Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry (original) (raw)
. Author manuscript; available in PMC: 2009 Apr 17.
Published in final edited form as: J Immunol Methods. 2007 Aug 8;327(1-2):63–74. doi: 10.1016/j.jim.2007.07.011
Abstract
Traditionally, the identification and quantification of eosinophils in inflammatory tissues and exudates has been primarily based upon morphologic criteria and manual counting. In this study, we describe a new flow cytometry-based assay to enumerate eosinophils present in murine bronchoalveolar lavage fluid (BAL) and lung parenchyma obtained from the normal/non-inflamed respiratory tract, following experimentally-induced allergic pulmonary inflammation, and during experimental infection with respiratory syncytial virus (RSV). By using a murine Siglec-F-specific antibody in combination with antibodies directed to CD45 and CD11c, we demonstrate that eosinophils can be distinguished from other cell types in the BAL fluid and lung parenchyma based upon their distinct CD45+ Siglec-F+ and CD11clow/− staining profile. In the BAL fluid, this flow cytometry-based method of eosinophil identification/quantitation yields results comparable to the standard morphology-based method without the potential observer bias or staining artifacts inherent in morphology-based quantitation. Furthermore, this flow cytometry-based method can be directly adapted to enumerate eosinophils infiltrating the inflamed lung parenchyma, thereby obviating the need for quantitative morphometry of tissue sections.
Keywords: Eosinophils, Detection, Quantitation, Siglec-F, Flow Cytometry
1. Introduction
Eosinophils are multifunctional granulocytes derived from myeloid progenitor cells in the bone marrow. Eosinophils likely play a beneficial role in host defenses against parasitic infections in the human (Klion and Nutman 2004), but have also been implicated in the development and enhancement of inflammation and injury in allergic diseases (Rothenberg and Hogan 2006). Eosinophils secrete a variety of granule-associated pro-inflammatory cytotoxic proteins including major basic protein, eosinophil cationic protein, and eosinophil peroxidase all of which can contribute to airway damage and alterations in airway structure (MacKenzie et al. 2001), (Humbles et al. 2004).
Allergic diseases of the respiratory tract are characterized by these increased accumulations of eosinophils in inflammatory infiltrates. Therefore, this granulocyte subset not only contributes to the pathogenesis of disease, but also serves as a marker for the development and expression of allergic inflammation during the type II (CD4+ Th2) T cell responses to antigen. Eosinophil quantitation is a standard procedure in the analysis of inflammation and type II responses in murine models of allergic airway sensitization (e.g. chicken egg albumin administration), and in murine models of respiratory virus infection where type II CD4+ T cell responses play a prominent role [e.g. challenge Respiratory Syncytial Virus (RSV) infection of immune mice (Openshaw et al. 1992), (Srikiatkhachorn and Braciale 1997), (Johnson et al. 1998), (Alwan et al. 1994), (Varga et al. 2000), (Varga et al. 2001)].
In these and other murine models of pulmonary eosinophilia, the detection and quantitation of eosinophils in fluids such as bronchoalveolar lavage (BAL) fluid or in lung tissue has primarily been based on morphologic criteria (i.e. the identification and enumeration of stained granulocytes with prominent eosinophilic granules). This morphologic approach has several drawbacks -- including variability in the staining of this leukocyte subset in BAL fluid or tissue, errors in inflammatory cell distribution inherent in quantitative morphometry based on sampling of lung tissue from several different sites, and reliance on the subjective interpretation of cell morphology in the quantitation of inflammatory cells.
Until recently, the application of more rigorous and quantitative techniques (e.g. flow cytometry) to identify and quantitate eosinophils has been hampered by the fact that these granulocytes expressed multiple cell surface markers (e.g. CD45, CD11b, etc.) that are shared by other cells of hematopoietic origin normally present in inflammatory infiltrates -- particularly in the respiratory tract (e.g. alveolar macrophages, neutrophils, etc.). Recently, a member of the sialic acid binding immunoglobulin-like lectin (Siglec) family (Varki and Angata 2006), Siglec-F, has been demonstrated to be displayed on the surface of murine eosinophils (Aizawa et al. 2003) (Zhang et al. 2004).
In this report, we describe a flow cytometry-based method for identifying and quantitating eosinophils based on the selective expression of Siglec-F (and differential display of other cell markers) by eosinophils. We demonstrate the application of this method for eosinophil enumeration in both the BAL fluid and lung tissue from mice undergoing experimental allergic inflammation and respiratory virus infection. This method provides a simple and rapid technique for quantitating eosinophils free of sampling bias and variability inherent in morphologic quantitation, particularly in tissues.
2. Methods
2.1 Mice
Female BALB/c AnNTac (H-2d) mice aged 12 weeks used for all experiments were purchased from Taconic Farms (Germantown, NY) and housed in a specific pathogen-free environment. All animal experiments were performed in accordance with protocols approved by the University of Virginia Animal Care and Use Committee.
2.2 OVA sensitization and challenge
The protocol followed was as previously described (Delayre-Orthez et al. 2004). Briefly, mice were sensitized by intraperitoneal injections of 50 μg chicken egg ovalbumin (OVA, Grade VI, Sigma, St. Louis, MO) adsorbed with 2 mg aluminum hydroxide (Sigma, St. Louis, MO) in sterile PBS on days 1 and 7. On days 18, 19, 20, and 21 after the initial sensitization, mice were anesthetized with Halothane (Halocarbon Laboratories, Rivers Edge, NJ) and then intranasally challenged with 10 μg OVA in sterile PBS. At day 22, mice were sacrificed and BAL fluid and tissue samples were collected.
2.3 Viruses and Infection of Mice
The recombinant vaccina virus expressing the G protein of RSV (vvG) was a gift from J. L. Beeler, Food and Drug Administration, National Institutes of Health. The A2 strain of RSV (a gift of P.L. Collins, National Institute of Allergy and Infectious Disease, National Institutes of Health) was grown in HEp-2 cells (American Type Culture Collection, Manassas, VA) and titered for infectivity. Groups of four mice were vaccinated with 3×106 PFU vvG in a 10μL volume via scarification at the base of the tail with a 25G needle. After a minimum of three weeks, these mice were anesthetized with Halothane (Halocarbon Laboratories, Rivers Edge, NJ) and then intranasally challenged with 2.16 x106 PFU RSV in a 50μL volume. At various times post-RSV infection, mice were sacrificed and BAL fluid and lung samples were collected for further analyses.
2.4 Preparation of Lung Mononuclear Cell Suspensions
The lungs were flushed via the right ventricle with approximately 4mL of 1× PBS with 10 U/mL Heparin (Sigma, St. Louis, MO) to remove blood. The lungs were then removed aseptically and separated away from the heart, thymus, and bronchial lymph nodes. Lungs were minced and then digested in 183U/mL collagenase (Sigma, St. Louis, MO) in RPMI media (GIBCO BRL, Gaithersburg, MD) containing 10U/mL penicillin (Gibco BRL, Gaithersburg, MD) and 10μg/mL streptomycin (Gibco BRL, Gaithersburg, MD) for 30 minutes at 37°C. Afterwards, the minced lung tissue was passed through a wire screen followed by a quick centrifugation at 440 × g to remove particulates. Residual red blood cells were lysed using ammonium chloride. Lung mononuclear cell suspensions were counted using a hemacytometer.
2.5 Preparation of BAL Cell Suspensions
Mice were sacrificed and an incision was made in the skin over the larynx. A syringe was inserted at the incision site and three 1mL aliquots of complete media were flushed through the lungs. BAL mononuclear cell suspensions recovered were counted using a hemacytometer.
2.6 Automated Cell Separation
Single cell lung suspensions were positively or negatively selected for expression of CD11c or Siglec-F using the AutoMACS Separator (Miltenyi Biotec GmbH, Auburn, CA) according to manufacturer’s protocol. To separate Siglec-F+ CD11c− cells, single cell lung suspensions were first negatively selected for CD11c using anti-CD11c microbeads (N418, Miltenyi Biotec GmbH, Auburn, CA). Next, the recovered CD11c− population was incubated with PE conjugated anti-Siglec-F antibody (E50-2440, BD Pharmingen, San Diego, CA), followed by anti-PE microbeads (Miltenyi Biotec GmbH, Auburn, CA) and then positive selection to yield CD11c− Siglec-F+ cells.
To separate Siglec-F+ CD11c+ cells, single cell lung suspensions were first incubated with a FITC conjugated anti-CD11c antibody (HL3, BD Pharmingen, San Diego, CA) followed by anti-FITC microbeads (Miltenyi Biotec GmbH, Auburn, CA). Following positive selection, the anti-FITC microbeads were removed from the CD11c+ population using the MultiSort kit (Miltenyi Biotec GmbH, Auburn, CA) in accordance with the manufacturer’s protocol. Next, the recovered CD11c+ population was incubated with PE conjugated anti-Siglec-F antibody (E50-2440, BD Pharmingen, San Diego, CA), followed by anti-PE microbeads (Miltenyi Biotec GmbH, Auburn, CA) resulting, after positive selection, in the enrichment of CD11c+ Siglec-F+ cells.
2.7 Differential Cell Counts
250μL of a single cell suspension of washed and resuspended BAL fluid was placed on a microscope slide and cytospun (Cytospin2, Shandon, Pittsburgh, PA) at 750rpm for 5 minutes at room temperature and then stained using Diff-Quik (Baxter Healthcare, Miami, FL) according to manufacturer’s recommendation. A differential cell count was performed on 300–400 cells in at least five different fields based on standard morphological criteria and staining properties at 40X magnification. Counts were completed in duplicate by two independent counters with the average of all counts shown. Cells with epithelial cell or fibroblast morphology were excluded to obtain total leukocyte counts in BAL fluid for eosinophil percentage estimates in Table 1.
Table 1.
Comparison of the morphologic and flow cytometric methods for eosinophil quantitation in the BAL fluid.
| Sample | Morphologic Counta(% leukocytes) | Flow Cytometryb(% CD45+) |
|---|---|---|
| 1. Day 5 vvG/RSV | 2.30 | 2.84 |
| 2. Day 5 vvG/RSV | 2.97 | 2.77 |
| 3. Day 5 vvG/RSV | 3.48 | 3.62 |
| 4. Day 5 vvG/RSV | 2.99 | 3.54 |
| Mean | 2.94 ± 0.24c | 3.20 ± 0.22c |
| 5. Day 7 vvG/RSV | 7.16 | 8.34 |
| 6. Day 7 vvG/RSV | 7.45 | 7.81 |
| 7. OVA-sensitized | 57.8 | 62.80 |
| 8. OVA-sensitized | 50.1 | 53.00 |
| 9. Normal/Non-inflamed | NDd | 0.25 |
| 10. Normal/Non-inflamed | NDd | 0.15 |
2.8 Flow Cytometric Analysis
For multicolor FACS analysis, approximately 2×106 cells from harvested lung or BAL fluid cell suspensions were incubated for 5 minutes with Fc receptor (2.4G2) blocking agent in 100μL of FACS buffer [1X PBS supplemented with 2% heat-inactivated fetal calf serum (Atlanta Biologicals, Norcross GA) and 0.02% NaN3] to prevent nonspecific binding. Next, specific monoclonal antibodies (mAbs) were directly added to the samples and incubated for 45 minutes in the dark at 4°C. Antibodies used were at a final concentration of 1μg/100μL and were PE-conjugated anti-Siglec-F (E50-2440), PerCP-Cy5.5 conjugated CD45 (30-F11), FITC-conjugated CD11c (HL3), and APC-conjugated CD11b (M1/70). All mAbs were purchased from BD Pharmingen (San Diego, CA). Stained cells were fixed and lysed with FACS lysing solution (Becton Dickinson, San Jose, CA) then washed and resuspended in FACS buffer. Stained cell suspensions were analyzed using a Becton Dickinson FACScalibur flow cytometer (Mountain View, CA) and FlowJo software (TreeStar).
3. Results
3.1 Identification of Siglec-F expressing cells in the normal and inflamed murine respiratory tract
Eosinophils are typically identified and quantified in tissues and organs like the murine respiratory tract based on morphological and staining criteria. In most solid organs, eosinophil numbers are enumerated by quantitative tissue morphometry while, in the murine lungs, the development of pulmonary eosinophilia can also be determined by differential counts of cells obtained from the bronchoalveolar lavage (BAL) fluid. Since Siglec-F has been demonstrated to be expressed by murine eosinophils (Aizawa et al. 2003) (Zhang et al. 2004), we examined the usefulness of Siglec-F expression as a marker in the identification and quantitation of eosinophils in the normal and inflamed murine lungs.
For this analysis, and as a ready source of eosinophils, we obtained total lung cell suspensions and BAL fluid from mice undergoing experimentally induced allergic pulmonary inflammation using the standard OVA allergen sensitization and re-challenge model (Delayre-Orthez et al. 2004). In parallel, we also obtained total lung suspensions and BAL fluid from normal/non-inflamed mice. These cell suspensions of lung parenchyma and of BAL fluid were then stained with antibodies to Siglec-F and other cell surface markers and subjected to flow cytometric analysis.
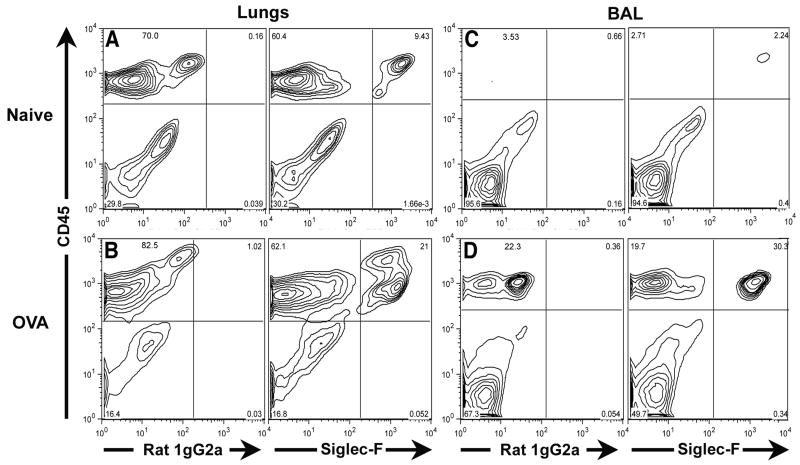
In the initial analysis, we stained total lung cell suspensions with antibodies to the leukocyte common antigen CD45 and to Siglec-F. As shown in Figure 1A (for normal/non-inflamed lung cells) and 1B (for allergen inflamed lung cells), Siglec-F expression was restricted to CD45+ lung cells (i.e. cells of hematopoietic origin). The antibody to Siglec-F bound to the CD45+ cells with a fluorescent intensity that was well above the background staining of the Siglec-F isotype control antibody. Analysis of BAL fluid from the normal and allergic lungs likewise demonstrated Siglec-F expression only by cells of hematopoietic origin (Figure 1 C, D). Siglec-F+ cells in both the normal/non-inflamed and inflamed lungs represented a distinct population of CD45+ cells. The finding (Figure 1A, C) that Siglec-F+ cells were present in the normal/non-inflamed lung parenchyma and BAL fluid (which express minimal numbers of eosinophils by standard morphologic criteria) indicated that at least one other cell type of hematopoietic origin expresses Siglec-F.
Figure 1. Identification of Siglec-F expressing cells in the lungs and BAL fluid of normal/non-inflamed and of OVA challenged and sensitized mice.
Single cell suspensions were prepared from the lungs (Figure 1A) and BAL fluid (Figure 1C) of normal/non-inflamed mice or from the lungs (Figure 1B) and BAL fluid (Figure 1D) of mice sensitized and challenged with OVA. The expression profiles of CD45 and Siglec-F (or the relevant isotype control, rat IgG2a) in these populations were subsequently analyzed by flow cytometry. Data shown is representative of at least three independent experiments.
3.2 Differential expression of CD11c distinguishes Siglec-F+ eosinophils and pulmonary macrophages
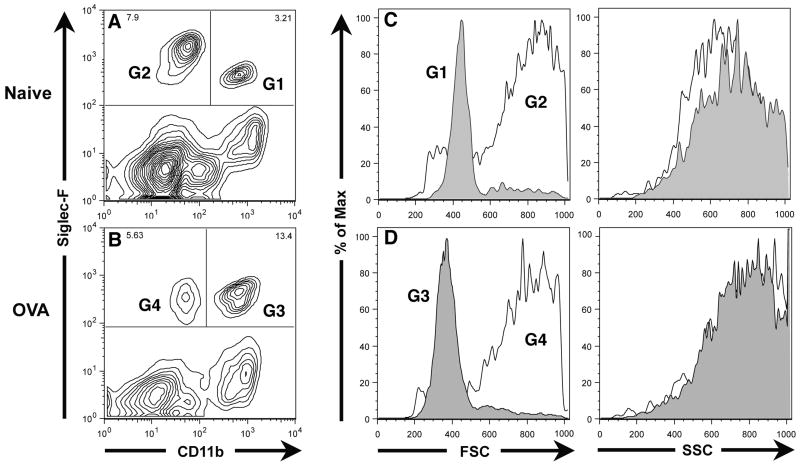
To better define the CD45+ Siglec-F+ cell types in the normal and inflamed respiratory tract, we surveyed the CD45+ Siglec-F+ cells in total lung cell suspensions for expression of other cell surface markers. One such useful marker was the integrin CD11b which is displayed by murine neutrophils and monocytes/macrophages. As shown in Figure 2, staining of CD45+ lung parenchyma cells for the expression of CD11b and Siglec-F in the normal/non-inflamed (Figure 2A) and inflamed/allergic (Figure 2B) lungs revealed two distinct populations of CD45+ Siglec-F+ cells. One of these populations had little or no CD11b expression above the background observed with the isotype control antibody and is represented by gate G2 in the normal/non-inflamed lung (Figure 2A, gate G2) and by gate G4 in the allergic/inflamed lungs (Figure 2B, gate G4). There was also a distinct population of Siglec-F+ CD11bhi cells in both the normal/non-inflamed (Figure 2A, gate G1) and in the allergic/inflamed lungs (Figure 2B, gate G3). These Siglec-F+ CD11bhi cells represented a minor fraction of the total Siglec-F+ cells in the normal/non-inflamed lung (i.e. approximately 30%) (Figure 2A), but were the major subset of the Siglec-F+ cells (i.e. approximately 70%) detected in the inflamed/allergic lungs exhibiting pulmonary eosinophilia (Figure 2B). Because of their marked enrichment in the lung parenchyma of mice undergoing allergic pulmonary inflammation, the Siglec-F+ CD11bhi cells in the normal/non-inflamed lung parenchyma (Figure 2A, gate G1) and in the inflamed lungs (Figure 2B, gate G3) likely represented eosinophils. The Siglec-F+ CD11blow/− cells were the dominant Siglec-F+ cell population in the normal/non-inflamed lung parenchyma (Figure 2A, gate G2) and were also represented in the parenchyma of the inflamed allergic lungs (Figure 2B, gate G4).
Figure 2. Identification of Siglec-F and CD11b expressing cells in the lungs of normal/non-inflamed and of OVA challenged and sensitized mice.
Single cell suspensions prepared from lungs harvested from either normal/non-inflamed mice (Figure 2A, C) or mice sensitized and challenged with OVA (Figure 2B, D) were gated on the CD45+ population and then analyzed for Siglec-F and CD11b expression as well as for forward and side scatter properties. Data shown is representative of at least three independent experiments.
It is noteworthy that the Siglec-F+ CD11blow/− cell population in both the non-inflamed (Figure 2A, gate G2) and inflamed (Figure 2B, gate G4) lung parenchyma appear to have high auto-fluorescence -- as the background staining for the CD11b marker using the isotype control antibody was comparable to the specific antibody (data not shown). This high auto-fluorescence is characteristic of alveolar macrophages. When the forward and side scatter properties of this Siglec-F+ CD11blow/− cell population from the normal/non-inflamed (gate G2 analyzed in Figure 2C) and the inflamed (gate G4 analyzed in Figure 2D) lung parenchyma were analyzed, the broad forward scatter and complex side scatter properties of these cells were characteristic of alveolar macrophages. By contrast, the Siglec-F+ CD11bhi cells (gates G1 and G3 analyzed in Figure 2C and 2D respectively) exhibited more uniform forward scatter but equally complex side scatter profile consistent with that of eosinophils.
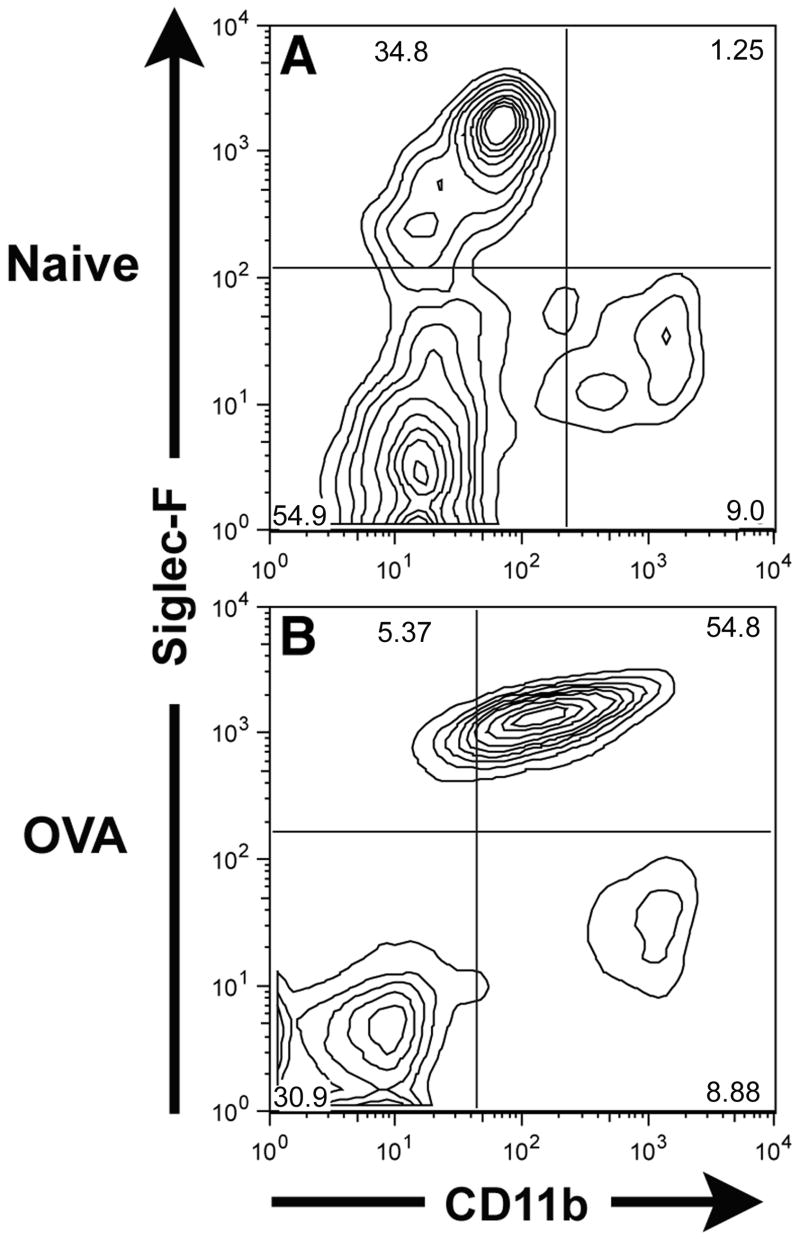
When CD11b expression was examined on CD45+ Siglec-F+ cells harvested from normal/non-inflamed BAL fluid, only the Siglec-F+ CD11blow/− population was observed (Figure 3A). This would be expected if the cells were alveolar macrophages, as this cell type is the dominant cell population in the BAL fluid from the normal murine respiratory tract. The absence of a detectable Siglec-F+ CD11b+ population (characteristic of eosinophils) most likely reflects low levels of eosinophils present in the BAL fluid of normal/non-inflamed mice. Unexpectedly, we observed in the BAL fluid from the allergic/inflamed lungs that the Siglec-F+ cells could not be distinguished into subsets based on CD11b expression (Figure 3B). While this result could mean there was only a single population of Siglec-F+ cells, it is more likely that the level of CD11b expression is altered on one or both of these Siglec-F+ BAL fluid cell populations in response to inflammation. Thus, while CD11b expression may be a useful marker to distinguish Siglec-F+ subsets in the absence of inflammation, it may not be an effective marker to distinguish between these two populations in the inflamed respiratory tract. We therefore further determined if another marker also expressed on alveolar macrophages could be used to discriminate Siglec-F+ cell subpopulations particularly in the inflamed lungs.
Figure 3. Identification of Siglec-F and CD11b expressing cells in the BAL fluid of normal/non-inflamed and of OVA challenged and sensitized mice.
Single cell suspensions prepared from BAL fluid harvested from either normal/non-inflamed mice (Figure 3A) or mice sensitized and challenged with OVA (Figure 3B) were gated on the CD45+ population and then analyzed for Siglec-F and CD11b expression. Data shown is representative of at least three independent experiments.
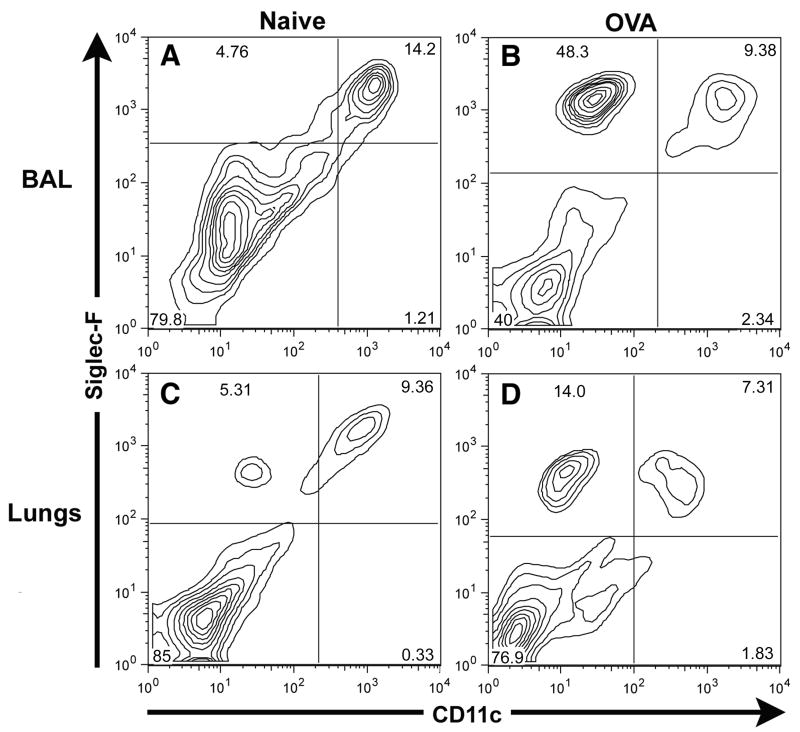
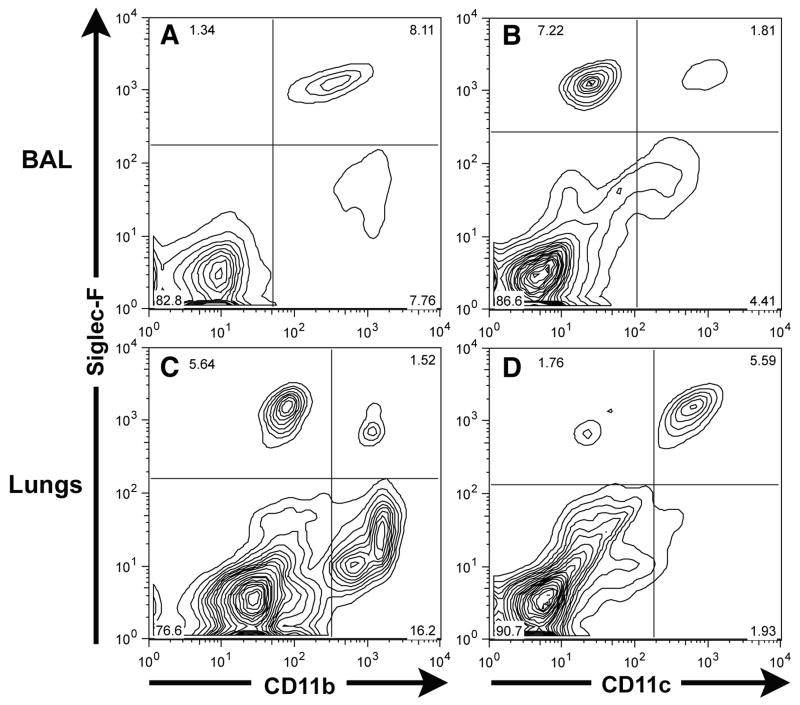
The □2 integrin, CD11c, is expressed on murine dendritic cells and is prominently expressed by murine alveolar macrophages. When we analyzed CD45+ Siglec-F+ cells in the BAL fluid from normal and inflamed lungs, we detected a predominant BAL fluid population of Siglec-F+ CD11c+ cells present in normal/non-inflamed lungs (Figure 4A) – the expected phenotype of alveolar macrophages. In contrast, while there was likewise a distinct population of Siglec-F+ CD11c+ cells in the BAL fluid taken from allergic mice, there was a markedly increased population of Siglec-F+ CD11clow/− cells in the allergic BAL fluid which represented the majority of Siglec-F+ cells in the BAL fluid from the allergic/inflamed respiratory tract (Figure 4B). These Siglec-F+ CD11clow/− cells had the same forward and side scatter properties as the Siglec-F+ CD11b+ lung parenchymal cells described above (Figure 2, gates G1, G3) and hence had the expected scatter properties of eosinophils. The CD11c marker could also effectively distinguish two distinct subsets of Siglec-F+ cells in the normal (Figure 4C) and allergic (Figure 4D) lung parenchyma. Thus, CD11c expression by Siglec-F+ cells in both the BAL fluid and lung parenchyma provided a more reliable means to discriminate eosinophil and macrophage populations than CD11b.
Figure 4. CD11c can distinguish between the Siglec-F+ eosinophil and alveolar macrophage populations in BAL fluid and lung parenchyma of normal/non-inflamed and of OVA challenged and sensitized mice.
Single cell suspensions of BAL fluid from normal/non-inflamed Mice (Figure 4A), of BAL fluid from mice sensitized and challenged with OVA (Figure 4B), of lung parenchyma from normal/non-inflamed mice (Figure 4C), and of lungs from mice sensitized and challenged with OVA (Figure 4D) were gated on the CD45+ population and then analyzed for Siglec-F and CD11c expression. Data shown is representative of at least three independent experiments.
3.3 Morphologic identification of Siglec-F+ pulmonary cell populations
The above results suggested that there are two distinct subsets of Siglec-F+ cells in the murine respiratory tract: CD11clow/− Siglec-F+ CD45+ cells likely representing eosinophils and CD11c+ Siglec-F+ CD45+ cells likely representing alveolar/pulmonary macrophages. To directly examine the relationship between these cell surface phenotypes and eosinophil and alveolar/pulmonary macrophage morphology, we employed a two-step magnetic bead-based separation method for isolating these two cell types in cell suspensions obtained from either normal/non-inflamed or allergic lungs (see Materials and Methods). Briefly, (see Figure 5A) total lung cell suspensions were separated into CD11c+ and CD11c− populations. Then, each of these populations was selected for Siglec-F expression yielding a CD11c+ Siglec-F+ and CD11c− Siglec-F+ population. The purity of these two populations after magnetic bead separation was typically greater than 95% as measured by flow cytometry. Single cell suspensions of these two populations were subjected to cytospin and Wright-Giemsa stain. As Figure 5B demonstrates, the CD11c+ Siglec-F+ population had the characteristic morphologic features of pulmonary/alveolar macrophages including numerous intracellular vesicles and a large cytoplasm to nucleus ratio. By contrast, the CD11c− Siglec-F+ population (Figure 5C) exhibited the multi-lobed nuclei and eosinophilic granules characteristic of eosinophils. Analysis of the forward and side scatter properties of these two cell populations (Figure 5D) demonstrates the complex forward and side scatter properties of the Siglec-F+ CD11c+ cells characteristic of alveolar macrophages (solid line) and the uniform forward scatter and complex side scatter of the Siglec-F+ CD11c− cells characteristic of eosinophils (shaded).
Figure 5. Morphologic examination of lung derived Siglec-F+ cells.
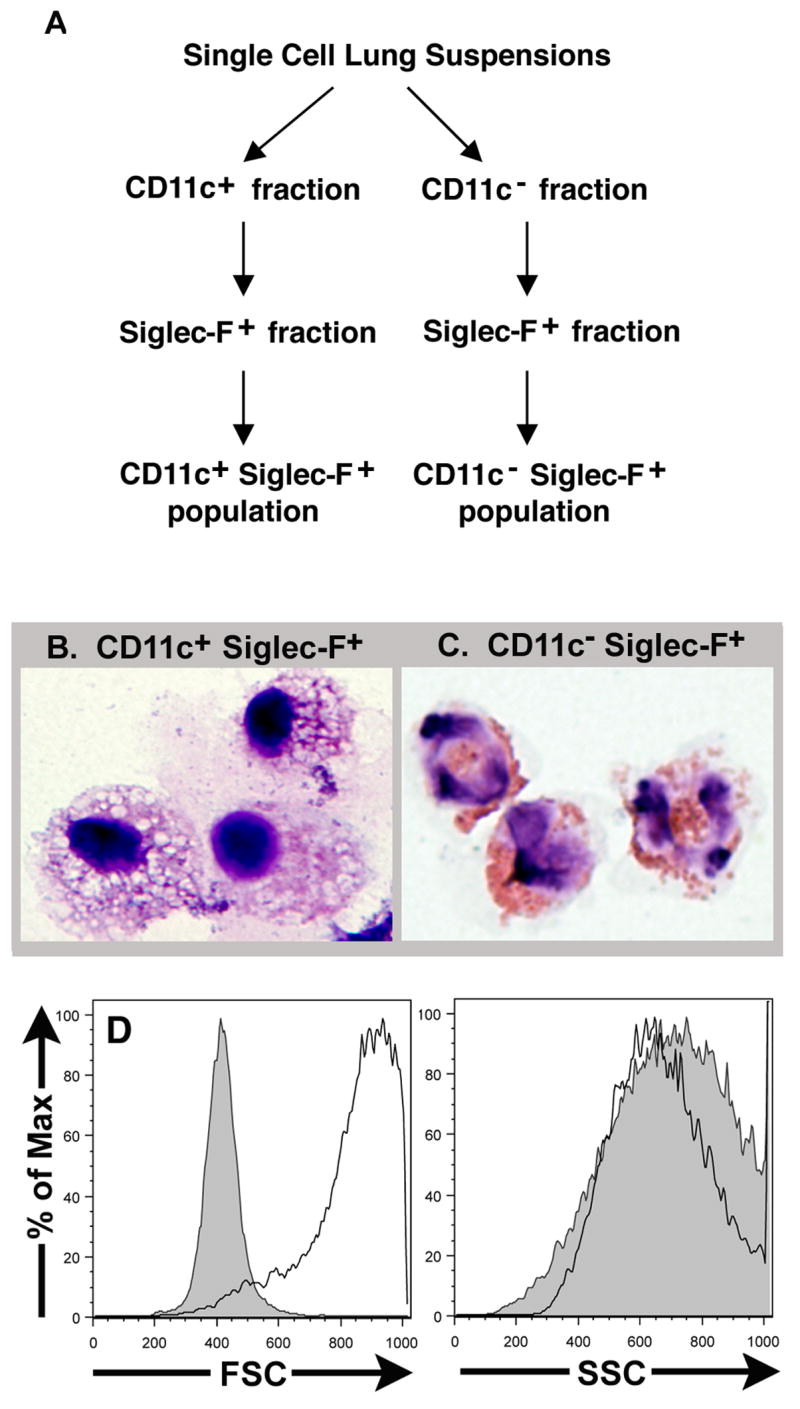
Single cell suspensions prepared from the lungs of normal/non-inflamed and OVA sensitized and challenged mice were first separated into CD11c+ and CD11c− fractions and then into Siglec-F+ fractions using a microbead based separation assay (Figure 5A). The CD11c+ Siglec-F+ fraction isolated from normal/non-inflamed lungs (Figure 5B) and CD11c− Siglec-F+ fraction isolated from allergic lungs (Figure 5C) were subject to cytospin and Wright Geimsa staining. The forward and side scatter properties of the CD11c+ Siglec-F+ (solid line) and the CD11c− Siglec-F+ (shaded) populations were analyzed using flow cytometry (Figure 5D).
3.4 Identification of CD11clow/− Siglec-F+ eosinophils in the respiratory tract during experimental respiratory virus infection
To further evaluate the utility of this cytofluorometric-based approach to identify and quantify eosinophils in the respiratory tract, we applied this method to the analysis of pulmonary eosinophilia in a model of enhanced pulmonary inflammation/injury induced during challenge infection with RSV. In this model, mice previously immunized with a recombinant vaccinia virus expressing the gene encoding the RSV-G glycoprotein will, upon challenge intranasal RSV infection, develop a CD4+ T cell Th2 type response in the lungs characterized by elevated levels of eosinophils in the BAL fluid and lung parenchyma (Openshaw et al. 1992), (Alwan et al. 1994), (Varga et al. 2000).
For this analysis, mice were immunized with the RSV-G gene expressing vaccinia virus vector and, three or more weeks later, the mice underwent challenge intranasal infection with RSV. Seven days post RSV infection, BAL fluid and lungs were harvested and cell suspensions were prepared from total lung tissue. Cells were stained with antibodies to CD45, CD11b, CD11c and Siglec-F. Cells present in the BAL fluid and in samples of lung cell suspensions were simultaneously examined for expression of CD45, Siglec-F, and either CD11b or CD11c. As Figure 6A demonstrates, and as likewise observed in the BAL fluid from allergic inflamed lungs (Figure 3B), the CD11b maker does not distinguish subsets of CD45+ Siglec-F+ cells in the BAL fluid of RSV infected mice. In contrast, staining of CD45+ Siglec-F+ BAL fluid cells with CD11c revealed the two distinct populations of Siglec-F+ cells, the major population of Siglec-F+ CD11clow/− eosinophils and the Siglec-F+ CD11c+ population of alveolar macrophages (Figure 6B). However, lung cell suspensions from the RSV-inflamed lung parenchyma when examined at day seven post-infection for Siglec-F expression in combination with either CD11b (Figure 6C) or CD11c (Figure 6D) did contain two distinct Siglec-F+ cell types.
Figure 6. Discrimination of Siglec-F+ populations using CD11b and CD11c in a murine model of RSV vaccine-enhanced disease.
Single cell suspensions generated from the BAL fluid (Figures 6A, 6B) and lungs (Figures 6C, 6D) harvested on day 7 following RSV infection in RSV G-primed mice were gated on the CD45+ population. Siglec-F and CD11b expression as well as Siglec-F and CD11c expression was analyzed. Data shown is representative of at least three independent experiments.
3.5 Quantitation of pulmonary eosinophils by morphologic and flow cytometric based methods
Our analysis of surface marker expression by cells isolated from the normal/non-inflamed and inflamed/infected BAL fluid and lung tissue suggested that eosinophils present in these sites can be readily identified based on flow cytometric criteria (i.e. eosinophils are CD45+ Siglec-F+ CD11clow/−). To further establish the utility of this method in quantitating eosinophil content in BAL fluid, we compared the determination of the percentage of eosinophils in BAL fluid exudates from chicken OVA allergen sensitized mice and RSV G-primed, RSV-challenged infected mice using the flow cytometry-based method with using the standard differential count of stained BAL fluid cells in cytospin preparations. Percentages of eosinophils were determined by the morphologic method as the percentage of eosinophils among total leukocytes in BAL fluid cell preparations. In parallel, the percentages of eosinophils were determined by the flow cytometry-based assay as the percentage of eosinophils among the total CD45+ cells in stained BAL fluid cell preparations.
Table 1 shows the results of this analysis for 10 independent samples of BAL fluid including four samples harvested from RSV G-primed RSV-challenged mice five days post-RSV infection (Table 1, samples 1–4), two BAL fluid samples harvested from RSV G-primed RSV-challenged mice seven days post-RSV infection (Table 1, samples 5–6) when higher levels of pulmonary eosinophilia are achieved, and two BAL fluid samples harvested from OVA sensitized and challenged mice (Table 1, samples 7–8). As controls, BAL fluid samples harvested from the lungs of normal/non-inflamed mice were included (Table 1, samples 9–10).
As Table 1 demonstrates, the percentage of eosinophils in the BAL fluid as determined by flow cytometry directly paralleled and closely correlated with the percentages enumerated by morphology under all experimental conditions. As expected, there was marked eosinophilia in the BAL fluid of mice experiencing ovalbumin induced allergic airway inflammation (Table 1, samples 7–8) as well as increased levels of pulmonary eosinophilia in RSV G-primed mice at day 7 (Table 1, samples 5–6) compared to day 5 (Table 1, samples 1–4) by either method. Also noteworthy is the fact that again, as expected, no eosinophils were detected by morphology in the BAL fluid of normal/non-inflamed mice and only a negligible number of eosinophils was detected in the BAL fluid of these mice by the more sensitive flow cytometry-based method (Table 1, samples 9–10).
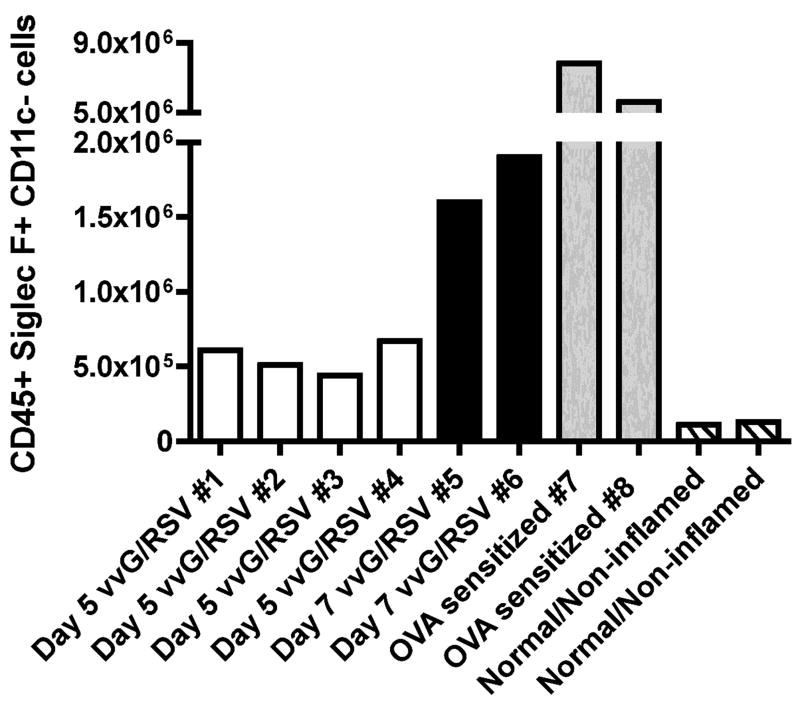
In parallel with the above analysis, we also used the flow cytometry-based approach to determine the total number of eosinophils present in the CD45+ fraction of total lung cell suspensions. Again, ten independent cell suspensions were generated from lung parenchyma harvested from four RSV G-primed RSV-challenged mice five days post-RSV infection (Figure 7, samples 1–4), two RSV G-primed RSV-challenged mice seven days post-RSV infection (Figure 7, samples 5–6), and two OVA sensitized and challenged mice (Figure 7, samples 7–8). As a control, we included lung parenchyma samples harvested from normal/non-inflamed mice (Figure 7, samples 9–10). The number of eosinophils present in the lung parenchyma was calculated by multiplying the percentage of Siglec-F+ CD11clow/− eosinophils observed in the CD45+ cell population by flow cytometry with the total number of cells counted in the lung cell suspension using a hemacytometer.
Figure 7. The quantification of eosinophils in whole lung tissue using the flow cytometric-based assay.
Single cell lung suspensions were generated from lung parenchyma harvested from RSV G-primed mice infected with RSV for 5 days (samples 1–4, white bars), from RSV G-primed mice infected with RSV for 7 days (samples 5–6, black bars), from OVA challenged and sensitized mice (samples 7–8, grey bars), and from normal/non-inflamed mice (samples 9–10, striped bars). Surface expression of CD45, Siglec-F, and CD11c was analyzed by flow cytometry. The number of eosinophils indicated was calculated by multiplying the percentage of Siglec-F+ CD11clow/− CD45+ eosinophils by the total number of cells counted in the lung suspension.
As shown in Figure 7, the total numbers of eosinophils present in the lung parenchyma largely paralleled the corresponding flow based-data from the BAL fluid. The greatest number of eosinophils detected was again observed in the lung parenchyma of mice experiencing ovalbumin induced allergic airway inflammation (Figure 7, samples 7–8). RSV-G primed mice infected with RSV for 7 days (Figure 7, samples 5–6) likewise exhibited greater numbers of pulmonary eosinophils than RSV-G primed mice infected with RSV for 5 days (Figure 7, samples 1–4). There is also evidence for a low-level of eosinophil detection in the parenchyma of the normal/non-inflamed mice in spite of the paucity of the eosinophils detected in the BAL fluid. These results suggest that the flow cytometry-based approach described here can also be used as a rapid and sensitive method to identify and quantitate eosinophils in lung parenchyma.
4. Discussion
The development and regulation of a CD4+ T cell Th2 type immune response has been extensively investigated in various murine models of allergic disease and experimental respiratory infection. Studies have shown that CD4+ T cells polarized toward a Th2 type phenotype secrete cytokines (e.g. IL-4 and IL-5) which serve as potent eosinophil chemoattractants (Lampinen et al. 2004). As a result, eosinophils constitute a prominent cell type observed during a Th2 type response and the presence of an eosinophil infiltrate at the site of inflammation is one of the most frequently used indicators for the development of such an immune response.
Traditionally, the detection and quantitation of eosinophils at sites of allergic inflammation in tissues has relied on histology (i.e. quantitative morphometric analyses of the stained tissue sections). This process can be laborious and requires adequate sampling -- that is, it requires a sufficient number of tissue sections from different areas to reflect the extent of the eosinophil infiltration in the entire tissue. Identification and assessment of eosinophil numbers in inflammatory exudates, such as the BAL fluid, is feasible only in a limited number of sites, and is similarly complex, like tissue analysis relying on the morphology of stained cells to identify and quantitate eosinophils. Quantitation of eosinophils by manual count in either whole tissues or inflammatory exudates can be further complicated by subjective interpretation of cell morphology and by artifacts acquired during the cell preparation, tissue fixation, or the staining process.
Because of the limitations of the morphologic-based method of eosinophil quantitation, we sought to develop an unbiased, less labor intensive, and more representative method for the identification and quantification of eosinophils in both inflammatory exudates and whole tissue which would make use of the sensitivity of flow cytometry and capability of this technique to accurately discriminate cell populations based on surface marker expression. We chose Siglec-F as a potential marker amenable to this flow-based approach since it has documented to be present on the surface of eosinophils and alveolar macrophages (Sung et al. 2006).
In this study, we used Siglec-F in combination with CD45 and CD11c to identify and quantitate eosinophils in the inflamed lungs and BAL fluids using flow cytometry. We found that expression of Siglec-F, when used in conjunction with CD45 and CD11c, identified eosinophils in both the BAL fluid and lungs of uninflamed, allergen inflamed, and RSV-infected mice based on their distinct Siglec-F+ CD11clow/− phenotype. These Siglec-F+ CD11clow/− cells displayed the low forward light scatter and high side light scatter properties as well as the cell morphology characteristic of eosinophils. In contrast, we found that the Siglec-F+ CD11c+ cell population also identified using this method is abundantly represented in the BAL fluid of normal/non-inflamed animals, and has high forward and side scatter properties as well as morphology consistent with that of alveolar macrophages.
Since CD11c is also expressed on dendritic cells, we analyzed the Siglec-F+ CD11c+ cells (and Siglec-F+ CD11clow/− cells) for the expression of other cell surface markers known to be expressed by dendritic cells. Neither population expressed high levels of MHC II, CD86, or CD40 when compared to the relevant isotype controls, suggesting that these populations did not include conventional Siglec-F− CD11c+ dendritic cells (Sung et al. 2006) (TS Kim, unpublished observations).
We also evaluated the effectiveness of the integrin, CD11b, to distinguish eosinophils from cells of monocyte/macrophage lineage present in the normal/non-inflamed or inflamed/infected lung parenchyma. While we could distinguish Siglec-F+ CD11b+ eosinophils from Siglec-F+ CD11b− pulmonary macrophages in the parenchyma of allergic inflamed and day 7 RSV-infected lungs, we were unable to discriminate distinct subsets of Siglec-F+ cells in the airway exudates (BAL fluid) of allergic or day 7 RSV infected animals. It is also noteworthy that CD11b could not be reliably used to distinguish eosinophils and macrophages in the lung parenchyma at an earlier time (i.e. day 5 during RSV infection) (W. Stevens, data not shown). These findings are consistent with an earlier report demonstrating upregulation of CD11b expression on pulmonary macrophages following infection with Streptococcus pneumoniae (Kirby et al. 2006). This variability in CD11b expression by monocyte/macrophage populations is perhaps not unexpected and reinforces the view that CD11b expression is variable depending on anatomic location (BAL fluid versus lung parenchyma) and degree of inflammation (normal/non-inflamed versus inflamed).
In summary, in this report, we describe a flow cytometry-based method to identify and quantitate murine eosinophils in tissue (i.e. lung parenchyma) and inflammatory exudates (i.e. BAL fluid) based on the cell surface expression of the lectin Siglec-F in combination with cell surface markers CD45 and CD11c. This method was applicable in two diverse models of eosinophilic infiltration of the respiratory tract, ovalbumin-induced allergic airway inflammation and intranasal infection of vaccinated animals with RSV. This method allows rapid, accurate, and unbiased quantitation of eosinophils in tissues where conventional histologic-based approaches have provided limited quantitative information.
Abbreviations
BAL
Bronchoalveolar lavage fluid
OVA
Chicken ovalbumin
vvG
Recombinant vaccina virus expressing the G protein of respiratory syncytial virus
RSV
Respiratory Syncytial Virus
Siglec
Sialic acid binding immunoglobulin-like lectin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82(5):521–30. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179(1):81–9. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delayre-Orthez C, de Blay F, Frossard N, Pons F. Dose-dependent effects of endotoxins on allergen sensitization and challenge in the mouse. Clin Exp Allergy. 2004;34(11):1789–95. doi: 10.1111/j.1365-2222.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305(5691):1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72(4):2871–80. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis. 2006;193(2):205–13. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113(1):30–7. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59(8):793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167(6):3146–55. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4(4):493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Braciale TJ. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71(1):678–85. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176(4):2161–72. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15(4):637–46. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J Immunol. 2000;165(11):6487–95. doi: 10.4049/jimmunol.165.11.6487. [DOI] [PubMed] [Google Scholar]
- Varki A, Angata T. Siglecs--the major subfamily of I-type lectins. Glycobiology. 2006;16(1):1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34(4):1175–84. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]