Lowering Blood Pressure Blocks Mesangiolysis and Mesangial Nodules, but Not Tubulointerstitial Injury, in Diabetic eNOS Knockout Mice (original) (raw)
Abstract
Recently, we and others reported that diabetic endothelial nitric oxide synthase knockout (eNOSKO) mice develop advanced glomerular lesions that include mesangiolysis and nodular lesions. Interestingly, insulin treatment lowered blood pressure and prevented renal lesions, raising the question as to whether these beneficial effects of insulin were due to its ability to lower either high glucose levels or high blood pressure. We, therefore, examined the effect of lowering blood pressure using hydralazine in this diabetic eNOSKO mouse model. Hydralazine treatment significantly blocked the development of mesangiolysis and microaneurysms, whereas tubulointerstitial injury was not prevented in these mice. Additionally, hydralazine did not reduce expression levels of either tubulointerstitial thrombospondin-1 or transforming growth factor-β despite controlling blood pressure. On the other hand, the critical role of high glucose levels on the development of tubulointerstitial injury was suggested by the observation that serum glucose levels were correlated with tubulointerstitial injury, as well as with the expression levels of both transforming growth factor-β and thrombospondin-1. Importantly, controlling blood glucose with insulin completely blocked tubulointerstitial injury in diabetic eNOSKO mice. These data suggest that glomerular injury is dependent on systemic blood pressure, whereas hyperglycemia may have a more important role in tubulointerstitial injury, possibly due to the stimulation of the thrombospondin-1-transforming growth factor-β pathway in diabetic eNOSKO mice. This study could provide insights into the pathogenesis of advanced diabetic nephropathy in the presence of endothelial dysfunction.
Diabetic nephropathy is pathologically characterized by glomerular hypertrophy, glomerular basement membrane thickening and mesangial expansion, and later by mesangiolysis and Kimmelstiel-Wilson nodules.1,2 While numerous diabetic models have been able to reproduce the early mesangial changes, until recently, a model of advanced diabetic nephropathy has been lacking. Recently, we and others have reported that diabetic endothelial nitric oxide synthase knockout (eNOSKO) mice develop severe glomerular lesions, which resemble advanced lesions of human diabetic nephropathy.1,2 Diabetic eNOSKO mice exhibit mesangiolysis, Kimmelstiel-Wilson-like nodules and glomerular capillary microaneurysms. Diabetic eNOSKO mice also develop worsening hypertension in association with renal injury.1,2 Importantly, insulin treatment can control blood glucose, significantly reduce blood pressure, and prevent glomerular injury. This raises the question as to whether the beneficial effects of insulin on renal injury are due to controlling blood glucose and/or lowering blood pressure.
Blood pressure control is considered a key recommendation for preventing the progression of diabetic renal disease.3 However, the role of blood pressure control in the presence of endothelial dysfunction is not well understood. For example, Chen et al have examined the role of hypertension in apo E/eNOS double knockout mice and found that lowering blood pressure with hydralazine did not prevent the development of atherosclerosis and aneurysms.4
Given this finding, we examined if lowering in blood pressure through the use of hydralazine could block the development of advanced diabetic nephropathy, including glomerular and tubulointerstitial lesions, in the presence of endothelial dysfunction. In addition, we also evaluated the role of blood glucose on tubulointerstitial injury in this model.
Materials and Methods
Diabetes was induced in 8-week-old male C57BL/6J-Nos3tm1Unc (eNOSKO mice; Jackson Laboratory, Bar Harbor, ME) with intraperitoneal injections of streptozotocin (100 mg/dl/day for 2 consecutive days).2 Blood glucose higher than 200 mg/dl was regarded as a diabetic state. A total of four groups with 12 mice per group were studied, including 1) non-diabetic diabetes mellitus (DM) eNOSKO, 2) non-DM eNOSKO with hydralazine, 3) DM-eNOSKO, and 4) DM-eNOSKO with hydralazine. Hydralazine was administered as 60 to 80 mg/kg body weight/day in the drinking water at 4 weeks. In addition, we reevaluated diabetic eNOSKO mice from our previous study to examine the effect of insulin on tubulointerstitial injury in this model (DM-eNOSKO with insulin treatment).2 For blood sugar control, a single insulin pellet (Linshin Canada Inc, Ontario, Canada) was implanted subcutaneously for 5 months. Blood glucose was monitored every 2 weeks and if the fasting blood glucose was >200 mg/dl, an additional insulin pellet was inserted. Systolic blood pressure was assessed using a tail cuff sphygmomanometer (Visitech BP2000; Visitech Systems, Apex, NC). Blood urea nitrogen, urinary albumin excretion and urinary albumin/creatinine ratio were measured as described previously.2 All animal experiments were performed in accordance with the Animal Care and Use Committee of the University of Florida.
Kidneys were fixed in Fekete’s and embedded in paraffin for periodic acid-Schiff staining and immunohistochemistry. A polyclonal rabbit anti-human fibronectin antibody (1:200, Sigma-Aldrich, St. Louis, MO), polyclonal rabbit anti-mouse collagen IV antibody (Chemicon International, Temecula, CA), rabbit anti-human TGF- β polyclonal antibody (Santa Cruz biotechnology, Santa Cruz, CA), polyclonal goat anti-human collagen III antibody (Southern Biotechnology Associates, Birmingham, AL), polyclonal goat anti-rat vascular endothelial growth factor (VEGF) antibody (R&D Systems, Minneapolis, MN), mouse monoclonal thrombospondin-1 (TSP-1) antibody5 and rat anti-mouse CD34 antibody2 were used for immunohistochemistry. Negative controls were performed by the replacement of primary antibodies with species-matched antibodies.
The mesangial area was determined by assessing the periodic acid-Schiff-positive and nuclei-free area in the mesangium. The glomerular area was also treated along the outline of capillary loop. These areas were measured using the AxioVision image analysis computer program (Carl Zeiss, Thrornwood, NY). Semiquantitative analysis for glomerular mesangiolysis was performed with 100 glomeruli in randomly selected fields for each subject. Mesangiolysis and nodular formation was assessed by scoring 100 glomeruli/mouse. The percentage of atrophic tubules (ie, tubular dilation, detachment of tubular epithelial cells, and condensation of tubular nuclei) was assessed by scoring 400 renal cortical tubules in randomly selected fields for each subject.6 All quantifications were performed in a blinded manner by two independent investigators.
Real-Time PCR
The mRNA extraction and cDNA synthesis were performed by using an RNeasy Mini kit and QuantiTect Reverse Transcription Kit (Qiagen Science, Valencia, CA) according to the manufacturer’s instructions. Real time PCR was performed for VEGF mRNA expression with whole kidneys as described previously.2
Western Blot Analysis
Whole mouse kidneys were snap-frozen in liquid nitrogen for protein isolation. Western blot analysis was performed as described previously.7 The blots were subsequently incubated with a polyclonal goat anti-rat VEGF antibody, mouse monoclonal TSP-1 antibody, or a monoclonal anti-mouse β-actin antibody (Sigma-Aldrich, St. Louis, MO), followed by incubation with peroxidase-conjugated rabbit IgG, goat IgG, or mouse IgG (DakoCytomation, Carpinteria, CA). Proteins were visualized with an enhanced chemiluminescence detection system (Amersham Pharmacia, Piscataway NJ). The density of each band was measured using the public domain NIH Image program.
Statistical Analysis
All values are expressed as mean ± SD. Statistical analysis was performed with unpaired, two-tailed Student’s _t_-test for single comparisons or analysis of variance with posthoc test using Tukey’s method for multiple comparisons. A P value of <0.05 was considered significant.
Results
General Characteristics
As shown in Table 1, streptozotocin induced hyperglycemia in eNOSKO mice. Hydralazine did not alter the level of blood glucose in these mice. The diabetic state resulted in body weight loss, but interestingly, weight loss was noted to occur to a lesser degree in the hydralazine-treated group, despite the high levels of blood glucose. Diabetes induced renal hypertrophy (as evidenced by kidney/body weight ratio) was not completely blocked by hydralazine. In terms of renal function, diabetes significantly reduced creatinine clearance (Ccr) while Blood urea nitrogen level tended to be increased. However, Ccr was partially, but significantly improved by hydralazine treatment. Similarly, the high level of urinary albumin excretion observed in DM-eNOSKO mice was significantly blocked by hydralazine treatment.
Table 1.
General Characteristics of Control and Diabetic Mice
| Non-DM | DM | |||
|---|---|---|---|---|
| Control | Hydralazine | Control | Hydralazine | |
| Body weight (g) | 27.9 ± 2.3 | 25.7 ± 4.7 | 21.5 ± 4.4* | 24.1 ± 2.4 |
| Blood glucose (mg/dl) | 121 ± 7 | 111 ± 38 | 329 ± 63† | 388 ± 56‡ |
| K/B weight (10−3) | 4.8 ± 0.6 | 5.3 ± 0.4 | 8.7 ± 1.4† | 7.8 ± 2.0‡ |
| BUN (mg/dl) | 29 ± 3.0 | 32 ± 1.8 | 35 ± 5.6 | 32 ± 6.3 |
| Ccr (ml/min) | 0.13 ± 0.064 | 0.17 ± 0.082 | 0.048 ± 0.018* | 0.089 ± 0.038§¶ |
| Urine-albumin (μg/day) | 73 ± 43 | 66 ± 18 | 273 ± 78∥ | 57 ± 31** |
| Urine-alb/Cre | 0.16 ± 0.12 | 0.17 ± 0.07 | 13.2 ± 5.8∥ | 1.28 ± 1.18** |
Blood Pressure
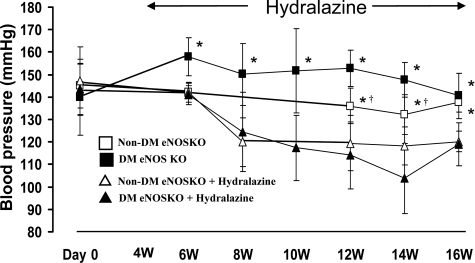
Blood pressure was elevated in diabetic eNOSKO mice as early as 6 weeks and lasted for 10 weeks (Figure 1). Hydralazine markedly reduced blood pressure to levels as low as 100mmHg, which was lower than that observed in the non-diabetic eNOSKO. This blood pressure level was equivalent to that present in non-diabetic wild-type mice (data not shown).
Figure 1.
Time course of blood pressure in eNOS KO mice. Hydralazine treatment begins from 4 weeks. White square, non-diabetes; white triangle, non-diabetes with hydralazine treatment; black square, diabetes; black triangle, diabetes with hydralazine treatment. *P < 0.01 vs. diabetes with hydralazine treatment; †P < 0.05 vs. diabetes. n = 5/non-diabetes group. n = 10/diabetes group.
Glomerular Histology
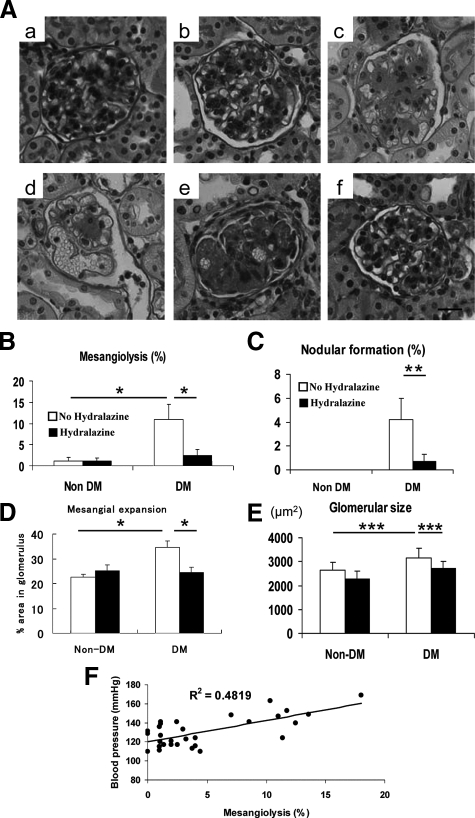
Compatible with previous reports,1,2 diabetic eNOSKO mice developed early lesions, including mesangial expansion and collagen deposition, as well as advanced lesions, such as mesangiolysis, glomerular microaneurysms and Kimmelstiel-Wilson-like nodular lesions (Figure 2A). Interestingly, hydralazine markedly prevented the development of these lesions, despite the presence of high glucose (Figure 2, B and C). The finding that mesangiolysis positively correlated with blood pressure suggests that the development of mesangiolysis was dependent on blood pressure in this model (Figure 2F). In addition, the early lesions, including glomerular hypertrophy, mesangial expansion, and the deposition of extracellular matrix, were also prevented by hydralazine (Figure 2, D and E). On the contrary, hydralazine did not alter glomerular morphology in non-diabetic mice (Figure 2A).
Figure 2.
Glomerular lesions in diabetic eNOS KO mice. The representative glomerular injuries are shown (A). Compared with glomerulus in non-diabetes eNOSKO (a) and in non-diabetes with hydralazine (b), diabetes induces mesangial expansion (c), capillary microaneurysm (d), and nodular lesions (e). Hydralazine blocks the development of these lesions (f). Scale bar = 10 μm. Diabetic condition induces mesangiolysis (B), nodular lesions (C), mesangial expansion (D), and glomerular hypertrophy (E), all of which are blocked by hydralazine treatment. The development of mesangiolysis is correlated with blood pressure (F). Data are shown as means and SD. *P < 0.001; **P < 0.01; ***P < 0.05. n = 5/non-diabetes group. n = 10/diabetes group.
The Deposition of Extracellular Matrix in Glomerulus
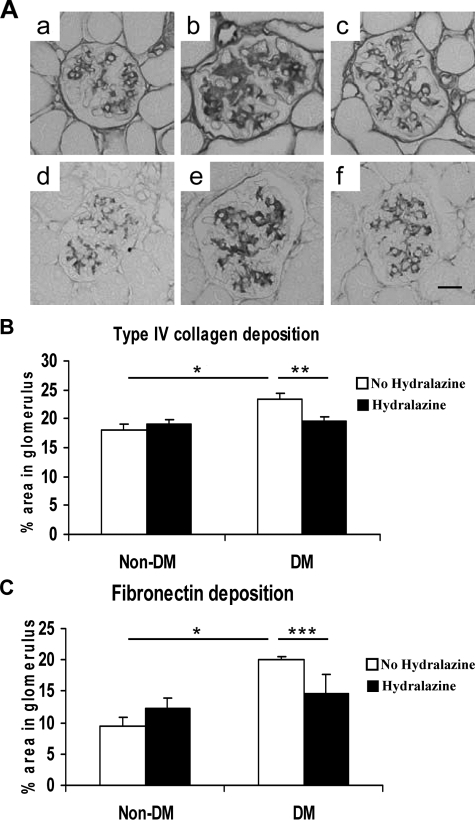
Diabetes resulted in increased collagen IV and fibronectin deposition in the mesangium of eNOSKO mice. These changes were significantly prevented by hydralazine treatment (Figure 3, A–C).
Figure 3.
The depositions of extracellular matrix in glomeruli. The representative features of extracellular matrix deposition in glomerulus are shown (A). Immunohistochemical staining shows the pattern of collagen IV (a, b, c) and fibronectin (d, e, f) depositions. Compared with the non-diabetic condition (a, d), diabetes increases the collagen IV (d) and fibronectin (e) deposition, both of which are blocked by hydralazine treatment (c, f). Bar: 10 μm. Quantitative analysis is shown for collagen IV deposition (B) and fibronectin (C). Data are shown as means and SD. *P < 0.001; **P < 0.005; ***P < 0.05. n = 5/non-diabetes group. n = 10/diabetes group.
Tubulointerstitial Histology
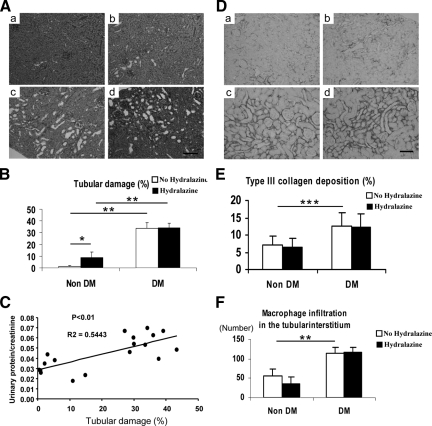
Although lowering blood pressure prevented the development of glomerular injury, renal function was not completely improved. Therefore, we examined the tubulointerstitial injury that occurs in this model. As shown in Figure 4A, diabetic eNOSKO mice developed severe tubular damages with tubular dilation and detachment of tubular epithelial cells, suggesting that tubulointerstitial injury was not dependent on blood pressure (Figure 4, A and B). In fact, no correlation was observed between blood pressure and tubular damage (data not shown) while tubular injury correlated with urinary protein excretion (Figure 4C).
Figure 4.
Tubulointerstitial injury in diabetic eNOS KO mice. In periodic acid-Schiff staining, the representative features of tubulointerstitial injury in non-diabetes (a), non-diabetes with hydralazine (b), diabetes (c) and diabetes with hydralazine (d) are shown (A). Scale bar = 50 μm. The quantitative analysis for tubulointerstitial injury with periodic acid-Schiff staining shows that diabetes induces tubular damage, which is not prevented by hydralazine treatment (B). Tubular injury is positively correlated with urinary protein excretion (urinary protein/urinary creatinine) (C). Type III collagen deposition in non-diabetes (a), non-diabetes with hydralazine (b), diabetes (c) and diabetes with hydralazine (d) are shown (D). Scale bar = 50 μm. It is quantitatively demonstrated that type III collagen deposition (E) and F4/80-positive macrophage infiltration (F) are prominent in diabetes while hydralazine has no effect. Data are shown as means and SD. *P < 0.01; **P < 0.001; ***P < 0.05. n = 10/group.
To confirm tubulointerstitial injury, we examined type III collagen deposition and macrophage infiltration. Diabetic eNOSKO mice showed increased tubulointerstitial collagen III deposition, which was not improved by hydralazine (Figure 4, D and E). Similarly, while F4/80 positive macrophage infiltration in the tubulointerstitium was prominent in the diabetic condition, hydralazine had no effect (Figure 4F). These data also suggest that the tubulointerstitial damage was not dependent on blood pressure in this model. Importantly, the tubulointerstitial injury could account for the impaired renal function despite controlled blood pressure.
Role of VEGF, TSP-1, and TGF-β in Tubulointerstitial Injury
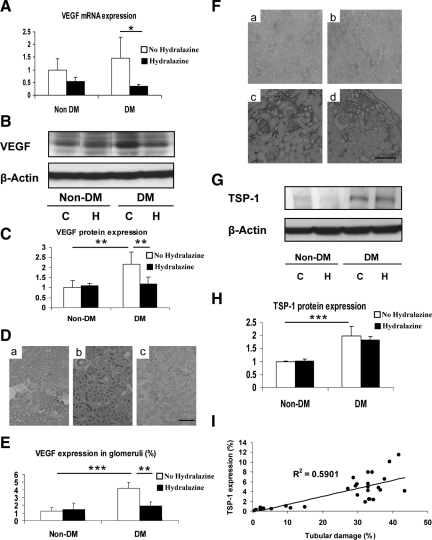
We next attempted to seek the potential mechanisms for the tubulointerstitial injury. First, we investigated VEGF and TSP-1 expression, both of which are important pathogenic mediators of diabetic nephropathy. As shown in Figure 5, A–C, VEGF mRNA and protein expression were increased in the whole kidney of diabetic eNOSKO mice. VEGF was predominantly located in apical membrane in tubular epithelial cells (Figure 5D) as well as glomerular podocyte (data not shown). Interestingly, hydralazine treatment significantly reduced VEGF expression in the tubules and glomeruli (Figure 5E), suggesting that VEGF could be a mediator for glomerular injury, but not tubulointerstitial injury in this model.
Figure 5.
VEGF and TSP-1 expressions in diabetic eNOS KO mice. Real-time PCR for VEGF mRNA (A) and Western blotting for VEGF protein (B, C) in whole kidney demonstrated that VEGF expression is increased in diabetic eNOSKO mice. However, an increase in VEGF expression in diabetic eNOSKO mice is prevented by hydralazine treatment. In C, the quantitative result is expressed as the relative ratio of VEGF to β-actin. In D (scale bar = 50 μm), VEGF protein expression in immunohistochemistry demonstrated that compared with non-diabetic animal (a), diabetes exhibits intensive signal for VEGF in apical membrane of tubular cells (b). This intensive signal cannot be observed in diabetic eNOSKO mice with hydralazine treatment (c). Quantitative analysis shows that VEGF expression in glomeruli is high in diabetes, but it is significantly blocked by hydralazine treatment (E). F: TSP-1 protein expression is examined using immunohistochemistry (scale bar = 20 μm). TSP-1 protein expression is examined using immunohistochemistry (scale bar = 20 μm) (F). Compared with non-diabetic eNOSKO mice (a) or non-diabetic eNOSKO mice with hydrazaline treatment (b), diabetic eNOSKO mice exhibit intensive signal for TSP-1 in tubulointerstitium (c). This intensive signal does not demonstrate any difference in diabetic eNOSKO mice with hydrazaline treatment (d) compared with diabetic eNOSKO mice (c). Western blotting demonstrates the upregulation of TSP-1 in whole kidney in diabetic eNOSKO mice, but hydralazine has no effect on TSP-1 expression (G). TSP-1 protein expression is quantified with Western blotting (H). In G, the quantitative result is expressed as the relative ratio of TSP-1 to β-actin. Tubulointerstitial injury is positively correlated with TSP-1 expression in all groups (I). Data are shown as means and SD *P < 0.05; **P < 0.01; ***P < 0.001. n = 5/non-diabetes group. n = 10/diabetes group.
TSP-1 expression was also induced in the diabetic eNOSKO mice as evidenced by immunohistochemistry (Figure 5F) and Western blotting (Figure 5, G and H). However, TSP-1 expression, in contrast to VEGF, was not prevented by hydralazine treatment. Furthermore, TSP-1 expression correlated with tubulointerstitial injury (Figure 5I), suggesting that TSP-1 could have a role in the tubulointerstitial injury independently of blood pressure in this model.
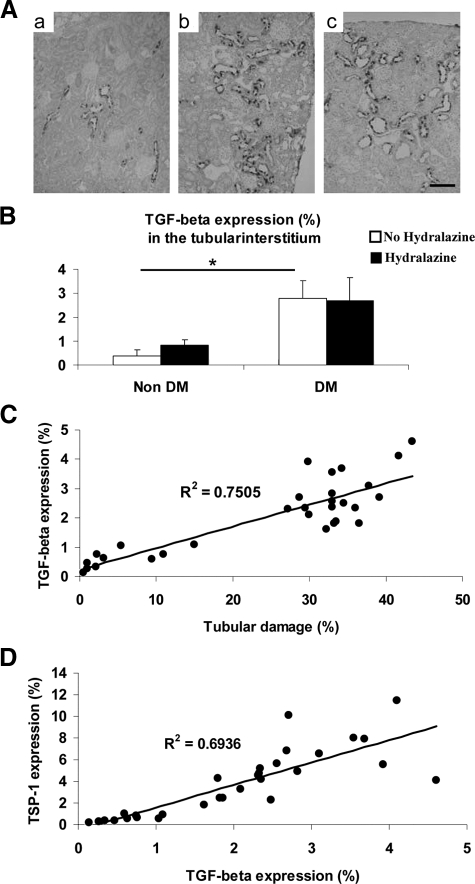
Since TSP-1 is known to activate TGF-β, which is one of the most important mediators in diabetic nephropathy, we next examined the role of TGF-β in this model. As shown in Figure 6, A–B, TGF-β expression was up-regulated in damaged tubules in diabetic mice. Hydralazine did not prevent TGF-β induction despite controlling blood pressure. Finally, TGF-β expression correlated with tubulointerstitial injury (Figure 6C), and with TSP-1 expression (Figure 6D). These data suggest that tubulointerstitial injury may be mediated by TGF-β, which could be activated by TSP-1.
Figure 6.
A: Tubular TGF-β expression in diabetic eNOS KO mice. Tubular TGF-β protein expression is examined using immunohistochemistry (scale bar = 20 μm). Compared with non-diabetic animals (a), diabetic mice exhibit intensive signal for TGF-β in damaged tubular cells (b). B: This intensive signal does not show any difference between diabetic eNOSKO mice with hydralazine treatment and diabetic eNOSKO mice (c). TGF-β protein expression is significantly increased in diabetic eNOSKO mice with/without hydralazine treatment in immunohistochemistry. TGF-β positively correlates with tubulointerstitial injury (C) and with TSP-1 expression (D) in all groups. Data are shown as means and SD. *P < 0.01. n = 5 for non-DM group. n = 10 for DM group.
Role of Glucose in Tubulointerstitial Injury
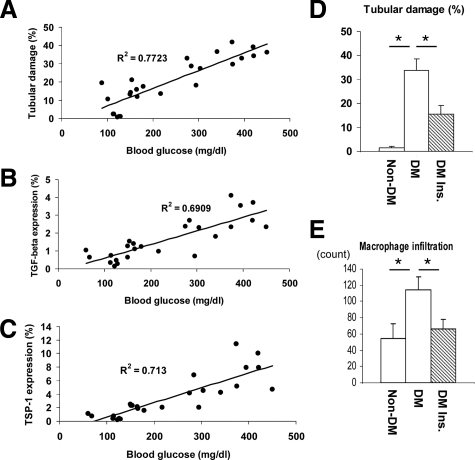
The evidence that hydralazine did not improve tubulointerstitial injury indicates that this form of injury might occur secondary to hyperglycemia in this model. Compatible with our assumption, we found that the tubulointerstitial injury in this model was positively correlated with blood glucose level (Figure 7A). Interestingly, TGF-β as well as TSP-1 expression correlated with blood glucose (Figure 7, B and C), suggesting glucose may be responsible for the tubulointerstitial injury via TSP-1-TGF-β pathway. To confirm this finding, we revisited our previous study in which diabetic eNOSKO mice were treated by insulin to maintain normal glucose level (Table 2). Histological analysis demonstrated that insulin treatment significantly prevented the development of tubulointerstitial injury along with a reduction in macrophage infiltration (Figure 7, D and E). These data suggest that tubulointerstitial injury in this model is likely mediated by blood glucose, but not blood pressure.
Figure 7.
The effect of high glucose on tubulointerstitial injury in diabetic eNOS KO mice. Blood glucose positively correlates with tubulointerstitial injury (A), TGF-β expression (B), and TSP-1 expression (C) in non-DM (n = 5), DM (n = 10), and DM-Ins (Insulin treatment) (n = 10) groups. Insulin treatment significantly blocks the development of tubulointerstitial injury (D) as well as macrophage infiltration in tubulointerstitium (E). Data are shown as means and SD, * P < 0.05.
Table 2.
General Characteristics of Diabetic eNOS KO Mice with Insulin at 3 Months
| Body weight | Blood glucose | Systemic BP | K/B weight (10−3) | BUN | |
|---|---|---|---|---|---|
| DM with insulin | 26.2 ± 2.2 | 90 ± 40 | 138 ± 14 | 4.8 ± 0.7 | 17.1 ± 3.0 |
Discussion
The major finding in this study is the histological evidence that lowering blood pressure blocked the development of advanced diabetic glomerular injury in the presence of eNOS deficiency. On the other hand, the tubulointerstitial injury was not dependent on blood pressure, and appeared to be due to the effects of hyperglycemia to stimulate the TSP-1-TGF-β pathway. A marked reduction of Ccr in diabetic eNOSKO was also observed. While it is possible that the reduction in Ccr could be secondary to dehydration (from the glycosuria), the fact that hydralazine treatment improved the renal function suggests that the reduced GFR was likely due to the renal injury.
Our study confirms a key role for blood pressure control to protect the glomerulus from diabetic injury in the eNOSKO mice. This protection could be potentially due to an improvement in glomerular hemodymanics. While controlling blood pressure was protective, it is also known that most hypertensive animal models do not produce mesangiolysis and Kimmelstiel-Wilson-like nodular lesions.8,9 Previous studies have suggested that endothelial dysfunction may alter autoregulation, and consequently predispose the animal to glomerular hypertension.10,11 In this process, endothelial NO is an indispensable factor to regulate the intraglomerular pressure by modulating afferent and efferent arterioles.12,13 This could also explain why only a mild elevation of blood pressure (15–20 mmHg) observed in this model could have such a strong impact on the glomerulus to cause advanced lesions. This is also indicating that lowering blood pressure may help prevent glomerular injury, and indeed targeting lower blood pressures may be required in this setting. In addition, endothelial dysfunction was shown to be associated with glomerular injury, including glomerular microangiopathy and mesangiolysis, in other animal models.14,15 These studies implicate the importance of endothelial dysfunction as an accelerating factor for renal injury.
Despite the importance of blood pressure on the glomerular injury in this model, we found that blood pressure control did not improve the tubulointerstitial damage even though albuminuria was reduced. In contrast, tubulointerstitial injury correlated with blood glucose levels. Glucose is known to enter tubular cells via Glut-1, an insulin-independent glucose transporter.16 Therefore, a high level of glucose could theoretically stimulate tubular cells in the absence of insulin, causing tubulointerstitial injury independently of blood pressure. Consequently, high glucose can stimulate the expressions of TSP-1 and TGF-β in tubulointerstitium.17,18,19 In addition, Wang et al demonstrated that glucose-induced TGF-β/TSP-1 expression was negatively regulated by nitric oxide in mesangial cell.20 Given that, NO deficiency might contribute to tubular TGF-β and TSP-1 expression in this model.
An alternative possibility is that the tubulointerstitial injury occurred secondary to the streptozotocin, as streptozotocin is known to be nephrotoxic. However, the observation that the tubulointerstitial injury correlated with blood glucose levels and that insulin treatment improved the glucose levels in concert with reducing the renal injury suggests that the renal injury was secondary to the diabetic state. Alternatively, it is possible that insulin could be working via actions independent of glucose. For example, insulin can stimulate nitric oxide release from endothelial cells. Tubular cells also possess the insulin receptor and insulin can exert a variety of actions in tubular cells, including an inhibition of gluconeogenesis and modulation of sodium and phosphate transport.21 Recently a protective role of insulin in tubular cells has been shown, as insulin stimulates PI3-Kinase/AKT pathway and inhibits apoptosis.22
TGF-β is one of the most important mediators in diabetic nephropathy.23,24 However, TGF-β, which is secreted as an inactive form, can be converted to its active form by TSP-1. By binding to both the latency-associated protein and the mature TGF-β, TSP-1 induces the conformational changes of TGF-β, which allows it to bind its receptor.25,26 Recently, Daniel et al examined the role of TSP-1 in diabetic TSP-1 knockout mice.27 They found that the development of diabetic nephropathy was significantly attenuated in these mice. Interestingly, although TGF-β expression was increased, its activation was blocked in these mice, confirming that the TGF-β activation is mediated by TSP-1. On the other hand, we have previously demonstrated that TGF-β, in turn, stimulates TSP-1 expression via Smad2 activation in proximal tubular cells.5 Thus, once tubular cells are stimulated by high glucose, both TGF-β and TSP-1 are induced and likely create a vicious cycle to induce tubulointerstitial injury in vivo.
In this study, we found that tubular VEGF expression was enhanced in diabetic conditions, but the behavior of VEGF was different from that observed with TSP-1/TGF-β. In fact, lowering blood pressure reduced VEGF expression. These data suggests that tubular VEGF expression is regulated by blood pressure, but not glucose. The association of VEGF expression with blood pressure has been shown in the DOCA salt hypertensive rats in which tubular VEGF expression was also reduced by lowering blood pressure with spironolactone.28 Recently Advani et al demonstrated that renal VEGF expression was up-regulated in spontaneously hypertensive rat and transgenic (mRen-2)27 rats.29 Given the fact that VEGF121 administration lowered blood pressure in the rat,30 the induction of VEGF could be a compensatory mechanism in response to hypertension in these animal models.
While VEGF may not mediate tubulointerstitial injury in this model, it is known that VEGF mediates early diabetic nephropathy in several diabetic animal models.31,32 The association of renal VEGF with human diabetic nephropathy is also documented.33,34 Recent evidence has demonstrated that the balance between VEGF and TSP-1 could be important to determine the fate of renal injury.35,36 Compatibly, we found that hydralazine reduced tubular VEGF expression while TSP-1 expression in tubulointerstitial lesion was still sustained. Therefore, the imbalance of VEGF with TSP-1 may account for the tubulointerstitial injury in this model.
In conclusion, our study demonstrates that in the presence of endothelial dysfunction, blood pressure is critical for the development of glomerular lesions while blood glucose may be more critical as a mediator of tubulointerstitial injury. Nevertheless, insulin treatment was able to prevent both lesions. These results may provide insights into the effects of these key treatments on diabetic nephropathy in humans.
Acknowledgments
We are grateful to Dr. Richard J. Johnson (Division of Nephrology in University of Florida, Gainesville, FL) for his ongoing consul and advice.
Footnotes
Address reprint requests to Takahiko Nakagawa: Division of Renal disease and Hypertension, University of Colorado Denver, PO Box C281, Aurora, CO 80045. E-mail: takahiko.nakagawa@uchsc.edu.
Supported by the Juvenile Diabetes Research Foundation (JDRF), by NIH grant DK-52121, and by generous funds from Gatorade.
References
- Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170:1473–1484. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson M, Johnson RJ, Croker B. Diabetic eNOS knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- Parving HH, Hovind P, Rossing K, Andersen S. Evolving strategies for renoprotection: diabetic nephropathy. Curr Opin Nephrol Hypertens. 2001;10:515–522. doi: 10.1097/00041552-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Chen J, Kuhlencordt PJ, Astern J, Gyurko R, Huang PL. Hypertension does not account for the accelerated atherosclerosis and development of aneurysms in male apolipoprotein e/endothelial nitric oxide synthase double knockout mice. Circulation. 2001;104:2391–2394. doi: 10.1161/hc4501.099729. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lan HY, Glushakova O, Zhu HJ, Kang DH, Schreiner GF, Bottinger EP, Johnson RJ, Sautin YY. Role of ERK1/2 and p38 mitogen-activated protein kinases in the regulation of thrombospondin-1 by TGF-beta1 in rat proximal tubular cells and mouse fibroblasts. J Am Soc Nephrol. 2005;16:899–904. doi: 10.1681/ASN.2004080689. [DOI] [PubMed] [Google Scholar]
- Kosugi T, Yuzawa Y, Sato W, Arata-Kawai H, Suzuki N, Kato N, Matsuo S, Kadomatsu K. Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab Invest. 2007;87:903–913. doi: 10.1038/labinvest.3700599. [DOI] [PubMed] [Google Scholar]
- Kosugi T, Yuzawa Y, Sato W, Kawai H, Matsuo S, Takei Y, Muramatsu T, Kadomatsu K. Growth factor midkine is involved in the pathogenesis of diabetic nephropathy. Am J Pathol. 2006;168:9–19. doi: 10.2353/ajpath.2006.050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ME, Allen TJ, O'Brien RC, Macmillan PA, Clarke B, Jerums G, Doyle AE. Effects of genetic hypertension on diabetic nephropathy in the rat-functional and structural characteristics. J Hypertens. 1988;6:1009–1016. doi: 10.1097/00004872-198812000-00009. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Wilkinson-Berka JL, Allen TJ, Cooper ME, Skinner SL. A new model of diabetic nephropathy with progressive renal impairment in the transgenic (mRen-2)27 rat (TGR). Kidney Int. 1998;54:343–352. doi: 10.1046/j.1523-1755.1998.00019.x. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol. 2001;281:F38–F47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- Edwards RM, Trizna W. Modulation of glomerular arteriolar tone by nitric oxide synthase inhibitors. J Am Soc Nephrol. 1993;4:1127–1132. doi: 10.1681/ASN.V451127. [DOI] [PubMed] [Google Scholar]
- Patzak A, Kleinmann F, Lai EY, Kupsch E, Skelweit A, Mrowka R. Nitric oxide counteracts angiotensin II induced contraction in efferent arterioles in mice. Acta Physiol Scand. 2004;181:439–444. doi: 10.1111/j.1365-201X.2004.01316.x. [DOI] [PubMed] [Google Scholar]
- Hohenstein B, Braun A, Amann KU, Johnson RJ, Hugo CP. A murine model of site-specific renal microvascular endothelial injury and thrombotic microangiopathy. Nephrol Dial Transplant. 2008;23:1144–1156. doi: 10.1093/ndt/gfm774. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Alpers CE, Pippin J, Shankland SJ, Adler S, Kurokawa K, Couser WG, Johnson RJ. A new model of renal microvascular endothelial injury. Kidney Int. 1997;52:182–194. doi: 10.1038/ki.1997.318. [DOI] [PubMed] [Google Scholar]
- Heilig C, Zaloga C, Lee M, Zhao X, Riser B, Brosius F, Cortes P. Immunogold localization of high-affinity glucose transporter isoforms in normal rat kidney. Lab Invest. 1995;73:674–684. [PubMed] [Google Scholar]
- Han DC, Isono M, Hoffman BB, Ziyadeh FN. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J Am Soc Nephrol. 1999;10:1891–1899. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chuang LY, Guh JY, Liu SF, Hung MY, Liao TN, Huang YL. Thrombospondin-1 mediates distal tubule hypertrophy induced by glycated albumin. Biochem J. 2004;379:89–97. doi: 10.1042/BJ20031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung S, Lee CY, Zhang Q, Lau SK, Tsang RC, Chan TM. Elevated glucose induction of thrombospondin-1 up-regulates fibronectin synthesis in proximal renal tubular epithelial cells through TGF-beta1 dependent and TGF-beta1 independent pathways. Nephrol Dial Transplant. 2006;21:1504–1513. doi: 10.1093/ndt/gfl017. [DOI] [PubMed] [Google Scholar]
- Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem. 2002;277:9880–9888. doi: 10.1074/jbc.M108360200. [DOI] [PubMed] [Google Scholar]
- Hammerman MR. Interaction of insulin with the renal proximal tubular cell. Am J Physiol. 1985;249:F1–F11. doi: 10.1152/ajprenal.1985.249.1.F1. [DOI] [PubMed] [Google Scholar]
- Meier M, Nitschke M, Hocke C, Kramer J, Jabs W, Steinhoff J, Schutt M. Insulin inhibits caspase-3 activity in human renal tubular epithelial cells via the PI3-kinase/Akt pathway. Cell Physiol Biochem. 2008;21:279–286. doi: 10.1159/000129386. [DOI] [PubMed] [Google Scholar]
- Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- Iwazu Y, Muto S, Fujisawa G, Nakazawa E, Okada K, Ishibashi S, Kusano E. Spironolactone suppresses peritubular capillary loss and prevents deoxycorticosterone acetate/salt-induced tubulointerstitial fibrosis. Hypertension. 2008;51:749–754. doi: 10.1161/HYPERTENSIONAHA.107.104901. [DOI] [PubMed] [Google Scholar]
- Advani A, Kelly DJ, Advani SL, Cox AJ, Thai K, Zhang Y, White KE, Gow RM, Marshall SM, Steer BM, Marsden PA, Rakoczy PE, Gilbert RE. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci USA. 2007;104:14448–14453. doi: 10.1073/pnas.0703577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- Kanesaki Y, Suzuki D, Uehara G, Toyoda M, Katoh T, Sakai H, Watanabe T. Vascular endothelial growth factor gene expression is correlated with glomerular neovascularization in human diabetic nephropathy. Am J Kidney Dis. 2005;45:288–294. doi: 10.1053/j.ajkd.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, Han KH, Ji YH, Cha DR. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. 2005;67:167–177. doi: 10.1111/j.1523-1755.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: i. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]