α-Synuclein Aggregation and Ser-129 Phosphorylation-dependent Cell Death in Oligodendroglial Cells (original) (raw)
Abstract
Multiple system atrophy is a neurodegenerative disorder characterized by accumulation of aggregated Ser-129-phosphorylated α-synuclein in oligodendrocytes. p25α is an oligodendroglial protein that potently stimulates α-synuclein aggregation in vitro. To model multiple system atrophy, we coexpressed human p25α and α-synuclein in the rat oligodendroglial cell line OLN-93 and observed a cellular response characterized by a fast retraction of microtubules from the cellular processes to the perinuclear region followed by a protracted development of apoptosis. This response was dependent on phosphorylation at Ser-129 in α-synuclein as demonstrated by site-directed mutagenesis. Treatment of the cells with the kinase inhibitor 2-dimethylamino-4,5,6,7-tetrabromo-1H benzimidazole that targets kinases like casein kinase 2, and polo-like kinases abrogated the toxicity. The polo-like kinase inhibitor BI 2536 caused apoptosis in the model. Ser-129 phosphorylation was linked to the formation of phosphorylated oligomers detectable by immunoblotting, and their formation was inhibited by 2-dimethylamino-4,5,6,7-tetrabromo-1H benzimidazole. The process of microtubule retraction was also dependent on aggregation as demonstrated by the protective effect of treating the cells with the specific peptide inhibitor of α-synuclein aggregation ASI1D and the non-selective inhibitors Congo Red and baicalein. The fast microtubule retraction was followed by the development of the apoptotic markers: activated caspase-3, phosphatidylserine externalization, nuclear condensation, and fragmentation. These markers could all be blocked by the inhibitors of phosphorylation, aggregation, and caspase-3. Hence, the model predicts that both Ser-129 phosphorylation and aggregation control the toxic α-syn pathway in oligodendroglial cells and may represent therapeutic intervention points in multiple system atrophy.
α-Synuclein (α-syn)2 is a soluble protein localized in presynaptic terminals (1). It accumulates as insoluble aggregates in cytoplasmic inclusions in α-synucleinopathies, among which Parkinson disease (PD) and dementia with Lewy bodies are the predominant members (2-4). Mutations in the α-syn gene leading to amino acid substitutions (A30P, A53T, and E46K) (5-7), and multiplications of the α-syn gene (8,9) are associated with rare familial forms of PD and Lewy bodies.
Multiple system atrophy (MSA) represents a special case among the α-synucleinopathies. The histological hallmark of MSA is the presence of α-syn-containing inclusions in oligodendrocytes, referred to as glial cytoplasmic inclusions (10,11). Clinically, MSA presents with parkinsonism, ataxia, and autonomic failure, which signify the wide distribution of the degenerative processes (12). The degeneration of oligodendrocytes has been studied using transgenic mice expressing human α-syn under the control of oligodendroglial promoters (13-15). These models have demonstrated that expression of α-syn in oligodendrocytes results in formation of α-syn-containing glial cytoplasmic inclusions, myelin damage, and cellular degeneration. Still, the mechanisms involved in oligodendroglial degeneration are unclear.
The oligodendroglial protein, p25α, is a potent inducer of α-syn aggregation in vitro (16). Recently, it was shown that p25α redistributed from the myelin sheets to the expanding cell bodies before accumulation and subsequent fibrillization of α-syn in MSA (17).
In the present study we generated a cellular model for MSA based on coexpression of human α-syn and human p25α in the rat OLN-93 oligodendrocyte cell line. Coexpression of the two proteins caused a rapid disorganization of the microtubular (MT) cytoskeleton within 24 h with retraction of MT from the cellular processes to the perinuclear region. MT retraction was coupled to a protracted development of apoptosis. The fast process of MT retraction in the cells was accompanied by accumulation of soluble α-syn oligomers, which were phosphorylated at Ser-129. Inhibition of Ser-129 phosphorylation, α-syn aggregation, and caspase activation abrogated the MT retraction and accompanying apoptosis. Our results suggest that specific signaling pathways amenable for treatment at several levels may be involved in α-syn-dependent degeneration in MSA.
MATERIALS AND METHODS
_Reagents_—Dulbecco's modified Eagle's medium was from Lonza (Verviers, Belgium). FuGENE 6 transfection reagent was purchased from Roche Applied Science. Affinity purified rabbit antibodies toward human α-syn (ASY1), human p25α (p25α1), and β-synuclein (BSY1) have been described previously (16,18,19). Polyclonal rabbit IgG against Ser-129-phosphorylated α-syn (anti-Ser(P)-129) was a kind gift from Prof. Takeshi Iwatsubo, Department of Neuropathology and Neuroscience, University of Tokyo, Japan. Monoclonal α-tubulin antibody was obtained from Sigma, and cleaved caspase-3 (Asp-175) rabbit mAb was from Cell Signaling Technology (Danvers, MA). Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 568-conjugated goat anti-rabbit IgG were from Invitrogen. Caspase-3 inhibitor, Ac-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO), as well as caspase-3 substrate, Ac-DEVD-7-amido-4-methylcoumarin were purchased from Bachem (Weil am Rhein, Germany). ASI peptides were prepared as previously described (20). Baicalein (a flavonoid isolated from the roots of Scutellaria baicalensis) was a kind gift from Prof. Daniel Otzen, Interdisciplinary Nanoscience Centre, University of Aarhus, Denmark. Congo Red and the casein kinase 2 (CK2) inhibitors 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT), 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), and emodin were from Sigma. BI 2536 was purchased from Axon Medchem BV, Gronningen, The Netherlands.
Plasmids and Transfection_—pcDNA3.1 zeo(-) plasmid expressing human wild type (wt) α-syn was constructed by PCR with pET-11d vector containing the human wt α-syn gene as a template (18). Similarly, pcDNA3.1 zeo(-) plasmid expressing human p25α was produced by PCR with pET-11d vector containing the human p25α gene as a template (16). The products were inserted into pcDNA3.1 zeo(-) vectors, which were transformed into competent_Escherichia coli DH5α cells to select positive clones for sequencing. The chosen clones were cultured, and plasmid DNA was purified. pTRUF20 plasmid expressing human wt α-syn was kindly provided by Dr. Deniz Kirik, Wallenberg Neuroscience Center, Division of Neurobiology, Lund University, Sweden (21). α-Syn S129A and S129D cDNA was produced by PCR-based mutagenesis with pET11d containing human wt α-syn as a template. The resulting products were inserted into pTRUF20 expression vectors. All constructs were confirmed by sequencing. Transient transfections were performed with FuGENE 6 transfection reagent according to the manufacturer's protocol.
_Cell Cultures and Chemicals_—OLN-93 is an immortalized oligodendroglia cell line derived from primary Wistar rat brain glial cultures (22). These cells were engineered to express the longest human tau isoform (Tau40) establishing a stable cell line, OLN-t40 (23). Additionally, a stable cell line, OLN-AS, was established by lentiviral transduction of α-syn into OLN-t40 cells (24). All cells were kept at 37 °C under 5% CO2 and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. OLN-t40 and OLN-AS cells were maintained in 50 μg/ml Geneticin.
For inhibition of caspase-3 activity, cells were treated with 20 μm concentrations of the caspase inhibitor Ac-DEVD-CHO for 1 h before transfection with p25α. For inhibition of α-syn aggregation, cells were treated with ASI peptides at 10 μm or baicalein at 100 μm for 1 h before transfection with p25α. For the Congo Red experiment, the cells were treated with 20 μm Congo Red 7 days before transfection with p25α. For inhibition of kinase activity, 1-10 μm DMAT, DRB, or emodin were added to the cells 4 h after transfection with p25α. For induction of stress responses, cells were treated with 1.5 μm rotenone or 50 μg/ml tunicamycin for 24 h before analysis.
_Western Blot Analysis and Immunoprecipitation_—Cells were harvested in lysis buffer A (50 mm Tris pH 7.4, 150 mm NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 1 mm EDTA, Complete protease inhibitor mixture (Roche Applied Science)). The protein concentration of lysates was determined using bicinchoninic acid (BCA) protein assay kit (Sigma). Lysates were resolved on 10-16% polyacrylamide gels (15-30 μg/lane) and transferred to nitrocellulose membranes. The membranes were blocked in 10 mm Tris, pH 7.4, 150 mm NaCl (Tris-buffered saline) supplemented with 0.1% Tween 20 and 5% nonfat milk and then probed with the different antibodies. After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG, and proteins were visualized with enhanced chemiluminescence using a Fuji LAS-3000 imager. Protein bands were quantified by the Multi-Gauge software (Fuji LAS-3000).
For detection of phosphorylated α-syn, cells were treated with 0.5 μm okadaic acid for 2 h and then lysed in lysis buffer B (10 mm Tris, pH 7.4, 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 25 mm β-glycerophosphate, 50 mm NaF, 0.2 mm Na2VO3, Complete protease inhibitor mixture). α-Syn was immunoprecipitated from cell lysates using ASY1 antibody immobilized on CNBr-activated Sepharose. The resulting immunoprecipitated material was washed three times in lysis buffer B and separated by SDS-PAGE. Western blotting was performed as described above using anti-Ser(P)-129 and ASY1 antibodies.
_Immunocytochemistry_—Cells were cultured on poly-l-lysine-coated coverslips for 24 h followed by treatment with various reagents and/or transfection. For analysis, cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 30 min, and blocked in 3% bovine serum albumin solution for 20 min at room temperature. Cell preparations were incubated with primary antibodies for 1 h at room temperature, and proteins were visualized by Alexa Fluor 488- or -568-conjugated secondary antibodies. Nuclei were stained by 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI). Signals were analyzed on a fluorescence microscope (Axiovert 200M, Zeiss, Germany) equipped with an ApoTome.
_Assessment of Microtubule Retraction and Apoptosis_—OLN-AS cells were immunostained for α-tubulin and p25α, counterstained with DAPI, and analyzed by fluorescence microscopy. Assessment of MT retraction in OLN-93 cells cotransfected with α-syn and p25α was performed using antibodies against α-syn, p25α, and α-tubulin. Cells were counter-stained with DAPI and analyzed by fluorescence microscopy. MT retraction was defined by a retraction of MT from the cellular processes to the perinuclear region resulting in an intense tubulin staining surrounding the nucleus. MT retraction was quantified by counting p25α-positive cells with a clear perinuclear localization of MT compared with the total number of p25α-positive cells. In each experiment, 120-200 transfected cells localized in five randomly chosen microscopic fields were examined at 100 times magnification. Cells were counted blindly by Christine L. Kragh and another investigator. The variation between the two investigators ranged from 0 to 10% in individual experiments.
Nuclear morphology was evaluated by DAPI staining, and apoptotic cells were identified by a condensed or fragmented nucleus. Apoptosis elicited by coexpressing α-syn and p25α was additionally detected by immunostaining for cleaved caspase-3 and by annexin-V fluorescein isothiocyanate apoptosis detection kit (Sigma). Cells were washed in annexin-V binding buffer containing 10 mm Hepes, 140 mm NaCl, and 2.5 mmCaCl2, and annexin-V staining was performed according to the manufacturer's protocol. Signals were analyzed by fluorescence microscopy.
_Flow Cytometry_—OLN-93 cells were seeded in poly-l-lysine-coated wells. The cells were transfected with FuGENE 6 transfection reagent. After 24 h of transfection the cells were detached with phosphate-buffered saline, 0.1% EDTA, fixed in 2% paraformaldehyde, and permeabilized with 0.2% saponin, and nonspecific binding sites were blocked in 5% goat serum. The analysis was performed on a FACSdiva (BD Biosciences). Cell debris and aggregated cells were eliminated from the analysis by using a gate-on-forward and side-ways scatter based on a TO-PRO staining of the cell nuclei (Invitrogen). The analysis of α-syn expression was based on immunostaining of intracellular α-syn by ASY1 and secondary anti-rabbit Alexa Fluor 488.
α_-Synuclein Small Interfering RNA (siRNA) Silencing_—siRNA targeting human α-syn was from Dharmacon (Lafayette, CO). Non-targeting siRNA (siControl) was included as a negative control. Transfection of siRNA into OLN-AS cells was performed using Dharmafect Transfection Reagent 4 according to the manufacturer's instructions. In brief, 50 nm siRNA was transfected into OLN-AS cells using 1 μl of Dharmafect Transfection Reagent 4 for cells in each well of 24-well plates. The expression level of α-syn was evaluated by immunofluorescence microscopy and Western blot analysis using ASY1 antibody. Cells were treated with siRNA for 96 h followed by transiently transfection with p25α, and MT retraction was quantified 24 h after p25α transfection.
_Caspase-3 Activity Assay_—Caspase-3 activity was determined by a fluorogenic assay using Ac-DEVD-7-amido-4-methylcoumarin as a substrate. Briefly, cells were grown in 96-well plates and transfected with p25α for 24 h. Lysis buffer (10 mm Tris pH 7.5, 100 mm NaCl, 1 mm EDTA, 0.5% Triton X-100) was added directly to the wells and incubated for 1 h at room temperature. Subsequently, reaction buffer containing 10 mm PIPES, pH 7.4, 2 mm EDTA, 0.1% CHAPS, 4 mm dithioerythritol, 8 μm Ac-DEVD-7-amido-4-methylcoumarin was added to the wells, and the plates were incubated for 3 h at room temperature. Fluorescence was measured with a Wallac Victor3 1420 multilabel counter (PerkinElmer Life Sciences) (excitation at 355 nm and emission at 460 nm), and measurements were obtained with 1-s integration. Measurements were performed in triplicate.
Statistics_—Each experiment was repeated at least three times unless otherwise stated. All comparisons were performed as differences between two groups and were analyzed by Student's t test for unpaired data. A_p value < 0.05 was considered significant.
RESULTS
Coexpression of α_-Synuclein and p25_α_Causes Microtubule Relocalization to the Perinuclear Region_—To model the cellular effects of α-syn-dependent degeneration in MSA, we studied the effect of coexpressing α-syn and p25α in rat oligodendroglial OLN-93 cells. We used three different lines; wt OLN-93 cells, OLN-t40 cells, expressing human tau40, and OLN-AS cells, expressing both human tau40 and α-syn. Our attempt to generate stable OLN cell lines expressing α-syn in the absence of tau was not successful because these lines were not viable for higher passage numbers.
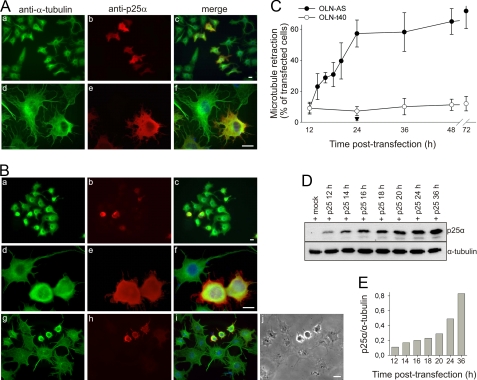
First, we used the OLN-AS cells because these cells only required a single transfection to obtain the coexpression. Coexpression of α-syn and p25α in these cells resulted in a retraction of cellular processes as evidenced by phase contrast microscopy (Fig. 1_Bj_). Moreover, analyzing the cells by immunofluorescence microscopy using anti-α-tubulin and anti-p25α antibodies revealed a relocalization of MT from the cellular processes to the perinuclear region (Fig. 1_B_,a-i). Anti-α-tubulin immunofluorescence revealed the relocalization to be a progressive process as most p25α-expressing cells displayed intact MT in their cellular processes 12 h after transfection (Fig. 1_A_,a-f) as compared with 24 h after transfection (Fig. 1_B_,a-f). The phenotype with perinuclear MT localization allowed the quantification of the process and revealed that p25α in the OLN-AS cells caused a rapid and almost linear induction of MT retraction commencing 12 h after transfection and reaching a plateau after 24 h (Fig. 1_C_). The cellular p25α expression increased in the period from 12 to 36 h as demonstrated by immunoblotting (Fig. 1_D_). Quantification of the expression level of p25α normalized to the level of tubulin demonstrates that it requires a doubling of the tolerable p25α level present at 12 h to obtain the half-maximal MT retraction at 16 h (Fig. 1_E_). However, the cellular p25α level continues to increase in the plateau phase and acquires an 8-fold increase at 36 h after transfection, thus indicating a low threshold level for initiation of MT retraction.
FIGURE 1.
Coexpression of α-synuclein and p25α causes retraction of microtubule to the perinuclear region. A and_B_, α-syn-expressing OLN-AS cells were transiently transfected with p25α for 12 h (A) or 24 h (B) and subjected to immunofluorescence microscopy using anti-α-tubulin and anti-p25α antibodies or to phase contrast microscopy (Bj). Overlay with nuclear DAPI staining is shown in c, f, and i. The gradual MT retraction from processes to the perinuclear region upon coexpression of p25α and α-syn is evident. Scale bars, 20 μm (apply to_panels A_ and B). MT retraction to the perinuclear region is quantified in panel C and used as a measure in the following figures.C, development of MT retraction among p25α-transfected OLN-AS cells (filled circles) and OLN-t40 cells (open circles) and mock-transfected OLN-AS cells (filled triangle) was quantified by calculating the percentage of p25α expressing cells, which demonstrated MT retraction. The points represent the mean ± 1 S.D. from five microscopic fields in one of three representative experiments. Note the absent MT retraction in mock-transfected OLN-AS cells and in p25α-transfected OLN-t40 cells. D, the expression of p25α in OLN-AS cells was analyzed by Western blotting using anti-p25α. α-tubulin was included as a loading control. E, quantification of the ratio between p25α and tubulin from panel D.
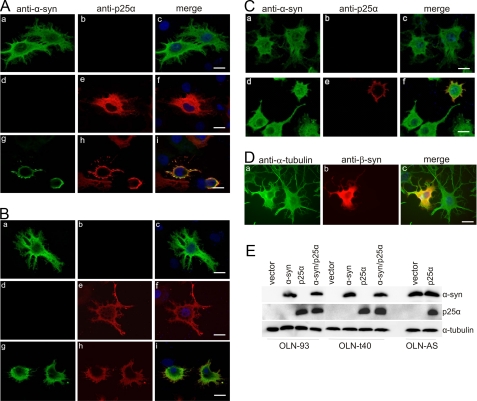
To assure that the MT retraction was specific for the coexpression of α-syn and p25α, we performed a series of control experiments. First, the cells tolerated expression of either protein as demonstrated in OLN-93 cells, wherein expression of either α-syn or p25α resulted in a diffuse distribution of the proteins in the cell body and cellular processes (Fig. 2_A_,a-f). However, coexpression of α-syn and p25α resulted within 24 h in a rearrangement of the antigens from the cellular processes to the perinuclear region with only marginal antigens remaining in the short residual processes (Fig. 2_A_,g-i). Second, the cells tolerated coexpression of α-syn and tau40 (Fig. 2_B, a-c_), p25α and tau40 (Fig. 2_B, d-f_), α-syn and β-synuclein (Fig. 2_D_), and α-syn and CRMP-2 (data not shown) in OLN-t40 and OLN-AS cells. Thus, the MT retraction was specific for coexpression of α-syn and p25α and was not merely due to additional transgenic expression of a second protein with α-syn. Immunoblot analysis of total cell lysates 24 h post-transfection confirmed the expression of exogenous protein in the three cell lines (Fig. 2_E_).
FIGURE 2.
Retraction of cellular processes is selective for coexpression of α-synuclein and p25α. A and B, OLN-93 (A) and tau-expressing OLN-t40 cells (B) were transiently transfected with human α-syn and empty vector (a-c), p25α and empty vector (d-f), and α-syn and p25α (g-i). Cells were examined 24 h after transfection by immunofluorescence microscopy using anti-α-syn and anti-p25α antibodies. Single transfections with either protein resulted in a diffuse distribution in cellular processes and cytoplasm (A and B, a-f). Coexpression of the two proteins resulted in retraction of the proteins from the cellular processes (A and B, g-i).C, OLN-AS cells retract their processes upon expression of p25α (d-f), whereas transfection with an empty vector (a-c) has no effect. Immunofluorescence microscopy was performed using rabbit anti-α-syn (ASY1), rat anti-p25α antibodies, and DAPI staining of the nuclei. D, expression of human β-synuclein in the OLN-AS cells for 24 h did not cause MT retraction. The β-synuclein-expressing cells were analyzed using anti-α-tubulin and anti-β-synuclein as primary antibodies. Note the retraction of cellular processes is selective for coexpression of α-syn and p25α (A, g-i; B, g-i;C, d-f) and does not occur upon coexpression of α-syn and tau40 (B, d-f) and α-syn and β-synuclein (D, a-c).Scale bars, 20 μm (apply to panels A-D). E, cell lysates were prepared 24 h after transfection, and 20 μg of protein was analyzed by Western blotting using ASY1 and anti-p25α antibodies. α-Tubulin was included as a loading control.
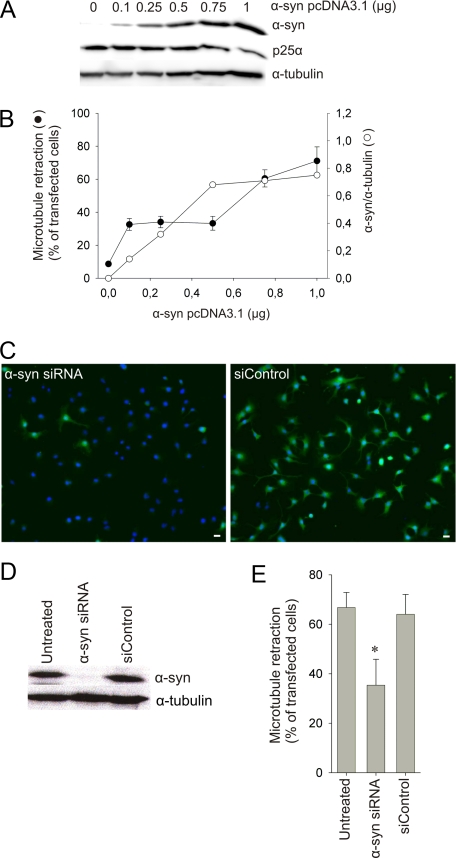
p25_α_-mediated Microtubule Retraction Requires Low Levels of α_-Synuclein_—The α-syn dependence of the p25α-mediated MT retraction was demonstrated by the absent effect of p25α expression in the OLN-93 and OLN-t40 cells devoid of α-syn (Fig. 2, A and_B_). However, transient expression using pcDNA3.1 vectors in OLN-93 and OLN-t40 cells caused a high expression of α-syn. To evaluate the levels of α-syn sufficient to elicit the degeneration, we cotransfected OLN-93 cells with a constant concentration of p25α vector and an increasing amount of α-syn vector. The variation in the concentration of the α-syn vector is complemented with empty pcDNA3.1 vector to ensure a constant level of vector DNA.Fig. 3, A and_B_, demonstrates that it was possible to lower the α-syn expression by 80% by reducing the concentration of the α-syn vector from 1 to 0.1 μg. The 80% reduction only caused a reduction of MT retraction from 70 to ∼35% (Fig. 3_B_). This suggests the existence of a low threshold for toxic cytosolic α-syn in the presence of p25α because substituting the α-syn vector with the empty pcDNA3.1 vector completely abrogated the MT retraction. The experimental approach of diluting the vector did not allow us to reproducibly lower the α-syn level to less than 20% so we chose siRNA to silence α-syn expression in the OLN-AS cells. A pool of four different siRNAs targeting human α-syn or a non-targeting control was transfected into OLN-AS cells for 96 h before analysis. This allowed us to lower the expression level by 90%, whereas the non-targeting siRNA control did not have any effect (Fig. 3, C and_D_). Fig. 3_E_ demonstrates that the 90% reduction in the α-syn levels only caused a 55% reduction in MT retraction. This is similar to the effect obtained by lowering the amount of α-syn expression vector (Fig. 3_B_) and, thus, corroborates the presence of a low threshold for α-syn expression to cause MT retraction.
FIGURE 3.
A low concentration of α-synuclein is sufficient for p25α-induced microtubule retraction. A and_B_, OLN-93 cells were cotransfected with 1 μg of p25α vector and the indicated concentrations of α-syn vector complemented with empty vector control to assure equal DNA load (in 6-well plates). A, cell lysates were prepared 24 h after transfection, and 30 μg of protein was analyzed by Western blotting using ASY1 and anti-p25α antibodies. α-Tubulin was included as a loading control. B, quantification of the ratio between α-syn and tubulin from panel A (open circles) and quantification of cotransfected cells showing MT retraction (filled circles) are shown as the mean ± 1 S.D. from five microscopic fields in one of three representative experiments. C and_D_, OLN-AS cells were transfected with siRNA targeting human α-syn or with a non-targeting control (siControl). The expression level of α-syn was analyzed by immunofluorescence microscopy (C) and Western blot analysis (D) 96 h after siRNA-transfection. One representative experiment of three is demonstrated.E, OLN-AS cells were transfected with siRNA for 96 h followed by transfection with p25α. The number of cells displaying MT retraction was quantified 24 h after transfection with p25α. Bars represent the mean ± 1 S.D. of three independent experiments. RNAi-mediated silencing of α-syn causes a significant reduction in the amount of p25α-positive cells displaying MT retraction (p < 0.05 with respect to untreated cells).
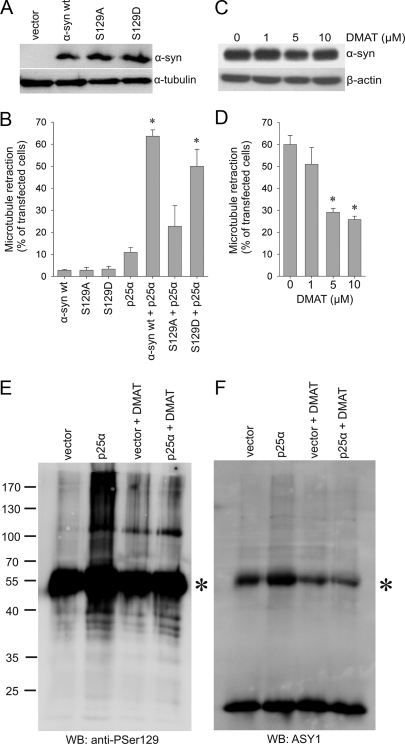
Microtubule Retraction Is Dependent on Phosphorylation at Ser-129 in α_-Synuclein_—Phosphorylation at Ser-129 in α-syn hallmarks α-syn pathology in tissue (25,26), and inhibition of the phosphorylation by site-directed mutagenesis to create α-syn S129A completely abrogates α-syn toxicity in a transgenic Drosophila model for PD (27). To investigate a possible role of Ser-129-phosphorylation in α-syn-dependent degeneration, we created a S129A mutant of α-syn. For comparison, we also generated a S129D mutant in which serine was converted to aspartate to mimic the negatively charged phosphoserine residue. pTRUF20 expression vectors carrying wt or mutant α-syn were transiently transfected into OLN-93 cells along with p25α. Coexpression of the phosphorylation-deficient S129A and p25α did not cause a level of MT retraction, which was significantly different from the cells expressing only p25α (Fig. 4_B_). This contrasts the significant MT retraction stimulated by coexpressing p25α with either wt α-syn or S129D α-syn (Fig. 4_B_; supplemental Fig. 1). Western blot analysis demonstrated that all three constructs (wt α-syn, S129D, and S129A) expressed similar levels of α-syn (Fig. 4_A_). This was confirmed by fluorescence-activated cell sorter analysis of α-syn expression at the single cell level, which revealed a coefficient of variance between the three α-syn constructs of 0.10 and 0.14 in two experiments (data not shown). Hence, elimination of the phosphorylation site at Ser-129 abrogates the p25α-stimulated MT retraction and, thus, suggests a critical role for Ser-129 phosphorylation in the process.
FIGURE 4.
Microtubule retraction is dependent on phosphorylation at Ser-129 in α-synuclein. A, OLN-93 cells were transfected with α-syn wt, α-syn S129A, or α-syn S129D for 24 h, and 20 μg of protein was subjected to immunoblotting using ASY1 and anti-α-tubulin antibodies. B, OLN-93 cells were transfected with p25α or α-syn (wt, S129A, or S129D) or cotransfected with p25α and the α-syn variants as indicated. At 24 h post-transfection cells were subjected to immunofluorescence microscopy with ASY1, anti-p25α, and anti-α-tubulin antibodies. MT retraction in the single and cotransfected cells was quantified, and bars represent the mean ± 1 S.D. of three independent experiments. Coexpression of α-syn wt and p25α or α-syn S129D and p25α caused significant MT retraction (p < 0.05 compared with cells cotransfected with p25α and empty vector), whereas coexpression of α-syn S129A and p25α had no significant effect on MT retraction. C, the kinase inhibitor DMAT does not affect α-syn expression as revealed by ASY1 and anti-β-actin immunoblotting of lysates from OLN-AS cells. D, OLN-AS cells transfected with p25α were treated with the indicated concentrations of DMAT and subjected to anti-p25α and anti-α-tubulin immunofluorescence microscopy to quantify MT retraction.Bars represent the mean ± 1 S.D. of three independent experiments. DMAT caused a dose-dependent attenuation of the MT retraction (p < 0.05 with respect to untreated cells). E and_F_, anti-Ser(P)-129 (E) and ASY1 (F) immunoblotting (WB) of α-syn immunoprecipitates from OLN-AS cells transfected with an empty vector or with p25α in the presence or absence of 5 μm DMAT. IgG bands are indicated by as asterisk. Note the enhanced anti-Ser(P)-129 (E)- and ASY1 (F)-positive high molecular weight smear in cells coexpressing α-syn and p25α that is reduced by treatment with DMAT. Molecular size markers are shown to the_left_. The experiment was repeated twice with similar results.
_Kinase Inhibition Abrogates the Process of Microtubule Retraction_—The search for kinases targeting α-syn has been focusing on kinases directed against Ser-129 given its relation to pathological α-syn aggregates (25,26). Several kinases have been demonstrated to phosphorylate Ser-129 including CK1, CK2, G protein-coupled receptor kinase 2 (GRK2), GRK5, dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A), and polo-like kinases (PLK)1-3 (27-32).3 CK2 was identified as being responsible for the phosphorylation at Ser-129 in α-syn in rat brain extracts (33). However, silencing PLK2 in human cortical cultures reduced Ser-129 phosphorylation by 70%, and PLK2 and -3 were more efficient than CK2 in phosphorylating Ser-129 in vitro (29). Accordingly, we tested the effect of the four kinase inhibitors emodin, DRB, DMAT, and BI 2536 on the process of MT retraction in our model. The former three inhibitors target CK2, whereas the latter two inhibit PLKs, although they are also effective toward other kinases such as the Dyrk1A and PIM kinases, cyclin-dependent kinase 7 (CDK7), CDK8, CDK9, and tyrosine kinase p56lck (34-36). The PLK inhibitor BI 2536 inhibited Ser-129 phosphorylation of endogenous α-syn in HEK292 cells and human cortical cultures with an IC50 of about 50 nm (29). Therefore, we first treated OLN-93 cells with 25 nm BI 2536 for 24 h, which resulted in mitotic arrest and activation of caspase-3 in about 40% of the cells (supplemental Fig. 2). However, this was not unexpected as BI 2536 was developed as an inhibitor of tumor growth and targets centrosome function (37,38). Second, we tested whether it was possible to treat the cells for 4 or 8 h as this was considered the minimum requirement in our assay if MT retraction was to be scored at 16 h post-transfection. The treatment resulted in a time-dependent development of mitotic arrest using 5-100 nm BI 2536 (data not shown) so further studies using this inhibitor in our proliferating cell line were terminated. By contrast, when culturing OLN-AS cells with up to 10 μm DMAT, emodin, and DRB for 24 h, we observed no toxic effects on cell viability and proliferation (data not shown). Fig. 4_D_ demonstrates that DMAT causes a dose-dependent albeit incomplete reduction in MT retraction of about 50%. Increasing the DMAT concentration above 5 μm did not lead to further protection. Similar data were obtained for emodin and DRB (data not shown). The expression level of α-syn was unaffected by the presence of up to 10 μm DMAT (Fig. 4_C_). This demonstrates that the protective effect was not caused by a stimulation of α-syn catabolism.
Western blot analysis demonstrated soluble Ser-129-phosphorylated α-syn species as a high molecular weight smear in α-syn immunoprecipitates from detergent extracts prepared from OLN-AS cells transfected with p25α (Fig. 4_E_). The anti-Ser(P)-129 immunoblotting was a sensitive method to detect the α-syn aggregates as compared with the weaker signal obtained by a high concentration of the high affinity ASY1 antibody (Fig. 4, E versus F). Phosphorylation of Ser-129 could be inhibited by treating the cells with DMAT, and this treatment also reduced the formation of the oligomers detectable by the ASY1 antibody (Fig. 4, E and_F_). Hence, kinases sensitive to DMAT, DRB, and emodin are critical for Ser-129 phosphorylation, oligomer formation, and MT retraction.
Inhibitors of α_-Synuclein Aggregation Block the Process of Microtubule Retraction_—The presence of soluble P-Ser-129 α-syn aggregates correlated to the altered morphology of the cells so we hypothesized that the MT retraction was caused by α-syn aggregation.
Immunofluorescence microscopic analyses of OLN-93, OLN-t40, and OLN-AS cells coexpressing α-syn and p25α did not reveal any consistent pattern of α-syn-positive inclusions using ASY1 (Fig. 2, A-C), anti-Ser(P)-129, or a panel of four novel monoclonal α-syn antibodies (data not shown). However, the analyses were complicated by the rounding of the cells, which caused the fluorescence signal to accumulate around the nucleus. Both confocal microscopic and ApoTome sectioning of the images were performed. Inconsistently, small highly intense ASY1 immunoreactive puncta could be observed in the cells, but this was observed in α-syn-expressing cells both in the absence and presence of p25α4 (data not shown). Thioflavin S staining did not reveal any positive staining of the p25α-expressing cells (data not shown). It was also analyzed whether the solubility and proteinase K sensitivity of α-syn in detergent extracts changed during the process of MT retraction. However, we were not able to detect insoluble α-syn material by centrifugation after transfection with p25α or a significant difference in proteinase K sensitivity between cells transfected with p25α or an empty control vector (data not shown).
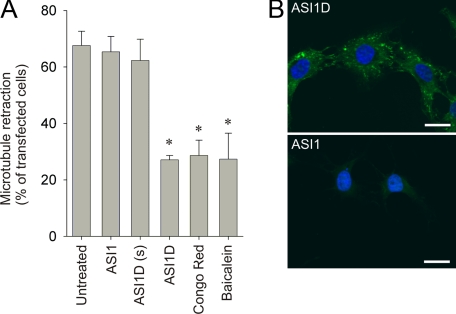
To demonstrate a functional role of α-syn aggregates in the process of MT retraction, we analyzed whether three inhibitors of α-syn aggregation, Congo Red, ASI1D peptide, and baicalein, could reduce the number of cells with MT retraction. Congo Red has been shown to inhibit the toxicity of α-syn oligomers toward the proteasome in vitro (39) and attenuate toxicity in animal models of aggregate-dependent neurodegenerative diseases (for review, see Ref. 40). Culturing OLN-AS cells with 20 μm Congo Red for 7 days before transfection with p25α reduced MT retraction by more than 50% (Fig. 5_A_). The Congo Red pretreatment had no detectable effect on their proliferative capacity and MT organization (data not shown). The ASI1 peptide corresponds to residues 68-75 within the hydrophobic NAC domain of α-syn and is a potent inhibitor of α-syn aggregation in vitro. Fusion of six arginine residues to the C terminus of ASI1 produces a cell-permeable inhibitor of α-syn aggregation (ASI1D) (20). ApoTome sectioning at the midnuclear plane demonstrates that ASI1D was internalized in the OLN-AS cells, whereas the cell-impermeable ASI1 peptide was not (Fig. 5_B_). Treatment of the OLN-AS cells with 10 μm concentrations of the ASI1D peptide before p25α transfection significantly reduced MT retraction (Fig. 5_A_). The cell-impermeable variant, ASI1, and a cell-permeable control peptide, containing a scrambled ASI1 sequence with the same poly-Arg carrier, had no effect on MT retraction (Fig. 5_A_). Treating the OLN-AS cells with up to 50 μm concentrations of the three ASI1 peptide variants had no detectable effects on the cell viability and proliferation (data not shown). Moreover, the flavonoid baicalein has been demonstrated to inhibit fibrillation of α-syn in vitro (41,42), and treatment of OLN-AS cells with 100 μm baicalein before p25α transfection significantly reduced the number of cells with MT retraction (Fig. 5_A_). To ensure that the protection of the anti-aggregatory compounds are specific to cellular responses to aggregated α-syn and not a general response to cellular stress, we treated OLN-AS cells with rotenone (1.5 μm) or tunicamycin (50 μg/ml) in the presence or absence of the ASI1D peptide or baicalein. Rotenone induces oxidative stress by inhibiting complex I of the mitochondrial respiratory chain (43), and tunicamycin stimulates unfolded protein response (44). MT retraction in the absence and presence of ASI1D was 95 ± 8.2 versus 92 ± 9.4% for rotenone and 89 ± 7.2 versus 86 ± 8.0% for tunicamycin (mean ± S.D. from two independent experiments). Similar results were obtained with baicalein (data not shown). Hence, the anti-aggregatory compounds are specific toward stress responses induced by aggregated α-syn and provide indirect evidence for a functional role of α-syn aggregation in the process of MT retraction.
FIGURE 5.
Inhibition of α-synuclein aggregation attenuates microtubule retraction. A, OLN-AS cells were pretreated with cell impermeable (ASI1) and cell-permeable (ASI1D and scrambled ASI1D(s)) peptide inhibitors of α-syn aggregation for 1 h before transfection with p25α. Alternatively, cells were treated with Congo Red for 7 days or baicalein for 1 h before transfection with p25α. MT retraction was quantified 24 h after transfection. Bars represent the mean ± 1 S.D. of three independent experiments. Congo Red, baicalein, and the cell-permeable inhibitor ASI1D caused a significant reduction in the number of p25α-positive cells displaying MT retraction (p < 0.05 with respect to untreated cells). B, internalization of biotinylated ASI1D was confirmed by ApoTome sectioning at the midnuclear plan. Cells were treated with biotinylated ASI1D (upper figure) or ASI1 (lower figure) for 1 h at 37 °C, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100. Cells were incubated with Alexa Fluor 488-conjugated streptavidin and visualized under a fluorescence microscope equipped with an ApoTome for optical sectioning. Scale bar, 20 μm.
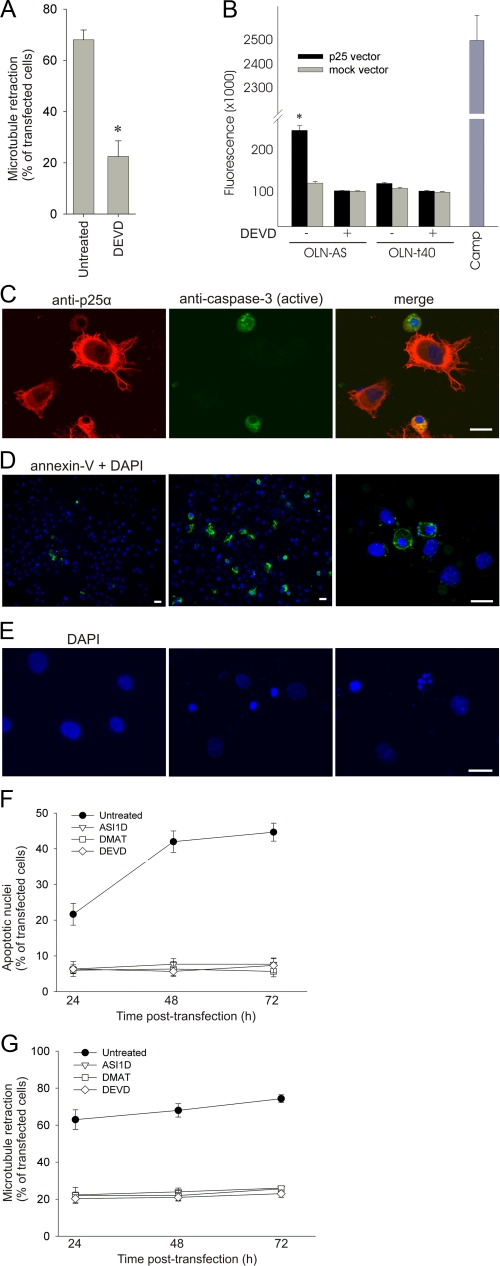
α_-Synuclein-dependent Microtubule Retraction and Apoptosis Are Coupled Processes_—Immunoreactivity for active caspases has been demonstrated in degenerating cells in neurodegenerative diseases associated with protein aggregation, suggesting that their role is more protracted that simply just executing the ultimate apoptotic process (45). We tested the effect of the caspase-3 inhibitor Ac-DEVD-CHO on MT retraction in OLN-AS cells transfected with p25α and observed a 60% reduction in MT retraction (Fig. 6_A_). Time course experiments demonstrated that caspase-3 inhibition blocked the development of MT retraction at all time points between 12 and 24 h (data not shown). The activity of caspase-3 in the cell extracts was measured using a fluorescent caspase-3 substrate. The inhibitor Ac-DEVD-CHO (20 μm) was included in parallel for each sample to determine the level of nonspecific background (Fig. 6_B_). A significant activation of caspase-3 was observed in OLN-AS cells upon expression of p25α. Caspase-3 activation was dependent on the coexpression of p25α and α-syn, as it was not observed in either OLN-t40 cells transfected with p25α or in mock-transfected OLN-AS cells. The caspase-3 activation elicited by p25α expression was compared with the level of activation induced by the topoisomerase inhibitor camptothecine, a well known inducer of apoptosis. Camptothecine induced caspase-3 activation to a level of 2.6 · 106 units as compared with 2.5 · 105 units induced by p25α (Fig. 6_B_). Adjusting for the transfection efficiency of about 25%, the cellular caspase-3 activation induced by p25α was ∼40% of that induced by camptothecin. This low activation may explain why we were incapable of demonstrating significant active caspase-3 immunoreactivity before 24 h (data not shown), whereupon it became apparent (Fig. 6_C_). Hence, low level caspase activation is a prerequisite for the rapid α-syn aggregate-dependent MT retraction.
FIGURE 6.
Microtubule retraction precedes development of apoptotic characteristics. A, OLN-AS cells were treated with a peptide aldehyde caspase-3 inhibitor Ac-DEVD-CHO (20 μm) before transfection with p25α. The number of transfected cells with MT retraction was quantified 24 h after transfection. Bars represent the mean ± 1.S.D. of three independent experiments. Inhibition of caspase-3 caused a significant reduction in MT retraction (p < 0.05 with respect to untreated cells). B, lysates from OLN-AS and OLN-t40 cells transfected with either mock or p25α vector for 24 h were analyzed for caspase-3 activity using Ac-DEVD-amido-4-methylcoumarin as substrate. Concentrations of released fluorescent products were measured in arbitrary units, and bars represent the mean ± 1 S.D. of triplicate. One of three representative experiments is shown. The presence or absence of the caspase-3 inhibitor, Ac-DEVD-CHO (20 μm), is indicated by a + or - below the bar chart. Cells treated with camptothecin (CAMP) were included as a positive control. OLN-AS cells expressing p25α demonstrate a significant increase in caspase-3 activity (p < 0.05) as compared with mock-transfected OLN-AS cells and p25α-transfected OLN-t40 cells. C, OLN-AS cells were transfected with p25α for 24 h and stained with anti-p25α and an antibody specific for active caspase-3. D, OLN-AS cells were transfected with either an empty vector (left) or p25α vector (middle and right) and stained with Annexin-V-fluorescein isothiocyanate and DAPI 24 h after transfection. E, OLN-AS cells were transfected with either an empty vector (left) or with p25α (middle and right). Apoptotic cells were identified by a condensed (middle) or fragmented (right) nuclei. Scale bars, 20 μm. F and G, the population of OLN-AS cells with apoptotic nuclei (F) and MT retraction (G) was quantified 24, 48, and 72 h after p25α-transfection in the absence or presence of ASI1D, DMAT, and DEVD. Points represent the mean ± 1 S.D. from five microscopic fields in one of three representative experiments. The development of MT retraction during the first 24 h is displayed inFig. 2.
Cellular apoptosis is considered a swift process where active caspase-3 orchestrates a range of irreversible molecular changes. Phosphatidylserine (PS) is externalized to the outer leaflet of the plasma membrane, and chromatin is condensed, fragmented, and cleaved between the nucleosomes in the nucleus. The time course for the development of these changes upon p25α expression was determined by immunofluorescence microscopy, where we observed active caspase-3 in a proportion of the cells (Fig. 6_C_), PS externalization as demonstrated by its binding of annexin-V-fluorescein isothiocyanate (Fig. 6_D_), and nuclear chromatin condensation as demonstrated by DAPI staining (Fig. 6_E_). Moreover, DNA fragmentation was visualized after 72 h of transfection by gel electrophoresis (data not shown). Quantification of chromatin condensation by DAPI staining demonstrates a slow induction with an ∼15% increase above background at 24 h which increases to 40% at 48 h (Fig. 6_F_). This development mirrors the advance in caspase-3 activation and PS externalization (data not shown). The slow induction of apoptotic markers differs from the rapid MT retraction that essentially occurs between 12 and 24 h, whereupon it remains constant (Figs.1_D_ and6_G_). Remarkably, all these cellular responses, MT retraction, visible caspase-3 activation, nuclear condensation, and PS externalization, were all inhibited by incubating the cells in the presence of the aggregation inhibitor ASI1D, the kinase inhibitor DMAT, and the caspase-3 inhibitor Ac-DEVD-CHO (Fig. 6, F and_G_, and data not shown). Expression of β-synuclein in OLN-AS cells for 48 h was included as a control for processes independent of α-syn aggregation, but it did not elicit any apoptotic effects (data not shown), which was analogous to its absent ability to induce MT retraction (Fig. 2_D_). Accordingly, caspase-3 activation is a prerequisite for both the early α-syn aggregation-dependent MT relocalization and the ensuing slow development of apoptosis. Both processes are sensitive to inhibitors of α-syn aggregation and phosphorylation, indicative of their functional coupling.
DISCUSSION
Cellular models for α-syn-dependent neurodegeneration are in high demand for the development of novel neuroprotective drugs against α-synucleinopathies. The most frequent strategy has been transgenic approaches to model the gain-of-function operating in the PARK1 and PARK4 families, which carry missense mutations and multiplications of the α-syn gene (46). However, phenotypes obtained with respect to α-syn aggregation and cell death have only been marginally successful, and many cell lines tolerate transgenic expression of human wt and mutant α-syn (46). Different strategies have been employed to overcome this tolerance, e.g. by the fusion of a truncated enhanced green fluorescent protein molecule to the C terminus of α-syn (47) or coexpression of α-syn with proteins able to stimulate aggregation, such as synphilin (48).
We recently demonstrated that p25α is a potent stimulator of α-syn aggregation and colocalizes with α-syn in Lewy bodies and glial cytoplasmic inclusions in PD and MSA, respectively (16). To model α-syn-dependent cytopathology in MSA, we coexpressed α-syn and p25α in the OLN-93 cell line (22). Coexpression of α-syn and p25α caused a retraction of MT from the cellular processes to the perinuclear region within 24 h after transfection. The early phase was followed by a more protracted development of apoptotic markers within the following 48 h with microscopically detectable caspase-3 activation, externalization of PS, and nuclear chromatin condensation. The fast MT retraction was dependent on coexpression of α-syn and p25α, as OLN-93 and OLN-t40 cells tolerated α-syn expression and only displayed a marginal effect of expressing p25α. This contrasts with recent data in HeLa and normal rat kidney cells where the expression of p25α induced MT reorganization and apoptosis (49). A possible explanation may be that OLN cells better tolerate p25α, which is normally expressed in oligodendrocytes (50,51).
Transgenic expression of cytosolic proteins by strong promoters always holds the risk of eliciting a nonspecific and potentially toxic response mediated by the heat shock factor 1 pathway (52). Several experiments were conducted to exclude nonspecific cellular stress as a cause of the MT retraction. First, the effect of p25α was specific toward α-syn as evidenced by absent MT retraction when coexpressing p25α with tau40 in the OLN-t40 cell line. Second, coexpression of proteins other than p25α with α-syn in OLN-93 cells was tolerated as exemplified by human β-synuclein and human CRMP-2. The α-syn level sufficient to trigger the process of MT retraction was investigated by two experimental approaches. First, OLN-93 cells were transfected with a constant concentration of p25α expression vector and an increasing amount of α-syn vector and, second, by silencing α-syn in OLN-AS cells transfected with p25α. The former approach yielded a dose-dependent reduction of 80% and the latter a 90% reduction in the α-syn level. Both approaches only reduced the MT retraction by about 50%, although complete absence of cellular α-syn by omitting the α-syn vector reduced the MT retraction to background levels. An ongoing study where α-syn is coexpressed with p25α in neuronal SHSY5Y cells does not show a strong phenotype (data not shown). This suggests the existence of a low tolerable threshold for α-syn in oligodendroglial cells expressing p25α and thereby a pivotal role for α-syn aggregates in triggering MSA-associated degeneration. This is in agreement with transgenic mice models of MSA (14,15).
Phosphorylation of α-syn at Ser-129 has been demonstrated to play a toxic role in the α-syn transgenic Drosophila model (27), although this effect was recently challenged in a rat model (53). Our model supports a critical role for phosphorylation at Ser-129 in mediating α-syn-dependent MT retraction as judged experimentally by the absent MT retraction upon expressing the S129A mutant. This is comparable with the role of Ser-129 phosphorylation in mediating α-syn inclusion formation (54) and the unfolded protein response in SHSY5Y cells (55). Expression of the phosphomimetic S129D mutant did not cause MT retraction in the OLN cells to the same extent as the wild type protein. An explanation may be that S129D is a poor substitute for Ser-129-phosphorylated α-syn (56).
Several kinases have been shown to phosphorylate Ser-129 including CK1, CK2, GRK2, GRK5, Dyrk1A, PLK1-3 (27-32).3 However, the kinase(s) responsible for the Ser-129 phosphorylation in our model is still unclear. CK2 and PLK2 have recently raised interest because CK2 is the major contributor of Ser-129 phosphorylation in rat brain extracts (33) and PLK2 is the predominant kinase responsible for this phosphorylation in human cortical cultures (29). It should be kept in mind that the Ser-129 phosphorylation in human brain almost exclusively is associated with pathological α-syn inclusions (25,26) and, thus, may be caused by a kinase up-regulated under pathological conditions. The three inhibitors DMAT, emodin, and DRB inhibit the MT retraction in micromolar concentrations, and they are all capable of inhibiting CK2 (57,58). However, DMAT was recently demonstrated to inhibit PLK2 in cells and in vitro in micromolar concentrations (29), but such an effect has not been reported for emodin and DRB. The PLK inhibitor BI 2536 inhibits Ser-129 phosphorylation in human cortical cells, but we found that it was toxic to our cells. This is likely because our cell line is proliferating and BI 2536 is an efficient PLK1 inhibitor resulting in mitotic arrest (37). The inhibitory profiles of the three active compounds DMAT, DRB, and emodin also support other potential candidates. DMAT inhibits Dyrk1A (35), which binds and phosphorylates α-syn (30), and PIM-1, which is induced in the brain upon seizures and long term potentiation (59,60). DRB inhibits cyclin-dependent kinase 7 (36), which is increased in brain during aging and in Alzheimer disease (61). Emodin inhibits p56lck (34), which is expressed in neurons (62) and protects retinal cells (63). The identification of the cytotoxic Ser-129-directed kinase pathway in the model will, thus, require the use of a broader panel of inhibitors followed by a siRNA approach toward selected candidates.
It should be kept in mind that the OLN model is based on an oligodendroglial cell line. Thus, it may better reflect the molecular events occurring in MSA as compared with PD and dementia with Lewy bodies and may, therefore, involve different kinases, although α-syn present in inclusions in both types of diseases is strongly phosphorylated at Ser-129 (64,65).
The role of Ser-129 phosphorylation in promoting α-syn aggregation is unclear as in vitro phosphorylation of this residue has been demonstrated to both promote and prevent aggregation (26,56). However, S129A mutagenesis and kinase inhibition by DMAT inhibited development of Ser-129-phosphorylated oligomers and the process of MT retraction in the OLN cell model and, thus, provides a direct link between phosphorylation, aggregation, and MT retraction in this model. Our results thereby corroborate_in vivo_ observations in the Drosophila model (27,66). High molecular weight α-syn species have previously been demonstrated in cell extracts after oxidative stress (67,68), treatment with polyunsaturated fatty acids (69), and upon expression of α-syn fusion proteins capable of forming bimolecular fluorescence complementation (70). However, the present report is to our knowledge the first to demonstrate that the soluble α-syn oligomers are phosphorylated at Ser-129.
The functional roles of the α-syn aggregates were further corroborated by the protective effect of the α-syn aggregation inhibitors Congo Red, baicalein, and the peptide ASI1D. Congo Red and baicalein are nonspecific inhibitors of amyloid-type aggregation and inhibit α-syn aggregation (41,42,71). ASI1D is a cell-permeable peptide inhibitor of α-syn aggregation (20). We verified that the protective effect of ASI1D was not caused by the hexa-Arg membrane-translocating peptide because a scrambled, non-inhibitory ASI1 segment attached to the hexa-Arg peptide was unable to protect the cells. Moreover, the ASI1D effect was not a general cytoprotective result because it did not protect OLN cells toward the stress elicited by mitochondrial complex I inhibition induced by rotenone and unfolded protein stress induced by tunicamycin. Previous cellular studies supporting a causal role of α-syn aggregates in triggering dysfunction are based on the protective effectonα-syn-dependent cytotoxicity of directly expressing the chaperone HSP70 (72) or indirectly by geldanamycin treatment (47,73).
The early MT retraction detected before 24 h could be blocked by the caspase-3 inhibitor Ac-DEVD-CHO, although we were unable to detect significant caspase-3 cleavage by immunofluorescence microscopy before 24 h. After 24 h the cells gradually developed the apoptotic markers; that is, cleaved caspase-3, nuclear condensation, and PS externalization. These apoptotic responses could be blocked by inhibiting α-syn aggregation, Ser-129 phosphorylation, and caspase-3 activation. The protracted cellular responses may be caused by the low cellular caspase-3 activity, which after 24 h of transfection was ∼40% that of the level induced by camptothecine. A low caspase-3 activity is in agreement with the demonstration of in situ activated caspase-3 in brain tissue from AD and PD patients (74). The slow induction of cell death in this model may enable more detailed studies of the early cellular responses to intracellular α-syn oligomers. Cellular investigations of the signaling pathways stimulated in α-syn transgenic cells have shown stimulation of the unfolded protein response in SHSY5Y cells (75). The cellular responses to accumulation of unfolded protein in the endoplasmic reticulum and cytosol involve the expression of the chaperones BIP and HSP70 (76), but we did not detect any increase hereof by immunoblotting when comparing OLN-AS cells transfected with p25α or an empty vector (data not shown). This discrepancy may relate to the experimental systems of oligodendroglial versus neuronal cells. However, it may also be due to a requirement for toxic α-syn aggregates in mediating the toxicity as our study is the first to validate that the response can be attenuated by inhibitors of aggregation. The sensitivity of different α-syn-based models to inhibitors of α-syn aggregation may represent an unbiased strategy for comparing the involvement of aggregated versus other forms of α-syn-dependent stress and, thus, be rewarding when considering the large interest in identifying specific toxic α-syn species (77,78).
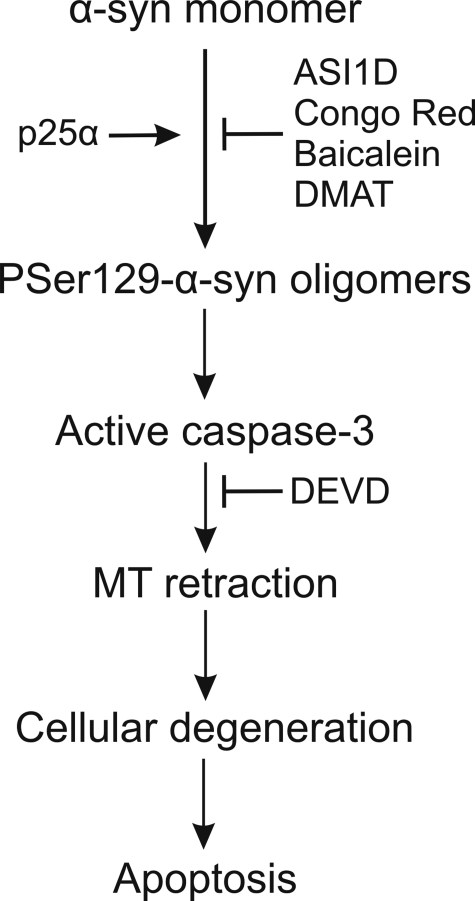
Conclusively, we have developed an oligodendroglial model that supports a hypothesis where coexpressing p25α with α-syn stimulates the formation of toxic Ser-129-phosphorylated oligomers, which trigger cellular processes leading to caspase-3 activation (Fig. 7). The precise role of p25α is yet unclear, but the preventive effect of kinase inhibition on oligomer formation suggests a role in facilitating nucleation of small α-syn aggregates, which present as better substrates for Ser-129 reactive kinases. The Ser-129 phosphorylation of these nuclei stimulates their potential for triggering α-syn aggregation. Importantly, the cell death requires only low concentrations of α-syn and can be blocked at different levels, proposing a signaling pathway with several targets amenable to pharmacological manipulation.
FIGURE 7.
Model for development of α-synuclein-mediated cytotoxicity. Proaggregatory factors such as p25α nucleate monomeric α-syn and thereby convert the monomers into better substrates for Ser-129-reactive kinases. The Ser-129 phosphorylation increases the nucleating activity of the α-syn aggregates, which favors the formation of cytotoxic Ser(P)-129 oligomers. These oligomers subsequently affect vital cellular signaling pathways that ultimately initiate slow activation of caspase-3 and its downstream prodegenerative effects comprising early MT dysfunction and subsequent apoptosis.
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank Jette B. Lauridsen and Lis Hygom for excellent technical assistance, Dr. Takeshi Iwatsubo for the anti-Ser(P)-129 antibody, Dr. Frederik Vilhardt for expert assistance with the fluorescence-activated cell sorter analyses, Dr. Deniz Kirik for the pTRUF20 plasmid, and Dr. Justus Daechsel for the CRMP-2 vector.
*
This work was supported by the Lundbeck Foundation, Danish Research Council for Health and Disease Grant 271-05-0166, the Aarhus University Research Foundation, The Danish Parkinson's Disease Foundation, Wacherhausen Foundation, National Health and Medical Research Council Grant 275528, Nordic Center of Excellence for Neurodegenerative Disorders, the Foundation of 2/7-1984 to Treatment of Parkinson's Disease, and the innovation consortium CureND.
The on-line version of this article (available athttp://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
2
The abbreviations used are: α-syn, α-synuclein; CK, casein kinase; DMAT, 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole; DRB, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole; wt, wild type; siRNA, small interfering RNA; PIPES, 1,4-piperazinediethanesulfonic acid; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; PLK, polo-like kinase; PD, Parkinson disease; MSA, Multiple system atrophy; MT, microtubular; DAPI, 4′,6-diamidine-2′-phenylindole dihydrochloride; Dyrk1A, dual-specificity tyrosine phosphorylation-regulated kinase 1A; PS, phosphatidylserine.
3
M. K. Mbefo, K. E. Paleologou, A. Boucharaba, A. Oueslati, D. Olschewski, H. Hirling, and H. A. Lashuel. α-Synuclein in Health and Disease, Lausanne, Switzerland, September 24-26, 2008, p. 21 (abstr.).
4
Riedel, M., Goldbaum, O., and Richter-Landsberg, C. (2009) J. Mol. Neurosci. in press.
References
- 1.Maroteaux, L., Campanelli, J. T., and Scheller, R. H. (1988) J. Neurosci. Res. 8 2804-2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gai, W. P., Power, J. H., Blumbergs, P. C., Culvenor, J. G., and Jensen, P. H. (1999) J. Neurochem. 73 2093-2100 [PubMed] [Google Scholar]
- 3.Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997) Nature 388 839-840 [DOI] [PubMed] [Google Scholar]
- 4.Wakabayashi, K., Yoshimoto, M., Tsuji, S., and Takahashi, H. (1998) Neurosci. Lett. 249 180-182 [DOI] [PubMed] [Google Scholar]
- 5.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., and Riess, O. (1998) Nat. Genet. 18 106-108 [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997) Science 276 2045-2047 [DOI] [PubMed] [Google Scholar]
- 7.Zarranz, J. J., Alegre, J., Gomez-Esteban, J. C., Lezcano, E., Ros, R., Ampuero, I., Vidal, L., Hoenicka, J., Rodriguez, O., Atares, B., Llorens, V., Tortosa, E. G., del Ser, T., Munoz, D. G., and de Yebenes, J. G. (2004) Ann. Neurol. 55 164-173 [DOI] [PubMed] [Google Scholar]
- 8.Farrer, M., Kachergus, J., Forno, L., Lincoln, S., Wang, D. S., Hulihan, M., Maraganore, D., Gwinn-Hardy, K., Wszolek, Z., Dickson, D., and Langston, J. W. (2004) Ann. Neurol. 55 174-179 [DOI] [PubMed] [Google Scholar]
- 9.Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., Lincoln, S., Crawley, A., Hanson, M., Maraganore, D., Adler, C., Cookson, M. R., Muenter, M., Baptista, M., Miller, D., Blancato, J., Hardy, J., and Gwinn-Hardy, K. (2003) Science 302 841. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger, K. A. (2003) Movement Disorders 18 Suppl. 6, S2-S12 [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi, K., and Takahashi, H. (2006) Neuropathology 26 338-345 [DOI] [PubMed] [Google Scholar]
- 12.Burn, D. J., and Jaros, E. (2001) Mol. Pathol. 54 419-426 [PMC free article] [PubMed] [Google Scholar]
- 13.Kahle, P. J., Neumann, M., Ozmen, L., Muller, V., Jacobsen, H., Spooren, W., Fuss, B., Mallon, B., Macklin, W. B., Fujiwara, H., Hasegawa, M., Iwatsubo, T., Kretzschmar, H. A., and Haass, C. (2002) EMBO Rep. 3 583-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shults, C. W., Rockenstein, E., Crews, L., Adame, A., Mante, M., Larrea, G., Hashimoto, M., Song, D., Iwatsubo, T., Tsuboi, K., and Masliah, E. (2005) J. Neurosci. 25 10689-10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazawa, I., Giasson, B. I., Sasaki, R., Zhang, B., Joyce, S., Uryu, K., Trojanowski, J. Q., and Lee, V. M. Y. (2005) Neuron 45 847-859 [DOI] [PubMed] [Google Scholar]
- 16.Lindersson, E., Lundvig, D., Petersen, C., Madsen, P., Nyengaard, J. R., Hojrup, P., Moos, T., Otzen, D., Gai, W. P., Blumbergs, P. C., and Jensen, P. H. (2005) J. Biol. Chem. 280 5703-5715 [DOI] [PubMed] [Google Scholar]
- 17.Song, Y. J. C., Lundvig, D. M. S., Huang, Y., Gai, W. P., Blumbergs, P. C., Hojrup, P., Otzen, D., Halliday, G. M., and Jensen, P. H. (2007) Am. J. Pathol. 171 1291-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen, P. H., Islam, K., Kenney, J., Nielsen, M. S., Power, J., and Gai, W. P. (2000) J. Biol. Chem. 275 21500-21507 [DOI] [PubMed] [Google Scholar]
- 19.Li, J.-Y., Jensen, P. H., and Dahlström, A. (2002) Neuroscience 113 463-478 [DOI] [PubMed] [Google Scholar]
- 20.El-Agnaf, O. M. A., Paleologou, K. E., Greer, B., Abogrein, A. M., King, J. E., Salem, S. A., Fullwood, N. J., Benson, F. E., Hewitt, R., Ford, K. J., Martin, F. L., Harriot, P., Cookson, M. R., and Allsop, D. (2004) FASEB J. 18 1315-1317 [DOI] [PubMed] [Google Scholar]
- 21.Kirik, D., Rosenblad, C., Burger, C., Lundberg, C., Johansen, T. E., Muzyczka, N., Mandel, R. J., and Bjorklund, A. (2002) J. Neurosci. 22 2780-2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter-Landsberg, C., and Heinrich, M. (1996) J. Neurosci. Res. 45 161-173 [DOI] [PubMed] [Google Scholar]
- 23.Goldbaum, O., Oppermann, M., Handschuh, M., Dabir, D., Zhang, B., Forman, M. S., Trojanowski, J. Q., Lee, V. M. Y., and Richter-Landsberg, C. (2003) J. Neurosci. Res. 23 8872-8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uryu, K., Richter-Landsberg, C., Welch, W., Sun, E., Goldbaum, O., Norris, E. H., Pham, C. T., Yazawa, I., Hilburger, K., Micsenyi, M., Giasson, B. I., Bonini, N. M., Lee, V. M., and Trojanowski, J. Q. (2006) Am. J. Pathol. 168 947-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson, J. P., Walker, D. E., Goldstein, J. M., de Laat, R., Banducci, K., Caccavello, R. J., Barbour, R., Huang, J. P., Kling, K., Lee, M., Diep, L., Keim, P. S., Shen, X. F., Chataway, T., Schlossmacher, M. G., Seubert, P., Schenk, D., Sinha, S., Gai, W. P., and Chilcote, T. J. (2006) J. Biol. Chem. 281 29739-29752 [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara, H., Hasegawa, M., Dohmae, N., Kawashima, A., Masliah, E., Goldberg, M. S., Shen, J., Takio, K., and Iwatsubo, T. (2002) Nat. Cell Biol. 4 160-164 [DOI] [PubMed] [Google Scholar]
- 27.Chen, L., and Feany, M. B. (2005) Nat. Neurosci. 8 657-663 [DOI] [PubMed] [Google Scholar]
- 28.Arawaka, S., Wada, M., Goto, S., Karube, H., Sakamoto, M., Ren, C. H., Koyama, S., Nagasawa, H., Kimura, H., Kawanami, T., Kurita, K., Tajima, K., Daimon, M., Baba, M., Kido, T., Saino, S., Goto, K., Asao, H., Kitanaka, C., Takashita, E., Hongo, S., Nakamura, T., Kayama, T., Suzuki, Y., Kobayashi, K., Katagiri, T., Kurokawa, K., Kurimura, M., Toyoshima, I., Niizato, K., Tsuchiya, K., Iwatsubo, T., Muramatsu, M., Matsumine, H., and Kato, T. (2006) J. Neurosci. 26 9227-9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inglis, K. J., Chereau, D., Brigham, E. F., Chiou, S. S., Schobel, S., Frigon, N. L., Yu, M., Caccavello, R. J., Nelson, S., Motter, R., Wright, S., Chian, D., Santiago, P., Soriano, F., Ramos, C., Powell, K., Goldstein, J. M., Babcock, M., Yednock, T., Bard, F., Basi, G. S., Sham, H., Chilcote, T. J., McConlogue, L., Griswold-Prenner, I., and Anderson, J. P. (2009) J. Biol. Chem. 284 2598-2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, E. J., Sung, J. Y., Lee, H. J., Rhim, H., Hasegawa, M., Iwatsubo, T., Min, d. S., Kim, J., Paik, S. R., and Chung, K. C. (2006) J. Biol. Chem. 281 33250-33257 [DOI] [PubMed] [Google Scholar]
- 31.Okochi, M., Walter, J., Koyama, A., Nakajo, S., Baba, M., Iwatsubo, T., Meijer, L., Kahle, P. J., and Haass, C. (2000) J. Biol. Chem. 275 390-397 [DOI] [PubMed] [Google Scholar]
- 32.Pronin, A. N., Morris, A. J., Surguchov, A., and Benovic, J. L. (2000) J. Biol. Chem. 275 26515-26522 [DOI] [PubMed] [Google Scholar]
- 33.Ishii, A., Nonaka, T., Taniguchi, S., Saito, T., Araic, T., Mann, D., Iwatsubo, T., Hisanaga, S., Goedert, M., and Hasegawa, M. (2007) FEBS Lett. 581 4711-4717 [DOI] [PubMed] [Google Scholar]
- 34.Jayasuriya, H., Koonchanok, N. M., Geahlen, R. L., McLaughlin, J. L., and Chang, C. J. (1992) J. Nat. Prod. 55 696-698 [DOI] [PubMed] [Google Scholar]
- 35.Pagano, M. A., Bain, J., Kazimierczuk, Z., Sarno, S., Ruzzene, M., Di, M. G., Elliott, M., Orzeszko, A., Cozza, G., Meggio, F., and Pinna, L. A. (2008) Biochem. J. 415 353-365 [DOI] [PubMed] [Google Scholar]
- 36.Rickert, P., Corden, J. L., and Lees, E. (1999) Oncogene 18 1093-1102 [DOI] [PubMed] [Google Scholar]
- 37.Lenart, P., Petronczki, M., Steegmaier, M., Di, F. B., Lipp, J. J., Hoffmann, M., Rettig, W. J., Kraut, N., and Peters, J. M. (2007) Curr. Biol. 17 304-315 [DOI] [PubMed] [Google Scholar]
- 38.Steegmaier, M., Hoffmann, M., Baum, A., Lenart, P., Petronczki, M., Krssak, M., Gurtler, U., Garin-Chesa, P., Lieb, S., Quant, J., Grauert, M., Adolf, G. R., Kraut, N., Peters, J. M., and Rettig, W. J. (2007) Curr. Biol. 17 316-322 [DOI] [PubMed] [Google Scholar]
- 39.Lindersson, E., Beedholm, R., Højrup, P., Moos, T., Gai, W. P., and Jensen, P. H. (2004) J. Biol. Chem. 279 12924-12934 [DOI] [PubMed] [Google Scholar]
- 40.Frid, P., Anisimov, S. V., and Popovic, N. (2007) Brain Res. Rev. 53 135-160 [DOI] [PubMed] [Google Scholar]
- 41.Kostka, M., Hogen, T., Danzer, K. M., Levin, J., Habeck, M., Wirth, A., Wagner, R., Glabe, C. G., Finger, S., Heinzelmann, U., Garidel, P., Duan, W., Ross, C. A., Kretzschmar, H., and Giese, A. (2008) J. Biol. Chem. 283 10992-11003 [DOI] [PubMed] [Google Scholar]
- 42.Zhu, M., Rajamani, S., Kaylor, J., Han, S., Zhou, F., and Fink, A. L. (2004) J. Biol. Chem. 279 26846-26857 [DOI] [PubMed] [Google Scholar]
- 43.Gille, G., Hung, S. T., Reichmann, H., and Rausch, W. D. (2004) Ann. N. Y. Acad. Sci. 1018 533-540 [DOI] [PubMed] [Google Scholar]
- 44.Oda, T., Kosuge, Y., Arakawa, M., Ishige, K., and Ito, Y. (2008) Neurosci. Res. 60 29-39 [DOI] [PubMed] [Google Scholar]
- 45.Troy, C. M., and Salvesen, G. S. (2002) J. Neurosci. Res. 69 145-150 [DOI] [PubMed] [Google Scholar]
- 46.Cookson, M. R., and van der Brug, M. (2008) Exp. Neurol. 209 5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLean, P. J., Klucken, J., Shin, Y., and Hyman, B. T. (2004) Biochem. Biophys. Res. Commun. 321 665-669 [DOI] [PubMed] [Google Scholar]
- 48.Engelender, S., Kaminsky, Z., Guo, X., Sharp, A. H., Amaravi, R. K., Kleiderlein, J. J., Margolis, R. L., Troncoso, J. C., Lanahan, A. A., Worley, P. F., Dawson, V. L., Dawson, T. M., and Ross, C. A. (1999) Nat. Genet. 22 110-114 [DOI] [PubMed] [Google Scholar]
- 49.Lehotzky, A., Tirian, L., Tokesi, N., Lenart, P., Szabo, B., Kovacs, J., and Ovadi, J. (2004) J. Cell Sci. 117 6249-6259 [DOI] [PubMed] [Google Scholar]
- 50.Goldbaum, O., Jensen, P. H., and Richter-Landsberg, C. (2008) Glia 56 1736-1746 [DOI] [PubMed] [Google Scholar]
- 51.Skjoerringe, T., Lundvig, D. M. S., Jensen, P. H., and Moos, T. (2006) J. Neurochem. 99 333-342 [DOI] [PubMed] [Google Scholar]
- 52.Hirsch, C., Gauss, R., and Sommer, T. (2006) Trends Cell Biol. 16 657-663 [DOI] [PubMed] [Google Scholar]
- 53.Gorbatyuk, O. S., Li, S., Sullivan, L. F., Chen, W., Kondrikova, G., Manfredsson, F. P., Mandel, R. J., and Muzyczka, N. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 763-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, W. W., Margolis, R. L., Li, X. J., Troncoso, J. C., Lee, M. K., Dawson, V. L., Dawson, T. M., Iwatsubo, T., and Ross, C. A. (2005) J. Neurosci. Res. 25 5544-5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugeno, N., Takeda, A., Hasegawa, T., Kobayashi, M., Kikuchi, A., Mori, F., Wakabayashi, K., and Itoyama, Y. (2008) J. Biol. Chem. 283 23179-23188 [DOI] [PubMed] [Google Scholar]
- 56.Paleologou, K. E., Schmid, A. W., Rospigliosi, C. C., Kim, H. Y., Lamberto, G. R., Fredenburg, R. A., Lansbury, P. T., Jr., Fernandez, C. O., Eliezer, D., Zweckstetter, M., and Lashuel, H. A. (2008) J. Biol. Chem. 283 16895-16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raaf, J., Brunstein, E., Issinger, O. G., and Niefind, K. (2008) Chem. Biol. 15 111-117 [DOI] [PubMed] [Google Scholar]
- 58.Sarno, S., Ruzzene, M., Frascella, P., Pagano, M. A., Meggio, F., Zambon, A., Mazzorana, M., Di, M. G., Lucchini, V., and Pinna, L. A. (2005) Mol. Cell. Biochem. 274 69-76 [DOI] [PubMed] [Google Scholar]
- 59.Feldman, J. D., Vician, L., Crispino, M., Tocco, G., Baudry, M., and Herschman, H. R. (1998) J. Neurosci. Res. 53 502-509 [DOI] [PubMed] [Google Scholar]
- 60.Konietzko, U., Kauselmann, G., Scafidi, J., Staubli, U., Mikkers, H., Berns, A., Schweizer, M., Waltereit, R., and Kuhl, D. (1999) EMBO J. 18 3359-3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu, X., Rottkamp, C. A., Raina, A. K., Brewer, G. J., Ghanbari, H. A., Boux, H., and Smith, M. A. (2000) Neurobiol. Aging 21 807-813 [DOI] [PubMed] [Google Scholar]
- 62.Omri, B., Crisanti, P., Marty, M. C., Alliot, F., Fagard, R., Molina, T., and Pessac, B. (1996) J. Neurochem. 67 1360-1364 [DOI] [PubMed] [Google Scholar]
- 63.Omri, B., Blancher, C., Neron, B., Marty, M. C., Rutin, J., Molina, T. J., Pessac, B., and Crisanti, P. (1998) Oncogene 16 2351-2356 [DOI] [PubMed] [Google Scholar]
- 64.Iwatsubo, T. (2007) Neuropathology 27 474-478 [DOI] [PubMed] [Google Scholar]
- 65.Yoshida, M. (2007) Neuropathology 27 484-493 [DOI] [PubMed] [Google Scholar]
- 66.Periquet, M., Fulga, T., Myllykangas, L., Schlossmacher, M. G., and Feany, M. B. (2007) J. Neurosci. 27 3338-3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee, H. J., Khoshaghideh, F., Patel, S., and Lee, S. J. (2004) J. Neurosci. 24 1888-1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu, J., Kao, S. Y., Lee, F. J., Song, W., Jin, L. W., and Yankner, B. A. (2002) Nat. Med. 8 600-606 [DOI] [PubMed] [Google Scholar]
- 69.Assayag, K., Yakunin, E., Loeb, V., Selkoe, D. J., and Sharon, R. (2007) Am. J. Pathol. 171 2000-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Outeiro, T. F., Putcha, P., Tetzlaff, J. E., Spoelgen, R., Koker, M., Carvalho, F., Hyman, B. T., and McLean, P. J. (2008) PLoS ONE 3 e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masuda, M., Suzuki, N., Taniguchi, S., Oikawa, T., Nonaka, T., Iwatsubo, T., Hisanaga, S., Goedert, M., and Hasegawa, M. (2006) Biochemistry 45 6085-6094 [DOI] [PubMed] [Google Scholar]
- 72.Klucken, J., Shin, Y., Masliah, E., Hyman, B. T., and McLean, P. J. (2004) J. Biol. Chem. 279 25497-25502 [DOI] [PubMed] [Google Scholar]
- 73.Auluck, P. K., Meulener, M. C., and Bonini, N. M. (2005) J. Biol. Chem. 280 2873-2878 [DOI] [PubMed] [Google Scholar]
- 74.Jellinger, K. A., and Stadelmann, C. (2000) J. Neural Transm. Suppl. 59 95-114 [DOI] [PubMed] [Google Scholar]
- 75.Smith, W. W., Jiang, H. B., Pei, Z., Tanaka, Y., Morita, H., Sawa, A., Dawson, V. L., Dawson, T. M., and Ross, C. A. (2005) Hum. Mol. Genet. 14 3801-3811 [DOI] [PubMed] [Google Scholar]
- 76.Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H., and Ron, D. (2000) Mol. Cell 5 897-904 [DOI] [PubMed] [Google Scholar]
- 77.Lee, V. M., and Trojanowski, J. Q. (2006) Neuron 52 33-38 [DOI] [PubMed] [Google Scholar]
- 78.Volles, M. J., and Lansbury, P. T., Jr. (2003) Biochemistry 42 7871-7878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]