Leptin controls adipose tissue lipogenesis via central, STAT3–independent mechanisms (original) (raw)
. Author manuscript; available in PMC: 2009 Apr 22.
Published in final edited form as: Nat Med. 2008 Jun 1;14(6):667–675. doi: 10.1038/nm1775
Abstract
Leptin (encoded by Lep) controls body weight by regulating food intake and fuel partitioning. Obesity is characterized by leptin resistance and increased endocannabinoid tone. Here we show that leptin infused into the mediobasal hypothalamus (MBH) of rats inhibits white adipose tissue (WAT) lipogenesis, which occurs independently of signal transducer and activator of transcription-3 (STAT3) signaling. Correspondingly, transgenic inactivation of STAT3 signaling by mutation of the leptin receptor (s/s mice) leads to reduced adipose mass compared to db/db mice (complete abrogation of leptin receptor signaling). Conversely, the ability of hypothalamic leptin to suppress WAT lipogenesis in rats is lost when hypothalamic phosphoinositide 3-kinase signaling is prevented or when sympathetic denervation of adipose tissue is performed. MBH leptin suppresses the endocannabinoid anandamide in WAT, and, when this suppression of endocannabinoid tone is prevented by systemic CB1 receptor activation, MBH leptin fails to suppress WAT lipogenesis. These data suggest that the increased endocannabinoid tone observed in obesity is linked to a failure of central leptin signaling to restrain peripheral endocannabinoids.
The increased prevalence of obesity worldwide is associated with an epidemic of type 2 diabetes mellitus. It is well established that a tight correlation exists between body adiposity and glucose homeostasis. Thus, understanding the mechanisms by which adipose tissue metabolism is regulated should help to elucidate the link between obesity and type 2 diabetes.
Endocannabinoids are lipid mediators that are produced on demand from membrane phospholipid precursors in the central nervous system and in peripheral tissues. Endocannabinoids have myriad functions, including the regulation of food intake and lipogenesis in adipose tissue and liver through activation of cannabinoid receptor type 1 (CB1, encoded by Cnr1). In obesity and insulin resistance, endocannabinoid abundance is increased in WAT, yet it is unclear at present what causes this increased endocannabinoid tone1,2. CB1 inhibition in subjects with obesity and type 2 diabetes induces weight loss and a marked improvement of cardiovascular risk factors3, yet the clinical utility of CB1 antagonists is hampered by an increased risk of anxiety or depression that is attributed to their central actions.
Leptin, a hormone secreted from adipose tissue, has potent effects on insulin action and carbohydrate and lipid metabolism that are largely independent of its effects on feeding behavior4,5. Leptin regulates fuel partitioning by promoting lipid oxidation and protein synthesis and by curtailing lipogenesis, resulting in a selective loss of adiposity while preserving lean body mass6,7. In particular, systemic leptin has these effects, in a weight- and calorie-independent manner, by depleting triglyceride content in the WAT, but without causing a concomitant rise in circulating free fatty acids (FFA) as a result of an increase in mitochondrial oxidation, an induction of futile metabolic pathways and a suppression of lipogenesis in this tissue6,7.
We have previously shown that the acute effects of leptin on regulation of food intake, hepatic glucose production and gonadotropin secretion are STAT3 dependent. In this study, we hypothesized that the ability of leptin to regulate WAT metabolism is chiefly mediated by hypothalamic leptin signaling via the STAT3 pathway. While we found that MBH leptin does indeed regulate WAT metabolism, unexpectedly we found it does so independently of STAT3 signaling. Thus, we consequently investigated which of the proximal leptin receptor (LRb) signaling pathways are required for controlling WAT metabolism. Finally, as obesity is characterized by leptin resistance and an increased endocannabinoid tone, we hypothesized that central leptin is able to restrain peripheral endocannabinoid tone.
RESULTS
MBH leptin suppresses WAT lipogenesis independently of STAT3
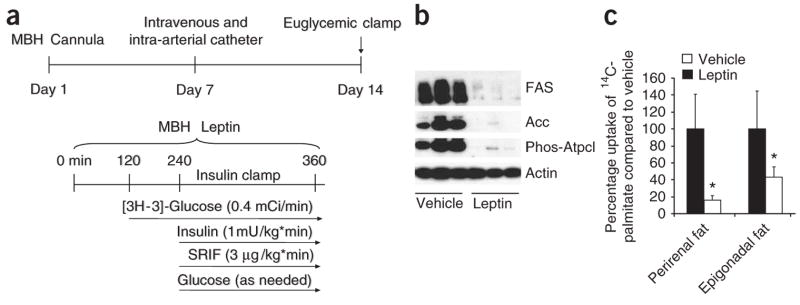
To characterize the contributions of hypothalamic leptin signaling to the control of WAT lipogenesis, we infused leptin directly into the MBH of conscious Sprague Dawley rats via stereotaxically implanted cannulae. Because insulin and glucose can also alter WAT metabolism, and centrally administered leptin can affect both of these parameters4,8–10, all rats received a 6-h infusion of leptin or vehicle within the MBH while the circulating glucose and insulin levels were kept constant at basal levels in all groups by a pancreatic basal insulin clamp (Fig. 1a). Central administration of leptin markedly down-regulated the protein expression of acetyl-CoA carboxylase (Acc, encoded by Acaca) and fatty acid synthase (FAS, encoded by Fasn) in epidydimal fat, the major visceral fat depot in rodents (Fig. 1b). FAS is a crucial enzyme for de novo lipogenesis, and its downregulation probably contributes to the inhibitory effect of leptin on adipose tissue lipogenesis.
Figure 1.
MBH leptin regulates adipose tissue lipogenesis. (a) Experimental protocol for catheter implantation and euglycemic clamp studies. To control for circulating glucose and insulin concentrations, rats underwent pancreatic basal-insulin clamp studies during a 6-h infusion of MBH leptin. (b) Protein expression of key lipogenic enzymes and the activation state of Atpcl, as assessed by a phosphospecific antibody, are reduced by MBH infusion of leptin. (c) 14C-palmitate incorporation is suppressed by MBH leptin in two different visceral WAT depots: the perirenal and epigonadal fat depots. *P < 0.05 versus MBH vehicle–treated group.
Malonyl-CoA has a fundamental role in substrate partitioning. Its cellular abundance potently regulates lipid oxidation through the inhibition of the mitochondrial enzyme carnitine palmitoyltransferase-1 as well as de novo lipogenesis through the provision of a key substrate. Acc is responsible for the formation of malonyl-CoA from acetyl-CoA. Thus, a decrease in the activity of Acc favors lipid oxidation over lipogenesis by a lowering of cellular malonyl-CoA. Acetyl-CoA is a substrate for both the FAS and the Acc reactions. ATP citrate lyase (Atpcl) catalyzes the formation of acetyl-CoA from citrate in the cytosol. Phosphorylation of Ser454 on Atpcl abolishes the homotropic allosteric regulation by citrate and enhances the catalytic activity of the enzyme, and we found that MBH administration of leptin markedly suppressed this phosphorylation (Fig. 1b). The leptin-induced decreases in the expression of Acc and FAS and in Atpcl phosphorylation and activity probably contribute to the central action of leptin on adipose tissue cellular malonyl-CoA11,12 and on fatty acid oxidation and biosynthesis. The suppression of lipogenic enzyme expression occurred as early as 3 h after the beginning of MBH leptin infusions and also occurred in mice that were not clamped (data not shown).
More prolonged administration of systemic leptin has been shown to induce STAT3 phosphorylation in WAT, which is believed to be the result of paracrine leptin signaling via LRb in adipocytes13. We hypothesized that central leptin signaling could induce STAT3 activation in WAT. MBH leptin increased both total STAT3 levels as well as STAT3 phosphorylation in WAT (Supplementary Fig. 1a,b online). Expression of the STAT3 target gene suppressor of cytokine signaling-3 (Socs3), which is also a key inhibitor of STAT3 signaling, was increased threefold by MBH leptin (Supplementary Fig. 1c), indicating that STAT3 signaling can be induced by the central action of leptin. Likewise, interleukin-6 (IL-6), a known inducer of STAT3 activation via the IL-6 receptor expressed on adipocytes14, was found to be upregulated by MBH leptin in WAT (Supplementary Fig. 1d). However, expression of tumor necrosis factor-α, which is commonly upregulated in the obese state, was not induced in WAT by MBH leptin (data not shown).
WAT obtains fatty acids not only from de novo lipogenesis, but also from circulating very low density lipoprotein particles after hydrolyzation via lipoprotein lipase. To test whether central leptin suppresses the uptake of circulating FFA into WAT, we infused leptin into the MBH for 4 h and administered intravenous 14C-palmitate as a primed infusion for the last 60 min of the study. The incorporation of 14C-palmitate into triglycerides in epidydimal and perirenal fat depots was markedly suppressed by MBH leptin, indicating that not only de novo lipogenesis but also FFA uptake into WAT is suppressed by MBH leptin (Fig. 1c). Thus, leptin has potent effects on de novo lipogenesis as well as on FFA uptake and utilization via either neuronal or humoral signaling from the MBH to the periphery.
Previous work from our laboratories has shown that the ability of leptin to suppress hepatic glucose production and food intake as well as to restore the luteinizing hormone surge in fasted female rats requires intact central STAT3 signaling10. To address the role of hypothalamic STAT3 signaling in mediating the effects of central leptin on WAT metabolism, we used a cell-permeable phosphopeptide inhibitor of STAT3 signaling (STAT3 PI) that we have shown to specifically inhibit STAT3 signaling of the long form of the leptin receptor10. Sprague Dawley rats received MBH infusions of vehicle, leptin or leptin plus the STAT3 PI for 6 h. Circulating glucose and insulin concentrations were controlled by a euglycemic clamp at basal insulin levels (Fig. 1a). The inhibition of the STAT3 signaling pathway by the STAT3 PI did not prevent central leptin from suppressing the protein expression of the lipogenic genes Fasn and Acaca or the activation state of Atpcl in WAT (Fig. 2a). The suppression of lipogenic protein expression was mirrored by a suppression of lipogenic gene expression (Fig. 2b), suggesting that the effects of central leptin are at least partially mediated by transcription.
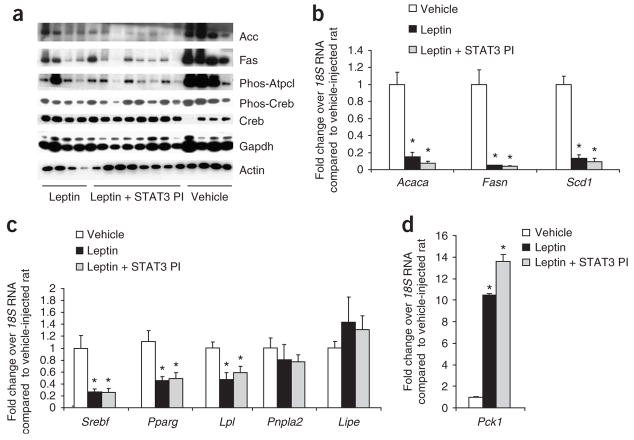
Figure 2.
The regulation of adipose tissue lipogenesis by MBH leptin is STAT3 independent. (a) Western blot analysis of WAT protein extracts derived from clamped rats. (b) Fold change in mRNA levels of the SREBP1c gene targets Acaca, Fasn and Scd1 after treatment with leptin, leptin plus STAT3 PI or a vehicle control in WAT as determined by real-time PCR. mRNA levels of each gene were normalized to their respective_18S_ RNA levels and then normalized again to the level of the vehicle control. (c,d) Fold change in mRNA levels in WAT after treatment with leptin, leptin plus STAT3 PI or a vehicle control of Srebf, Pparg and its target gene Lpl and the lipases Pnpla2 and Lipe (c) and of Pck, the rate-limiting enzyme of glyceroneogenesis (d). *P < 0.05 versus MBH vehicle–treated group.
Because the sterol regulatory element–binding protein-1c (Srebp1c, encoded by Srebf) has a major role in the transcriptional regulation of both Acc and FAS, we next asked whether MBH leptin reduced the expression of Srebf in adipose tissue. The MBH infusion of leptin decreased adipose tissue Srebf mRNA fourfold compared with the MBH infusion of vehicle (Fig. 2b). Stearoyl-CoA desaturase (Scd1), a lipogenic enzyme catalyzing the synthesis of monounsaturated fatty acids, is regulated by Srebp1c in adipocytes15. Scd1 has been shown to be a target of systemic leptin action in the liver and WAT16,17. Scd1 mRNA expression was suppressed by central leptin (Fig. 2b). Collectively, these results indicate that MBH leptin suppresses the expression of Srebp1c in adipose tissue, leading to the marked downregulation of several Srebp1c targets such as Scd1, FAS and Acc. Notably, the coadministration of the STAT3 PI into the MBH did not modify the effects of leptin on lipogenic gene and protein expression, whereas the effects on hepatic glucose fluxes were completely blunted by the inhibition of MBH STAT3 signaling (data not shown and ref. 10).
As cyclic AMP signaling has a major role in the regulation of adipose tissue lipid metabolism, partly via transcriptional regulation, we postulated that central leptin could regulate the expression of lipogenic genes through the activation of protein kinase A (PKA), cAMP response element-binding protein (CREB) or both. However, MBH leptin decreased CREB Ser133 phosphorylation (Fig. 2a) and did not modify PKA phosphorylation (data not shown).
Peroxisome proliferator–activated receptor-γ (PPAR-γ, encoded by Pparg) is a master regulator of adipogenesis18. However, recent evidence also supports a key role for this transcription factor in the regulation of lipogenesis in mature adipocytes19. This is illustrated by the fact that Pparg haplo-insufficiency leads to decreased lipogenesis and increased lipid oxidation in mouse adipose tissue20. Thus, we examined whether central leptin also regulates the expression of PPAR-γ in adipose tissue. MBH leptin suppressed Pparg mRNA expression (Fig. 2c). The abundance of the well-established PPAR-γ target gene lipoprotein lipase (Lpl)21 was also decreased in rats receiving MBH leptin (Fig. 2c). This is consistent with our finding that MBH leptin suppresses the uptake of 14C-palmitate into WAT (Fig. 1c). Lipolysis in WAT is principally regulated by two lipases, adipose triglyceride lipase (Atgl, encoded by Pnpla2), which hydrolyzes the first ester bond of triglycerides22,23, and hormone-sensitive lipase (Hsl, encoded by Lipe), which favors diacylglycerides. MBH leptin failed to alter the expression of these lipolytic enzymes (Fig. 2c). Net lipolysis is an equilibrium between triglyceride breakdown and fatty acid re-esterification. The latter requires glycerol-3-phosphate, the product of glyceroneogenesis, for which the key enzyme is phosphoenolpyruvate carboxykinase (Pepck, encoded by Pck1). MBH leptin markedly increased the expression of Pck1 (Fig. 2d), showing that futile pathways are induced by the central actions of leptin that are predicted to contribute to the weight loss observed after systemic leptin treatment24. In summary, our results indicate that the effects of central leptin in modulating WAT metabolism do not require the activation of the STAT3 pathway in the MBH. Furthermore, the decrease in the expression of Srebp1c and PPAR-γ probably has a key role in mediating the acute effects of leptin on adipose tissue lipid homeostasis.
MBH leptin requires PI3K signaling to suppress WAT lipogenesis
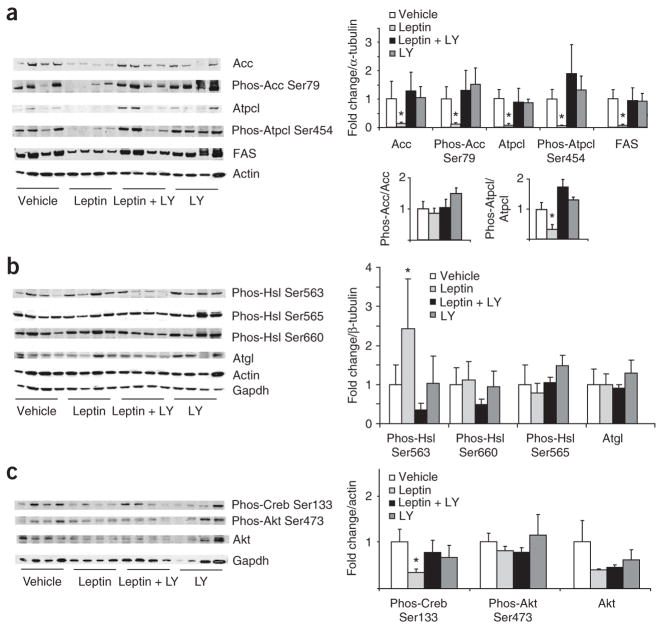
Besides the STAT3 signaling pathway, LRb also has been shown to signal through the insulin receptor substrate–phopshatidylinositide 3-kinase (PI3K) pathway that LRb shares with the insulin receptor signaling pathway. We next tested whether the effects of MBH leptin on adipose tissue lipogenesis are dependent on the central activation of PI3K signaling. Inhibition of the PI3K signaling of MBH leptin with the PI3K inhibitor LY294002 prevented the suppression of Acc and FAS expression (Fig. 3a). Although total Atpcl abundance was markedly decreased by MBH leptin, which is consistent with it being a target of Srebp1c, MBH leptin lowered Atpcl phosphorylation to an even greater degree as total Atpcl (Fig. 3a). Thus, MBH leptin regulates both Atpcl activity and Atpcl protein abundance. In contrast, MBH leptin did not change the ratio of phospho-Acc to total Acc (Fig. 3a), indicating that MBH leptin regulates Acc mainly by lowering Acc protein in WAT.
Figure 3.
PI3K signaling is required for the central effects of leptin on WAT metabolism. (a) Left, western blot analysis of WAT lysates from rats that received 4-h MBH infusions of either vehicle, leptin, leptin plus the PI3K inhibitor LY294002 (LY) or LY294002 alone. Right, quantification of the infrared scanned images normalized over α-tubulin and expressed as fold change compared to vehicle. Lower right, phospho-Acc is normalized to total Acc and phospho Atpcl is normalized to total Atpcl. (b) Left, western blot analyses of the indicated Hsl phosphorylations, Atgl and the two housekeeping genes actin and Gapdh. Right, the corresponding quantification of the western blot data normalized over alpha tubulin and expressed as fold change compared to vehicle. (c) Left, western blot analysis of Creb and Akt activation, as assessed by phosphospecific antibodies. Right, quantification of western blot analysis. *P < 0.05 versus MBH vehicle–treated group.
Leptin is known to induce lipolysis in WAT7,25. Thus, we examined whether the key lipolytic enzyme Hsl is post-translationally regulated by central leptin signaling, as we were unable to detect differences in the expression of this gene (Fig. 2b). Hsl is known to have three major phosphorylation sites. Activated PKA phosphorylates Hsl at Ser563 and Ser660, which stimulates Hsl activity26. In contrast, AMP-activated protein kinase (AMPK) phosphorylates Hsl at Ser565, which reduces Hsl phosphorylation at Ser563 by PKA and inhibits Hsl activity26. Recent work indicates that phosphorylation at Ser600 by mitogen-activated protein kinases p42 and p44 also enhances the enzymatic activity of Hsl27. Using phospho-specific antibodies, we found that MBH leptin stimulated the phosphorylation of Hsl on Ser563, but had no effect on phosphorylation of Ser660 or Ser565 (Fig. 3b). The fact that the phosphorylation of Ser565 of Hsl was not changed by MBH leptin is consistent with our finding that AMPK and p42 and p44 mitogen-activated protein kinase activity was not altered (data not shown). As in Figure 2, we confirmed in this series of experiments that MBH leptin decreased CREB Ser133 phosphorylation (Fig. 3c). This suggests either that the activation of Hsl is unlikely to be the result of an increase in cAMP signaling but is rather induced by an alternative signaling pathway of unclear identity or that CREB Ser133 phosphorylation is not a sensitive marker of cAMP signaling in WAT. The fact that the ratio of phospho-Acc over total Acc was not changed by MBH leptin is consistent with our finding that AMPK, which regulates Acc phosphorylation, is not altered by MBH leptin (Fig. 3c). To test whether MBH leptin changes the activity of the insulin signaling effectors Akt and glycogen synthase kinase-3, we measured Akt Ser473 (Fig. 3c) and glycogen synthase kinase-3 Ser9 phosphorylation (data not shown), which were not affected by MBH leptin. Thus, the regulation of WAT lipogenesis by MBH leptin occurs independently of insulin levels (as this was controlled for by the euglycemic clamp studies) and of modulation of insulin signaling in WAT.
s/s mice have lower adiposity than db/db mice
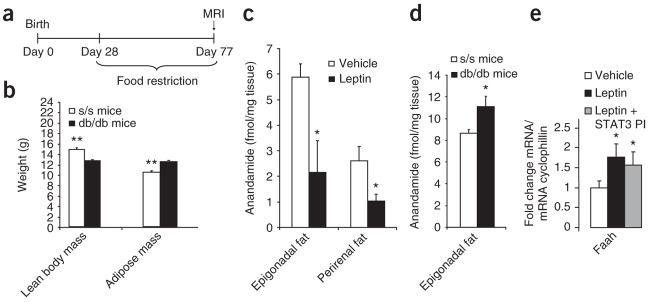
In mice carrying a gene encoding a leptin receptor mutant for Tyr1138 (s/s mice) leptin fails to activate STAT328, yet, unlike db/db mice that lack all LRb signaling, s/s mice are able to signal through STAT3-independent pathways of LRb. When s/s mice are pair-fed to the level of control mice from the time of weaning, they do not develop hyperglycemia or glucose intolerance at least up to 8 weeks of age29. On the contrary, pair-fed db/db mice develop moderate hyperglycemia29, yet both s/s and db/db mice have the same degree of severe hepatic insulin resistance10. To test whether adipose mass is differentially regulated by STAT3-independent pathways of LRb signaling in these two mutant mouse strains, we restricted the food intake of both s/s and db/db mice to 3.8 g/d (the average food intake of wild-type (WT) mice) starting, before both strains developed hyperphagia, at ~4 weeks of age (Fig. 4a). At 11 weeks of age (after 7 weeks of caloric restriction and equal caloric intake) s/s and db/db mice were studied by clamp studies and magnetic resonance imaging. Mice of both strains had very similar body weights, both heavier than WT mice, and a marked increase in fat mass largely accounted for the difference in body weight compared to WT mice. However, in s/s mice, whole-body adiposity was significantly reduced with a compensatory increase in the lean body mass compared with age-matched and pair-fed db/db mice (Fig. 4b). These discrepancies in body composition, independent of caloric intake, between db/db and s/s mice illustrate how potently leptin influences the partitioning of fuel storage via STAT3-independent mechanisms. Of note, caloric restriction also reduced the lean body mass of both s/s and db/db mice compared to WT mice (lean body mass, 14 grams; Fig. 4b), showing that leptin signaling is crucial for the preservation of lean body mass during caloric restriction. Of note, in spite of the equally severe hepatic insulin resistance between these two strains, the s/s mice have significantly improved glucose homeostasis compared to db/db mice10. This improvement, however, is likely due to the differences in body composition resulting from the STAT3-independent LRb signaling that remains in the s/s mice.
Figure 4.
Leptin regulates adiposity and WAT anadamide independently of Stat3 signaling. (a) Experimental protocol for pair-feeding of db/db and s/s mice. (b) Body composition of pair-fed db/db and s/s mice, as assessed by MRI. (c) Anandamide abundance in epidydimal and perirenal fat depots from rats that were infused with MBH leptin or vehicle for 4 h. *P < 0.05. (d) Anandamide levels in epidydimal adipose tissue from clamped db/db and s/s mice as previously described10. (e) Faah mRNA expression in epidydimal fat from clamped rats treated with MBH vehicle, leptin or leptin plus STAT3 inhibitor. MBH leptin induces FAAH in WAT, an effect that is not blocked by inhibition of STAT3 signaling. *P < 0.05, **P < 0.01.
Central leptin suppresses anandamide levels in WAT
Endocannabinoids like anandamide have been shown to increase lipogenesis by activating CB1 receptors30. CB1 antagonists, conversely, improve dyslipidemia and hyperglycemia in individuals with obesity, type 2 diabetes or both, largely independently of their ability to transiently reduce food intake31,32. Leptin has been shown to suppress hypothalamic endocannabinoid levels33 and CB1-mediated endocannabinoid tone in the lateral hypothalamus34. Recent results indicate that human adipocytes do synthesize and secrete endocannabinoids35. As CB1 activation is known to increase lipogenesis36, we asked whether central leptin suppresses endocannabinoid levels in WAT. To this end, we determined the concentration of anandamides in the two largest visceral fat depots of rodents, the epigonadal and the perirenal fat depots, in rats treated with MBH vehicle or leptin for 3 h. Anandamide concentrations were significantly reduced in rats given MBH leptin infusions compared to MBH vehicle–infused rats (Fig. 4c), whereas 2-arachidonoylglycerol concentrations remained unchanged (data not shown). Next, because s/s mice, in whom only STAT3-independent leptin signaling occurs, had lower adiposity than db/db mice, we measured the endocannabinoid abundance in WAT of s/s and db/db mice. We found that s/s mice have lower anandamide levels in visceral (epigonadal) adipose tissue (Fig. 4d), whereas 2-arachidonoylglycerol levels tended to be lower without reaching statistical significance (P = 0.06). This confirms in a genetic model that endocannabinoid levels in WAT are regulated by STAT3-independent pathways. The major metabolizing enzyme of anandamide (but not of 2-arachidonoylglycerol) is fatty acid amidohydrolase (Faah), which we found to be induced by MBH leptin in a STAT3-independent fashion in WAT, as assessed by quantitative PCR (qPCR; Fig. 4e). Thus, the induction of Faah expression may be one of the mechanisms by which MBH leptin suppresses WAT endocannabinoid tone.
To assess whether the suppression of WAT lipogenesis by MBH leptin depends on lowered endocannabinoid tone, we repeated the MBH infusions of leptin while systemically activating CB1 signaling with the CB1 agonist Win 55,212-2 in Sprague Dawley rats. As CB1 receptors are also expressed in the pancreas, where CB1 agonists could potentially regulate insulin secretion, we performed euglycemic clamps in this series of studies to control circulating insulin levels. We administered Win 55,212-2 by intraperitoneal injection at a dose of 3 mg/kg body weight concomitantly with the start of the MBH leptin infusions (Supplementary Fig. 2a online). After 6 h, we killed the rats and analyzed lipogenic enzyme expression in WAT by western blot analyses. MBH leptin was unable to suppress FAS, Acc and Atpcl when Win 55,212-2 was given intraperitoneally; there was also no induction of Hsl Ser563 phosphorylation, indicating that MBH leptin loses the ability to affect WAT metabolism when the endocannabinoid tone is maintained pharmacologically with a CB1 agonist (Supplementary Fig. 2b,c).
MBH leptin curbs WAT lipogenesis via sympathetic innervation
Next, we examined whether the regulation of WAT lipogenesis by MBH leptin requires intact autonomic innervation. First, we performed surgical denervation of the right epidydimal fat pads in Sprague Dawley rats, leaving the contralateral fat pad innervation intact as an internal control. Food intake and body weight were unaltered compared to rats that received sham surgery over the next 7 d (data not shown). One week after denervation, we killed the rats and dissected the epidydimal and inguinal fat pads. Denervation of fat pads has been described to increase the size of the fat pads37,38. Consistent with these reports, the weight of the denervated right epidydimal fat pad had increased by ~40% compared to the contralateral pad37,38 (Supplementary Fig. 3a online), whereas the size of the inguinal fat pads (intact innervation) was unaffected (Supplementary Fig. 3b). Furthermore, surgical denervation resulted in an ~85% reduction in norepinephrine abundance in the denervated epidydimal fat pads (Supplementary Fig. 3c), indicating that the denervation was successful37,38.
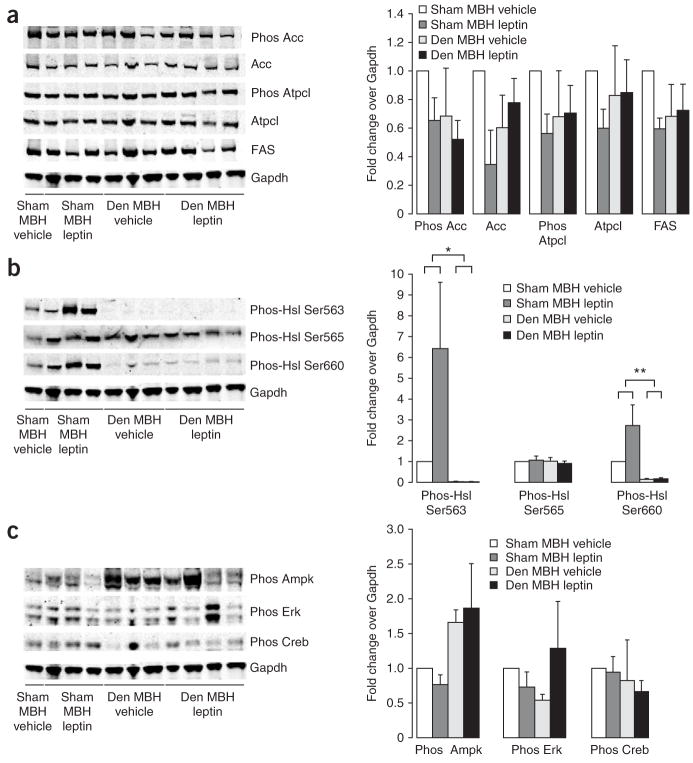
Having established a model of surgical denervation of the epidydimal fat pad, we infused leptin into the MBH of Sprague Dawley rats 7 d after both epidydimal fat pads had been denervated and compared lipogenic protein expression in WAT to that in rats that had undergone sham surgery. MBH leptin did not suppress lipogenic protein expression in denervated epidydimal fat pads (Fig. 5a), indicating that the brain control of WAT lipogenesis by central leptin requires intact autonomic innervation. Notably, the post-translational activation of Hsl by phosphorylation at Ser563 and Ser660 almost completely depended on intact innervation, as not only was the increase by MBH leptin blocked, but baseline Hsl activation was already markedly suppressed (Fig. 5b). Of note, although denervated fat pads tend to increase in size compared to non denervated fat pads (Supplementary Fig. 3a), lipogenic protein expression was not increased and tended to be decreased compared to that in MBH vehicle–infused rats (Fig. 5c). Thus, the increase in fat-pad size seen after denervation is most likely due to a loss of Hsl activation and not due to an increase in de novo lipogenesis. We also assessed AMPK phosphorylation and again found that MBH leptin did not affect AMPK activation in the sham group, yet AMPK phosphorylation tended to be upregulated in denervated fat pads (P = 0.06; Fig. 5c). Notably, although Hsl Ser565 is a known target of activated AMPK, its phosphorylation was not affected by either MBH leptin or denervation (Fig. 5b). Although the marked suppression of norepinephrine indicates that cAMP signaling is suppressed in denervated fat pads (Supplementary Fig. 3c), Creb phosphorylation again was affected neither by MBH leptin nor by denervation (Fig. 5c). Thus, CREB Ser133 phosphorylation is not a sensitive marker for β-adrenergic signaling in WAT. Denervation abrogated the suppression of anandamide by MBH leptin (Supplementary Fig. 3d), whereas denervation by itself did not change WAT anandamide compared to intact innervation (13.5 ± 1.5 fmol/mg tissue versus 12.1 ± 1.0 fmol/mg tissue).
Figure 5.
Control of WAT lipogenesis by MBH leptin requires intact autonomic innervation. (a) Lipogenic protein expression in denervated WAT of rats that were infused with either MBH vehicle or leptin as assessed by western blot analyses. MBH leptin failed to suppress lipogenic protein expression in denervated (Den) epidydimal fat pads, as compared to those in rats that had undergone sham surgery. (b) Hsl activation after MBH vehicle and leptin infusions in sham and denervated WAT. (c) MBH leptin did not affect Ampk activation in the sham group, whereas Ampk phosphorylation was upregulated in the denervated fat pads. Neither Creb phosphorylation nor Erk phosphorylation were affected by MBH leptin or by denervation. *P < 0.05, **P < 0.01.
There is considerable debate as to whether WAT is only innervated by sympathetic fibers or also by parasympathetic efferents39,40. We performed pharmacological sympathectomy by injecting 6-hydroxydopamine (6-OHDA) into the neurovascular bundles supplying the epidydimal fat pads to test whether the sympathetic nervous system is required for the regulation of lipogenesis by MBH leptin. One week after this treatment, we infused either vehicle or leptin into the MBH for 3 h and then killed the rats. Food intake and body weight were not affected by this treatment (data not shown). Norepinephrine abundance was significantly reduced by 6-OHDA treatment (28.8 pg/mg tissue versus 4.9 pg/mg tissue, P < 0.01, n = 4 per group), which was comparable to the change caused by surgical denervation. Of note, MBH leptin did not suppress lipogenic enzyme expression (Supplementary Fig. 4a online). Anandamide levels in WAT were not suppressed by MBH leptin after sympathectomy by 6-OHDA (Supplementary Fig. 4b), indicating that the sympathetic nervous system mediates the effects of MBH leptin on adipose tissue metabolism and endocannabinoid tone.
DISCUSSION
Leptin has a pivotal role in the regulation of energy homeostasis by curtailing food intake and regulating substrate fluxes. Notably, leptin reduces adiposity without decreasing lean body mass. The selective effects of leptin on adipose tissue metabolism through the regulation of substrate partitioning complement the action of leptin on food intake and energy balance. Our study identifies the MBH as an important anatomical location for mediating the central effects of leptin on adipose tissue metabolism. It will be useful to map out other brain areas where leptin receptors are expressed and to study their participation in the brain control of WAT metabolism. The major role that the brain plays in the control of WAT metabolism by leptin is highlighted by the recent finding that the reconstitution of LRb signaling solely in neurons in db/db mice is sufficient to completely reverse the obese phenotype41. Furthermore, deletion of peripheral LRbs, including WAT LRbs, in lean C57 mice (while central LRbs are left intact) does not result in obesity42. These results do not rule out that under certain circumstances LRb signaling in adipocytes participates in adipose tissue metabolism, but they do emphasize the role of brain control of WAT metabolism.
A dichotomy in LRb signaling pathways exists with regard to specific biological parameters. Whereas the hypothalamic regulation of food intake, hepatic glucose fluxes and reproductive function depends on intact STAT3 signaling10, the control of adipose tissue metabolism by leptin is STAT3 independent (Fig. 6). This is not to say that LRb-induced STAT3 signaling is sufficient for mediating the effects of leptin on food intake, glucose fluxes and gonadotropin secretion, as the effects of leptin on food intake also require PI3K signaling43 and possibly the mammalian target of rapamycin pathway44. However, the fact that STAT3 signaling is dispensable for the acute regulation of WAT lipogenesis by MBH leptin is unique. In fact, the ability of leptin to suppress WAT lipogenesis is lost when leptin-induced PI3K signaling is inhibited in the MBH. In this regard, it is noteworthy that in the early stages of diet-induced obesity, the activation of PI3K by leptin seems to be impaired, whereas leptin-induced STAT3 activation is still normal45. Therefore, the ability of leptin to regulate WAT metabolism may be an early manifestation of leptin resistance, although this hypothesis remains to be tested.
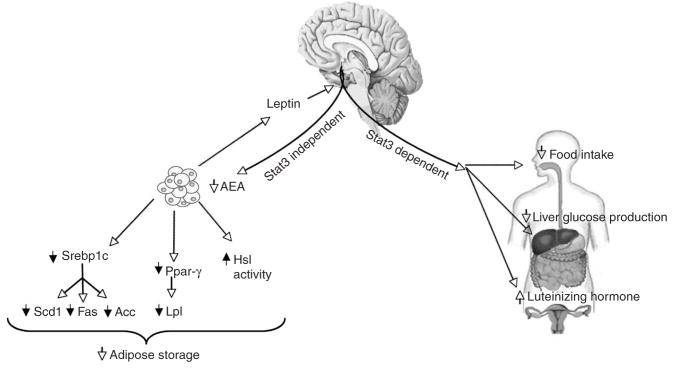
Figure 6.
STAT3-dependent and STAT3-independent pathways of leptin signaling. Our previous data show that the effects of central leptin on the regulation of food intake, liver glucose production and luteinizing hormone secretion are STAT3-dependent. MBH leptin controls adipose tissue metabolism and anandamide levels independently of STAT3 signaling, leading to a decrease in body adiposity.
We identify several biochemical mechanisms by which MBH leptin regulates fuel partitioning in WAT: suppression of the mRNA and protein expression of key de novo lipogenic enzymes, activation of lipolysis by post-translational activation of Hsl, induction of futile cycles as indicated by increased Pepck mRNA expression, and suppression of both palmitate uptake into WAT and LPL expression. These observed weight- and food intake–independent metabolic actions of central leptin were not due to changes in circulating insulin and glucose concentration, as we had controlled for both of these parameters by performing euglycemic clamp studies. On the contrary, surgical denervation and pharmacological sympathectomy of fat pads obliterated the ability of MBH leptin to suppress lipogenesis in WAT. This demonstrates that the acute regulation of adipose tissue metabolism by central leptin crucially depends on the sympathetic nervous system. However, it is likely that the brain-sympathetic nervous system control of WAT that we have delineated is not the only mechanism by which leptin regulates adiposity and that, instead, redundant pathways participate in the regulation of adipose tissue metabolism by leptin. These may account for the observation that weeklong treatment with high circulating doses of leptin can reduce fat pad size, even if the fat pad is denervated46,47. One of the humoral mechanisms by which leptin reduces adipose depot size may be its ability to suppress insulin secretion.
We found that MBH leptin induced STAT3 activation and Socs3 expression in WAT, which is likely to be mediated by IL-6 (Supplementary Fig. 1). The magnitude of the Socs3 induction is moderate compared to what has been seen previously after a 2-week induction of hyperleptinemia by adenoviral overexpression13. The mRNA expression of Ppargc1a, encoding Pgc1-α, an important regulator of mitochondrial biogenesis, was not significantly induced by MBH leptin (data not shown). It is likely that the effects on Socs3, AMPK and Pgc1-α expression in the WAT that were described after 2-week induction of hyperleptinemia are due to the marked weight loss that these rats experienced7. Our experimental design was chosen to detect changes of MBH leptin that occurred independently of food intake. The fact that, in rodents on a high-fat diet, leptin-induced STAT3 activation and Socs3 expression is lower than in rodents on a low fat diet13 may be a consequence of their central leptin resistance, which would need to be examined in future studies. Thus, systemic leptin can induce Socs3 expression via central pathways and does not necessarily require increased circulating leptin concentrations to regulate body fat mass. Lep mRNA levels in WAT were not affected by MBH leptin, although local increases in leptin abundance may still occur through increased leptin secretion by adipocytes. Notably, our results show that MBH leptin is able to induce IL-6 expression in WAT. The role of IL-6 in metabolism has been somewhat controversial. Although IL-6 abundance is increased in obesity and type 2 diabetes, IL-6 is induced by exercise and has been shown to increase lipolysis in humans, whereas the absence of IL-6 in mice leads to an obese and insulin-resistant phenotype48. It is possible that the stimulation of IL-6 in WAT by MBH leptin in part mediates the lipolytic effects of systemic leptin administration.
A key result of these studies is the demonstration that peripheral endocannabinoid tone is under the control of central leptin signaling. Again, the sympathetic nervous system seems to play a major part in this control of WAT endocannabinoid tone. This mechanism has clinical relevance, as CB1 inhibition has emerged as a promising pharmacological approach to improve the metabolic syndrome3. CB1 activation has been shown to induce Srebp1c30. Consequently, the downregulation of Srebp1c by MBH leptin could be a result of the suppressed anandamide in WAT. The effect of anandamide on PPAR-γ signaling is twofold: it can increase PPAR-γ expression and it can serve as a PPAR-γ ligand49, although only at concentrations ~100 times higher than are required for CB1 activation (half-maximal effective concentration, 8 μM versus 70 nM), as has been shown in human adipocytes2,50. Thus, the suppression of anandamide by MBH leptin could contribute to the suppression of lipogenesis by a reduction in PPAR-γ activation and Srebp1c expression in WAT. Our studies do not unequivocally show that the suppression of peripheral endocannabinoids mediates the inhibition of WAT lipogenesis, as the systemically administered CB1 agonist can also have central effects. However, they do establish that the ability of leptin to regulate WAT lipogenesis is lost when the suppression of the systemic endocannabinoid tone is prevented. Future studies using tissue-specific _Cnr1_-knockout mice or peripherally restricted cannabinoid ligands should be helpful in delineating the role of central versus peripheral endocannabinoid signaling. On the basis of our results, one might predict that restoring hypothalamic leptin signaling in diet-induced obesity would reduce the systemic endocannabinoid tone.
In conclusion, we have identified a neural pathway linking the hypothalamus to WAT that probably has a major role in mediating the selective impact of leptin on adipose tissue rather than on muscle mass. Additionally, we provide evidence that the peripheral endocannabinoid tone is under the control of MBH leptin signaling. As obesity is commonly associated with hypothalamic leptin resistance, it is likely that the increased endocannabinoid tone seen in obesity is caused by a failure of central pathways to restrain the endocannabinoid tone.
METHODS
Animals
We housed 10-week-old male Sprague Dawley rats (Charles River Breeding Laboratories) in individual cages in a temperature- and light (12-h light-dark cycle)-controlled facility. We stereotaxically fit the rats with indwelling intrahypothalamic cannulae 2 weeks before euglycemic clamp study, followed 1 week later by intracarotid arterial and intrajugular venous catheterization for infusion and blood sampling, respectively. We allowed rats to recover for 5 d after their last surgery and required them to reach their presurgical body weights before studying them.
Mice with the ObrS1138 mutation (s/s mice) on the C57BL/6 genetic background have been previously described (see Supplementary References online (ref. 1)). and were provided by M. Myers. C57BL/6 db/db mice were obtained from the Jackson Laboratory or were generously provided by R. Harris. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine and Mount Sinai School of Medicine.
Pair-feeding studies
Up to 4 weeks of age, the body weight of s/s and db/db mice does not differ from that of WT mice, after which both mutant strains become progressively hyperphagic and obese. To assess the consequences of lifelong deficiency of all LRb signaling and the isolated obliteration of the STAT3 pathway of LRb signaling, we pair-fed db/db and s/s mice to the daily food intake of WT mice, starting at 4 weeks of age (average of 3.8 g/day, Test Diet 5001). When the mice had reached 11 weeks of age, we studied s/s and db/db mice by MRI followed by clamp studies (see also Fig. 4a).
Implantation of chronic cannulae and intrahypothalamic infusions
We selected stereotaxic coordinates according to the rat brain atlas (Supplementary References (ref. 2)) and experimentally modified them to fit the anatomy of Sprague Dawley rats. Briefly, we placed the rats in a stereotaxic frame (Harvard/American Scientific Institution) under ketamine-xylazine anesthesia. We targeted the MBH bilaterally with a dual-guide, 26-guage cannula system (Plastics One) directed to stereotaxic coordinates 3.3 mm posterior to bregma and 9.6 mm below the surface of the skull. The center-center distance between each guide of the cannula was 0.8 mm. The MBH infusions at a rate of 0.1 μl per h per side lasted for either 6 h (Figs. 1 and 2 and Supplementary Fig. 5) or 4 h (Figs. 3 and 5). The following doses were given per rat: leptin (33 ng/h), Stat3 PI (2.5 pmol/h) and LY294002 (9 pmol/h). The vehicle was artificial CSF (Harvard). We initially validated the anatomical placement of the bilateral cannulae by histochemistry and by sampling of specific nuclei after infusion of tracer (H3 glucose). Routinely, we infused 1 μl of food dye after killing the rat and immediately before removing the brain, followed by anatomical sampling of either the whole hypothalamus or a wedge resection of the MBH.
Denervation procedures
For surgical denervation, we identified the vascular strand innervating the epigonadal fat pad after laparotomy. We separated the fascia containing the nerve bundle from the vessels and dissected it, followed by local application of phenol. We performed pharmacological sympathectomy by injecting 6-OHDA into the nerve bundle innervating the epigonadal fat pad.
Euglycemic and hyperinsulinemic clamp studies
We performed pancreatic-basal insulin clamp studies in rats as previously described (see Supplementary References (ref. 3)); for experimental protocol see Figure 2a and Supplementary Figure 2a. Leptin, leptin plus STAT3 PI or vehicle was infused for 6 h through the MBH cannula.
Analytical procedures
We measured plasma glucose by the glucose oxidase method (Glucose Analyzer II). We extracted endocannabinoids from 100 mg tissue and measured by HPLC–mass selective detector (HPLC-MSD) as previously described (see Supplementary References (ref. 4)) with the following modification. After the final evaporation step, 40–90 μl of clear oil remained, which we extracted with 60 μl of ice-cold methanol. After methanol evaporation, we determined the endocannabinoids by HPLC-MSD. We determined catecholamines as previously described4.
RNA extraction and quantitative real-time RT-PCR
We obtained total RNA from frozen tissue (~85–100 mg) with TRIzol reagent (Invitrogen; Fig. 2b,c) or with the RNeasy Lipid Tissue Mini kit (Valencia; Fig. 4 and Supplementary Fig. 1) according to the manufacturer’s instructions. After treatment with DNase I (Invitrogen), we used purified RNA as a template for first-strand cDNA synthesis with Superscript III (Invitrogen). We ran quantitative real-time RT-PCR either with LC-Fast Start DNA SYBR Green I chemistry (Roche Diagnostics) on a LightCycler 2.0 platform (Roche Diagnostics; Fig. 2b,c) or with SYBR GreenER qPCR SuperMix (QIAGEN) on a 7900HT sequence detection system (Applied Biosystems; Fig. 4 and Supplementary Fig. 1). Forward and reverse primer pairs are listed in Supplementary Table 1 online.
Western blot analyses
We homogenized WAT in 20 mM MOPS, 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 2 mM orthovanadate, 0.5% NP-40 and complete protease inhibitor cocktail (Roche) and centrifuged at 12,000_g_ for 15 min, and we harvested the supernatant while carefully avoiding the lipid layer on top. We measured protein concentration with a BCA protein quantification kit (Pierce). We separated protein extracts on 4–12% NuPAGE gels (Invitrogen) and blotted them onto Immobilon FL PVDF (Millipore). We blocked membranes at room temperature for 1 h in Odyssey LI-COR Blocking Buffer (LI-COR) and incubated them in primary antibodies against Acc, phospho-STAT3 Tyr705, phospho-Akt Ser473, Akt, phospho-Jnk1/2, (all Cell Signaling Technology), STAT3 (Santa Cruz Biotechnology) and Gapdh (Research Diagnostics) in 1:1 Blocking Buffer/TBS-T overnight at 4 °C. After four consecutive 5-min washes in TBS-T (0.1%), we incubated blots with Alexa Fluor 680–conjugated donkey antibody to goat IgG, Alexa Fluor 680–conjugated donkey antibody to mouse IgG (Molecular Probes) or IR Dye 800–conjugated goat antibody to rabbit IgG (Rockland) for 1 h at 18 °C in blocking buffer containing 0.1% TBS-T and 0.1% SDS. After three washes in TBS-T and a final wash in TBS, we scanned the blots with the LI-COR Odyssey (LI-COR) and quantified them with Odyssey 2.0 software on the basis of direct fluorescence measurement (Figs. 3, 5 and 6 and Supplementary Figs. 1 and 2) or developed them with horseradish peroxidase–coupled secondary antibodies (Figs. 1 and 2).
14C-palmitate storage in WAT triglycerides
We prepared infusates daily in quantities sufficient to perform experiments in two rats. We added 150 μl of ethanol containing ~8 × 107 d.p.m. 14C-palmitate drop-wise to 0.6 ml of continuously stirred 4% (w/v) essentially FFA-free BSA (Sigma) in normal saline. The infusate was made up to a final volume of ~2 ml by addition of normal saline. At t = 0 min, we initiated an MBH infusion of leptin or artificial cerebrospinal fluid. We initiated a primed-continuous infusion of 14C-palmitate (GE Healthcare; 20 μCi bolus; 0.4 μCi/min) at t = 180 min and maintained it until we killed the rats at t = 240 min. In some initial studies, we obtained samples for the 14C-palmitate specific activity every 5 min for the last 60 min of the study. The steady state was reached after ~10 min of 14C-palmitate infusion. In the subsequent studies, we determined the serum specific activity of 14C-palmitate at the end of the study after Folch extraction and divided it by the FFA concentration in the same sample. We determined incorporated 14C-palmitate after Folch extraction of adipose tissue and expressed it as d.p.m. per gram tissue × specific activity.
Statistics
All values are presented as the mean ± s.e.m. Comparisons among groups were made using analysis of variance followed by unpaired, nonparametric Student’s _t_-test. Differences were considered statistically significant at P < 0.05.
Supplementary Material
nm1775-S1
Acknowledgments
We wish to thank B. Liu, S. Gaveda and C. Baveghems for technical assistance, S. Chua for helpful discussions and M. Myers (University of Michigan, Ann Arbor) for the s/s mice. Some of the db/db mice were a gift from R. Harris (University of Georgia, Athens). This work was supported by grants to L.R. (NIH DK048321), C.B. (NIH DK074873) and G.J.S. (NIH DK066618) from the US National Institutes of Health, the Skirball Institute for Nutrient Sensing and the New York Obesity Research Center (NIH DK026687). C.B. is the recipient of a Junior Faculty Award and E.D.M. is the recipient of a Physician Scientist Training Award, both from the American Diabetes Association.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
E.D.M performed qPCR (Fig. 2), A.C. performed qPCR and western blots (Fig. 2), L.C. assisted with western blots and qPCR (Figs. 4 and 5 and Supplementary Figs. 1, 3 and 4), T.S. performed western blots (Figs. 5 and 6), A.P. performed and supervised clamp studies (Fig. 2), K.S. carried out MBH infusions and western blots (Fig. 3 and 5), B.C. performed some of the clamp studies (Fig. 5), J.H.-W. measured endocannabinoid and catecholamine levels, X.L. performed denervations, G.J.S. performed 6-OHDA injections, denervations and designed experiments (Fig. 5 and Supplementary Figs. 3 and 4), G.K. analyzed endocannabinoid and catecholamine levels and designed experiments (Fig. 4 and Supplementary Figs. 2 and 3), L.R. designed experiments (Figs. 1–3), and C.B. designed and performed experiments, supervised experimentation, analyzed the data, coordinated the project and wrote the manuscript.
References
- 1.Bluher M, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matias I, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 3.Woods SC. The endocannabinoid system: mechanisms behind metabolic homeostasis and imbalance. Am J Med. 2007;120:S9–S17. doi: 10.1016/j.amjmed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Barzilai N, et al. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. 1997;100:3105–3110. doi: 10.1172/JCI119865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimabukuro M, et al. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem. 1999;274:17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- 8.Muzumdar R, et al. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. FASEB J. 2003;17:1130–1132. doi: 10.1096/fj.02-0991fje. [DOI] [PubMed] [Google Scholar]
- 9.Fan W, et al. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- 10.Buettner C, et al. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha AK, et al. Malonyl-CoA regulation in skeletal muscle: its link to cell citrate and the glucose-fatty acid cycle. Am J Physiol. 1997;272:E641–E648. doi: 10.1152/ajpendo.1997.272.4.E641. [DOI] [PubMed] [Google Scholar]
- 12.Potapova IA, El Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry. 2000;39:1169–1179. doi: 10.1021/bi992159y. [DOI] [PubMed] [Google Scholar]
- 13.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin’s paracrine activity: Implications for treatment of human obesity. Proc Natl Acad Sci USA. 2005;102:18011–18016. doi: 10.1073/pnas.0509001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zvonic S, Baugh JE, Jr, Arbour-Reily P, Mynatt RL, Stephens JM. Cross-talk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280:33856–33863. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]
- 15.Tabor DE, Kim JB, Spiegelman BM, Edwards PA. Transcriptional activation of the stearoyl-CoA desaturase 2 gene by sterol regulatory element–binding protein/adipocyte determination and differentiation factor 1. J Biol Chem. 1998;273:22052–22058. doi: 10.1074/jbc.273.34.22052. [DOI] [PubMed] [Google Scholar]
- 16.Cohen P, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 17.Soukas A, Cohen P, Socci ND, Friedman JM. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegelman BM, Puigserver P, Wu Z. Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S8–S10. doi: 10.1038/sj.ijo.0801492. [DOI] [PubMed] [Google Scholar]
- 19.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2:282–286. doi: 10.1093/embo-reports/kve071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi T, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J Biol Chem. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 21.Kageyama H, et al. Lipoprotein lipase mRNA in white adipose tissue but not in skeletal muscle is increased by pioglitazone through PPAR-γ. Biochem Biophys Res Commun. 2003;305:22–27. doi: 10.1016/s0006-291x(03)00663-6. [DOI] [PubMed] [Google Scholar]
- 22.Schweiger M, et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 24.Reshef L, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 25.Reidy SP, Weber J. Leptin: an essential regulator of lipid metabolism. Comp Biochem Physiol A Mol Integr Physiol. 2000;125:285–298. doi: 10.1016/s1095-6433(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 26.Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg AS, et al. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal–regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- 28.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 29.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Osei-Hyiaman D, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 32.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. J Am Med Assoc. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 33.Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 34.Jo YH, Chen YJ, Chua SC, Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonthier MP, et al. Identification of endocannabinoids and related compounds in human fat cells. Obesity (Silver Spring) 2007;15:837–845. doi: 10.1038/oby.2007.581. [DOI] [PubMed] [Google Scholar]
- 36.Cota D, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cousin B, et al. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology. 1993;133:2255–2262. doi: 10.1210/endo.133.5.8404678. [DOI] [PubMed] [Google Scholar]
- 38.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol. 1998;275:R1488–R1493. doi: 10.1152/ajpregu.1998.275.5.R1488. [DOI] [PubMed] [Google Scholar]
- 39.Giordano A, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kreier F, Buijs RM. Evidence for parasympathetic innervation of white adipose tissue, clearing up some vagaries. Am J Physiol Regul Integr Comp Physiol. 2007;293:R548–R549. doi: 10.1152/ajpregu.00890.2006. [DOI] [PubMed] [Google Scholar]
- 41.de Luca C, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo K, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 43.Niswender KD, et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 44.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 45.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology. 2008;149:1121–1128. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang ZW, et al. Hyperleptinemia depletes fat from denervated fat tissue. Biochem Biophys Res Commun. 1999;260:653–657. doi: 10.1006/bbrc.1999.0918. [DOI] [PubMed] [Google Scholar]
- 47.Rooks CR, et al. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol. 2005;289:R92–R102. doi: 10.1152/ajpregu.00858.2004. [DOI] [PubMed] [Google Scholar]
- 48.Ruderman NB, et al. Interleukin-6 regulation of AMP-activated protein kinase: potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55:S48–S54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 49.Bouaboula M, et al. Anandamide induced PPARγ transcriptional activation and 3T3–L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 50.Pagano C, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via PI3-kinase– and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92:4810–4819. doi: 10.1210/jc.2007-0768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nm1775-S1