Five-Year Follow-Up of Patients With Advanced Chronic Lymphocytic Leukemia Treated With Allogeneic Hematopoietic Cell Transplantation After Nonmyeloablative Conditioning (original) (raw)
Abstract
Purpose
We reported encouraging early results of allogeneic hematopoietic cell transplantation (HCT) after nonmyeloablative conditioning in 64 patients who had advanced chronic lymphocytic leukemia (CLL). Here, we have extended the follow-up to a median of 5 years and have included data on an additional 18 patients.
Patients and Methods
Eighty-two patients, age 42 to 72 years, who had fludarabine-refractory CLL were conditioned with 2 Gy total-body irradiation alone or combined with fludarabine followed by HCT from related (n = 52) or unrelated (n = 30) donors.
Results
Complete remission (CR) and partial remission were achieved in 55% and 15% of patients, respectively. Higher CR rates were noted after unrelated HCT (67% v 48%). The 5-year incidences of nonrelapse mortality (NRM), progression/relapse, overall survival, and progression-free survival were 23%, 38%, 50%, and 39%, respectively. Among 25 patients initially reported in CR, 8% relapsed and 8% died as a result of NRM, whereas 84% have remained alive and in CR. Among 14 responding patients who were tested and who had molecular eradication of their disease, two died as a result of NRM, two relapsed, and 10 have remained negative. At 5 years, 76% of living patients were entirely well, whereas 24% continued to receive immunosuppression for chronic graft-versus-host disease; the median performance status in each group was 100% and 90%, respectively. Lymphadenopathy ≥ 5 cm, but not cytogenetic abnormalities at HCT, predicted relapse. In a risk-stratification model, patients who had lymphadenopathy less than 5 cm and no comorbidities had a 5-year OS of 71%.
Conclusion
Nonmyeloablative HCT resulted in a median survival of 5 years for patients who had fludarabine-refractory CLL with sustained remissions and in the continued resolution of chronic graft-versus-host disease in surviving patients.
INTRODUCTION
Graft-versus-leukemia effects appear to play a more important role than conditioning intensity in the control of minimal residual disease among patients with chronic lymphocytic leukemia (CLL).1-4 Because of high nonrelapse mortality (NRM) associated with conventional allogeneic hematopoietic cell transplantation (HCT) in patients who have CLL,5-11 reduced-intensity or truly nonmyeloablative conditioning regimens have been investigated. We have shown initial success of allogeneic HCT after nonmyeloablative conditioning in achieving disease control among 64 fludarabine-refractory CLL patients who also had acceptable NRM rates.12 The 2-year rates of overall survival (OS) and progression-free survival (PFS) were 60% and 52%, respectively. Other investigators have reported their experiences with reduced-intensity HCT for CLL, with 2-year rates of OS and PFS in the range of 51% to 80% and 34% to 67%, respectively.13-17 Here, we addressed two critical questions. One was the ability of this approach to provide long-term disease control and to improve survival in both patients with high-risk CLL, including fludarabine-refractory CLL, who otherwise have survival rates of 12 to 18 months with chemoimmunotherapy18-21 and in those who have high-risk genomic features associated with resistance to conventional therapy.22-26 The second question concerned chronic graft-versus-host disease (GVHD) in this elderly patient population. To this end, we updated results on the initial 64 patients with an additional 3 years of follow-up, and we described data from 18 additional patients. Also, we formulated a risk-stratification model on the basis of lymphadenopathy ≥ 5 cm and HCT-specific comorbidity index (HCT-CI) scores.
PATIENTS AND METHODS
Previously reported results from 64 patients with CLL were updated with a median follow-up of 5 years (range, 3.0 to 7.3 years) after nonmyeloablative HCT12 and were combined with those from an 18 additional patients who had a median follow-up of 1.6 years (range, 0.9 to 3.1 years). All patients were enrolled on multi-institutional trials conducted between December 1997 and January 2006 at 12 academic centers, and the Fred Hutchinson Cancer Research Center acted as the coordinating center. Protocols were approved by the institutional review boards of the Fred Hutchinson Cancer Research Center and the collaborating sites. All patients signed consent forms. Inclusion and exclusion criteria for protocols and the definition of fludarabine-refractoriness have been published.12 Conditioning regimens, postgrafting immunosuppression, HLA typing and matching, collection of hematopoietic cells, supportive care, analyses of donor chimerism, pre-transplant risk factors, post-transplant disease responses, and minimal residual disease monitoring by allele-specific complementary-determining region III (CRDIII) sequences were carried out as described.12 Pre-transplant comorbidities were scored by using an HCT-CI.27 Data were analyzed as of April 2007.
Cumulative incidence estimates were calculated for acute and chronic GVHD, relapse, relapse-related mortality, and NRM. OS and PFS rates were estimated by the Kaplan-Meier method. Deaths were treated as competing events in analyses of graft rejection, GVHD, and disease progression. Progression and NRM were the components of PFS and were treated as competing events. Hazard ratios were estimated from Cox regression models. Multivariate models were constructed in a stepwise fashion by using a threshold significance level of .10 for inclusion in the model. Multivariate P values for a variable were based on adjustment for all other variables in the model. All P values were derived from likelihood ratio statistics and were two-sided. Onset of chronic GVHD and cessation of all immunosuppressive agents were shown with prevalence curves, as described previously.28
RESULTS
Patient Characteristics and Disease Responses
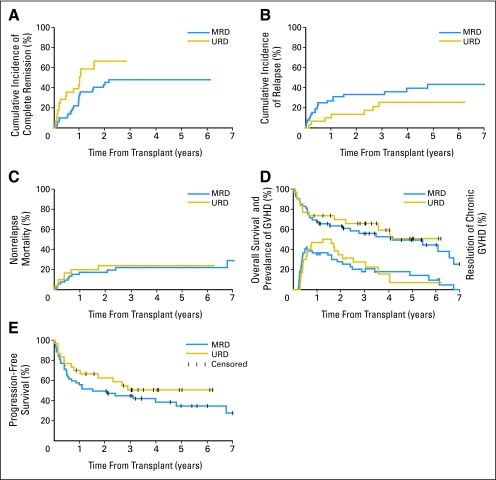
Patient and disease characteristics are listed in Table 1 and described in the Appendix (online only). Four patients had no measurable disease at HCT; one died as a result of relapse, two died as a result of NRM while in complete remission (CR), and one is alive in CR. Among 78 patients who had measurable disease at HCT, the 5-year cumulative probabilities for achieving CR and partial remission (PR) were 55% and 15%, respectively. Unrelated recipients had a statistically significantly higher 5-year rate of CR (67% v 48%; P = .04) compared with related recipients (Fig 1A). Table 2 lists the overall responses of the 82 patients.
Table 1.
Patients and Disease Characteristics
| Characteristic | Recipients | ||
|---|---|---|---|
| Related (n = 52) | Unrelated (n = 30) | All (N = 82) | |
| Diagnosis, % | |||
| CLL | 83 | 90 | 85 |
| CLL/SLL | 13 | 3 | 10 |
| CLL/PLL | 4 | 7 | 5 |
| Conditioning regimen, % | |||
| 2-Gy TBI | 25 | 0 | 16 |
| 2-Gy TBI + fludarabine | 75 | 100 | 84 |
| Age, years | |||
| Median | 55 | 57 | 56 |
| Range | 44-72 | 42-69 | 42-72 |
| Years from diagnosis to HCT | |||
| Median | 4.22 | 5.18 | 4.54 |
| Range | 0.9-24.7 | 0.5-10.8 | 0.5-24.7 |
| HCT-CI scores, % | |||
| 0 | 44 | 40 | 43 |
| 1-2 | 35 | 20 | 29 |
| ≥ 3 | 21 | 40 | 28 |
| Prior autologous HCT, % | 2 | 7 | 4 |
| Number of prior regimens, % | |||
| 1-2 | 25 | 20 | 23 |
| 3-4 | 48 | 37 | 44 |
| 5-6 | 17 | 20 | 18 |
| ≥ 7 | 10 | 23 | 15 |
| Fludarabine-refractory disease, % | 86 | 87 | 87 |
| Disease status at HCT, % | |||
| Responsive | |||
| CR | 4 | 7 | 5 |
| PR | 38 | 40 | 37 |
| Unresponsive | 48 | 40 | 45 |
| Untreated relapse | 10 | 13 | 11 |
| Disease burden, % | |||
| Lymph node size ≥ 5 cm | 27 | 20 | 24 |
| Marrow infiltration ≥ 50% | 57 | 30 | 47 |
| Lymphocyte count > 104/μL | 21 | 13 | 18 |
| β2 microglobulin > 2.5 μg/mL* | 50 | 50 | 50 |
| CD38+ expression† | 60 | 59 | 59 |
| Unfavorable cytogenetics‡ | 39 | 43 | 41 |
| Splenomegaly | 46 | 37 | 43 |
| Cell transplanted, median | |||
| CD34+ × 106/kg | 8.4 | 6.6 | 6.75 |
| CD3+ × 108/kg | 3.45 | 2.69 | 3.03 |
Fig 1.
Cumulative incidences and rates of (A) achieving complete remission, (B) disease progression or relapse, (C) nonrelapse mortality, (D) Kaplan-Meier estimates of overall survival and prevalence of chronic graft-versus-host disease, and (E) progression-free survival among recipients of related and unrelated grafts. MRD, matched related donor; URD, unrelated donor.
Table 2.
Disease Status at Study Enrollment and Disease Responses During the Study
| Disease Status at HCT (n = 82) | Disease Responses | Treatment Given to Alive Patients With PD/Relapse | ||
|---|---|---|---|---|
| No. and Type of Best Response After HCT (n = 82) | Outcomes at Last Contact (months after HCT) | |||
| Months Deceased per Status (n = 40) | Months Alive per Status (n = 42) | |||
| CR (n = 4)/PR (n = 32) | 24 CR | CR: 5, 7, 8, 8, 25, 29; relapse: 50, 80 | CR: 12, 13, 15, 19, 26, 37, 37, 39, 40, 48, 49, 50, 66, 73, 87; relapse*: 62 | R →R →CHOP |
| 4 PR | PD: 27 | PR: 19, 25; PD*: 18 | R | |
| 1 SD | — | SD: 36 | ||
| 2 NA | NA: 1, 5 | — | ||
| 5 PD | PD: 1, 4, 10, 34 | PD*: 19 | IF→ R | |
| Relapse (n = 9) | 7 CR | CR: 12 | CR: 26, 37, 42, 61, 61, 76 | |
| 1 PR | — | PR: 11 | ||
| 1 NA | NA: 1 | |||
| Refractory (n = 37) | 14 CR | CR: 3, 21, 82; relapse: 44 | CR: 37, 42, 43, 56, 60, 68, 74, 85, 86, 88 | |
| 8 PR | PR: 2, 5, 7; PD: 8, 19, 66 | PR: 33; PD*: 66 | Alemtuzumab | |
| 2 SD | SD: 2, 2 | — | ||
| 1 NA | NA: 0.4 | — | ||
| 12 PD | PD: 1, 3, 4, 4, 7, 7, 13, 43, 51, 74 | PD*: 34 | ESHAP | |
| PD: 61 | Alemtuzumab→ R |
Overall, 41 patients who had measurable disease at HCT experienced CR after HCT; 30 of these are alive and in CR, eight died as a result of NRM, and three experienced disease relapse. One of the latter three is currently alive and in CR after rituximab followed by one cycle of chemotherapy (follow-up 28 months); one died as a result of refractory CLL after two cycles of chemotherapy and two donor lymphocyte infusions (DLIs); and one died as a result of advanced GVHD after treatment with rituximab and DLI. Thirteen patients who had measurable disease at HCT achieved PR after HCT; four are alive and in PR at a median of 22 months, three died as a result of NRM while in PR at a median of 5 months after HCT; and six experienced disease progression. Four of the latter six died as a result of progressive disease, and two are alive in PR after treatment with alemtuzumab (n = 1; 65.6 months of follow-up) and rituximab (n = 1; 17.7 months of follow-up). Four patients were not assessed for disease response because they experienced early NRM. Three patients had stable disease; two died as a result of NRM 1.8 months (n = 1) and 2 months (n = 1) after HCT; and one, who was in PR at HCT, is alive with stable disease 36 months after HCT. Seventeen patients had disease progression; 14 died; and three are alive after being treated with rituximab (n = 1), rituximab and alemtuzumab (n = 1), or chemotherapy (n = 1).
In our initial report, 39 of 64 patients were alive at a median of 2 years. With 3 years of additional follow-up, two of 25 patients who were in CR experienced nonrelapse deaths while in CR, and two died as a result of relapse; the remaining 21 patients (84%) are alive and in CR. Among five patients who were in PR, one progressed and died, one progressed and is alive with disease, and three are alive in CR. Of two patients who had stable disease, one still has stable disease, and one has progressed. Six of seven patients who had relapse/progressive disease have died, and one is alive in CR after treatment with monoclonal antibody and chemotherapy.
Molecular monitoring was done in 14 patients after HCT. All 14 achieved molecular remissions. Two patients died as a result of NRM during molecular remission; two patients relapsed 32 and 39 months after HCT, respectively; and nine have sustained CR at a median of 73.4 months (range, 19 to 87.5 months).
Overall, six patients had disease relapse after 2 years. Five of the six experienced relapse of their original disease, and one patient experienced disease transformation to large-cell lymphoma.
Overall Outcomes
Cumulative incidences of NRM and relapse/progression and rates of OS and PFS at 5-years were 23%, 38%, 50%, and 39%, respectively. There were no statistically significant differences in NRM (22% v 24%; P = .97), relapse/progression (43% v 26%; P = .18), OS (49% v 51%; P = .57), or PFS (35% v 51%; P = .28) between related and unrelated recipients (Figs 1B, 1C, 1D, and 1E).
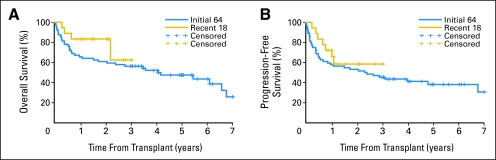
We analyzed the outcomes of the initial 64 patients separately from the 18 patients who recently received transplants (Appendix Fig A1, online only) and found 5-year NRM, relapse, OS, and PFS rates were 25%, 37%, 48%, and 38%, respectively, which were not different from the figures for the total 82 patients. For the additional 18 patients, the same outcomes were 11%, 31%, 83%, and 58%, respectively at 2 years. These results were not statistically significantly different from those in the original patients.
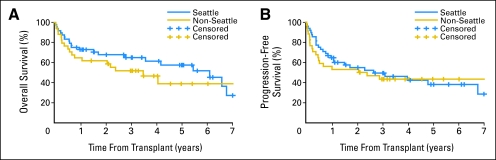
Because 59% of patients underwent transplantation at Seattle, WA, we compared outcomes of those patients to all other patients who underwent transplantation at nine collaborating institutions. Comparable 5-year OS rates (58% v 39%; P = .27) and PFS rates (38% v 43%; P = .69) were noted among patients from Seattle, WA compared with those not from Seattle, WA, respectively (Appendix Fig A2, online only).
Nonrelapse deaths (n = 19) included infections with acute (n = 4) or chronic (n = 6) GVHD, cardiac arrest (n = 2), pulmonary hemorrhage after rejection (n = 1), cerebrovascular stroke (n = 1), de novo metastatic lung cancer (n = 1), pneumonia (n = 1), sepsis (n = 1), grade 4 gut acute GVHD after DLI for low chimerism (n = 1), and multiorgan failure after open heart surgery (n = 1).
GVHD and Duration of Immunosuppressive Therapy
Cumulative incidences of grades 2, 3, and 4 acute GVHD were 39%, 14%, and 2%, respectively, among related recipients and 43%, 20%, and 3%, respectively, among unrelated recipients. Five-year cumulative incidences of chronic extensive GVHD were 49% for related and 53% for unrelated recipients (P = .95). The 5-year prevalence of patients alive after discontinuation of all immunosuppressive medications was 38% (35% for related and 44% for unrelated recipients; Fig 1D). The median duration of treatment for chronic GVHD was 25 months (range, 12 to 61 months), and the median interval between stoppage of all immunosuppressive medications and last follow-up was 19 months. The median performance status at last contact was 90% and 100% for patients with or without chronic GVHD, respectively.
Impact of Prognostic Factors on Outcomes
Multiple risk factors were analyzed for their effects on NRM, relapse, OS and, PFS by using univariate analyses. Age, donor type, CD34+ cell dose, CD3+ cell dose, CD5/CD19 coexpression of CD38 greater than 30%, cytogenetic risks, splenomegaly, disease status at HCT, and time between diagnosis and HCT were not significantly associated with outcomes at the .10 level of significance. Factors that had significant impact in univariate analyses were entered in multivariate analyses. Lymphadenopathy ≥ 5 cm at the time of HCT strongly predicted increased relapse, HCT-CI scores independently predicted NRM, and both were independent predictors for OS and PFS (Appendix Table A1, online only). Patients who had fludarabine-refractory CLL had a higher relapse hazard but had a trend toward lower NRM. Marrow infiltration with ≥ 50% CLL cells, circulating lymphocyte counts greater than 104/μL, β2 microglobulin greater than 2.5 μg/mL, and the number of preceding treatment regimens did not independently predictor outcomes.
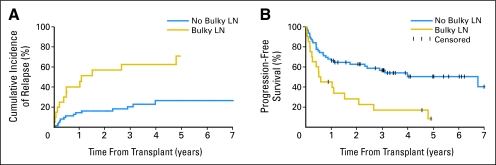
Relapse and PFS rates were comparable not only among patients who had refractory/untreated relapse and those who had CR/PR (P = .41 and P = .61, respectively), but also among those who had greater than 30% CD38 expression compared with ≤ 30% (P = .22 and P = .27, respectively) and among those who had favorable compared with unfavorable cytogenetics (P = .65 and P = .88, respectively). The outcomes of patients who had specific adverse cytogenetic abnormalities are listed in Table 3. There were higher 5-year relapse (71% v 27%; P = .0004) and lower PFS (8% v 50%; P = .002) rates among patients who had lymphadenopathy ≥ 5 cm or less than 5 cm, respectively (Fig 2).
Table 3.
Disease Responses and Outcomes Among Patients Diagnosed With Unfavorable Cytogenetics
| Abnormality | Patients Who Remained Alive | Patients Who Died | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR (No.) | PR (No.) | PD/Relapse (No.) | Median Interval (years) | CR/PR (No.) | NA (No.) | PD/Relapse (No.) | Median Interval (years) | |||
| From Diagnosis | From HCT | From Diagnosis | From HCT | |||||||
| del 17p (n = 7) | 4 | — | — | 6.2 | 3.3 | 1 | — | 2 | 3 | 0.3 |
| del 11q (n = 7) | 1 | 2 | 2 | 8.2 | 2.1 | 1 | — | 1 | 7.5 | 0.7 |
| tri 12 (n = 7) | 4 | — | — | 6.7 | 4.4 | — | 2 | 1 | 3.8 | 0.2 |
| Complex (n = 9) | 4 | — | — | 11.4 | 5.7 | 1 | — | 4 | 10.5 | 3.5 |
Fig 2.
Cumulative incidences of relapse and Kaplan-Meier estimates of progression-free survival among patients diagnosed with advanced chronic lymphocytic leukemia and treated with nonmyeloablative conditioning followed by related or unrelated allogeneic hematopoietic cell transplantation as stratified by lymph node (LN) diameter.
NRM and OS incidences and rates at 5 years were comparable among patients age ≥ 60 years versus younger than 60 years (P = .96 and P = .35, respectively) and among patients given 0 to 3 versus ≥ 4 prior chemotherapy regimens (P = .97 and P = .18, respectively). Patients who had HCT-CI scores of 0 versus 1 to 2 versus ≥ 3 had NRM incidences of 15%, 29%, and 29%, respectively, and OS rates of 55%, 52%, and 38%, respectively.
Risk-Stratification Model
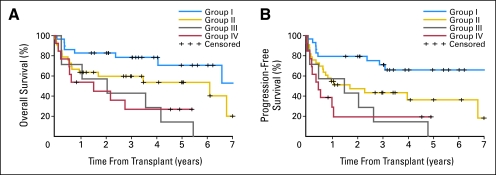
All patients then were stratified on the basis of the two statistically significant prognostic factors: lymphadenopathy ≥ 5 cm or less than 5 cm and HCT-CI scores of 0 or ≥ 1. Accordingly, patients could be divided into four groups with increasing risk of worse outcomes: group I included patients who had no comorbidities and lymphadenopathy less than 5 cm (n = 28); group II, patients with comorbidities only (n = 34); group III, patients with lymphadenopathy ≥ 5 cm only (n = 7); and group IV, patients with comorbidities and lymphadenopathy of ≥ 5 cm (n = 13). Patients in the four groups had 3-year OS rates of 78%, 60%, 43%, and 27%, respectively (Fig 3).
Fig 3.
Kaplan-Meier probabilities of survival among patients with advanced chronic lymphocytic leukemia who were treated with allogeneic nonmyeloablative hematopoietic cell transplantation (HCT) as stratified into four risk groups on the basis of consolidated HCT-specific comorbidity index sores and lymph node diameter. Group I included patients who had no comorbidities and who had lymphadenopathy of less than 5 cm (n = 28); group II, patients with comorbidities only (n = 34); group III, patients with lymphadenopathy of ≥ 5 cm only (n = 7); and group IV, patients with both comorbidities and lymphadenopathy of ≥ 5 cm (n = 13).
DISCUSSION
This report updated previously published data of a multicenter study of nonmyeloablative HCT for patients who had poor-risk CLL. After 3 additional years of follow-up, 21 (84%) of 25 patients who were in CR in the initial report12 have remained alive and in CR. The 5-year OS and PFS of the initially reported 64 patients were 48% and 38%, respectively, which confirms the long-term efficacy of the nonmyeloablative HCT. Moreover, conversion to CR was observed in three of five patients reported to have PR. This supported the hypothesis that graft-versus-leukemia (GVL) effects after a truly nonmyeloablative HCT were capable of inducing late disease responses in CLL. Results among the additional 18 patients confirm the previously reported results.
We also assessed the influence of poor prognostic features of CLL29 on HCT outcomes,30-34 and we detected no significant correlations between these parameters and GVL effects. This is particularly important as chemotherapy, monoclonal antibodies, and autologous HCT have been associated with limited efficacy in patients who have poor prognostic features (including fludarabine-refractoriness and unfavorable cytogenetics); median OS is usually less than 1 to 2 years.19,21,34-37 For example, treatment with fludarabine, cyclophosphamide, and rituximab combination in patients who have fludarabine-refractory versus fludarabine-sensitive CLL have resulted in 6% versus 33% CR rates, respectively.37 Patients with del(17p) and del(11q) had only 9 and 13 months of treatment-free intervals, respectively, in the hierarchical model of Dohner et al30 and had PFS intervals of 11 and 21.5 months, respectively, after fludarabine-based chemotherapy.26,38 These findings supported the assertion that alternative therapies need to be pursued for poor-risk patients. In our series, patients with fludarabine-refractory CLL achieved a 55% CR rate, and greater than 50% of patients with del(17p) or del(11q) have shown responses and were alive after 39 and 25 months, respectively, of median follow-up. However, results are limited by the small number of patients (n = 14) who have del(17p) or del(11q). Nevertheless, they confirm, with longer follow-up, our earlier results and those in other reports that show the efficacy of reduced-intensity HCT in CLL patients who have poor genomic features.39-41 Even though these patients had advanced CLL, poor-risk cytogenetic features of del(17p) and del(11q) were relatively under-represented (17%). This might be explained by the change in diagnostic standards and technology in the 10 years during which the current patients underwent transplantation. For example, even though all patients had conventional cytogenetics performed at the time of HCT, cells from 24% of patients were not analyzed by fluorescent in situ hybridization; thus, poor-risk genomic features might have been missed. Nevertheless, the majority of patients (87%) in this study had fludarabine-refractory CLL, which is associated with dismal outcome, and, to our knowledge, no reports have shown that such outcome is dependent on cytogenetic risks.
Chronic GVHD is a complication of allogeneic HCT. Information on the outcome of chronic GVHD among elderly patients who undergo nonmyeloablative HCT for CLL has been underreported in the literature. This report showed that, even in this older patient population, the prevalence of chronic GVHD and its resolution were comparable to those observed among younger patients treated with myeloablative HCT.42,43 Although approximately 50% of patients were affected by chronic GVHD, signs and symptoms of this complication eventually resolved in most patients, and immunosuppression was discontinued after a median of approximately 2 years. These findings, together with the normal to near-normal performance status of surviving patients, should encourage further accrual of older CLL patients to nonmyeloablative HCT protocols.
In agreement with our initial observations, CRs were more frequent among unrelated compared with related recipients, but they had comparable survivals. The overall long-term OS (51%) and PFS (51%) among current unrelated recipients seemed superior to the historical experience with myeloablative HCT, as reported by the International Blood and Marrow Transplantation Registry, which showed OS and PFS of 33% and 32%, respectively.6 Our results supported the suggestion that unrelated donor HCT should be considered in patients who lacked HLA-identical siblings.44
Despite reasonable long-term success, problems were identified among this patient cohort. NRM, although lower than after myeloablative HCT for younger patients,45 was still significant. Slightly more than half of the NRM was caused by GVHD and associated infections, whereas other causes of death were related to comorbidities. Efforts, which include optimizing GVHD prophylaxis and better understanding predictors of NRM among elderly patients given nonmyeloablative HCT, are underway to reduce NRM. Lymphadenopathy of ≥ 5 cm at HCT was a major cause of disease relapse/progression (71% at 5 years). For patients who have lymphadenopathy of ≥ 5 cm, more aggressive approaches are needed for cytoreduction, which thereby allow time for GVL effects to occur. One approach that we are currently exploring is combination of a radiolabeled anti-CD20 monoclonal antibody with nonmyeloablative conditioning regimen.46 Novel cyclin-dependent kinase inhibitors, such as flavopiridol,47,48 or immunomodulatory agents, such as lenalidomide,49 have shown activity in bulky fludarabine-refractory CLL and could be explored for administration before HCT. Moreover, even among patients who have lymphadenopathy of less than 5 cm, 27% progression/relapse rates have been seen at 5 years. Pre-emptive treatment with monoclonal antibodies after HCT might be useful to address this problem.15 Of note, 12 of 28 patients who progressed after HCT survived between 12.3 and 63.6 months after receiving treatment with monoclonal antibody (n = 3), antibody combined with DLI (n = 3), or antibody combined with chemotherapy (n = 5). Pre-emptive interventions also could be offered to patients in whom early progression is diagnosed by four-color flow cytometry, which is equally sensitive but simpler and cheaper than quantitative polymerase chain reaction.2,50
In most other reports on outcomes in patients with CLL who were given reduced-intensity conditioning, median follow-ups were shorter and reached a maximum of 2 years.13-17 A report from Spain described outcomes among 30 patients who underwent HCT from HLA-identical siblings.39 Median follow-up was 4 years, and OS and event-free survival (EFS) rates at 5 years were 72% and 70%, respectively.39 Only 37% of their patients were refractory to fludarabine compared with 86% of current patients, and 80% versus 42% had CR/PR at HCT, respectively. For myeloablative HCT, 5-year OS rates of 32% to 45%, respectively, and PFS rates of 32% to 42%, respectively, were reported.6,8,10,51 In general, patients in those reports were 10 years younger than these patients.
Extrapolation of these results to the general CLL population is difficult, as they include only those patients who were referred and enrolled onto the HCT protocols at the different institutions. The percentages of patients not referred for HCT because of lack of insurance coverage or rapidly progressing disease that did not allow time for transplantation preparation or donor search are unknown. Nevertheless, within those limitations, our results support the use of nonmyeloablative allogeneic HCT for patients who have fludarabine-refractory CLL.
Current findings of long-term disease control and resolution of chronic GVHD among a majority of patients supported the use of nonmyeloablative HCT for patients who have failed fludarabine. Early HCT should be considered for patients who have poor genomic features. This strategy would limit patient exposure to extensive courses of conventional treatment and would allow nonmyeloablative HCT to be offered at earlier and at more optimal disease stages. In support of this recommendation, these patients who had lymphadenopathy less than 5 cm and no comorbidities had 3-year and 5-year OS of 78% and 71%, respectively.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jerald Radich, Novartis (C), Bristol-Myers Squibb Oncology (C), Merck & Co (C) Stock Ownership: None Honoraria: Jerald Radich, Novartis, Bristol-Myers Squibb Oncology Research Funding: Jerald Radich, Novartis, Bristol-Myers Squibb Oncology Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mohamed L. Sorror, Rainer Storb, David G. Maloney
Financial support: Rainer Storb
Administrative support: Rainer Storb
Provision of study materials or patients: Mohamed L. Sorror, Brenda M. Sandmaier, Michael Maris, Judith Shizuru, Richard Maziarz, Edward Agura, Thomas R. Chauncey, Michael A. Pulsipher, Peter A. McSweeney, James C. Wade, Benedetto Bruno, Amelia Langston, Dietger Niederwieser, Karl G. Blume, Rainer Storb, David G. Maloney
Collection and assembly of data: Mohamed L. Sorror
Data analysis and interpretation: Mohamed L. Sorror, Barry E. Storer, Brenda M. Sandmaier, Karl G. Blume, Rainer Storb, David G. Maloney
Manuscript writing: Mohamed L. Sorror, Brenda M. Sandmaier, Rainer Storb, David G. Maloney
Final approval of manuscript: Mohamed L. Sorror, Barry E. Storer, Brenda M. Sandmaier, Judith Shizuru, Richard Maziarz, Edward Agura, Thomas R. Chauncey, Michael A. Pulsipher, James C. Wade, Benedetto Bruno, Amelia Langston, Jerald Radich, Dietger Niederwieser, Karl G. Blume, Rainer Storb, David G. Maloney
Acknowledgments
We thank the data coordinators, Jennifer Freese, Heather Hildebrant, and Gary Schoch, and the study nurses, Mary Hinds, John Sedgwick, Michelle Bouvier and Joanne Greene, for their invaluable help in making the study possible; Bonnie Larson, Helen Crawford, Karen Carbonneau, and Sue Carbonneau for assistance with manuscript preparation; and the transplantation teams.
Appendix
Patient and disease characteristics.
Diagnoses included chronic lymphocytic leukemia (85%), small lymphocytic lymphoma (10%), and prolymphocytic leukemia (5%). Patients had a median age of 56 years (range, 42 to 72 years). Most patients (87%) were refractory to fludarabine-based regimens, and 56% had unresponsive disease or were in untreated relapse at hematopoietic cell transplantation (HCT). Unrelated recipients had a greater median interval between diagnosis and HCT (5.18 v 4.22 years), greater numbers of preceding therapy regimens (four v three), and higher HCT-specific comorbidity index scores (≥ 3 in 40% of patients v in 21% of related recipients). Related recipients were more likely to have ≥ 50% marrow infiltration with chronic lymphocytic leukemia cells (57% v 30%), circulating lymphocyte counts greater than 104/μL (21% v 13%), and enlarged spleens (46% v 37%) compared with unrelated recipients. Both cohorts had comparable elevations of β2 microglobulin greater than 2.5 μg/mL (50% v 50%), CD5/CD19 coexpression of CD38 greater than 30% (60% v 59%), unfavorable cytogenetics (39% v 43%), and lymph node sizes of ≥ 5 cm (27% v 20%). Related recipients were given higher median numbers of CD34+ (8.4 v 6.6 × 106/kg) and CD3+ cells (3.45 v 2.69 × 108/kg) compared with unrelated recipients.
Forty-eight patients underwent transplantation at Seattle, WA; nine at Stanford, CA; seven at Leipzig, Germany; six at Portland, OR; four at Baylor, TX; three at Salt Lake City, UT; two at Boulder, CO; and one each at Milwaukee, WI; Torino, Italy; and Atlanta, GA.
Fig A1.
Kaplan-Meier probabilities of (A) overall and (B) progression-free survival among the initially reported 64 patients compared with the 18 patients who recently underwent transplantation; both groups were diagnosed with advanced chronic lymphocytic leukemia and were treated with allogeneic nonmyeloablative hematopoietic cell transplantation.
Fig A2.
Kaplan-Meier probabilities of (A) overall and (B) progression-free survival among patients who underwent transplantation at Seattle, WA (n = 48), compared with patients who underwent transplantation at other collaborating institutions (n = 34).
Table A1.
Multivariate Analysis of Risk Factors Among 82 Patients Diagnosed With Advanced CLL and Treated With Nonmyeloablative Conditioning and Allogeneic HCT From Related or Unrelated Donors
| Factor | Mortality (n = 40) | PFS (n = 46) | Relapse/Progression (n = 27) | NRM (n = 19) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| HCT-CI | ||||||||||||
| 0 (n = 36) | 1.0 | .75 | 1.0 | .19 | 1.0 | .70 | 1.0 | .14 | ||||
| 1,2 (n = 24) | 1.13 | 0.5 to 2.4 | 1.60 | 0.8 to 3.2 | 1.20 | 0.5 to 2.9 | 2.47 | 0.8 to 8.1 | ||||
| ≥ 3 (n = 22) | 2.43 | 1.1 to 5.5 | .03 | 2.14 | 1.0 to 4.6 | .05 | 1.62 | 0.6 to 4.4 | .35 | 3.34 | 1.0 to 11 | .05 |
| Bone marrow ≥ 50% | ||||||||||||
| No (n = 44) | 1.0 | .22 | 1.0 | .26 | 1.0 | .42 .99 | .51 | |||||
| Yes (n = 38) | 1.62 | 0.8 to 3.5 | 1.49 | 0.7 to 3.0 | 1.45 | 0.6 to 3.6 | 1.46 | 0.5 to 4.4 | ||||
| Bulky lymph nodes | ||||||||||||
| No (n = 62) | 1.0 | .006 | 1.0 | .01 | 1.0 | .008 | 1.0 | .60 | ||||
| Yes (n = 20) | 2.77 | 1.3 to 5.8 | 2.44 | 1.2 to 4.8 | 3.14 | 1.4 to 7.3 | 1.40 | 0.4 to 4.8 | ||||
| Lymphocytes > 1010 | ||||||||||||
| No (n = 67) | 1.0 | .34 | 1.0 | .25 | 1.0 | .35 | 1.0 | .58 | ||||
| Yes (n = 15) | 1.51 | 0.7 to 3.5 | 1.57 | 0.7 to 3.4 | 1.59 | 0.6 to 4.2 | 1.43 | 0.4 to 5.1 | ||||
| No. of prior regimens | ||||||||||||
| 0-3 (n = 39) | 1.0 | .37 | 1.0 | .68 | 1.0 | .72 | 1.0 | .77 | ||||
| ≥ 4 (n = 43) | 1.39 | 0.7 to 2.8 | 1.15 | 0.6 to 2.2 | 1.17 | 0.5 to 2.8 | 1.17 | 0.4 to 3.4 | ||||
| Fludarabine refractoriness | ||||||||||||
| No (n = 11) | 2.49 | 0.8 to 7.4 | 1.30 | 0.5 to 3.6 | 0.0 | Undefined | 3.13 | 0.9 to 11 | ||||
| Yes (n = 71) | 1.0 | .10 | 1.0 | .62 | 1.0 | .04 | 1.0 | .07 |
published online ahead of print at www.jco.org on September 15, 2008.
Supported by Grants No. CA78902, CA18029, and CA15704 from the National Institutes of Health, Bethesda, MD. (M.S); in part by the Paros Family Fund (M.L.S.); and by a grant from Ministero dell'Istruzione, dell'Università, della Ricerca, Italy (B.B.).
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA, and at the Tandem Bone Marrow Transplantation Meeting, February 8-12, 2007, Keystone, CO.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Esteve J, Montserrat E, Dreger P, et al: Stem cell transplantation (SCT) for chronic lymphocytic leukemia (CLL): Outcome and prognostic factors after autologous and allogeneic transplants. Blood 98:482a, 2001. (abstr 2013) [Google Scholar]
- 2.Bottcher S, Ritgen M, Pott C, et al: Comparative analysis of minimal residual disease detection using four-color flow cytometry, consensus IgH-PCR, and quantitative IgH PCR in CLL after allogeneic and autologous stem cell transplantation. Leukemia 18:1637-1645, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ritgen M, Stilgenbauer S, von Neuhoff N, et al: Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene status: Implications of minimal residual disease measurement with quantitative PCR. Blood 104:2600-2602, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Moreno C, Villamor N, Colomer D, et al: Clinical significance of minimal residual disease, as assessed by different techniques, after stem cell transplantation for chronic lymphocytic leukemia. Blood 107:4563-4569, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Michallet M, Archimbaud E, Bandini G, et al: HLA-identical sibling bone marrow transplantation in younger patients with chronic lymphocytic leukemia: European Group for Blood and Marrow Transplantation and the International Bone Marrow Transplant Registry. Ann Intern Med 124:311-315, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Pavletic SZ, Khouri IF, Haagenson M, et al: Unrelated donor marrow transplantation for B-cell chronic lymphocytic leukemia after using myeloablative conditioning: Results from the Center for International Blood and Marrow Transplant research. J Clin Oncol 23:5788-5794, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Pavletic S, Khouri I, King R, et al: HLA-matched unrelated donor (MUD) bone marrow transplantation for B-cell chronic lymphocytic leukemia: Results from the CLL Working Group, National Marrow Donor Program. Proc Am Soc Clin Oncol 19:4a, 2000. (abstr 8) [Google Scholar]
- 8.Doney KC, Chauncey T, Appelbaum FR: Allogeneic related donor hematopoietic stem cell transplantation for treatment of chronic lymphocytic leukemia. Bone Marrow Transplant 29:817-823, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Dreger P, Montserrat E: Autologous and allogeneic stem cell transplantation for chronic lymphocytic leukemia. Leukemia 16:985-992, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Khouri IF, Keating MJ, Saliba RM, et al: Long-term follow-up of patients with CLL treated with allogeneic hematopoietic transplantation. Cytotherapy 4:217-221, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gribben JG, Zahrieh D, Stephans K, et al: Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood 106:4389-4396, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Sandmaier BM, et al: Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol 23:3819-3829, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Schetelig J, Thiede C, Bornhauser M, et al: Evidence of a graft-versus-leukemia effect in chronic lymphocytic leukemia after reduced-intensity conditioning and allogeneic stem-cell transplantation: The Cooperative German Transplant Study Group. J Clin Oncol 21:2747-2753, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Dreger P, Brand R, Hansz J, et al: Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning. Leukemia 17:841-848, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Khouri IF, Lee MS, Saliba RM, et al: Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: Impact of rituximab on immunomodulation and survival. Exp Hematol 32:28-35, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Dreger P, Brand R, Milligan D, et al: Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: A population-matched analysis. Leukemia 19:1029-1033, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Brown JR, Kim HT, Li S, et al: Predictors of improved progression-free survival after nonmyeloablative allogeneic stem cell transplantation for advanced chronic lymphocytic leukemia. Biol Blood Marrow Transplant 12:1056-1064, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Montserrat E, Lopez-Lorenzo JL, Manso F, et al: Fludarabine in resistant or relapsing B-cell chronic lymphocytic leukemia: The Spanish Group experience. Leuk Lymphoma 21:467-472, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Perkins JG, Flynn JM, Howard RS, et al: Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: Implications for clinical trials in this patient population. Cancer 94:2033-2039, 2002 [PubMed] [Google Scholar]
- 20.O'Brien SM, Kantarjian HM, Cortes J, et al: Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol 19:1414-1420, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Keating MJ, Flinn I, Jain V, et al: Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood 99:3554-3561, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Cordone I, Masi S, Mauro FR, et al: p53 expression in B-cell chronic lymphocytic leukemia: A marker of disease progression and poor prognosis. Blood 91:4342-4349, 1998 [PubMed] [Google Scholar]
- 23.Byrd JC, Smith L, Hackbarth ML, et al: Interphase cytogenetic abnormalities in chronic lymphocytic leukemia may predict response to rituximab. Cancer Res 63:36-38, 2003 [PubMed] [Google Scholar]
- 24.Wiestner A, Rosenwald A, Barry TS, et al: ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood 101:4944-4951, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Byrd JC, Gribben JG, Peterson BL, et al: Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: Justification for risk-adapted therapy. J Clin Oncol 24:437-443, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Grever MR, Lucas DM, Dewald GW, et al: Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: Results from a prospective randomized U.S. intergroup phase III trial E2997. J Clin Oncol 25:799-804, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storb R, et al: Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106:2912-2919, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepe MS, Longton G, Pettinger M, et al: Summarizing data on survival, relapse, and chronic graft-versus-host disease after bone marrow transplantation: Motivation for and description of new methods. Br J Haematol 83:602-607, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Seymour JF, Robertson LE, O'Brien S, et al: Survival of young patients with chronic lymphocytic leukemia failing fludarabine therapy: A basis for the use of myeloablative therapies. Leuk Lymphoma 18:493-496, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Dohner H, Stilgenbauer S, Benner A, et al: Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343:1910-1916, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Krober A, Seiler T, Benner A, et al: V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood 100:1410-1416, 2002 [PubMed] [Google Scholar]
- 32.Hamblin TJ, Orchard JA, Ibbotson RE, et al: CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood 99:1023-1029, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Crespo M, Bosch F, Villamor N, et al: ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 348:1764-1775, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Montserrat E, Moreno C, Esteve J, et al: How I treat refractory CLL. Blood 107:1276-1283, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Keating MJ, O'Brien S, Kontoyiannis D, et al: Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma 43:1755-1762, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Byrd JC, Stilgenbauer S, Flinn IW: Chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 163-183, 2004 [DOI] [PubMed]
- 37.Wierda W, O'Brien S, Wen S, et al: Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 23:4070-4078, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Stilgenbauer S, Kröber A, Busch R, et al: 17p deletion predicts for inferior overall survival after fludarabine-based first line therapy in chronic lymphocytic leukemia: First analysis of genetics in the CLL4 trial of GCLLSG. Blood 106:212a, 2005. (abstr 715) [Google Scholar]
- 39.Caballero D, Garcia-Marco JA, Martino R, et al: Allogeneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of B-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-). Clin Cancer Res 11:7757-7763, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Khouri IF, Saliba RM, Admirand J, et al: Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol 137:355-363, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Schetelig J, van Biezen A, Caballero D, et al: Allogeneic hematopoietic cell transplantation for chronic lymphcytic leukemia (CLL) with 17p deletion: A retrospective EBMT analysis. Blood 110:22a-23a, 2007. (abstr 47) [Google Scholar]
- 42.Sorror ML, Leisenring W, Deeg HJ, et al: Twenty-year follow-up of a controlled trial comparing a combination of methotrexate plus cyclosporine with cyclosporine alone for prophylaxis of graft-versus-host disease in patients administered HLA-identical marrow grafts for leukemia. Biol Blood Marrow Transplant 11:814-815, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Sorror ML, Leisenring W, Deeg HJ, et al: Twenty-year follow-up in patients with aplastic anemia given marrow grafts from HLA-identical siblings and randomized to receive methotrexate/cyclosporine or methotrexate alone for prevention of graft-versus-host disease. Biol Blood Marrow Transplant 11:567-568, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Mielcarek M, Storer BE, Sandmaier BM, et al: Comparable outcomes after nonmyeloablative hematopoietic cell transplantation with unrelated and related donors. Biol Blood Marrow Transplant 13:1499-1507, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorror ML, Storer BE, Maloney DG, et al: Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood 111:446-452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopal AK, Rajendran JG, Pagel JM, et al: A phase II trial of 90Y-ibritumomab tiuxetan-based reduced intensity allogeneic peripheral blood stem cell (PBSC) transplantation for relapsed CD20+ B-cell non-Hodgkins lymphoma (NHL). Blood 108:98a, 2006. (abstr 316) [Google Scholar]
- 47.Grever MR, Lucas DM, Johnson AJ, et al: Novel agents and strategies for treatment of p53-defective chronic lymphocytic leukemia. Best Pract Res Clin Haematol 20:545-556, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Byrd JC, Lin TS, Dalton JT, et al: Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 109:399-404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chanan-Khan A, Miller KC, Musial L, et al: Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J Clin Oncol 24:5343-5349, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Rawstron AC, Villamor N, Ritgen M, et al: International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 21:956-964, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Toze CL, Galal A, Barnett MJ, et al: Myeloablative allografting for chronic lymphocytic leukemia: Evidence for a potent graft-versus-leukemia effect associated with graft-versus-host disease. Bone Marrow Transplant 36:825-830, 2005 [DOI] [PubMed] [Google Scholar]