61; P < 0.001) with the inverse variance method, whereas no heterogeneity was apparent among studies (I-squared = 0.0%; P = 0.599).
Figure 5.
Cumulative RR estimate of hospitalizations in active treatment groups when compared with control groups. P < 0.001 for the overall estimate by inverse variance method. Studies with no events in both groups (Table 3) were excluded. RR, relative risk.
NNT to prevent one hospitalization was 19.9 and 50.3 (95% CI 32.5, 61.9) hospitalizations were prevented in each 1000 patients treated; these data were based on a RR = 0.393 applied to the control group event rate.
Six-minute walk distance
Investigational treatments significantly improved exercise capacity as assessed by the 6MWD. The overall heterogeneity test provided statistically significant results (I-squared = 76.6%; P < 0.001). The weighted mean improvement of exercise capacity assessed by the random-effect model in patients allocated to active treatments in the 19 RCTs (83%) reporting this parameter (see Supplementary material online, Figure S1) was 35.61 m (95% CI 27.13, 44.08; P < 0.001) ranging from −10 to +108 m. This average improvement appears to be an increase of about 10.8% when compared with the mean baseline values. Heterogeneity was related to both, drug classes (P < 0.001) and to baseline exercise capacity (P = 0.001).
NYHA/WHO functional class
In the 13 RCTs (53%) reporting NYHA/WHO functional class data (see Supplementary material online, Figure S2), investigational treatments significantly improved this parameter by at least one functional class (RR 2.35; 95% CI 1.59, 3.48; P < 0.001). Statistical tests indicated the existence of heterogeneous study results (I-squared = 56.2%; P = 0.007) and data were assessed by the random-effect model. Heterogeneity was related to both, drug classes (P = 0.044) and to baseline exercise capacity (P = 0.086).
Haemodynamic parameters
Investigational treatments significantly improved haemodynamic parameters as assessed by right heart catheterization. The weighted mean reduction in right atrial pressure in patients allocated to active treatments in the 11 RCTs (48%) reporting this parameter (see Supplementary material online, Figure S3) was −1.84 mmHg (95% CI −1.89, −1.80, P < 0.001) ranging from 1.00 to −6.20 mmHg. The weighted mean reduction in mean pulmonary arterial pressure in patients allocated to active treatments when compared with treatment groups in the 13 RCTs (57%) reporting this parameter (see Supplementary material online, Figure S4) was −2.86 mmHg (95% CI −2.93, −2.77; P < 0.001) ranging from −1.00 to −9.30 mmHg. The weighted mean increase in cardiac index in patients allocated to active treatments in the 12 RCTs (52%) reporting this parameter (see Supplementary material online, Figure S5) was 0.18 L/min/m2 (95% CI 0.17, 0.19, P < 0.001) ranging from 0.00 to 1.10 L/min/m2. The weighted mean reduction in pulmonary vascular resistance in patients allocated to active treatments in the 13 RCTs (57%) reporting this parameter (see Supplementary material online, Figure S6) was −4.09 resistance units (95% CI −4.18, −3.99; P < 0.001) ranging from −1.40 to −7.50 resistance units. Statistical tests indicated the existence of heterogeneous study results for each of the haemodynamic parameters (I-squared ranged from 87.6% to 98.3%; P < 0.001) and data were assessed by the random-effect model.
Discussion
The results of this meta-analysis on RCTs performed in pulmonary arterial hypertension patients show that the mortality in the control groups is high, being approximately 3.8% in the 14.3 weeks of the mean observation period (about 1.1% per month). This confirms the severity of the condition even in the stable and selected patients population included in RCTs. A reduction in the overall mortality of 43% was observed in the patients randomized to the active treatments when compared with those randomized to the placebo control arms (21 RCTs); a reduction of 38% was also confirmed after the addition of the two remaining RCTs, which included a concurrently initiated active control arm. These results were observed even if the average duration of the RCTs was limited to 14.3 weeks and even with the inclusion of RCTs on compounds which were eventually not approved by the Regulatory Agencies because of lack of consistent efficacy such as the thromboxane synthase inhibitor, terbogrel9 and the oral available prostanoid, beraprost.22,25
Subgroups analysis according to the different classes of drugs or with baseline exercise capacity as assessed by 6MWD did not show statistically significant heterogeneity in the effects on mortality. These data suggest that the results have not been driven by one class of drugs or by a group of patients with a specific disease severity.
The reasons for these non-heterogeneous results among different classes of drugs targeting diverse pathobiological pathways are not clear and may include specific disease characteristics, such as a ceiling effect which can limit and homogenize the extent of the beneficial effects that any medical treatment can achieve. In addition, the statistical power of the meta-analysis might not be sufficient to show a difference among drug classes or disease severity groups.
The survival benefit as suggested by the NNT evaluation appears to be significant because 61.6 patients are needed to be treated for an average period of 14.3 weeks for preventing one death.
The favourable results on survival observed in the current meta-analysis when compared with a previous reported meta-analysis8 may be explained by different reasons including a more appropriate selection of the trials (excluding acute studies and studies with different pulmonary hypertension aetiologies) and a larger sample size of both, number of studies (+44%) and number of patients (+63%).
The rate of explicitly reported hospitalizations owing to pulmonary arterial hypertension observed in the control groups of eight studies of this meta-analysis appears high being approximately 8% in an average period of 14.3 weeks. The reduction by 61% in the rate of hospitalizations observed in the groups of patients randomized to the active treatments appears to support the clinical efficacy of the targeted treatments for pulmonary arterial hypertension: one hospitalization can be prevented treating 19.9 patients for the average observation period of 14.3 weeks. However, these data were reported only in 35% of the RCTs of this meta-analysis and a reporting bias based on whether results tended to be favourable cannot be excluded.
The meta-analysis has confirmed the improvement in exercise capacity as assessed by 6MWD observed in all but two of the 18 studies reporting this parameter. These results are not surprising as the 6MWD has represented the primary endpoint for the majority of the RCTs and both patients' sample size and statistical power were calculated according to the predicted change of this parameter. The weighted average improvement was about 10.8% when compared with baseline 6MWD but markedly heterogeneous results were observed among different studies ranging from −10 to +108 m.
About half of the RCTs of this meta-analysis have included WHO/NYHA functional class and cardiopulmonary haemodynamic data. The improvement of one functional class was observed more often in patients randomized to active treatments even if only about one-third of the subjects achieved this result. Also in this case a reporting bias based on whether results tended to be favourable cannot be excluded.
Statistically significant improvements in the haemodynamic data, including mean pulmonary arterial pressure, cardiac index, pulmonary vascular resistance, and right atrial pressure were observed. The weighted mean improvements of these parameters appear to be small to moderate ranging from a reduction of about −5% in pulmonary arterial pressure and an increase of 8% of the cardiac index to a reduction of about −29% of the pulmonary vascular resistance.
The limitations of this meta-analysis include the prolonged period of time between the publication of the first and the last RCT (about 18 years), the different duration of the trials (ranging from 8 to 36 weeks), the lack of blindness in some studies,17–19,30 the pooling of multiple active treatment arms (potential alteration of the trial structure), the report of secondary outcome parameters only in part of the RCTs (possible reporting bias), and potential heterogeneity in the conduct of the trials and in the definition of hospitalization for pulmonary arterial hypertension in different RCTs (no individual patients data were reviewed). On the other hand, this meta-analysis, which considered all randomized patients (intention-to-treat), also included studies with compounds which were eventually not approved because of lack of efficacy9,22,25 and doses of approved drugs which were not endorsed because less effective or for increased side effects.13,24,28,31
A publication bias, favouring the publication of positive studies, also cannot be excluded. The funnel-plot analysis (plots of effect estimates against standard error of the estimate) did not show asymmetry (see Supplementary material online, Figure S7) and a possible publication bias should not have influenced substantially the results of this meta-analysis.
In conclusion, the results of this meta-analysis suggest an improvement of survival in the patients treated with the targeted therapies approved for pulmonary arterial hypertension. A reduction in the hospitalization rate and favourable results on exercise capacity, functional capacity, and haemodynamics were also observed in the groups of RCTs reporting these data.
Despite these results, the current treatment strategy remains inadequate because the mortality rate continues to be high and the functional and haemodynamic impairments are still extensive in many patients. The non-equivocal progresses observed recently in the medical treatments of this condition are not yet sufficient. Additional efforts are required to explore new strategies including RCTs with initial combination therapy, with new classes of drugs, and with new designs including morbidity and mortality endpoints and prolonged observation periods.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funding to pay the Open Access publication charges for this article was provided by the Cardiovascular Department of the University of Bologna.
Conflict of interest: N.G. has participated in advisory board activities for Actelion, Pfizer, United Therapeutics, Eli-Lilly, Bayer-Schering, Encysive, and Glaxo-SmithKline, given paid lectures for Actelion, Pfizer, Bayer-Schering, and Encysive. The Institute of Cardiology of the University of Bologna has received research grants from Actelion, Pfizer, United Therapeutics, Eli-Lilly, Bayer-Schering, Encysive and Glaxo-Smith-Kline. A.M., L.N., M.P., M.L.B.R., and A.B. had nothing to be declared.
Supplementary Material
[Supplementary Data]
References
- 1.Galie N, Rubin L. Pulmonary arterial hypertension. Epidemiology, pathobiology, assessment and therapy. J Am Coll Cardiol. 2004;43:S1–S90. doi: 10.1016/j.jacc.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl. 12):S13–S24. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 5.Rich S. The value of approved therapies for pulmonary arterial hypertension. Am Heart J. 2007;153:889–890. doi: 10.1016/j.ahj.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Farber HW. The status of pulmonary arterial hypertension in 2008. Circulation. 2008;117:2966–2968. doi: 10.1161/CIRCULATIONAHA.108.782979. [DOI] [PubMed] [Google Scholar]
- 7.Ghofrani HA, Wilkins MW, Rich S. Uncertainties in the diagnosis and treatment of pulmonary arterial hypertension. Circulation. 2008;118:1195–1201. doi: 10.1161/CIRCULATIONAHA.106.674002. [DOI] [PubMed] [Google Scholar]
- 8.Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, Tognoni G. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007;153:1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Langleben D, Christman BW, Barst RJ, Dias VC, Galie N, Higenbottam TW, Kneussl M, Korducki L, Naeije R, Riedel A, Simonneau G, Hirsch AM, Rich S, Robbins IM, Oudiz R, McGoon MD, Badesch DB, Levy RD, Mehta S, Seeger W, Soler M. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am Heart J. 2002;143:E4. doi: 10.1067/mhj.2002.121806. [DOI] [PubMed] [Google Scholar]
- 10.Singh T, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J. 2006;151:851.e1–851.e5. doi: 10.1016/j.ahj.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Beghetti M, Gatzoulis MA, Granton J, Berger RMF, Lauer A, Chiossi E, Landzberg M for the Bosentan Randomized Trial of Endothelin Antagonist Therapy. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–1263. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 13.Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, Naeije R, Galie N. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 14.Hoeper M, Leuchte H, Halank M, Wilkens H, Meyer FJ, Seifarth HJ, Wensel R, Ripken F, Bremen H, Kluge S, Hoeffken G, Behr J. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Resp J. 2006;4:691–694. doi: 10.1183/09031936.06.00057906. [DOI] [PubMed] [Google Scholar]
- 15.Ghofrani HA, Wiedemann R, Rose F, Olschewski H, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136:515–522. doi: 10.7326/0003-4819-136-7-200204020-00008. [DOI] [PubMed] [Google Scholar]
- 16.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 17.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, Diehl JH, Crow J, Long W. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 18.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group [see comments] N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 19.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, Rich S, Barst RJ, Barrett PS, Kral KM, Jobsis MM, Loyd JE, Murali S, Frost A, Girgis R, Bourge RC, Ralph DD, Elliott CG, Hill NS, Langleben D, Schilz RJ, McLaughlin VV, Robbins IM, Groves BM, Shapiro S, Medsger TA., Jr Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial [see comments] Ann Intern Med. 2000;132:425–434. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 20.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 21.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ. Continuous Subcutaneous Infusion of Treprostinil, a Prostacyclin Analogue, in Patients with Pulmonary Arterial Hypertension. A double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 22.Galie N, Humbert M, Vachiery JL, Vizza CD, Kneussl M, Manes A, Sitbon O, Torbicki A, Delcroix M, Naeije R, Hoeper M, Chaouat A, Morand S, Besse B, Simonneau G. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomised, double-blind placebo-controlled trial. J Am Coll Cardiol. 2002;39:1496–1502. doi: 10.1016/s0735-1097(02)01786-2. [DOI] [PubMed] [Google Scholar]
- 23.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Sitbon O, Speich R, Hoeper M, Behr J, Winkler J, Seeger W for the AIR Study Group. Inhaled iloprost in severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 25.Barst RJ, McGoon M, Mc Laughlin VV, Tapson V, Rich S, Rubin L, Wasserman K, Oudiz R, Shapiro S, Robbins I, Channick R, Badesch BD, Rayburn BK, Flinchbaugh R, Sigman J, Arneson K, Jeffs R for the Beraprost Study Group. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2125. doi: 10.1016/s0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 26.Sastry BKS, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension. 1: A randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol. 2004;43:1149–1153. doi: 10.1016/j.jacc.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Barst RJ, Robbins IM, Channick RN, Galie N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 28.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 29.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G the Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin-Sing W, Banya WA, Westwood MA, Stefanidis A, Ng LL, Pennell DJ, Mohiaddin RH, Nihoyannopoulos P, Gibbs JS. Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) study. Am J Respir Crit Care Med. 2005;171:1292–1297. doi: 10.1164/rccm.200410-1411OC. [DOI] [PubMed] [Google Scholar]
- 31.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ for the Ambrisentan in Pulmonary Arterial Hypertension RD-BP-CMESAG. Ambrisentan for the treatment of pulmonary arterial hypertension. Results of the Ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, Efficacy (ARIES) Study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 32.Galie' N, Rubin LJ, Hoeper M, Jansa P, Al-Hiti H, Meyer GMB, Chiossi E, Kusic-Pajic A, Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 33.Simonneau G, Rubin L, Galie N, Barst RJ, Fleming T, Frost A, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch BD For the Pulmonary Arterial Hypertension combination Study of Epoprostenol Sildenafil (PACES) Study Group. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 34.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(Suppl. 12):S5–S12. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6 min walk: a new measure of exercise capacity in patients with chronic failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 36.Barst R, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski A, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl. [12 1]):S40–S47. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Petitti DB. Meta-analysis, decision analysis, and cost-effectiveness analysis. London, UK: Oxford University Press; 1994. [Google Scholar]
- 38.Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 39.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JPT, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplementary Data]