Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 2.
Published in final edited form as: Behav Brain Res. 2008 Oct 18;198(1):247–251. doi: 10.1016/j.bbr.2008.10.015
Abstract
The effects of abnormally high or low stress on learning are well established. The Barnes maze and Morris water maze are two commonly-used tests of spatial memory, of which the water maze is considered more stressful; however, until now this has not been demonstrated empirically. In the present study, mice matched for performance on commonly-used anxiety tasks were trained on either the Barnes maze or water maze or received no cognitive testing. Water-maze training induced greater increases in plasma corticosterone than did Barnes maze training, assessed 30 min. after the final session. Importantly, spatial learning was inversely correlated with corticosterone levels in the water maze but not the Barnes maze, suggesting that performance on the water maze may be more affected by test-induced stress even within wild-type subjects of the same age and gender. These findings are important when considering the appropriate cognitive tasks for any experiment in which stress responses may differ systematically across groups.
Keywords: Anxiety, stress, corticosterone, Barnes maze, Morris water maze, Elevated plus maze, Light-dark activity, social dominance, behaviour
The Barnes maze [3] and Morris water maze [19] are similar tasks in that they both measure the ability of a mouse to learn and remember the location of a target zone using a configuration of distal visual cues located around the testing area [11, 23]. Both tasks rely on hippocampal-dependent spatial reference memory and on the inherent tendencies of the subjects to escape from an aversive environment [2, 27]. It has been suggested that the Barnes maze is less anxiogenic [13, 21]; however, we know of no data that support this assumption.
Innate anxiety and cognitive ability differ considerably among mouse strains [5, 13, 18] highlighting the fact that the selection of a background strain and the choice of behavioural tasks are critical to the outcome of an experiment. The present experiment was designed to determine whether water-maze and Barnes-maze performance induce differential stress reactions, as assessed biochemically by serum corticosterone. Rats exhibit a robust increase in corticosterone on the first day of water maze testing that is stable and only slightly diminished throughout days of testing [1]. No such data are available for the Barnes maze. The demonstration of differential stress responses in mice performing the two behavioural tasks would have implications for the choice of background strain as well as the appropriate cognitive and control tasks to use for a particular study.
The subjects were thirty 7-week-old male, C57BL/6J mice obtained from Jackson Laboratory (Bar Harbor, ME, USA; stock #000664) and left undisturbed for 1 week before testing began. Mice were housed five per cage until 1 day before testing in the Barnes or water maze, when mice were singly housed to eliminate any additional stress that may arise from test order within the cage as other mice are removed for testing and then returned. Although individual housing itself may be stressful [9], all mice were treated identically and thus the effect of housing stress was constant across groups. Mice were housed in a temperature-controlled room under a 12-h light/dark cycle with free access to food and water. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Anxiety was tested as previously described, in a standard elevated plus maze [4, 12, 22] followed by the light-dark activity task [4, 12, 22] on separate days during the first week. In the elevated plus maze time spent in closed arms was calculated as the percent of total time on arms excluding time in the central area [7, 8]. The number of entries into arms and distance traveled were also recorded. In the light-dark task the measures of interest were latency to enter the dark compartment, time spent within each area, transitions between areas, and total distance traveled.
Social dominance was assessed in the second week using the tube test [14, 15, 17]. On the day before testing each mouse was given three habituation sessions to become accustomed to the act of running into the tube. During the test two mice from the same home-cage were placed at either end of a 30-cm long × 3.5-cm diameter clear tube. A subject was declared a “winner” when its opponent backed out of the tube. Each mouse was tested against each of the other four individuals from within its cage and trials were repeated with mice beginning from the opposite ends to avoid position bias. A score of 1 was awarded to the winner of a bout and a score of 0 was given to the loser. Thus each mouse was assigned a dominance score of between 0 (no wins) and 8 (no losses). The mouse with the highest score in each cage was assigned the ‘dominant’ status, if the highest score was shared between two mice they were designated ‘co-dominant’, and the lowest scoring mouse was the ‘subordinate’ mouse.
Mice were then matched according to an overall anxiety and dominance score calculated using _Z_-scores to combine performance on the anxiety-related measures in the elevated-plus and light-dark tasks and the social dominance test. _Z_-scores were calculated for time in closed arms and entries into closed arms in the plus maze and time in dark and latency to enter the dark in the light/dark task. Three groups were created; Water-maze, Barnes maze, or Naïve (no further testing) with 10 mice per group such that the mean _Z_-score for each group did not differ [F2, 27 = .001; _P_=.998] and with one dominant, two co-dominant and two subordinate mice in each group.
The Barnes and water mazes were used to assess spatial learning, as previously described in detail [4, 11, 12, 22]. In order to make fair comparisons between the two tasks, our usual procedures were modified to equate the number and duration of trials and number of testing days. The two mazes were of equal diameter and positioned in the same place in the same testing room so that visible spatial cues as well as other environmental factors (background noise and light level) were identical between the two tasks. Both tasks were conducted by the same experimenter 1 week apart but at the same time of day. For each task, six 1-min trials were run per day for 5 days, conducted in a massed fashion so that each mouse completed all of its daily trials before the next mouse was tested. Spatial learning in the Barnes maze was assessed using total and primary errors (errors committed before the first encounter with the escape hole). Escape latency and path length were also measured. In the water maze, escape latency and path length as well as mean distance from the platform (search error) were the variables of interest. Swim speed and swimming in the periphery (outer 8 cm of the pool) were also assessed in the water maze as non-cognitive control factors. On each testing day, the 10 mice in the test group were transported in their home cages approximately 10 meters on a cart from the colony room to the corridor outside the testing room, where they remained for the duration of the session. At the same time, five Naïve control mice were yoked to five of the tested mice. Yoked Naïve mice were transported with test mice to control for anxiety responses associated with removing the cages from the housing room and transporting them to the testing room.
Thirty minutes following the last trial on the fifth day of cognitive testing, mice were briefly anaesthetized using isoflurane and then sacrificed by decapitation. Trunk blood was collected and stored on wet ice for 1 hour, then spun in a centrifuge at 13,000 rpm for 20 minutes. Serum was collected from each sample and stored at -80 °C until used for analysis of corticosterone levels. Corticosterone was measured by radioimmunoassay [25] using an ImmuChem Double Antibody Corticosterone Kit (MP Biomedicals, Solon, OH, USA; Cat #07-120103) by Vanderbilt’s Hormone Assay Core. Mice were killed at 30 minutes after testing when serum corticosterone levels were expected to still be elevated even after 5 days of testing [1]. Although corticosterone levels may vary over time between testing and death, all animals were sacrificed at the same time interval following testing and thus any post-test variations in corticosterone magnitude was consistent across groups. Although corticosterone is not the only biological indicator of stress (e.g. adrenocorticotrophic hormone, adrenaline) it is a reliable marker that has often been used to indicate the stress following behavioural tasks (e.g. [1, 2]).
Statistical analyses were conducted using SPSS 14.0 for Windows. Corticosterone level was analyzed by univariate ANOVA with behavioural group (Water maze, Barnes maze or Naïve) as the between-groups factor. Follow-up comparisons were made using Bonferroni corrected t-tests. Task acquisition across the 5 days in the Barnes maze and water maze was analyzed separately within each task by repeated-measures ANOVA with session as the repeated measure. Comparisons of day 5 escape latencies between Barnes maze and water maze were determined using an independent-samples t-test. Relationships between corticosterone and behaviour, and among measures in the anxiety, social dominance, and spatial learning tasks were determined using bivariate correlations.
Anxiety and Social Dominance
None of the anxiety-related measures in the elevated plus maze was significantly correlated with any of the anxiety-related measures in the light/dark test (r’s <.272, p’s >.146), suggesting that they represent independent aspects of the psychological construct “anxiety”. The score on the social dominance tube test was unrelated to any of the anxiety measures in either test (r’s <.310, p’s >.095).
Spatial Learning
Mice improved significantly on both spatial learning tasks across the 5 days of testing. The water maze measures of search error, escape latency, and total path length all decreased, indicating good spatial learning [_F_s4, 36>11.7, _P_s <.001]. Similarly, total and primary errors decreased significantly in the Barnes maze [_F_s4, 36>14.915, _P_s <.001]. Escape latency and path length, which sometimes correlate with error measures in the Barnes maze, also decreased across training [_F_s4, 36>16.959, _P_s <.001]. On Day 5, escape latencies on the Barnes and water mazes were not statistically different, indicating that mice in each group spent approximately the same amount of time in the testing environments [t(18) =.454, P =.466].
Anxiety and Spatial Learning
To evaluate the relationship between stress and learning, correlative analyses were conducted on data from water-maze and Barnes-maze groups. In the Barnes maze, the unitary measures of escape latency and path length were both correlated with percent closed-arm entries in the plus maze (r’s >.685, p’s <.03). In the water maze, path length was associated with percent time in dark in the light-dark test (r =.739, p =.015). None of the other pre-test anxiety measures was significantly correlated with any other measure of performance in either the Barnes-maze (r’s <.50, p’s >.14) or water maze (r’s <.584, p’s >.075). Dominance score was not correlated with any of the water- or Barnes-maze measures (r’s <.432, p’s >.213).
Corticosterone and Spatial Learning
Post-test corticosterone levels were used as an indicator of stress during cognitive testing. Corticosterone levels differed significantly among the groups [F2, 27 = 73.264; _P_ <.001; Fig. 1]. Naïve animals had significantly lower corticosterone levels than both groups of experimental mice (_P_s <.001). The water maze test was the more stressful test as indicated by plasma corticosterone levels significantly higher than those of Barnes-maze-tested mice (_P_ <.001). The pre-test anxiety measures in the plus maze and light/dark chamber did not predict stress response in the cognitive tasks (r’s <.419, p’s >.228, selected data summarized in Table 1), and were not correlated with corticosterone levels in the Naïve group (r’s <.62, p’s >.056). Plasma corticosterone was not significantly related to any of the Barnes-maze acquisition measures (r’s <.478; _P_s >.162; Fig. 2). In contrast, corticosterone levels were positively and significantly correlated with day 5 search error, escape latency, and swim-path length in the water maze (r’s >.720; p’s <.02; Fig. 2). Peripheral swimming on day 1 is often considered an indicator of stress response to the testing environment. However, percent time spent swimming in the periphery of the maze on day 1 was not related to corticosterone [r =.261, p =.467] nor to any of the measures of learning in the water maze on day 5 [r’s <.286, p’s >.424]. Day 1 peripheral swimming was positively related to latency to enter the dark zone in the light-dark task (r =.791, p =.006) but none of the other pre-test anxiety measures was significantly related to peripheral swimming in the water maze (r’s <.554, p’s >.096).
Figure 1. Cognitive testing elicits corticosterone.
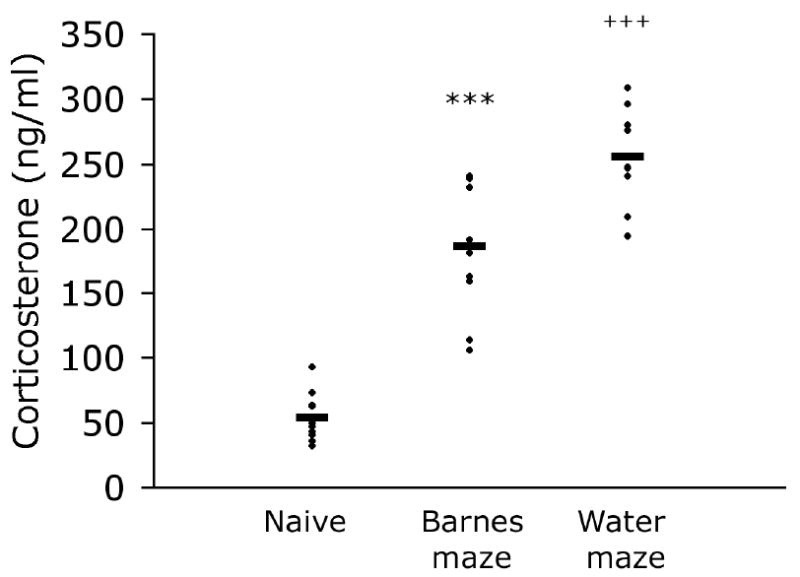
Corticosterone levels were significantly greater in the Barnes-maze group than in Naïve mice, and significantly greater in the Water-maze group than in either Barnes-maze or Naïve groups. Group means are represented by horizontal bars. *** P<.001 significantly different from Naïve mice; +++ P<.001 significantly different from both Naïve and Barnes-maze groups.
Table 1.
Correlations coefficient (Pearson’s r) values between corticosterone levels and selected anxiety measures and day 5 learning measures in the Barnes maze and water maze.
| Pct. time on closed arms | Pct. time in dark zone | Errors | Primary Errors | Escape Latency | Path Length | |
|---|---|---|---|---|---|---|
| Barnes Maze | -0.196_p_=.587 | 0.104_p_=.775 | 0.468_p_=.173 | 0.324_p_=.361 | 0.338_p_=.340 | 0.478_p_=.162 |
| Search Error | Escape latency | Path length | Thigmo-taxis | |||
| Water Maze | 0.265_p_=.460 | 0.326_p_=.228 | 0.721*_p_=.019 | 0.781**_p_=.008 | 0.778**_p_=.008 | 0.261_p_=.467 |
| Naive | -0.463_p_=.177 | 0.122_p_=.738 |
Figure 2. Corticosterone levels are associated with cognitive ability in the water maze but not the Barnes maze.
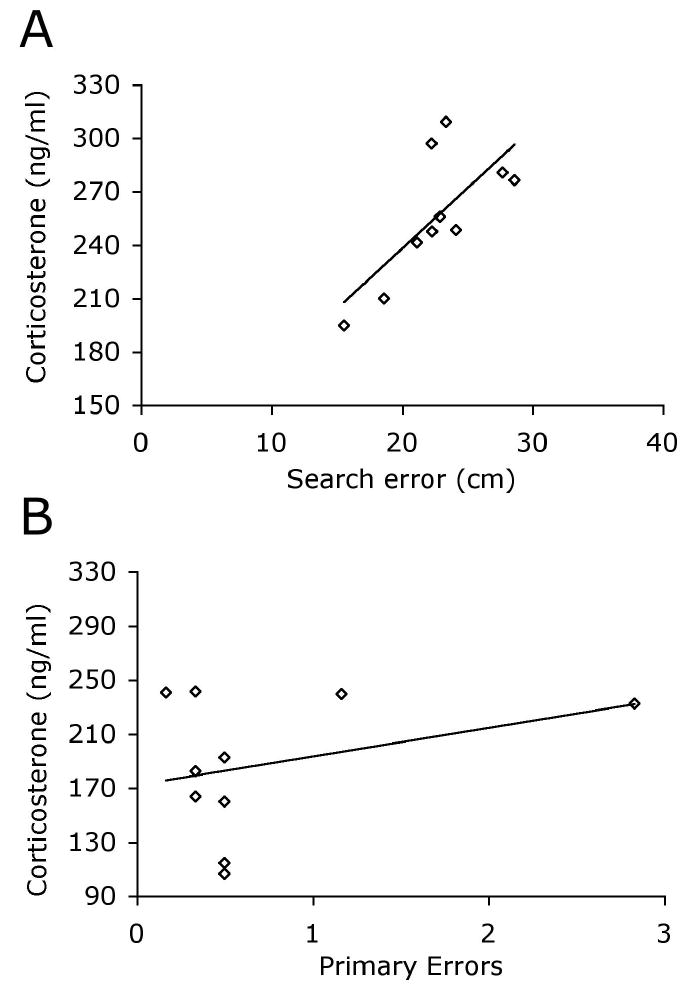
(A) Corticosterone levels were positively and significantly correlated with day 5 search error, path length and escape latency in the water maze (search error shown, r=.721, p=.019) and (B) were not related to any indices of learning in the Barnes maze, (primary errors shown, r=.324, p=.361).
The data presented here show greater levels of corticosterone in mice tested in the water maze than in the Barnes maze, with the lowest detected levels in naïve mice that did not undergo cognitive testing. These data support the contention that the water maze is more stressful than the Barnes maze, an idea that has often been posited but not demonstrated empirically. The controlled and highly similar testing environments and procedures suggest that the differences are likely due to the increased stress associated with swimming.
The majority of anxiety measures were unrelated to any of the measures in the Barnes or water maze, suggesting that pre-test anxiety does not predict spatial learning. Time in the dark was correlated with water maze path length, and percent entries into closed arms was related to path length and latency in the Barnes maze. However, unitary measures such as latency and path length are more susceptible to non-mnemonic influences and are not the best indicators of cognitive performance in learning tasks [16]. In the Barnes maze, mice can make a large number of errors in a very short time, and unitary measures often do not parallel choice measures such as total or primary errors [4, 11, 22]. Although choice measures are not typically used in the water maze, search error has been used as a measure of accuracy during acquisition [10]. In the present study, none of the pre-test anxiety measures was significantly correlated with search error in the water maze or with primary or total errors in the Barnes maze. Thus it is unlikely that pre-test anxiety affected spatial learning in any systematic manner.
Plasma corticosterone was not related to any of the anxiety measures on the elevated plus maze or light-dark tests conducted 2 to 3 weeks earlier, suggesting that pre-test anxiety levels cannot predict stress response in these spatial memory tasks. Similarly, Barnes-maze learning was not significantly associated with corticosterone levels. In contrast, corticosterone was the best predictor of water-maze performance, explaining 52-61% of the variance. The relationship between stress and learning, known as the Yerkes-Dodson Law, is well-established [26, 29]. Okuda et al. [20] showed that post-training injection of different doses corticosterone affected 24-hour retention on an object recognition task in an inverted-U-shaped fashion, suggesting that an optimal level of corticosterone will enhance memory and higher levels may impair it. Diamond et al. [6] demonstrated a similar dose effect on primed-burst potentiation (PBP), using a physiologically-relevant burst stimulus in anesthetized rats receiving exogenous corticosterone. A similar phenomenon may be in operation in the present study. The Barnes maze induced a modest increase in corticosterone that facilitated proficient learning. The water maze induced a modest increase in corticosterone in some of the mice but a larger increase in others. The negative correlation between learning and corticosterone specifically in the water maze suggests that the elevated corticosterone impaired learning in mice with a greater stress response. However, the data presented here are correlational and thus a causative direction cannot be drawn. It is also possible that the inverse relationship is also true; mice that took longer to locate the platform had to swim for longer which could, in turn, lead to the elevated corticosterone levels. This hormonal-feedback could then further inhibit learning in mice with the highest corticosterone levels. Although peripheral swimming is often used as an indicator of stress response in the water maze, our data showed no relationship between peripheral swimming in the water maze and other tests of anxiety or corticosterone level. Other behaviours such as passive floating can also present a problem with mice in the water maze [28] and should be carefully noted during experiments, especially as this will increase time spent in the pool and thus could potentially further elevate a stress response. Typically, such behaviours do not persist throughout a multiple training session experiment but if they do then such mice should probably be removed from the experiment or another spatial learning task should be used, such as the Barnes maze.
We took great care in equating the two spatial learning environments and test procedures. Importantly, our Barnes-maze protocol produces rapid escape learning without additional stress-inducing stimuli such as fans, loud buzzers, or bright lights, which are often used to increase motivation to escape [21, 24]. When conducted in this way the Barnes maze provides an alternative to the water maze in which stress responses will be less likely to interfere with assessment of spatial learning. This may be particularly important when using aged or physically-impaired animals, or mouse lines with abnormal responses to stress.
Acknowledgments
This work was supported by a grant from the NIH (AG022439 to Mike McDonald). The Vanderbilt Hormone Assay & Analytical Services Core is supported by NIH grants DK20593 to the Diabetes Research Training Center (DRTC) and DK59637 to the Mouse Metabolic Phenotyping Center (MMPC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguilar-Valles A, Sanchez E, de Gortari P, Balderas I, Ramirez-Amaya V, Bermudez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82:306–319. doi: 10.1159/000093129. [DOI] [PubMed] [Google Scholar]
- 2.Akirav I, Sandi C, Richter-Levin G. Differential activation of hippocampus and amygdala following spatial learning under stress. Eur J Neurosci. 2001;14:719–725. doi: 10.1046/j.0953-816x.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo A, Harrison FE, McCord M, Zhao J, Bruchey A, Davies SS, Jackson Roberts L, 2nd, Mathews PM, Matsuoka Y, Ariga T, Yu RK, Thompson R, McDonald MP. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 6.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- 8.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 9.Fitchett AE, Collins SA, Barnard CJ, Cassaday HJ. Subordinate male mice show long-lasting differences in spatial learning that persist when housed alone. Neurobiol Learn Mem. 2005;84:247–251. doi: 10.1016/j.nlm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 11.Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison FE, Yu SS, Van Den Bossche KL, Li L, May JM, McDonald MP. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 15.Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- 16.McDonald MP, Overmier JB. Present imperfect: a critical review of animal models of the mnemonic impairments in Alzheimer’s disease. Neurosci Biobehav Rev. 1998;22:99–120. doi: 10.1016/s0149-7634(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 17.Messeri P, Eleftheriou BE, Oliverio A. Dominance behavior: a phylogenetic analysis in the mouse. Physiol Behav. 1975;14:53–58. doi: 10.1016/0031-9384(75)90141-9. [DOI] [PubMed] [Google Scholar]
- 18.Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 19.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pompl PN, Mullan MJ, Bjugstad K, Arendash GW. Adaptation of the circular platform spatial memory task for mice: use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. J Neurosci Methods. 1999;87:87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 22.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 23.Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: dissociation of “proximal”- and “distal”-cue-based behaviors. Behav Neurosci. 1987;101:62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu K, Amagaya S, Ogihara Y. Analysis of corticosterone in the serum of mice and rats using high-performance liquid chromatography. J Chromatogr. 1983;272:170–175. doi: 10.1016/s0378-4347(00)86114-9. [DOI] [PubMed] [Google Scholar]
- 26.Teichner WH. Interaction of behavioral and physiological stress reactions. Psychol Rev. 1968;75:271–291. doi: 10.1037/h0020281. [DOI] [PubMed] [Google Scholar]
- 27.Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res Dev Brain Res. 2003;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- 29.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]