SASP reflects senescence (original) (raw)
Senescence is a permanent state of cell cycle arrest that—unlike quiescence—is unresponsive to growth factors. Originally described in terms of the replicative exhaustion of cultured fibroblasts (Hayflick & Moorhead, 1961), it has since been shown that senescence can occur prematurely upon oncogene induction and other cellular stresses. As it represents a permanent exit from the cell cycle, senescence has been implicated in several pathological contexts such as cancer, vascular diseases and other age-related conditions. This might sound as if the contribution of senescence is passive, but the recent discovery of the senescence-associated secretory phenotype (SASP)—also recently coined the “senescence messaging secretome” (SMS) by Daniel Peeper—suggests that senescence might have a more active and pathologically diverse role (Campisi & d'Adda di Fagagna, 2007; Kuilman & Peeper, 2009).
It has long been known within the field that the culture medium of senescent cells is enriched with secreted proteins (Krtolica & Campisi, 2002; Shelton et al, 1999). The SASP concept was first proposed by the Campisi group, when they realized that secreted factors from senescent fibroblasts promote the transformation of pre-malignant—but not of normal—mammary epithelial cells. They suggested that matrix metalloproteinase 3 (MMP3), was a candidate factor for this activity (Krtolica et al, 2001; Parrinello et al, 2005); MMP3 is a classical marker of senescence and is widely used to help confirm a senescence phenotype in vitro. This initial observation of SASP implies that senescence might not simply be a tumour suppressor mechanism, but rather a double-edged sword within the tumour microenvironment. What remained unclear, however, were the functional effects of SASP on the senescence phenotype itself. Kortlever et al (2006) identified plasminogen activator inhibitor 1 (PAI1), another classical marker of senescence, as having a crucial role in the induction of replicative senescence downstream of p53. Together, these early studies suggest that SASP components could have diverse effects through both autocrine and paracrine mechanisms. A series of recent papers (Acosta et al, 2008; Coppé et al, 2008; Kuilman et al, 2008; Wajapeyee et al, 2008), including one in this issue of EMBO reports by the group of David Bernard (Augert et al, 2009), have added various new members involved in SASP, and collectively reinforced the idea that senescence is both regulated by and regulates the extracellular environment (Fig 1).
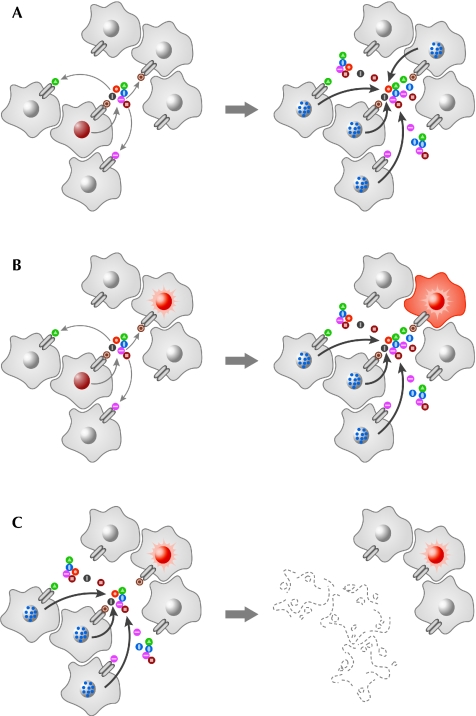
Figure 1.
The roles of senescence-associated secretory phenotypes. SASP forms part of the senescence mechanism to combat aberrant cellular proliferation, but the secretion of these factors into the extracellular environment can have several effects. For simplification, the figure only represents ligand-receptor signalling events. In addition to the roles illustrated here, SASP might also have undefined activities. (A) Oncogenic stress (brown nucleus) within one cell triggers SASP, which can induce senescence (blue dots in nuclei) in an autocrine and/or paracrine manner. These signals cause further secretion and, hence, the amplification of the phenotype (at least in the inflammatory network of SASP). (B) A neighbouring cell is predisposed to proliferation (represented by the bright red nucleus). The secreted factors cause senescence in many of the surrounding cells, but in this cell they lead to transformation. (C) SASP can also activate the innate immune system to eliminate senescent cells, which could remove the potentially tumorigenic factors from the microenvironment. SASP, senescence-associated secretory phenotype.
Senescence bypass screening is a powerful tool to identify new components of the senescence machinery. Some of these factors might be potential tumour suppressors, whereas others could be ‘context-dependent' tumour suppressors or even oncogenes. Among the recent series of papers, three groups have used genome-wide RNA interference (RNAi) screens and identified SASP components to be crucial regulators of senescence. Wajapayee et al (2008) identified insulin-like growth factor (IGF)-binding protein 7 (IGFBP7) as an essential gene for oncogenic B-RAF (BRAF; _BRAFV600E)_-induced senescence in human diploid fibroblasts (HDFs) and melanocytes. IGFBP7 belongs to a group of secreted proteins that bind to IGFs. The authors showed that IGFBP7 is both necessary and sufficient for senescence induction. Interestingly, IGFBP7 induces apoptosis—rather than senescence—in melanoma cell lines that harbour an activating BRAF mutation. Thus, IGFBP7 can induce senescence or apoptosis—two important tumour suppression mechanisms—in normal melanocytes or _BRAFV600E_-positive melanomas, respectively. Furthermore, Wajapeyee et al (2008) also showed that the promoter of IGFBP7 is widely hypermethylated in _BRAFV600E_-positive melanoma, suggesting that IGFBP7 is potentially a unique tumour suppressor and a promising therapeutic target, at least for _BRAFV600E_-positive melanomas.
The potentially tumour-suppressive action of IGFBP7 has been attributed to its ability to inhibit the BRAF–MEK–MAPK (mitogen-activated protein kinase) signalling pathway (Wajapeyee et al, 2008). IGFBP7 induction was also shown to be downstream of this pathway and, hence, a negative feedback loop exists. A similar feedback mechanism had previously been shown to be involved in the regulation of oncogene-induced senescence (OIS; Courtois-Cox et al, 2006), which implicates negative feedback loops in the oncogenic signalling pathways as powerful effector mechanisms in OIS. However, the molecular mechanism by which IGFBP7 inhibits the MAPK pathway was not shown. IGFBPs modulate IGF bioactivity by limiting its access to the IGF receptor; however, unlike other IGF-binding proteins, IGFBP7 has low affinity for IGF and whether the IGF–IGFR axis or other pathways are involved in the activity of IGFBP7 during OIS is not clear.
Two other studies that used senescence bypass screens found the receptors CXC chemokine receptor 2 (CXCR2) and phospholipase A2 receptor (PLA2R), which led them to the identification of their ligands as new components of SASP (Acosta et al, 2008; Augert et al, 2009). Interestingly, in a similar scenario to that of IGFBP7, there might be another potential tumour suppressor in the study of Augert et al (2009, in this issue). The authors found that knockdown of PLA2R prevents senescence, whereas its overexpression induces premature senescence. PLA2R is a receptor that binds to several secreted phospholipase A2 enzymes (sPLA2) and the authors showed that not only PLA2R, but also a number of sPLA2s, are upregulated in senescent cells. They focused on the most highly upregulated sPLA2, PLA2G2A, and showed that its overexpression is sufficient to induce senescence in a PLA2R-dependent manner. This result is rather surprising because—unlike in the mouse—the binding affinity of human PLA2R for human PLA2G2A is weak and whether they interact remains an open question (Hanasaki, 2004). Interestingly, PLA2G2A has been implicated in cancer; its expression is often high in early-stage tumours but rather decreased in late-stage tumours and—similar to _IGFBP7_—this correlates strongly with the hypermethylation of the PLA2G2A promoter in late-stage cancers (Ganesan et al, 2008). However, a few points remain to be clarified, such as the actual binding between PLA2G2A and PLA2R during senescence, the paracrine activity of PLA2G2A on senescence and the phenotype resulting from the re-introduction of PLA2G2A into PLA2G2A-negative cancers. Nonetheless, both Wajapayee et al (2008) and Augert et al (2009) highlight that SASP could reveal a promising pool of drug targets for cancer therapy.
Conversely, however, the implications for SASP in tumorigenesis can be more complex. Components of the inflammatory network have collectively been considered to be anti-cancer, as well as pro-cancer, mechanisms. The anti-cancer function is attributed to the ability of the immune system to clear both damaged and tumour cells, whereas the pro-cancer function of inflammation could be to provide an oncogenic microenvironment (de Visser et al, 2006). Two recent studies have shown that chemokine and inflammatory cytokine signals are crucial regulators of senescence (Acosta et al, 2008; Kuilman et al, 2008). Acosta et al (2008) found that knockdown of CXCR2 alleviates senescence and that senescent cells secrete interleukin-8 (IL-8) and growth-regulated oncogene-α (GROα), which are the ligands of CXCR2, thereby reinforcing the pro-senescence activity of CXCR2. A similar observation was made by Kuilman et al (2008), who searched for new pathways that mediate OIS by genome-wide transcription analysis. By using the power of bioinformatics, they identified the specific activation of the ‘inflammatory transcriptome', which they further narrowed down to interleukin-6 (IL-6). As in the case of IL-8/CXCR2, the cognate receptor of IL-6 (IL-6R/GP80) is also upregulated during senescence, and both IL-6 and its receptor are essential for OIS.
How is the inflammatory network in SASP regulated? Kuilman et al (2008) performed another microarray experiment with or without IL-6 knockdown under oncogenic stress. Surprisingly, an unbiased gene ontology analysis of the microarray data revealed that the depletion of IL-6 showed a strong suppression of the whole inflammatory response, including the levels of IL-8 and the transcription factor C/EBPβ (CCAAT/enhancer binding protein-β), which is important for the regulation of genes involved in the inflammatory response. The authors also confirmed that C/EBPβ directly regulates both IL-6 and IL-8 expression during OIS. These data suggest that there is a hierarchy in the OIS inflammatory network in which IL-6 has a central role, and IL-6 and C/EBPβ form a positive feedback loop to maintain and amplify the activity of the whole network. Interestingly, it has been shown that human PLA2G2A accumulates during inflammation and that PLA2R-mediated responses are involved in inflammatory cytokine production in mice during endotoxic shock (Hanasaki, 2004; Lambeau & Gelb, 2008), potentially suggesting cross-talk in the regulation of various types of SASP component. Therefore, feedback and amplification loops might exist beyond the inflammatory network.
The pro-tumorigenic effects of these factors have been studied in more detail by Coppé et al (2008), who took a proteomics approach in which the conditioned media from senescent HDFs was applied to antibody arrays. In agreement with the other studies discussed here, the SASP components identified included inflammatory and immune-modulatory cytokines and chemokines, growth factors, shed cell surface molecules and survival factors (Coppé et al, 2008). The conditioned media from senescent—but not from pre-senescent—HDFs induced the epithelial–mesenchymal transition (EMT), which has been linked to tumour invasiveness and metastasis, in non-aggressive human breast cancer cell lines. Among the SASP components identified, Coppé et al confirmed the crucial role of IL-6 and IL-8 for EMT induction.
Why do IL-6 and IL-8 exert apparently opposite effects on the cells from which they originate compared with their neighbouring cells? The maintenance of tissue homeostasis by the immune system is subject to finely tuned regulation. For example, inflammation contributes to efficient immune surveillance and tissue regeneration but, if it is persistent and excessive, it could damage the tissue and even the whole organism, as well as generate a hyper-proliferative tissue environment. An analogy could be applied to the paradoxical effect of SASP in tumorigenesis. It should be noted that the target cells of these opposite effects are different; IL-6/IL-8 autonomously reinforce the phenotype of senescent cells, which are in immediate danger, while enhancing tumorigenesis in neighbouring cells non-autonomously. Thus, similarly to actual inflammation, the oncogenic activity of SASP might be a side-effect caused by the persistent or excessive cell-autonomous tumour-suppressive activity.
If this is true, do cells have any regulatory mechanisms to keep this system in check? Xue et al (2007) have shown the first evidence that links SASP to the clearance of senescent cells in vivo. In mouse liver tumour models, senescence induced by the reactivation of p53 was accompanied by the induction of inflammatory cytokines that activated the innate immune response, leading to the clearance of the senescent cells. This mechanism would be an effective way to limit the persistent adverse effect of SASP. In addition, p53 also seems to contribute to limiting the excessive activation of SASP, as Coppé et al (2008) found that p53 is not required for SASP but, rather, suppresses its amplification, indicating a cell non-autonomous tumour suppressive activity of p53. Interestingly, both IL-8/CXCR2-mediated and PLA2R-mediated senescence are p53 dependent. Together, these data indicate that p53 could mediate the pro-senescence activity of SASP, as well as buffering excessive SASP activity. Thus, senescent cells seem to have mechanisms to regulate the ‘side effect' of SASP. However, it is also possible that SASP has other active roles both cell autonomously and non-autonomously. As discussed above, SASP involves a wide range of secreted factors and their receptors, and the combination of those factors varies depending on the situation (Shelton et al, 1999). Those unique combinations of the secreted factors or signalling pathways might create new and complex bioactivities and extracellular environments, which would be challenging to understand by the characterization of the individual factors alone. Senescence, once assumed to be a terminal state, might in fact be the beginning of a new state with a diversity of functions.

References
- Acosta JC et al. (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 [DOI] [PubMed] [Google Scholar]
- Augert A, Payré C, de Launoit Y, Gil J, Lambeau G, Bernard D (2009) The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep 10: 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740 [DOI] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K (2006) A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 10: 459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM (2006) Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6: 24–37 [DOI] [PubMed] [Google Scholar]
- Ganesan K et al. (2008) Inhibition of gastric cancer invasion and metastasis by PLA2G2A, a novel β-catenin/TCF target gene. Cancer Res 68: 4277–4286 [DOI] [PubMed] [Google Scholar]
- Hanasaki K (2004) Mammalian phospholipase A2: phospholipase A2 receptor. Biol Pharm Bull 27: 1165–1167 [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621 [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R (2006) Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8: 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Campisi J (2002) Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol 34: 1401–1414 [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98: 12072–12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9: 81–94 [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031 [DOI] [PubMed] [Google Scholar]
- Lambeau G, Gelb MH (2008) Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem 77: 495–520 [DOI] [PubMed] [Google Scholar]
- Parrinello S, Coppé JP, Krtolica A, Campisi J (2005) Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 118: 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton DN, Chang E, Whittier PS, Choi D, Funk WD (1999) Microarray analysis of replicative senescence. Curr Biol 9: 939–945 [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132: 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445: 656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]