Neural Response to Catecholamine Depletion in Unmedicated Subjects With Major Depressive Disorder in Remission and Healthy Subjects (original) (raw)
. Author manuscript; available in PMC: 2009 May 4.
Published in final edited form as: Arch Gen Psychiatry. 2008 May;65(5):521–531. doi: 10.1001/archpsyc.65.5.521
Abstract
Context
The pathophysiologic mechanism of major depressive disorder (MDD) has been consistently associated with altered catecholaminergic function, especially with decreased dopamine neurotransmission, by various sources of largely indirect evidence. An instructive paradigm for more directly investigating the relationship between catecholaminergic function and depression has involved the mood response to experimental catecholamine depletion (CD).
Objectives
To determine whether catecholaminergic dysfunction represents a trait abnormality in MDD and to identify brain circuitry abnormalities involved in the pathophysiologic mechanism of MDD.
Design
Randomized, double-blind, placebo-controlled, crossover, single-site experimental trial.
Setting
Psychiatric outpatient clinic.
Participants
Fifteen unmedicated subjects with MDD in full remission (hereinafter referred to as RMDD subjects) and 13 healthy controls.
Intervention
Induction of CD by oral administration of α-methylparatyrosine. Sham depletion used identical capsules containing hydrous lactose.
Main Outcome Measures
Quantitative positron emission tomography of regional cerebral glucose utilization to study the neural effects of CD and sham depletion. Behavioral assessments included the Montgomery-Asberg Depression Rating Scale and the Snaith-Hamilton Pleasure Scale (anhedonia).
Results
Depressive and anhedonic symptoms increased during CD to a greater extent in RMDD subjects than in controls. In both groups, CD increased metabolism in the anteroventral striatum and decreased metabolism in the orbital gyri. In a limbic-cortical-striatal-pallidal-thalamic network previously implicated in MDD, composed of the ventromedial frontal polar cortex, midcingulate and subgenual anterior cingulate cortex, temporopolar cortex, ventral striatum, and thalamus, metabolism increased in RMDD subjects but decreased or remained unchanged in controls. Metabolic changes induced by CD in the left ventromedial frontal polar cortex correlated positively with depressive symptoms, whereas changes in the anteroventral striatum were correlated with anhedonic symptoms.
Conclusions
This study provides direct evidence for catecholaminergic dysfunction as a trait abnormality in MDD. It demonstrates that depressive and anhedonic symptoms as a result of decreased catecholaminergic neurotransmission are related to elevated activity within the limbic-cortical-striatal-pallidal-thalamic circuitry.
The pathophysiologic mechanisms of major depressive disorder (MDD) consistently have been associated with altered catecholaminergic function, especially with decreased dopamine (DA) neurotransmission, by various sources of largely indirect evidence.1–4 An instructive paradigm for investigating the relationship between catecholaminergic function and depression more directly involves the mood response to catecholamine depletion (CD), achieved by administering α-methylparatyrosine (AMPT),5,6 a competitive inhibitor of the rate-limiting enzyme in catecholamine synthesis, tyrosine hydroxylase.7 Administration of AMPT decreases catecholamine transmission by depleting central DA and norepinephrine stores, evidenced by reduced concentrations of catecholamines and their metabolites in plasma, urine, and cerebrospinal fluid,8,9 and decreased occupancy of striatal DA receptors by DA.10
In subjects studied in the remitted phase of MDD (RMDD) who either were medicated with norepinephrine reuptake inhibiting antidepressant drugs (NRIs)11–13 or were drug free,5 AMPT administration produced marked depressive responses. This finding raised the possibility that manifesting depressive symptoms after AMPT administration constitutes a trait marker related to the vulnerability for developing depression.6
The current study evaluated the role of catecholaminergic function in the pathophysiologic mechanisms of depression by measuring cerebral metabolic effects of CD in unmedicated subjects with RMDD (hereinafter referred to as RMDD subjects) by means of positron emission tomography (PET) and fludeoxyglucose F 18, and related AMPT-induced metabolic changes to associated mood changes. Although a previous study12 assessed AMPT-induced metabolic changes in NRI-treated RMDD subjects, our study is, to our knowledge, the first to assess neurophysiologic effects of CD in unmedicated RMDD subjects and the first to compare AMPT’s metabolic effects in RMDD subjects against healthy controls. Because DA projections into the striatum inhibit glutamate release from afferent excitatory projections,14 we hypothesized that CD would disinhibit limbic-cortical-striatal-pallidal-thalamic circuits implicated in depression,15–17 as evidenced by increased glucose utilization18 in the amygdala, orbitomedial prefrontal cortex (PFC), ventral striatum, and medial thalamus, and that this effect would occur to a greater extent in RMDD subjects than in controls and be associated with a return of depressive symptoms.15
METHODS
PARTICIPANTS
Individuals aged 18 to 56 years either met DSM-IV criteria for MDD in full remission (n=15) or had no history of any psychiatric disorder and no major psychiatric condition in first-degree relatives (n=13). Diagnosis was established by the Structured Clinical Interview for DSM-IV19 and confirmed by an unstructured interview with a psychiatrist. The subjects were recruited through the outpatient clinical services of the National Institute of Mental Health and by advertisements in local newspapers and posters on the National Institutes of Health campus. Exclusion criteria included major medical illnesses, pregnancy, psychotropic drug exposure (including nicotine) within 3 months, substance abuse within 1 year, lifetime history of substance dependence, psychiatric disorders other than MDD, and structural brain abnormalities on magnetic resonance (MR) images. Inclusion criteria required that RMDD subjects had remained in remission without medications for 3 months or longer and had manifested depression onset before 40 years of age. Written informed consent was obtained as approved by the institutional review board of the National Institute of Mental Health.
EXPERIMENTAL DESIGN
In a randomized, double-blind, placebo-controlled, crossover design, subjects underwent 2 identical sessions separated by at least 1 week in which they received either AMPT or placebo. To reduce risk of adverse reactions, we used a body weight–adjusted AMPT dose of 40 mg/kg of body weight orally, to a maximum of 4 g, over 22 hours. Each session took 3 days and was performed on an inpatient basis at the National Institutes of Health Clinical Center. To reduce the risk of crystalluria during AMPT administration, subjects received sodium bicarbonate, drank at least 2 L of water daily, and underwent urinalysis twice daily. Behavioral ratings included the Montgomery-Asberg Depression Rating Scale (MADRS), Beck Anxiety Inventory (BAI), Snaith-Hamilton Pleasure Scale (SHAPS), and Stanford Sleepiness Scale. Blood samples were taken 30 hours after the first AMPT intake in each session to measure serum prolactin levels by means of an electrochemiluminescent immunoassay (Boehringer, Mannheim, Germany).
The PET images were obtained 30 hours after administration of the first AMPT or placebo dose, corresponding to the time when peak depressive responses were expected.5,12 Scanning was performed with a scanner in 3-dimensional mode (35 contiguous sections, 4.25 mm thick; 3-dimensional resolution, 6 mm full-width half-maximum) (GE Advance; GE Medical Systems, Waukesha, Wisconsin) and slow bolus (over 2 minutes) injection of fludeoxyglucose. To obviate the need for arterial blood sampling, cerebral glucose utilization was quantitated by a method that combines the left ventricular chamber time-tissue radioactivity data, measured by dynamic PET imaging of the heart, with venous blood sampling to provide the fludeoxyglucose input function.20,21 This method has previously been validated against more invasive approaches using arterial plasma sampling.20,21 During image processing, the left ventricular time-radioactivity curve was extended in time to include the period of the brain emission scan with venous blood samples obtained 25, 30, 35, and 50 minutes after fludeoxyglucose injection. The mean radioactivity of these samples was divided by the mean left ventricular radioactivity concentration between 25 and 35 minutes after injection. This ratio was used to scale the 50-minute venous sample concentration, which then was appended to the left ventricular curve to complete the input function used to generate parametric images of regional cerebral metabolic rates for glucose (rCMRglu), as described by Moore et al.21
To provide an anatomic framework for analysis of the PET images, structural MR images were acquired with a 3.0-T scanner (Signa; GE Medical Systems) and T1-weighted pulse sequence (magnetization prepared rapidly acquired gradient echo [MP-RAGE]; voxel size, 0.9×0.9×1.2 mm).
PET IMAGE ANALYSIS
The a priori hypothesis was tested by assessing differential changes in the AMPT vs placebo conditions by means of MR imaging–based analysis of the PET data in the amygdala, medial and lateral orbitofrontal cortex (OFC), anteroventral striatum (accumbens area), and medial thalamus. Regions of interest (ROIs) were selected according to the findings and model of Drevets et al,16 as originally proposed on the basis of DA depletion by Swerdlow and Koob.17 The ROIs were predefined on an anatomic MR imaging template (using anatomic boundaries and methods described by Drevets et al22 and Neumeister et al20) and transferred to the coregistered PET image by means of imaging software (MEDx; Medical Numerics Inc, Sterling, Virginia). Average rCMRglu per voxel was obtained for gray-matter voxels within each ROI by multiplying the PET image by a binary mask of gray matter obtained from the segmented anatomic MR image.20 Whole-brain metabolism was obtained with an MR imaging–based template of whole-brain gray plus white matter.
To assess differential effects of CD on metabolism between groups, the rCMRglu was compared between drug and placebo conditions by means of repeated-measures analyses of variance for 2 within-subjects factors (drug: AMPT vs placebo; laterality: left vs right) and 1 between-subjects factor (group: RMDD vs control). The Shapiro-Wilks test was applied to ensure that the data were normally distributed. In regions where results of analysis of variance indicated significant main effects or interactions, specific contrasts were performed by paired or unpaired t tests, depending on the type of comparison being made. All P values were 2-tailed. Analyses were performed with SPSS version 13.01 statistical software (SPSS Inc, Chicago, Illinois).
POST HOC VOXELWISE ANALYSIS
To assess differential metabolic changes across conditions in other regions, voxelwise analysis of the PET data was performed post hoc by means of Statistical Parametric Mapping software (SPM2) (Wellcome Department of Imaging Neuroscience, London, England) in the high-level mathematics environment MATLAB 6.0 (Math Works Inc, Natick, Massachusetts). The PET images were coregistered to the MR images and spatially normalized to the Montreal Neurological Institute brain template by means of SPM2. Images were filtered with a 6-mm gaussian smoothing kernel to compensate for anatomic variability and misalignment error arising during spatial normalization. Montreal Neurological Institute coordinates were nonlinearly translated to the stereotaxic spatial array of Talairach and Tournoux23 (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/mni2tal.m). Normalized rCMRglu was compared between drug and placebo conditions for the entire group, and then differences between conditions were compared between groups. To assess metabolic correlates of CD-induced psychiatric symptoms, changes in depression, anxiety, and anhedonia ratings for each session (maximum score minus baseline score) were entered as additional regressors in the model. The difference between the within-session rating change for each subject under AMPT vs placebo was calculated to reflect the CD-induced effect on each symptom cluster (depression, anxiety, and anhedonia). The statistical models applied to compare normalized rCMRglu included main effects of placebo vs drug, behavioral rating, and subject. The significance threshold for the voxelwise contrasts and correlational analyses was set at P<.001 for a minimum cluster of 10 voxels (based on the “expected voxels per cluster” threshold computed by SPM2 for our data set, as provided in the SPM output file).
RESULTS
BEHAVIORAL RESPONSE TO CD
The clinical and demographic characteristics of the study samples and the clinical ratings at baseline and during scanning appear in Table 1, Table 2, and Figure 1. Peak depressive responses were found approximately 36 hours after the first AMPT dose, coinciding approximately with fludeoxyglucose scanning. Depression ratings (MADRS) increased in both the RMDD and control samples under AMPT vs placebo (P<.001 and _P_=.007, respectively). Using a relapse criterion of a total MADRS score greater than 10 (ie, exceeding the most common operationally defined upper limit for remission24), only 1 of 17 patients (6%) and none of the 13 control subjects had an increase in MADRS score above this level with placebo. In contrast, 12 of 17 patients (71%) and 2 of 13 controls (15%) had an increase in MADRS score to a level outside the remission range with AMPT. The difference between the drug and placebo conditions reached significance for patients (_P_<.001) but not controls (_P_=.48) by McNemar test. The differential “relapse rate” between groups was significant under AMPT (_P_=.004) but not placebo (_P_>.99) by Fisher exact test. Of crucial importance, none of the subjects showed persistence of the depressive symptoms experienced under AMPT at the 96-hour follow-up interview.
Table 1.
Demographic and Clinical Characteristics of Unmedicated Subjects With RMDD and Healthy Controls
| Characteristic | RMDD Subjects (n=15) | Controls (n=13) |
|---|---|---|
| Sex, No. F/M | 14/1 | 12/1 |
| Age, mean (SD), y | 39 (11) | 39 (12) |
| Age at onset, mean (SD), y | 24 (8.3) | NA |
| Major depressive episodes, mean (SD), No. | 2.7 (1.4) | 0 |
| Time in remission, mo | ||
| Mean (SD) | 46 (45) | NA |
| Range | 8-240 | NA |
| Antidepressant drug–naive, No. | 2 | 13 |
| Previous use of NRIs and TCAs, No. | 4 | 0 |
| Time medication free, mo | ||
| Mean (SD) | 35 (28) | NA |
| Range | 7-118 | NA |
| First-degree relative(s) with mood disorder, No. | 14 | 0 |
| Remote (> 1 y ago) history of alcohol abuse, No. | 3 | 1 |
| History of drug abuse, No. | 0 | 0 |
| Montgomery-Asberg Depression Rating Scale score at study entry, mean (SD) | 2.3 (2.1) | 0.5 (1.2) |
Table 2.
Depression, Anxiety, and Anhedonia Ratings at Baseline and Immediately Before PET Scanning Classified by Diagnosis and Treatment Condition
| RMDD Subjects (n=15) | Controls (n=13) | |||
|---|---|---|---|---|
| Characteristic | AMPT | Placebo | AMPT | Placebo |
| Montgomery-Asberg Depression Rating Scale | ||||
| Baseline | ||||
| Mean (SD) | 1.7 (1.7)a | 2.0 (2.5)a | 0.46 (1.1)a | 0.46 (0.88)a |
| Range | 0–6 | 0–8 | 0–3 | 0–3 |
| Scan initiation | ||||
| Mean (SD) | 12 (5.0)a,b | 2.7 (3.3) | 5.9 (4.1)a,c | 1.9 (2.3) |
| Range | 4–24 | 0–12 | 0–13 | 0–7 |
| Beck Anxiety Inventory | ||||
| Baseline | ||||
| Mean (SD) | 1.9 (2.6)a | 1.7 (1.5)a | 0.15 (0.38)a | 0.38 (1.1)a |
| Range | 0–10 | 0–5 | 0–1 | 0–4 |
| Scan initiation | ||||
| Mean (SD) | 9.5 (11)b | 1.9 (2.5) | 1.8 (4.6) | 0.9 (2.2) |
| Range | 0–37 | 0–9 | 0–16 | 0–6 |
| Snaith-Hamilton Pleasure Scale | ||||
| Baseline | ||||
| Mean (SD) | 51 (4.5) | 51 (4.3) | 51 (5.0) | 51 (4.6) |
| Range | 42–56 | 42–56 | 43–56 | 42–56 |
| Scan initiation | ||||
| Mean (SD) | 44 (9.7)a,b | 49 (4.7) | 50 (5.0)a | 51 (5.4) |
| Range | 26–56 | 42–56 | 43–56 | 42–56 |
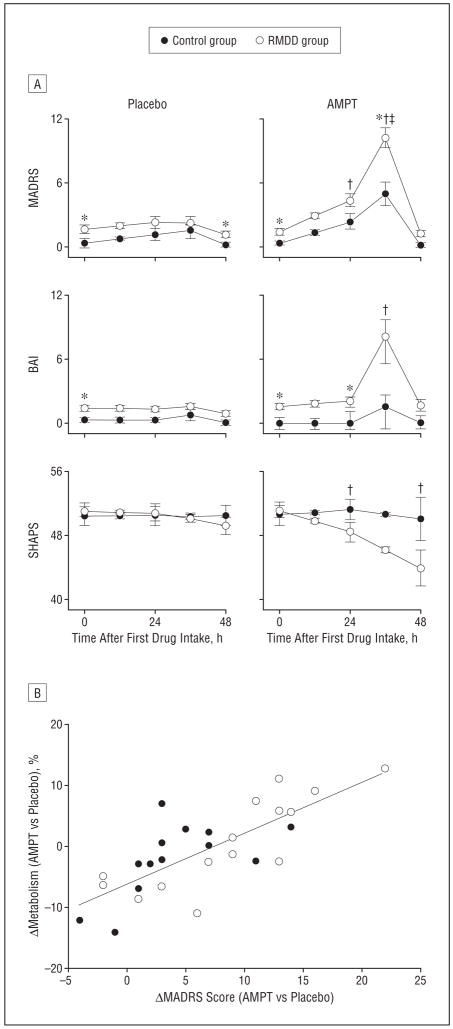
Figure 1.
Behavioral response to catecholamine depletion and relationship to brain metabolism in the ventromedial prefrontal cortex. A, Behavioral response to catecholamine depletion with α-methylparatyrosine (AMPT) and placebo in unmedicated subjects with major depressive disorder in remission (RMDD subjects) and healthy control subjects. BAI indicates Beck Anxiety Inventory; MADRS, Montgomery-Asberg Depression Rating Scale; and SHAPS, Snaith-Hamilton Pleasure Scale. *Significant diagnosis effect (RMDD subjects vs controls, P<.05). †Significant treatment effect for RMDD subjects. ‡Significant treatment effect for controls. B, Positive relationship between AMPT-induced changes in normalized metabolism (regional/global cerebral metabolic rates for glucose) in the ventromedial frontal polar cortex (at x=−4, y=54, z=−8) and the corresponding changes in depressive symptoms (_r_=0.77, P<.001). The difference between the within-session MADRS score change for each subject in the AMPT session vs the placebo session was calculated to reflect the magnitude of the AMPT-induced effect on depression ratings. The regression line (determined from the Pearson correlation coefficient) represents regression on data from RMDD subjects and control subjects together (N=28).
Under AMPT, the pleasure ratings assessed by SHAPS were reduced in the RMDD subjects (P<.001) but remained unchanged in controls (_P_=.76). The increases in the depression and anhedonia ratings in RMDD subjects exceeded the corresponding changes in controls treatment×diagnosis interactions:MADRS, _F_1,117.8=5.86, _P_=.02; SHAPS, _F_1,39.1=5.01, P =.03). Anxiety ratings (BAI) also increased (_P_=.003) in the RMDD group under AMPT vs placebo, but the corresponding changes in controls were not significant (_P_=.95). The treatment×diagnosis interaction for anxiety approached significance (BAI, _F_1,40.4=3.78, _P_=.06). Sleepiness ratings increased in both groups under AMPT vs placebo, and no treatment ×diagnos is interaction was evident(Stanford Sleepiness Scale, F1,106.6=0.78, _P_=.38). There was a trend for a correlation between AMPT-induced depressive symptoms as measured with the MADRS and AMPT-induced sleepiness as measured with the Stanford Sleepiness Scale(_r_=0.35, _P_=.06).The AMPT-induced changes on MADRS scores correlated with corresponding changes in BAI(_r_=0.40, _P_=.03)and SHAPS scores(_r_=−0.59, _P_=.001). There was a significant increase in the serum prolactin level after AMPT (mean [SD], 33.3 [13.6] vs 7.7 [4.0] μg/L [to convert to picomoles per liter, multiply by 43.478];P<.001), while there was no effect of diagnosis (_P_=.80) and no diagnosis×treatment interaction(_P_=.99)regarding serum prolactin concentration.
CEREBRAL GLUCOSE METABOLISM CHANGES UNDER CD
Whole-brain metabolism did not differ between groups at baseline, or between placebo and AMPT conditions (_P_>.25 for main effects of group, main effects of treatment, and group×treatment interactions; Table 3). Regional analyses thus were performed with normalized (regional/global) data to reduce variability introduced by nonspecific fluctuations in global activity.
Table 3.
Whole-Brain Absolute CMRglu and Normalized Regional Metabolism (Regional/Global CMRglu) by Diagnosis and Treatment Condition
| CMRglu, mg/min/mL, Mean (SD) | ||||
|---|---|---|---|---|
| RMDD Subjects | Controls | |||
| Region | AMPT | Placebo | AMPT | Placebo |
| Whole brain | 0.068 (0.013) | 0.071 (0.014) | 0.074 (0.025) | 0.076 (0.010) |
| Normalized (regional/whole-brain CMRglu) in regions of primary interest | ||||
| Amygdala | ||||
| Left | 0.792 (0.070) | 0.785 (0.071) | 0.809 (0.131) | 0.823 (0.092) |
| Right | 0.782 (0.094) | 0.806 (0.056) | 0.834 (0.078) | 0.843 (0.053) |
| Anteroventral striatuma | ||||
| Left | 1.410 (0.166) | 1.300 (0.102) | 1.410 (0.094) | 1.350 (0.151) |
| Right | 1.390 (0.128) | 1.280 (0.129) | 1.370 (0.100) | 1.310 (0.138) |
| Medial thalamusb | ||||
| Left | 1.310 (0.120) | 1.340 (0.151) | 1.280 (0.122) | 1.330 (0.156) |
| Right | 1.320 (0.191) | 1.290 (0.175) | 1.240 (0.131) | 1.340 (0.118) |
| Orbitofrontal cortexc | ||||
| Left | 1.100 (0.065) | 1.120 (0.059) | 1.130 (0.058) | 1.170 (0.078) |
| Right | 1.080 (0.063) | 1.100 (0.074) | 1.120 (0.050) | 1.160 (0.057) |
Under AMPT vs placebo, both groups showed increased metabolism in anteroventral striatum (_F_1,26=27.39, P<.001) and decreased metabolism in OFC (_F_1,26=26.83, P<.001; Table 3). In both regions, metabolism was higher on the left than the right (anteroventral striatum, _F_1,26=5.48, P =.03; OFC, _F_1,26=5.75, P =.02). In OFC, RMDD subjects showed lower metabolism than did controls under both placebo and AMPT (_F_1,26=4.34, P =.047). Treatment×diagnosis×laterality interactions were evident in the medial thalamus (_F_1,26=4.67, P =.04), accounted for by higher metabolism under placebo vs AMPT in controls in the right medial thalamus.
VOXELWISE ANALYSES
Baseline (Placebo Condition): RMDD Group vs Controls
Under placebo, regional metabolism differed between the RMDD subjects and controls in several regions (presented as t values and stereotaxic coordinates [x, y, z; interpreted as described in a footnote to Table 4] for the peak voxel t value, and the cluster size [kE] of contiguous voxels for which P<.01). Metabolism was decreased (_P_uncorrected<.001) in the RMDD vs control groups in right dorsolateral PFC (_t_=4.51; x=34, y=20, z=47; kE=64), right temporopolar cortex (t =3.90; x=46, y=16, z=−26; kE=50), right middle temporal cortex (t =3.84; x=60, y=−50, z=8; kE=23), and ventromedial frontal polar cortex (_t_=3.71; x=6, y=46, z=−21; kE=24). Metabolism was increased significantly (P<.001) in the RMDD vs control groups in the right parahippocampal cortex (_t_=3.91; x=22, y=−37, z=−10; kE=22), dorsal anterior cingulate cortex (_t_=3.91; x=−2, y=14, z=42; kE=142), left inferior parietal cortex (_t_=3.83; x=−54, y=−43, z=45; kE=101), and right dorsal frontal polar cortex (t =3.81; x=28, y=53, z=18; kE=61).
Table 4.
Regional Effects of Catecholamine Depletion With AMPT on Normalized rCMRglu for All Subjects Identified by Voxelwise Analysisa
| Brain Regions | Coordinates,b x/y/z | t Value |
|---|---|---|
| Metabolic Increases Under AMPT vs Placebo | ||
| L anteroventral striatum | −24/6/−4 | 8.53c,d |
| −12/14/3 | 5.51c,d | |
| R anteroventral striatum | 32/−4/2 | 6.51c,d |
| 28/12/−1 | 6.00c | |
| 10/12/3 | 5.99c | |
| L putamen | −30/−2/9 | 5.57c,d |
| Dorsomedial superior frontal gyrus (SMA) | 4/−11/61 | 5.72c,d |
| R precentral gyrus | 8/−26/64 | 5.27d |
| 38/−24/60 | 3.94d | |
| R postcentral gyrus | 20/−42/61 | 4.92d |
| R midcingulate gyrus | 2/−17/51 | 4.53d |
| R hippocampus/parahippocampal gyrus | −26/−34/−10 | 4.34d |
| L precentral gyrus | −4/−32/55 | 4.33d |
| −26/−14/65 | 4.00d | |
| Metabolic Decreases Under AMPT vs Placebo | ||
| R lateral cerebellum | 42/−71/−25 | 7.97c,d |
| L lateral cerebellum | −32/−75/−28 | 5.51c,d |
| Medial occipital cortex | −4/−94/−2 | 6.24c,d |
| L medial orbital gyrus | −16/36/−20 | 5.83d |
| R lateral orbital gyrus | 28/40/−16 | 5.50d |
| R inferior parietal lobe | 50/−60/28 | 4.90d |
| L occipital cortex | −26/−75/43 | 4.63d |
| Medial parietal cortex (precuneus) | 4/−72/32 | 4.50d |
| R frontal polar cortex | 22/60/3 | 4.14d |
Effect of AMPT vs Placebo
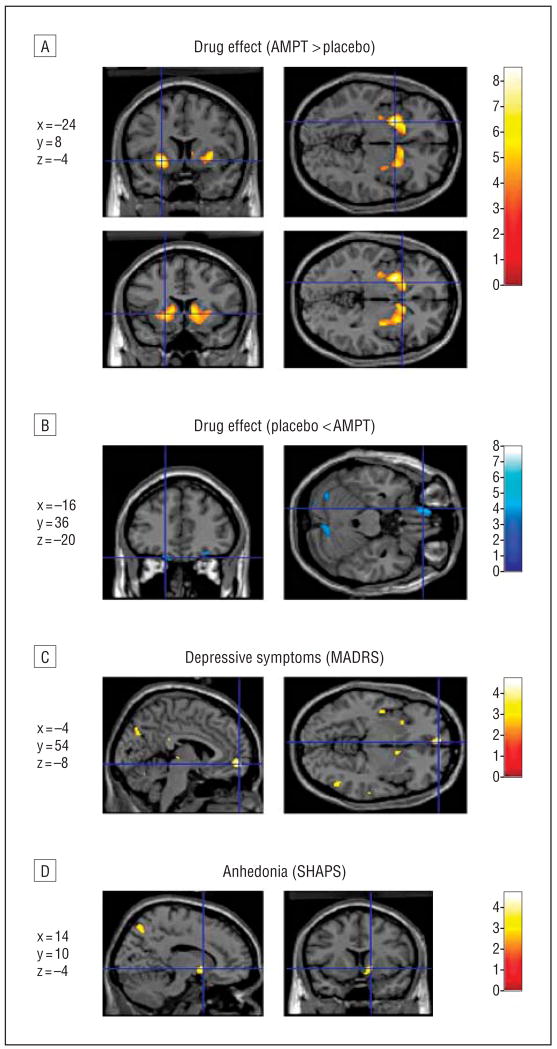
Consistent with the ROI data (Table 3), voxelwise analysis of the effect of AMPT vs placebo in the entire subject sample showed bilateral increases in metabolism in the ventral striatum, with the peak effect size located in the anteroventral putamen (Figure 2A). This effect remained significant after correcting for multiple comparisons (Table 4). Metabolism also increased (_P_uncorrected<.001) under AMPT vs placebo in bilateral precentral gyrus, dorsomedial superior frontal cortex (supplementary motor area), hippocampus and parahippocampus, and midcingulate gyrus. Metabolism decreased (_P_uncorrected<.001) under AMPT vs placebo in left medial orbital and right lateral orbital gyri (consistent with the ROI analysis; Figure 2B), bilateral cerebellum, bilateral occipital cortex, right inferior parietal cortex, medial parietal cortex, and right frontal polar cortex.
Figure 2.
Image sections obtained with Statistical Parametric Mapping software (SPM2) (Wellcome Department of Imaging Neuroscience, London, England) illustrating α-methylparatyrosine (AMPT)-induced metabolic changes and correlations between AMPT-induced symptoms and regional metabolism displayed on an anatomic magnetic resonance image of the brain in the SPM2 analyses of the combined samples (N=28). A, Metabolism increased after AMPT treatment in the bilateral anteroventral striatum, as shown by voxel t values (in color bar) corresponding to P<.001; the crosshair of the lower images does not correspond to a particular peak. B, Reductions in metabolism after AMPT treatment in the left orbitofrontal cortex, as shown by voxels with t corresponding to P<.001 in the medial orbital gyrus. C, Area where changes in metabolism correlated with AMPT-induced depressive symptoms (rated by the Montgomery-Asberg Depression Rating Scale [MADRS]) in the ventromedial frontal polar cortex, as shown by voxel t values corresponding to P<.001 in the correlational analysis. D, Area where glucose utilization correlated with AMPT-induced anhedonia (rated by negative scores from the Snaith-Hamilton Pleasure Scale [SHAPS]) in the right accumbens area, shown as voxel t values corresponding to P<.005. Stereotaxic coordinates corresponding to the horizontal and vertical axes (shown in blue) are listed to the left of each image set and are interpreted as in Table 4.
Differential Effects of AMPT vs Placebo Across Groups
Differential effects of AMPT-induced changes in metabolism between groups are given in Table 5. Under AMPT, metabolism increased in RMDD subjects, but decreased in controls, in ventromedial frontal polar cortex, right thalamus, left ventral striatum (ventral putamen), infralimbic cortex (posterior subgenual anterior cingulate cortex [sgACC]), left superior temporal gyrus, left inferior parietal lobe, left precentral gyrus, and vicinity of the posterior hypothalamus. In the midcingulate gyrus, metabolism increased under AMPT in RMDD subjects but did not change significantly in controls. Metabolism decreased under AMPT in the RMDD group, but increased in controls, in the bilateral cerebellum and occipital cortex and left postcentral gyrus.
Table 5.
Differential Effects of Catecholamine Depletion With AMPT on Normalized rCMRglu Between RMDD Subjects and Healthy Controls Identified by Voxelwise Analysisa
| Brain Regions | Coordinates, x/y/z | t Value |
|---|---|---|
| Metabolism increased in RMDD subjects, unchanged in controls | ||
| Midcingulate gyrus | 2/−17/51 | 3.94 |
| Metabolism increased in RMDD subjects, decreased in controls | ||
| L ventromedial frontal polar cortex | −6/60/−11 | 4.85 |
| −18/61/−13 | 3.49 | |
| 6/49/−23 | 3.49 | |
| Vicinity of posterior hypothalamus | −2/−14/−4 | 4.27 |
| R thalamus | 16/−16/1 | 3.92 |
| L ventral putamen | −32/−19/1 | 3.65 |
| Posterior subgenual anterior cingulate cortex (infralimbic cortex) | −4/13/−12 | 3.60 |
| L superior temporal gyrus (on temporal pole) | −51/14/−1 | 3.56 |
| L inferior parietal lobe | −53/−26/23 | 3.51 |
| L precentral gyrus | −55/2/11 | 3.39 |
| Metabolism decreased in RMDD subjects, increased in controls | ||
| R lingual gyrus | 10/−80/−8 | 4.49 |
| L cerebellum | −14/−63/−7 | 4.43 |
| R occipital cortex | 36/−85/4 | 3.79 |
| R cerebellum | 42/−44/−21 | 3.60 |
| L postcentral gyrus | −48/−9/50 | 3.56 |
| L lingual gyrus | −12/−72/0 | 3.46 |
| L occipital cortex | −18/−91/12 | 3.29 |
CORRELATIONS BETWEEN REGIONAL CEREBRAL METABOLISM AND SYMPTOM RATINGS
Table 6 shows regions where metabolic changes under AMPT correlated with corresponding changes in mood, anxiety, and an hedonic symptoms. Depression ratings (MADRS) correlated positively with metabolic changes in left ventromedial frontal polar cortex (Figure 1B and Figure 2C), left superior temporal gyrus, left posterior insula, right inferior parietal lobe, right middle temporal gyrus, medial parietal cortex, right ventro lateral PFC, and left superior parietallobe at _P_uncorrected<.001. No areas were identified where metabolic changes correlated inversely with changes in depression ratings at this significance threshold.
Table 6.
Regions Where Catecholamine Depletion–Induced Changes in Metabolism Correlated Positively With Corresponding Changes in Mood, Anxiety, and Anhedonic Symptoms in Subjects With RMDD and Controlsa
| Brain Regions | Coordinates, x/y/z | t Value |
|---|---|---|
| Montgomery-Asberg Depression Rating Scale | ||
| Ventromedial PFC | −4/54/−8 | 6.01a |
| L superior temporal gyrus | −59/−26/14 | 5.12 |
| L posterior insula | −42/−10/−1 | 4.92 |
| R inferior parietal lobe | 62/−39/28 | 4.78 |
| R middle temporal gyrus | 53/−62/1 | 4.72 |
| Medial parietal cortexb | −2/−68/33 | 4.60 |
| 14/−63/57 | 4.57 | |
| R ventrolateral PFC | 42/44/18 | 4.55 |
| L superior parietal lobe | −28/−60/47 | 4.05 |
| Beck Anxiety Inventory | ||
| Medial cerebellum | −8/−71/−13 | 4.87 |
| R medial parietal cortexb | 10/−63/57 | 4.66 |
| L fusiform gyrus | −46/−67/−13 | 4.36 |
| R medial thalamus | 6/−23/9 | 3.85 |
| R superior temporal gyrus | 61/−2/7 | 3.75 |
| Anterior cingulate cortex | −2/21/27 | 3.65 |
| Posterior hypothalamus | 2/−14/−4 | 3.42 |
| R parahippocampal gyrus | 32/−17/−19 | 3.35 |
| Snaith-Hamilton Pleasure Scale (Negative Correlation) | ||
| R precentral gyrus | 32/−14/65 | 5.24 |
| R dorsolateral PFC | 36/8/47 | 4.11 |
| Medial parietal cortexb | −12/−61/56 | 4.93 |
| 4/−70/46 | 4.54 | |
| 2/−32/53 | 3.81 | |
| Midcingulate gyrus | 4/−8/41 | 4.35 |
| L medial orbital gyrus/accumbens area | −14/17/−9 | 4.18 |
| L thalamus | −18/−25/12 | 4.08 |
| L superior temporal gyrus | −59/−42/19 | 3.91 |
| −63/−36/15 | 3.68 | |
| R middle temporal gyrus | 53/−64/7 | 3.77 |
| R inferior parietal lobule | 48/−36/48 | 3.77 |
| 62/−42/24 | 3.65 | |
| R superior temporal gyrus | 53/−13/10 | 3.72 |
| R accumbens area | 14/10/−4 | 3.71 |
| L posterior cingulate cortex | −12/−39/35 | 3.64 |
Changes in anxiety symptoms (BAI) correlated positively with metabolic changes in left medial cerebellum, right medial parietal cortex (precuneus), left fusiform gyrus, right medial thalamus, right superior temporal gyrus, anterior cingulate cortex, right parahippocampal gyrus, and vicinity of posterior hypothalamus. Changes in BAI scores correlated negatively with metabolic changes in the left temporopolar cortex (Table 6, footnote).
The AMPT-induced metabolic changes correlated positively with changes in anhedonia ratings (SHAPS) in the left medial orbital gyrus, bilateral accumbens area (anteroventral striatum; Figure 2D), right precentral gyrus, right dorsolateral PFC, posterior and midcingulate gyrus, left thalamus, medial and inferior parietal cortex, left superior temporal gyrus, and right middle temporal gyrus (Table 6). No region was identified where changes in metabolism correlated negatively with changes in anhedonia ratings at _P_uncorrected<.001.
In post hoc analyses limited to the women (ie, excluding the male subject from each group), the results were nearly identical. The behavioral and neuroimaging results also remained essentially unchanged when the subjects with past alcohol abuse (Table 1) were excluded from analysis.
COMMENT
This study is the first, to our knowledge, to compare the effects of CD on depression-related symptoms and neurophysiologic characteristics between unmedicated RMDD subjects and healthy controls. The CD induced greater increases in depressive, anxiety, and anhedonic symptoms in RMDD subjects than those in controls. In both groups, AMPT administration resulted in increased metabolism in the anteroventral striatum and decreased metabolism in the OFC. The most significant positive correlation between AMPT-induced changes in depression ratings and corresponding increases in metabolism appeared in the ventromedial frontal polar cortex.
Administration of AMPT evoked significantly more depressive symptoms in the RMDD group than in controls, although, in contrast to most previous studies,25 we found a minor but statistically significant effect of AMPT on mood in the healthy controls. The RMDD subjects described their CD-associated depressive symptoms as qualitatively similar to those experienced during major depressive episodes. Moreover, RMDD subjects, but not controls, showed increased anhedonia ratings under AMPT. These findings suggested that depressive and anhedonic responses during CD reflected a biological vulnerability in some RMDD subjects. It is noteworthy that AMPT-induced anxiety symptoms were nearly as prominent as AMPT-induced depressive symptoms, although none of the RMDD subjects had a comorbid anxiety disorder. This observation appears consistent with evidence from family and twin studies showing important nonspecific genetic and environmental factors underlying both depression and anxiety,26,27 and from studies of the pathological DA depletion state of Parkinson disease, which showed increased rates of anxiety as well as depressive symptoms.28
Although our study assessed the neurophysiologic effects of AMPT in unmedicated RMDD subjects, another study reported that AMPT-induced depressive symptoms were associated with decreased activity in the OFC, thalamus, dorsolateral PFC, and temporal cortex in medicated RMDD subjects.12 The previous study had a more balanced sex ratio (9 women and 9 men) but did not include a control group. Although we also found that AMPT resulted in reduced OFC metabolism in unmedicated RMDD subjects, we additionally demonstrated that this effect extended to healthy controls. We also demonstrated that, under AMPT, metabolism increased in both the RMDD and control groups in the anteroventral striatum, a region not specifically assessed by Bremner et al.12 Our results differed from theirs in the dorsolateral PFC, where we observed no significant change in metabolism under AMPT, and in the thalamus and temporal cortex, where we found that metabolism increased in RMDD in the right thalamus and left superior temporal gyrus. Although these differences in the results across studies may be accounted for by the differential sex proportions in the study samples (our sample was predominantly female), they may also reflect other experimental design differences. Bremner et al administered diphenhydramine hydrochloride as an active placebo, although this drug may have influenced cerebral metabolism via antihistaminergic and anticholinergic effects. They also studied RMDD subjects receiving NRI antidepressants, so the metabolic changes they observed may have included effects of CD on NRI-induced changes in catecholaminergic function. Finally, they included cigarette smokers, subjects with past alcohol or cocaine dependence, and RMDD subjects in remission for as little as 2 weeks.
A difference between our study and all previous studies of AMPT effects in mood disorders was that we used a slightly lower, body weight–adjusted dose to reduce risk of adverse reactions (eg, dystonia). Although some previous studies observed adverse events in response to AMPT doses greater than 4 g,29 none of our subjects experienced serious adverse effects. Nevertheless, this lower AMPT dose may have influenced the sensitivity for detecting differences between groups.
Some limitations of our methods warrant comment. Healthy controls with a latent vulnerability to MDD could not be definitively excluded, so AMPT-induced mood symptoms in some controls might reflect undetected risk factors for depression. We also did not include a “positive” control group with psychiatric conditions other than MDD (eg, anxiety disorders), which would have helped to evaluate the specificity of the results for MDD. In addition, the specificity of our results was limited by AMPT’s effects of reducing the synthesis of norepinephrine as well as DA and of inducing sedation. Furthermore, sedation may have been interpreted as a mood-lowering effect by subjects, potentially reducing the specificity of mood ratings and interfering with the subject and rater blinding to the drug condition. However, the previous study of CD in unmedicated RMDD subjects demonstrated that AMPT resulted in significantly greater effects on the depressed mood and anxiety items of the depression rating scale than did the sedative diphenhydramine (administered as an active placebo).5 In our study, the effect of sedation was partly controlled by comparison with healthy subjects, who experienced a degree of sedation similar to that experienced by RMDD subjects. In addition, our cross-sectional design could not establish whether the depressive response to AMPT in RMDD reflected an endophenotypic vulnerability to depression or a consequence of illness.
Finally, although the PET-fludeoxyglucose technique allowed us to address our primary aim of assessing neurophysiologic responses to CD by using measures that were unaffected by nonspecific changes in cerebral blood flow and vascular tone, this method could not provide specific biochemical information about catecholamine concentrations. Instead, we relied on the observation that AMPT produced a similar rise in serum prolactin levels in the RMDD and control samples to indicate that the AMPT effect on catecholamine synthesis was similar across groups.30 Nevertheless, a more selective method for assessing the depth of CD on intrasynaptic DA concentrations would potentially be afforded by measures obtained with PET using carbon 11–labeled raclopride; these measures are sensitive to endogenous DA levels.31
The generalizability of our results was limited by the small size and predominantly female sex composition of our samples. Moreover, a selection bias may have been introduced by requiring that RMDD subjects be in remission without medications for 3 months or longer, potentially explaining the relatively small number of past depressive episodes (mean [SD], 2.7 [1.4]).
Effects of AMPT in unmedicated RMDD subjects hold particular interest for elucidating the role of central catecholamine systems in conferring depressive vulnerability and maintaining symptom remission. Both our study and the other study that characterized AMPT effects in unmedicated RMDD patients5 observed that CD induced reemergence of depressive symptoms. These data suggest that MDD is associated with persistent vulnerability for developing depressive responses to reduced catecholamine neurotransmission. The variable mood response to CD across individuals (Figure 1B) further suggests that genetic and/or pathophysiologic variation exists in the dependence on catecholaminergic function for maintaining remission. The positive relationship between AMPT-induced changes in regional glucose metabolism in the ventromedial frontal polar cortex (Figure 1B) across diagnostic groups suggests qualitatively similar relationships between AMPT-induced mood change and associated alterations in local metabolic rates in both groups. However, we identified several other brain regions that showed metabolic changes that differed in direction in RMDD subjects vs controls (Table 5). These latter observations suggest that RMDD is additionally associated with specific neural responses to CD. Taken together, our findings indicate that both qualitative and quantitative differences exist in the neurophysiologic response to AMPT between RMDD subjects and controls.
We hypothesized that vulnerability to CD arises because reduced dopaminergic function would disinhibit limbic-cortical-striatal-pallidal-thalamic circuits implicated in depression.16 Dopaminergic projections from the substantia nigra and ventral tegmental area to the striatum, amygdala, and PFC compose an important inhibitory input into these structures.16,32 In the striatum, dopaminergic projections synapse onto axon terminals of afferent glutamatergic neurons, and DA release inhibits glutamate release from these neurons.14 Reducing DA input into the striatum thus disinhibits efferent neural transmission from the striatum.33 The AMPT-induced elevation of anteroventral striatal metabolism was compatible with this hypothesis (Tables 3 and 4 and Figure 2A). Metabolism increased 8.5% and 8.6% in the RMDD group vs 4.4% and 4.6% in controls in the left and right anteroventral striatum, respectively, although the differences between groups were not significant (_P_=.14).
In other regions of the limbic-cortical-striatal-pallidal-thalamic circuitry, however, AMPT-induced metabolic changes differed significantly between groups (Table 5), and these changes correlated with the depressive and anhedonic responses to AMPT in the RMDD sample (Table 6). The interaction analyses (Table 5) showed that, under AMPT, metabolism increased in RMDD subjects but decreased or remained unchanged in controls in the ventromedial frontal polar cortex, sgACC, midcingulate cortex, superior temporal gyrus, ventral striatum, and thalamus. In these regions, metabolism reportedly is elevated in currently depressed patients with MDD vs controls.16,34,35 Moreover, in RMDD samples imaged under tryptophan depletion,20,36 the depressive relapse induced putatively by reduced central serotonergic function also was associated with increased metabolism in the frontal polar cortex, sgACC, superior temporal gyrus, ventral striatum, and thalamus. Conversely, physiologic activity decreases in these regions after antidepressant treatment (reviewed by Drevets et al34) or deep brain stimulation of the sgACC.37 These regions, along with the hypothalamus (Table 5) and amygdala, share extensive anatomic interconnections to form part of an extended visceromotor network that modulates autonomic, neuroendocrine, and experiential aspects of emotional behavior.38
The central dopaminergic and noradrenergic systems participate in modulating anxiety responses to stress or threat (reviewed by Charney and Drevets39). For example, in the anterior cingulate cortex, which receives extensive dopaminergic innervation,40 AMPT-induced metabolic changes correlated positively with anxiety ratings (Table 6). Reduced DA transmission to the accumbens also may dysregulate stress or anxiety responses because DA release in this region correlated inversely with anxiety ratings in healthy humans during amphetamine challenge.22
Dopaminergic projections from the ventral tegmental area to the accumbens play major roles in learning associations between operant behaviors or sensory stimuli and reward and in mediating reinforcing properties of drugs of abuse and natural rewards.41 Reduced dopaminergic transmission into the accumbens during CD may partly underlie the anhedonic response to AMPT in RMDD (Table 6). The mechanisms by which CD resulted in anhedonia in RMDD subjects, but not controls, also may involve differential effects on the sgACC and ventromedial frontal polar cortex function (Table 5 and Table 6) because medial PFC neurons stimulate phasic DA release from the ventral tegmental area in rats.42
The AMPT-induced depressive symptoms may additionally or alternatively relate to reductions in norepinephrine synthesis. Dysfunction of the central noradrenergic system has been hypothesized to play a role in the pathophysiologic mechanisms of MDD on the basis of evidence of decreased norepinephrine metabolism, increased activity of tyrosine hydroxylase, and decreased density of norepinephrine transporter in the locus ceruleus in depressed patients.43 In addition, decreased neuronal counts in the locus ceruleus, increased α2-adrenergic receptor density, and decreased α1-adrenergic receptor density have been found in the brains of depressed suicide victims post mortem.44
An abnormality that conceivably may confer vulnerability to CD is the reduction in OFC metabolism in RMDD subjects under placebo. This baseline abnormality may reflect the neuropathologic changes found in the OFC post mortem in MDD.45 During depressive episodes, OFC metabolism is elevated to an extent that correlates inversely with depression severity, suggesting that this region functions to modulate symptoms.46 In contrast, depressive relapse under CD was associated with reduced OFC function, consistent with evidence that catecholaminergic transmission is necessary for optimal PFC function.47 Impaired baseline OFC function in RMDD thus may increase vulnerability for developing depressive symptoms during CD-associated reductions in OFC function. Compatible with this hypothesis, OFC activity is decreased in depressed vs nondepressed subjects with Parkinson disease.48,49
In conclusion, RMDD subjects manifest a diathesis to develop depressive relapse and altered visceromotor network physiologic processes as a result of decreased catecholaminergic neurotransmission. The association between depressive symptoms and metabolic changes supports hypotheses that dysmodulation of limbic-cortical-striatal-pallidal-thalamic circuits underlies the pathophysiologic mechanisms of depression,46 and that reduced catecholaminergic function constitutes one pathway through which dysmodulation of this circuit may arise. Our results encourage further research to characterize the neural and behavioral responses to AMPT as possible endophenotypic markers of depression and to elucidate the genetic factors that modulate these responses.50
Acknowledgments
Funding/Support: This study was supported by the Intramural Research Program of the National Institutes of Mental Health.
Footnotes
Author Contributions: Dr Hasler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
References
- 1.Goodwin FK, Bunney WE., Jr Depressions following reserpine: a reevaluation. Semin Psychiatry. 1971;3(4):435–448. [PubMed] [Google Scholar]
- 2.Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(suppl 6):3–8. [PubMed] [Google Scholar]
- 3.Lambert G, Johansson M, Agren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry. 2000;57(8):787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, Oren DA, Heninger GR, Charney DS. Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch Gen Psychiatry. 1999;56(5):395–403. doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- 6.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 7.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 8.Mignot E, Laude D. Study of dopamine turnover by monitoring the decline of dopamine metabolites in rat CSF after alpha-methyl-p-tyrosine. J Neurochem. 1985;45(5):1527–1533. doi: 10.1111/j.1471-4159.1985.tb07223.x. [DOI] [PubMed] [Google Scholar]
- 9.Stine SM, Krystal JH, Petrakis IL, Jatlow PI, Heninger GR, Kosten TR, Charney DS. Effect of alpha-methyl-para-tyrosine on response to cocaine challenge. Biol Psychiatry. 1997;42(3):181–190. doi: 10.1016/s0006-3223(96)00331-9. [DOI] [PubMed] [Google Scholar]
- 10.Verhoeff NP, Christensen BK, Hussey D, Lee M, Papatheodorou G, Kopala L, Rui Q, Zipursky RB, Kapur S. Effects of catecholamine depletion on D2 receptor binding, mood, and attentiveness in humans: a replication study. Pharmacol Biochem Behav. 2003;74(2):425–432. doi: 10.1016/s0091-3057(02)01028-6. [DOI] [PubMed] [Google Scholar]
- 11.Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, Charney DS. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53(2):117–128. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD, Vythilingam M, Chin KN, Vermetten E, Nazeer A, Oren D, Berman RM, Charney DS. Regional brain metabolic correlates of α-methylparatyrosine–induced depressive symptoms. JAMA. 2003;289(23):3125–3134. doi: 10.1001/jama.289.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado PL, Miller HL, Salomon RM, Licinio J, Heninger GR, Gelenberg AJ, Charney DS. Monoamines and the mechanism of antidepressant action: effects of catecholamine depletion on mood of patients treated with antidepressants. Psychopharmacol Bull. 1993;29(3):389–396. [PubMed] [Google Scholar]
- 14.Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42(4):653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 15.Drevets WC, Price JL. Neuroimaging and neuropathological studies of mood disorders. In: Licinio J, Wong ML, editors. Biology of Depression: From Novel Insights to Therapeutic Strategies. Vol. 1. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co; 2005. pp. 427–466. [Google Scholar]
- 16.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12 (9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swerdlow NR, Koob GF. Lesions of the dorsomedial nucleus of the thalamus, medial prefrontal cortex and pedunculopontine nucleus: effects on locomotor activity mediated by nucleus accumbens-ventral pallidal circuitry. Brain Res. 1987;412(2):233–243. doi: 10.1016/0006-8993(87)91129-2. [DOI] [PubMed] [Google Scholar]
- 18.Shulman RG, Hyder F, Rothman DL. Cerebral metabolism and consciousness. C R Biol. 2003;326(3):253–273. doi: 10.1016/s1631-0691(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 20.Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61(8):765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 21.Moore DF, Altarescu G, Barker WC, Patronas NJ, Herscovitch P, Schiffmann R. White matter lesions in Fabry disease occur in “prior” selectively hypometabolic and hyperperfused brain regions. Brain Res Bull. 2003;62(3):231–240. doi: 10.1016/j.brainresbull.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 23.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Georg Thieme Verlag; 1988. [Google Scholar]
- 24.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, Regier DA, Rosenbaum JF, Ray O, Schatzberg AF. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 25.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 26.Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women: phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch Gen Psychiatry. 1995;52 (5):374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 27.Merikangas KR, Risch NJ, Weissman MM. Comorbidity and co-transmission of alcoholism, anxiety and depression. Psychol Med. 1994;24(1):69–80. doi: 10.1017/s0033291700026842. [DOI] [PubMed] [Google Scholar]
- 28.Nuti A, Ceravolo R, Piccinni A, Dell’Agnello G, Bellini G, Gambaccini G, Rossi C, Logi C, Dell’Osso L, Bonuccelli U. Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol. 2004;11(5):315–320. doi: 10.1111/j.1468-1331.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- 29.de Haan L, Booij J, Lavalye J, van Amselsvoort T, Linszen D. Subjective experiences during dopamine depletion. Am J Psychiatry. 2005;162(9):1755. doi: 10.1176/appi.ajp.162.9.1755. [DOI] [PubMed] [Google Scholar]
- 30.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 31.Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17(3):162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 32.Graybiel AM. Basal ganglia: input, neural activity, and relation to the cortex. Curr Opin Neurobiol. 1991;1(4):644–651. doi: 10.1016/s0959-4388(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 33.Wooten GF, Collins RC. Metabolic effects of unilateral lesion of the substantia nigra. J Neurosci. 1981;1(3):285–291. doi: 10.1523/JNEUROSCI.01-03-00285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12(6):527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 35.Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx. 2006;3(1):22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS. Differential effects of 5-HT-TLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63(9):978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 37.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 39.Charney DS, Drevets WC. The neurobiological basis of anxiety disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 901–930. [Google Scholar]
- 40.Crino PB, Morrison JH, Hof PR. Monoaminergic innervation of cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston, MA: Birkhauser; 1993. pp. 285–312. [Google Scholar]
- 41.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 43.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE 2004. 2004;225:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 44.Pandey GN, Dwivedi Y. Noradrenergic function in suicide. Arch Suicide Res. 2007;11(3):235–246. doi: 10.1080/13811110701402587. [DOI] [PubMed] [Google Scholar]
- 45.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48(8):766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 46.Drevets WC, Gadde KM, Krishnan KRR. Neuroimaging studies of mood disorders. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2. Vol. 32. Oxford, England: Oxford University Press; 2004. pp. 461–490. [Google Scholar]
- 47.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205(4409):929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 48.Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrzeejewski PL, Dannals RF, Wagner HN, Jr, Robinson RG. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann Neurol. 1990;28(1):57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- 49.Ring HA, Bench CJ, Trimble MR, Brooks DJ, Frackowiak RS, Dolan RJ. Depression in Parkinson’s disease: a positron emission study. Br J Psychiatry. 1994;165(3):333–339. doi: 10.1192/bjp.165.3.333. [DOI] [PubMed] [Google Scholar]
- 50.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]