MMP9 modulates tight junction integrity and cell viability in human airway epithelia (original) (raw)
Abstract
The family of zinc- and calcium-dependent matrix metalloproteases (MMPs) play an important role in remodeling of the airways in disease. Transcriptional regulation by proinflammatory cytokines increases lymphocyte-derived MMP9 levels in the airway lumen of asthmatics. Moreover, the levels of the MMP9 inhibitor, tissue inhibitor of metalloprotease (TIMP1), are decreased leading to increased protease activity. The mechanism by which MMP9 activity leads to asthma pathogenesis and remodeling remains unclear. Using a model of well-differentiated human airway epithelia, we found that apical MMP9 significantly increases transepithelial conductance. Moreover, apical MMP9 treatment decreased immunostaining of tight junction proteins suggesting disruption of barrier function. Consistent with this, viruses gained access to the epithelial basolateral surface after MMP9 treatment, which increased infection efficiency. All of these effects were blocked by TIMP1. In addition, loss of epithelial integrity correlated with increased epithelial cell death. Thus we hypothesized that MMP9 exerts its effects on the epithelium by cleaving one or more components of cell-cell junctions and triggering anoikis. Taken together, these data suggest that a component of airway remodeling associated with asthma may be directly regulated by MMP9.
Keywords: protease, adhesion, cell death
the airway epithelium sits at the interface between the external environment and the body proper. With each inhaled breath, viruses, bacteria, pollutants, and allergens are introduced into the system, each with the potential to adversely affect the host. In the airways, mechanisms to reduce these hazards have evolved including mucociliary clearance, mucus secretion, as well as sneeze and cough. In addition, the polarized nature of the epithelium itself poses a structural barrier to inhaled challenges. At the apical pole of cell-cell interfaces, the tight junction, a large complex of proteins that functions as a fence, separates the apical and basolateral epithelial compartments and restricts flow through the paracellular space.
Two transmembrane components of tight junctions are occludin and the claudins. Both are tetraspanning proteins that extend their extracellular loops across neighboring cells. Claudins comprise a large family of 24 known members that form homo- and heterotypic associations with one another. In addition, claudins have recently been characterized as forming anion-/cation-selective pores within the paracellular space; selectivity is regulated by the specific claudins that associate across the membrane (4, 23, 25). Thus the claudin expression profile, as well as how claudins interact with one another, largely regulates the epithelial selectivity for cation/anion transport. In addition, together with occludin, the tight junction forms a seal, demarcating the apical and basolateral membranes. Electron microscopic analysis of the tight junction shows that this protein complex forms a continuous, anastomosing array of fibrils that circumscribes each epithelial cell (2, 3, 6). This circumferential seal serves as a barrier to inhaled components. Knockout studies have demonstrated that claudins are both necessary and sufficient for tight junction formation, but the role of occludin remains less clear. However, its involvement in barrier function is exemplified in a number of disease states associated with dysfunction of the blood-brain barrier; for example, Morgan et al. (31) found that inflammation associated with experimental autoimmune encephalomyelitis, a model of multiple sclerosis (MS), results in decreased phosphorylation of occludin that correlates with increased endothelial conductance and disease progression.
Similar to MS, increased inflammation and altered barrier function play important roles in asthma. Several studies have suggested that impaired epithelial barrier function and inadequate repair of damaged epithelia may be critical features of asthma. For example, even in relatively mild asthma, impairment of in vivo barrier function is evident as measured by clearance to the systemic circulation of inhaled Tc-99m-labeled DTPA (19). In addition, epithelial desquamation, previously thought to be an artifact of tissue processing, is now believed to be a structural alteration associated with other markers of stress and injury including expression of heat shock proteins, altered localization of the epidermal growth factor receptor, as well as markers of apoptosis and squamous metaplasia (8, 9, 34). These data suggest that epithelial damage and impaired barrier function are associated with asthma and increasing disease severity though the specific roles played by allergen- or viral-mediated injury, airway inflammation, and repair processes remain undefined.
Matrix metalloproteases (MMPs) are a large family of zinc- and calcium-dependent enzymes initially characterized by their capacity to degrade components of the ECM. MMP activity is not solely limited to the ECM but can also degrade other substrates. MMP activity is regulated at three levels: 1) gene transcription (growth factors, cytokines, and inflammatory components can each modulate MMP transcriptional activity); 2) processing (MMPs are synthesized as zymogens that require proteolytic cleavage of the prodomain for enzymatic activity); and 3) secretion of tissue inhibitors of metalloproteases (TIMPs), specific MMP inhibitors that bind in a 1:1 molar ratio and inactivate MMPs. Normally, these mechanisms strictly regulate MMP activity. However, in chronic inflammatory diseases, the environment may promote alterations in MMP activity.
In asthma, data suggest that airway inflammation supports increased MMP9 activity. MMP9, a 92-kDa protease also known as gelatinase B for its ability to cleave denatured collagen (i.e., gelatin), can be synthesized by a variety of cell types including neutrophils, macrophages, and epithelial cells. In addition, MMP9 knockout mice have less severe experimental arthritis and reduced experimental autoimmune encephalomyelitis suggesting a role for this MMP in inflammation and disease (13, 33). Consistent with this, immunostaining of bronchial biopsies as well as bronchoalveolar lavage fluid (BAL) studies have demonstrated increased MMP9 expression in the airways of asthmatic subjects (7, 10, 22, 29). Importantly, the ratio of MMP9 to TIMP1 was elevated in BAL from severe asthmatics (29), suggesting that MMP9 activity may become dysregulated in asthma both by increases in metalloprotease and decreases in its inhibitor. In addition, analysis of sputum collected during an asthma attack also demonstrates elevation of MMP9 (39, 40). Moreover, using a mouse model of asthma, Kumagai et al. (24) found that, similar to humans, antigen-sensitized mice responded to antigen challenge with an increase in BAL levels of MMP9. Together, these data suggest that MMP9 activity in the asthmatic lung is linked to airway inflammation; this may leave the airways primed for injury. However, the mechanism of MMP9-mediated airway injury remains unknown.
We hypothesized that MMP9 acts directly on the airway epithelium inducing epithelial damage and that this is one mechanism leading to the structural and functional changes associated with airway remodeling in asthma. To test this hypothesis, we used a primary model of well-differentiated human airway epithelia (HAE) grown at the air-liquid interface, a model that faithfully replicates in vivo airway epithelial structure and electrophysiological function (21). To assess the effects of MMP9 on epithelial health and barrier function, transepithelial conductance, resistance to viral infection, as well as immunolocalization of tight junction proteins were monitored. Our data suggest that MMP9 targets components at the tight junction and the adherens junction and that these effects on cell-cell junctions lead to anoikis, that is detachment-induced cell death, of airway epithelial cells.
MATERIALS AND METHODS
Primary cultures of well-differentiated HAE.
HAE from tracheas and bronchi of lungs removed for organ donation were isolated by enzyme digestion and cultured at the air-liquid interface as described previously (21). Briefly, airway epithelial cells were seeded onto collagen-coated semipermeable membranes (0.6 cm2, Millicell-HA; Millipore, Bedford, MA) and maintained at 37°C in a humidified atmosphere of 5% CO2 and air. Culture medium was as follows: 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12, 2% Ultroser G (BioSepra SA, Cergy-Saint-Christophe, France), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 μg/ml), fluconazole (2 μg/ml), and amphotericin B (1.25 μg/ml). After 24 h in culture, the medium is aspirated from the apical compartment, and the cells are grown at the air-liquid interface. Twenty-four to forty-eight hours postseeding, airway cells formed a confluent culture with electrically tight junctions. On day 3 postseeding, epithelia were generally flat and undifferentiated; by _days 3_-14, epithelial cells differentiate with a predominantly ciliated phenotype. Epithelia were studied at least 2 wk after seeding, when they had differentiated and had a ciliated apical surface.
Transepithelial conductance measurement.
Transepithelial electrical conductance was measured with a chopstick ohmmeter (World Precision Instruments, Sarasota, FL). PBS (300 μl) was applied apically for each measurement and then removed. An n of 6 epithelial cultures per group were measured at each time point.
MMP activation and epithelial treatment.
Recombinant human MMP2, MMP7, and MMP9 (R&D Systems, Minneapolis, MN) were activated with _p_-aminophenylmercuric acetate (APMA; Sigma, St. Louis, MO) at a final concentration of 1 mM at 37°C for 1 h (MMP2 and MMP7) or overnight (MMP9). Following activation, 2 μl of activated MMP (final concentration of 0.54 μM) was applied to the apical surface of epithelia and incubated at 37°C for different periods of time. Neutrophil elastase (0.54 μM) and trypsin (0.54 μM) treatments were performed as described for MMPs. An n of 6 epithelial cultures were treated for each condition; three different donors were studied.
Sphingomyelin-BODIPY assay.
Epithelia were chilled and maintained on ice for the duration of labeling. BODIPY-FL-C12-sphingomyelin (Invitrogen-Molecular Probes, Carlsbad, CA) was incubated with 10 mg of BSA in P buffer (145 mM NaCl, 10 mM HEPES, pH 7.4, 1 mM Na pyruvate, 10 mM glucose, 3 mM CaCl2) for 30 min on ice to generate sphingomyelin-BODIPY-BSA. Apical and basolateral surfaces of epithelia were washed with chilled P buffer three times. P buffer was aspirated from the apical surface, and the basolateral surfaces were maintained in P buffer for the duration of labeling. Sphingomyelin-BODIPY-BSA was applied to the apical surfaces of epithelia for 10 min after which it was aspirated and the apical surfaces washed twice with ice-cold P buffer. Epithelia were maintained on ice for 1 h and then fixed with 4% paraformaldehyde for 15 min. Epithelia were mounted onto glass slides, coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and analyzed, and images were acquired with an Olympus FluoView FV1000 confocal microscope. Four epithelia from each of three different donors were studied.
Epithelia were treated apically with active MMP9 overnight at 37°C before sphingomyelin-BODIPY labeling. Epithelia were treated apically with 8 mM EGTA in water for 5 min before sphingomyelin-BODIPY labeling.
Adenoviral infection.
Recombinant adenovirus carrying the enhanced green fluorescent protein (eGFP) transgene (Ad5-CMV-eGFP) was generated by the University of Iowa Gene Transfer Vector Core. Before infection, the apical surfaces of epithelia were pretreated with: 1) 8 mM EGTA in water for 5 min; 2) active MMP9 (0.54 μM) overnight at 37°C; 3) pro-MMP9 (0.54 μM) overnight at 37°C; 4) recombinant human TIMP1 (3.6 μM; R&D Systems) overnight at 37°C; or 5) overnight incubation with active MMP9 (0.54 μM) previously complexed to TIMP1 (3.6 μM) by incubation for 1–2 h at 37°C. Following pretreatment, epithelia were infected apically with virus at a multiplicity of infection of 100 for 1 h at 37°C. Virus was aspirated, and the apical surfaces were washed twice with media. Epithelia were returned to the incubator overnight. Infection efficiency was analyzed by looking for GFP-positive cells on the Olympus FluoView FV1000 confocal microscope. Three epithelia per condition from each of three different donors were studied.
Immunocytochemistry.
Airway epithelia were fixed with 4% paraformaldehyde for 15 min, washed extensively with PBS, and permeabilized with 0.2% Triton X-100 (Pierce, Rockford, IL). Nonspecific binding was blocked by a 1-h incubation in SuperBlock Blocking Buffer (Pierce), and the apical surfaces were incubated with primary antibody for 2 h at 37°C. Primary antibodies used were as follows: 1) rabbit anti-claudin-1 (1:100; Invitrogen-Zymed Laboratories, Carlsbad, CA); 2) rabbit anti-occludin (1:100; Invitrogen-Zymed Laboratories); and 3) rabbit anti-zonula occludens-1 (anti-ZO-1; 1:100; Invitrogen-Zymed Laboratories). Epithelia were counterstained with either ethidium bromide (Sigma, Minneapolis, MN) or TO-PRO-3 (Invitrogen-Molecular Probes) and analyzed on an Olympus FluoView FV1000 confocal microscope.
For immunostaining of activated caspase-3 (1:100 of rabbit anti-human antibody; R&D Systems), epithelia were fixed with 1% paraformaldehyde in methanol for 35 min at −20°C, washed with PBS, and permeabilized with 0.2% Triton X-100 (Pierce). Nonspecific binding was blocked with SuperBlock Blocking Buffer (Pierce) with 2% BSA for 1 h at room temperature. Primary antibody in SuperBlock with 2% BSA was applied apically, and epithelia were incubated overnight at 4°C. The following day, epithelia were washed with SuperBlock plus 2% BSA and incubated with secondary antibody (goat-anti-rabbit-A488, 1:200 in SuperBlock plus 2% BSA) for 3 h at room temperature protected from light. Following extensive washes with SuperBlock plus 2% BSA, epithelia were counterstained with TO-PRO-3 (Invitrogen-Molecular Probes), and inserts were then mounted onto glass slides and coverslipped using Vectashield mounting medium (Vector Laboratories). As a positive control for activated caspase-3 immunostaining, epithelia were treated apically with 100 μM staurosporine (Trevigen, Gaithersburg, MD) at 37°C for different periods of time. Four epithelia from each of six different donors were studied.
Assessment of cell loss.
Twenty-four hours following treatment, the apical surfaces of epithelia were washed to collect extruded cells. The PBS wash was then loaded into a cytospin apparatus (Shandon Cytospin 3) via a PBS prewetted filter card and spun at 1,000 rpm for 30 s and then spun onto a glass slide at 800 rpm for 5 min. The glass slides containing cells were then soaked in methanol for 20 min, washed with PBS, and coverslipped using Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories). The number of cells was then quantified by counting DAPI-positive nuclei.
As an additional quantification of cell loss, epithelia were processed for immunostaining as described above, and ZO-1 localization was used as an indication of cell borders. En face confocal images were taken of treated and control epithelia, and a grid system was used to quantify cell number. Fifty control and fifty MMP9-treated epithelial images were counted from five different epithelia.
Thrombin and sFasL treatment and control cell lines.
Epithelia were treated apically with 25 U/ml thrombin (Sigma, St. Louis, MO) and 1.1 μg/ml soluble Fas ligand (sFasL; R&D Systems) at 37°C for different periods of time. 293T cells served as a positive control cell line for thrombin activation of protease-activated receptor (PAR)-mediated apoptosis. A549 cells served as a positive control cell line for sFasL activation of Fas-mediated apoptosis. Three epithelia from each of eight different donors were studied.
RESULTS
MMP9 increases transepithelial conductance.
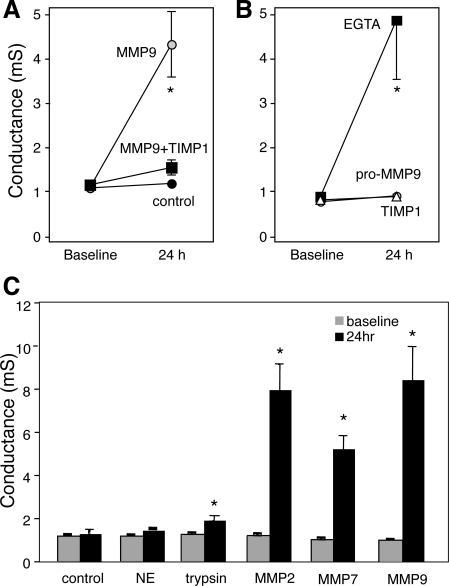
Reports associating increased MMP9 activity in the airway surface liquid (ASL) of inflamed lung (e.g., asthma, cystic fibrosis) with altered lung physiology in these conditions prompted us to determine the physiological and/or structural effect(s) of MMP9 on the airway epithelium. Using transepithelial electrical conductance, we tested whether MMP9 alters epithelial barrier function. Well-differentiated HAE grown at the air-liquid interface were treated apically with MMP9 (0.54 μM). Figure 1_A_ shows that, compared with control epithelia, MMP9 treatment increased conductance. Importantly, incubation of active MMP9 complexed with its inhibitor, TIMP1, blocks the effect. In addition, the MMP9-mediated compromise of barrier function is comparable to that resulting from EGTA treatment (Fig. 1_B_). By chelating calcium at the adherens junction, EGTA breaks down cell-cell junctions, leading to barrier function failure. These data suggest that MMP9 alters cell-cell adhesion. The MMP9 activity is required for this effect since the zymogen (proform) has no effect on conductance (Fig. 1_B_). In addition, treatment with TIMP1 also has no effect on conductance (Fig. 1_B_). HAE treated apically with 0.54 μM neutrophil elastase showed no change in conductance, whereas similar treatment with trypsin did lead to an increased conductance that was statistically significant (Fig. 1_C_). However, given the promiscuous nature of trypsin cleavage, this effect is likely to be nonspecific. Interestingly, apical treatment with either MMP2 or MMP7 at 0.54 μM also led to an increased conductance similar in scale to that following MMP9 treatment (Fig. 1_C_). Although the literature suggests a role for MMP9 in asthma, MMP2 may be involved in the resolution of inflammation rather than disease progression (12), and a function for MMP7 in asthma has not yet been identified. Therefore, we focused our studies on the effect of MMP9 on airway epithelia as it pertains to asthma.
Fig. 1.
Matrix metalloprotease-9 (MMP9) treatment results in increased transepithelial conductance. A: transepithelial electrical conductance increases following 24-h treatment with active MMP9. This effect is inhibited when MMP9 is complexed to its inhibitor, tissue inhibitor of metalloprotease (TIMP1). Control epithelial conductance does not change over this time period. B: transepithelial electrical conductance increases following EGTA treatment, whereas treatment with either pro-MMP9 or TIMP1 alone has no effect. C: similar to control epithelia, transepithelial electrical conductance does not change following 24-h treatment with neutrophil elastase (NE), however, trypsin as well as both MMP2 and MMP7 induce significant increases in conductance. *P < 0.05.
MMP9 increases permeability to macromolecules and viruses.
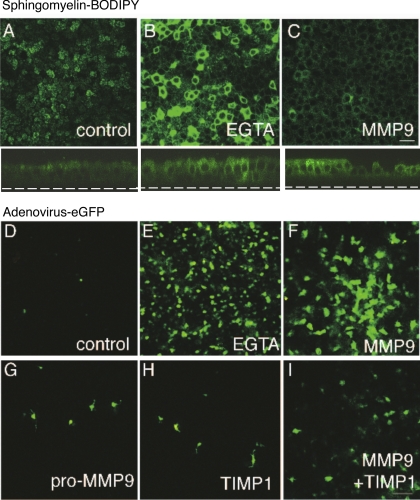
We next investigated whether increased electrical conductance correlates with increased epithelial permeability to macromolecules and viruses. HAE were apically treated with sphingomyelin conjugated to a green fluorophore, BODIPY. Sphingomyelin, a lipid component of the plasma membrane, easily intercalates into the outer leaflet of the bilayer where it freely diffuses and fluorescently labels the apical membrane. Tight junctions restrict the lipid from diffusing and labeling the basolateral membrane (Fig. 2_A_). When epithelial barrier function is impaired by EGTA (Fig. 2_B_) or MMP9 (Fig. 2_C_) treatment, diffusion of sphingomyelin is no longer limited, and the basolateral membrane becomes labeled. These data suggest that not only does MMP9 alter transepithelial electrical conductance, but also epithelial permeability to macromolecules and support our hypothesis that MMP9 weakens the barrier function of airway epithelia.
Fig. 2.
MMP9 treatment increases Epithelial permeability to macromolecules and viruses. A: sphingomyelin-BODIPY labels the apical surface of control epithelial, whereas pretreatment with EGTA (B) or MMP9 (C) leads to labeling of the basolateral membrane. Below A_–_C are the corresponding _x_-z images to better demonstrate membrane labeling. Scale bar, 10 μm. D: apical infection of control epithelia results in low infection efficiency as evidenced by few enhanced green fluorescent protein (eGFP)-positive cells. Pretreatment with either EGTA (E) or MMP9 (F) results in a significant increase in infection efficiency with many more cells expressing transgene. Pretreatment with either pro-MMP9 (G), TIMP1 (H), or active MMP9 complexed with TIMP1 (I) are similar to control with limited infection evident. Scale bar, 10 μm.
We and others have shown that basolateral localization of CAR, the coxsackievirus and adenovirus receptor, limits adenoviral infection of HAE. Therefore, we predicted that the effect of MMP9 on epithelial barrier function increases the vulnerability of the epithelium to adenoviral infection. To test this hypothesis, HAE were infected apically with a recombinant adenovirus containing the eGFP transgene. Figure 2_D_ shows that, in the absence of any treatment, airway epithelia are resistant to infection. Pretreatment with EGTA resulted in increased infection efficiency (Fig. 2_E_). Similarly, pretreatment with MMP9 increased infection (Fig. 2_F_). However, pretreatment with pro-MMP9, TIMP1, or the protease-inhibitor complex had little effect on infection efficiency, which was similar to control (Fig. 2, G_–_I). Taken together, these functional data suggest that MMP9 treatment increases infection efficiency by altering epithelial barrier function.
MMP9 alters the localization of tight junction proteins.
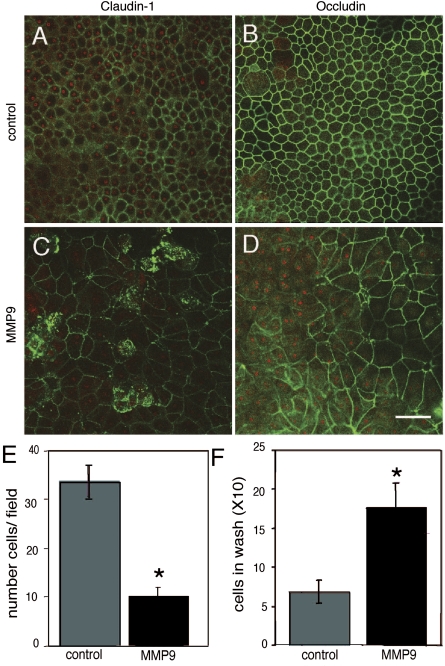
MMP9-induced impairment of barrier function suggests that it alters one or more components of cell-cell junctions. Because the tight junction is the apical-most complex, we first asked whether MMP9 modifies the localization of tight junction proteins. To test this possibility, HAE were treated with MMP9 and examined for localization of occludin and claudin-1 using specific antibodies and immunocytochemistry. Figure 3 shows en face confocal images of immunostained epithelia. Control epithelia demonstrate a chicken-wire pattern of staining typical of the tight junction (Fig. 3, A and B). MMP9 treatment changes this staining pattern (Fig. 3, C and D); although some cells retain membrane staining, intracellular immunofluorescence becomes apparent. These data suggest that MMP9 alters the localization of at least two components of the tight junction, claudin-1 and occludin.
Fig. 3.
MMP9 treatment alters claudin-1 and occludin immunolocalization and leads to cell loss. En face confocal images of immunolocalized claudin-1 (A) as well as occludin (B) in control epithelia demonstrate the characteristic chicken-wire pattern of staining (green). Following MMP9 treatment, this pattern is disrupted such that some claudin-1 (C) as well as occludin (D) staining is lost in some cells, whereas in others it becomes internalized. Red is ethidium bromide nuclear counterstain. Quantification of cell loss as assayed by counting cells per field (E) as well as the number of cells collected following an apical wash (F) suggest that MMP9 leads to cell death. Scale bar, 20 μm. *P < 0.05.
MMP9 results in cell loss.
In addition to altering the immunostaining pattern of tight junction components, we noticed that MMP9-treated epithelial cells were often larger and appeared fewer in number (Fig. 3, A and B, compared with Fig. 3, C and D); in some areas, epithelial cells were very large and disorganized relative to control. However, at no point were denuded areas apparent, but rather the surface remained covered by cells. Since the surface area of the filter support is constant, the larger cell size suggests a decrease in cell number. To quantify this effect, we used en face images of immunostained epithelia to quantify cell number. Figure 3_E_ shows that MMP9 treatment decreases cell number. As an additional test of cell loss, the apical surfaces of control and treated epithelia were washed to harvest and quantify dead cells. MMP9 led to an increased number of cells in the apical wash relative to control, suggesting that protease treatment increased cell loss from the epithelium (Fig. 3_F_).
Apical MMP9 treatment results in apoptosis of epithelial cells.
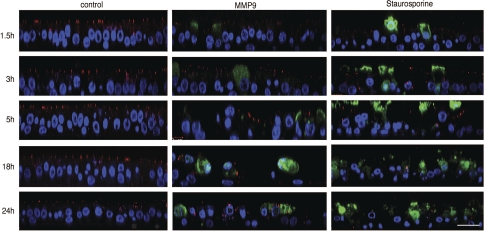
Decreased cell number following MMP9 treatment suggests the activation of cell death programs. To test this hypothesis, control and treated epithelia were immunostained using an activated caspase-3 antibody as a marker of apoptosis. In addition, epithelia were stained for ZO-1 to assess the state of the tight junction. Figure 4, left, shows a time series of confocal vertical sections from control epithelia. DAPI-stained nuclei show an organized epithelium and, at all times points examined, ZO-1 staining localizes to the apical-most pole of the epithelia. Importantly, no positive activated caspase-3 staining is evident, suggesting that under control conditions, airway epithelial turnover rate is very low. Figure 4, middle, shows vertical sections from MMP9-treated epithelia. Three significant changes relative to control are evident: 1) ZO-1 staining is absent in many regions, whereas in others it is no longer restricted to the apical-most aspect of the cells; 2) nuclei are not organized and are fewer in number; and 3) activated caspase-3-positively stained cells appear to be extruded apically. Figure 4, right, contains vertical sections of epithelia treated with staurosporine, a nonselective protein kinase inhibitor that induces apoptosis. This positive control demonstrated staining similar to that of MMP9-treated epithelia. Taken together, these data suggest that MMP9-mediated epithelial cell loss occurs via activation of apoptosis.
Fig. 4.
MMP9 treatment leads to cell death and apical extrusion. Vertical confocal images immunostained for zonula occludens-1 (ZO-1; red), activated caspase-3 (green), and counterstained with TO-PRO-3 (blue) were taken at different time points following treatment with MMP9 (middle) or staurosporine (right). MMP9 treatment leads to altered ZO-1 staining, activated caspase-3 staining of cells that appear to be extruded apically, and decreased TO-PRO-3-stained nuclei, suggesting loss of cells. A similar pattern of staining was evident following staurosporine treatment. Control, untreated epithelia (left) demonstrate ZO-1 localized at the apical-most aspect of cell-cell junctions where tight junctions reside; cells are tall and columnar. Scale bar, 20 μm.
MMP9-induced apoptosis is not mediated by Fas or PAR activation.
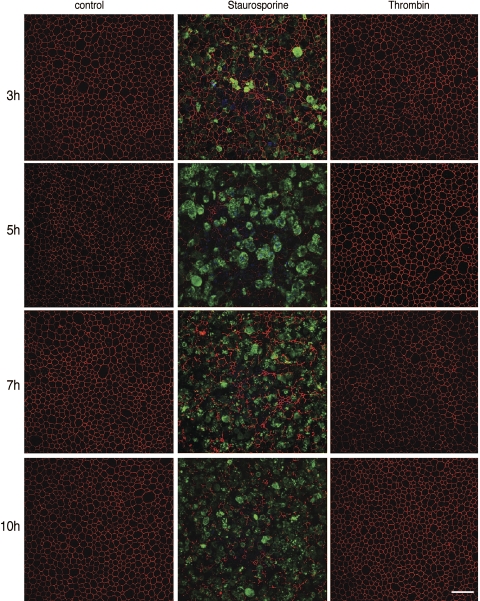
Various cellular signals can trigger apoptosis. We investigated apoptosis induced by both PARs and Fas because MMPs can cleave PAR and FasL (5, 18). We hypothesized that if apical thrombin and/or sFasL can recapitulate the effects of MMP9 on airway epithelia, activation of one of these pathways may explain the mechanism of MMP9-mediated disruption of epithelial integrity. We first investigated whether thrombin, a PAR ligand, triggers cell death. Figure 5 shows en face confocal images of control epithelia, epithelia treated with 25 U/ml thrombin, or staurosporine as a positive control. We found that, in contrast to staurosporine, thrombin failed to induce apoptosis in epithelia or change the tight junction staining profile. However, the same dose of thrombin induced apoptosis in 293T cells (Supplemental Fig. S1 available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). These data suggest that MMP9 cleavage of PAR from the apical surface is unlikely to be the mechanism of MMP9-induced cell death.
Fig. 5.
Thrombin treatment does not stimulate epithelial cell death. En face confocal images are of control, staurosporine and thrombin-treated epithelia immunostained for ZO-1 (red) and activated caspase-3 (green) at different time points posttreatment. Control epithelia demonstrate the characteristic pattern of ZO-1 staining and lack activated caspase-3 staining. Staurosporine treatment leads to a significant increase in activated caspase-3 staining, whereas thrombin treatment resembles that of control. Scale bar, 30 μm.
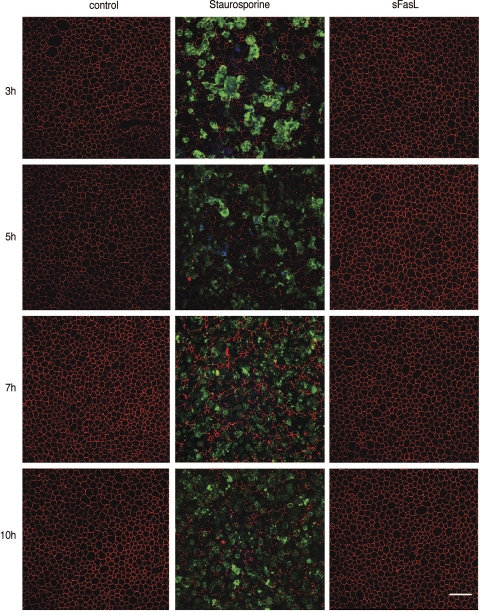
The classic Fas/FasL system involves activation of the Fas death receptor family and initiates cell death. FasL, either on the surface of cytotoxic T cells or in its cleaved sFasL, binds Fas triggering receptor/ligand clustering and subsequent activation of cell death. Two members of the MMP family, MMP3 and MMP7, cleave FasL, generating sFasL (35, 41). Thus we hypothesized that MMP9 activates cell death by cleavage of FasL. We tested whether sFasL activates cell death on HAE. Figure 6 shows en face confocal images at different time points posttreatment with MMP9, staurosporine, or sFasL. Treatment with sFasL had no effect on cell death or ZO-1 localization. In contrast, A549 cells that are responsive to sFasL demonstrated significant activated caspase-3 staining (Supplemental Fig. S2). Thus we conclude that, in HAE, apical sFasL does not trigger airway epithelial cell apoptosis. The lack of response could be due to the absence of Fas receptor, signaling pathway components, or other reasons beyond the scope of this study. However, the data suggest that MMP9-mediated cleavage of FasL is unlikely to be the mechanism for MMP9-induced apoptosis.
Fig. 6.
Soluble Fas ligand (sFasL) does not activate cell death in airway epithelia. En face confocal images are of epithelia treated with staurosporine or sFasL immunostained for ZO-1 (red) and activated caspase-3 (green) at different time points of treatment. Controls demonstrate a chicken-wire pattern of ZO-1 staining and lack staining for caspase-3, whereas staurosporine treatment leads to caspase-3 staining and altered ZO-1 localization. Epithelia treated with sFasL resemble controls. Scale bar, 30 μm.
MMP9 activates cell death via anoikis.
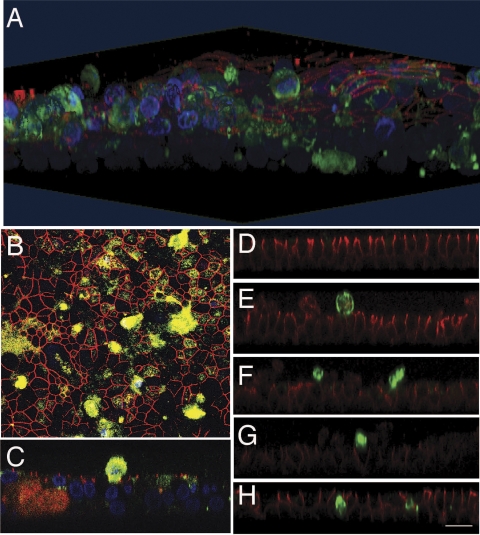
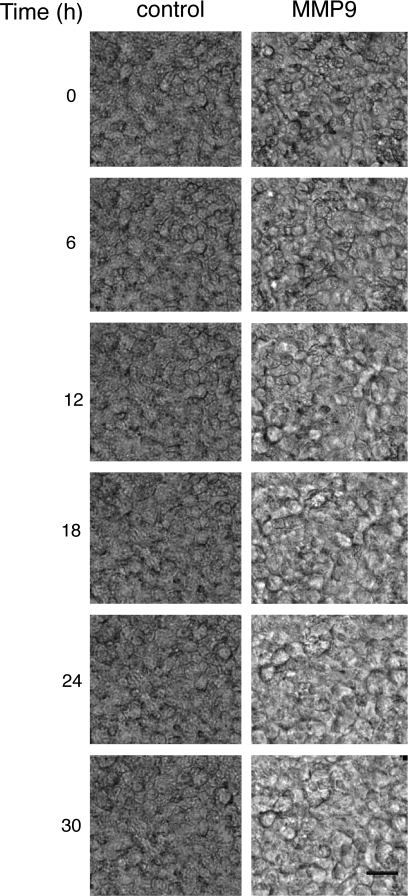
Our findings that MMP9 increases transepithelial conductance, alters the localization of occludin and claudin-1, and increases cell death suggest that the effect of MMP9 on the tight junction may activate cell death via a process called anoikis, programmed cell death due to cell detachment from the ECM (16, 36). Similar to MMP9, EGTA treatment of HAE also led to cell death. Figure 7, D_–_H, shows vertical confocal images of HAE at 0, 3, 6, 12, and 24 h post-EGTA treatment, respectively. As in MMP9-treated cells, EGTA treatment results in cell death (activated caspase-3-positive cells) with apical extrusion of dying cells. A three-dimensional reconstruction of an MMP9-treated epithelium stained for activated caspase-3, ZO-1, and DAPI further emphasizes this apical extrusion (Fig. 7_A_); a vertical section clearly demonstrates a dying extruded cell, above the plane of ZO-1 staining (Fig. 7_C_). When viewed en face, the extent of cell death is more evident, and many of the remaining cells appear to be “stretched” as if to fill the space once occupied by the extruded cells (Fig. 7_B_). Taken together, these data suggest that interventions leading to collapse of the epithelial barrier activate epithelial cell death. To investigate this effect over time, en face bright-field images were taken of MMP9-treated epithelia every 3 min for 30 h. Images were then consolidated into a movie (Supplemental Videos). Untreated control epithelia were imaged identically. Figure 8 shows a series of images from time 0 to 30 h. Whereas cells within control epithelia do not move or change shape significantly over the course of the experiment, apical extrusion of cells becomes apparent at ∼3 h in the MMP9 condition. Cellular extrusion and movement increase for the duration of the imaging such that by 30 h the size of cells increases significantly and cells are fewer in number compared with that of control. Taken together, these data suggest that MMP9 induces anoikis.
Fig. 7.
MMP9 and EGTA similarly stimulate airway epithelial cell death. A: confocal 3-dimensional reconstruction of an epithelium treated with MMP9 immunostained for ZO-1 (red) and activated caspase-3 (green) and counterstained with TO-PRO-3 (blue). Activated caspase-3-positively stained cells are extruded through the apical surface of the epithelium. B: en face confocal image of A, demonstrating that, in areas neighboring caspase-3 stained cells, cellular morphology appears stretched. C: vertical section of A, demonstrating a caspase-3-positive cell being extruded apically. D_–_H are vertical confocal images of an epithelium treated with EGTA and stained for E-cadherin (red) and activated caspase-3 (green) at different time points post-EGTA treatment (D, time 0; E, 3 h; F, 6 h; G, 12 h; H, 24 h). Similar to MMP9 treatment, EGTA leads to apical extrusion of caspase-3-positively stained cells. Scale bar, 20 μm.
Fig. 8.
MMP9 treatment leads to apical extrusion of airway epithelial cells. Time-lapse images taken at different time points over the course of 30 h of apical MMP9 treatment or untreated control. Over the course of the experiment, MMP9 treatment leads to apical extrusion of epithelial cells and increased cell size, whereas epithelial cells in the control epithelium do not appear to move and change shape significantly. Scale bar, 10 μm.
The adherens junction is altered by MMP9.
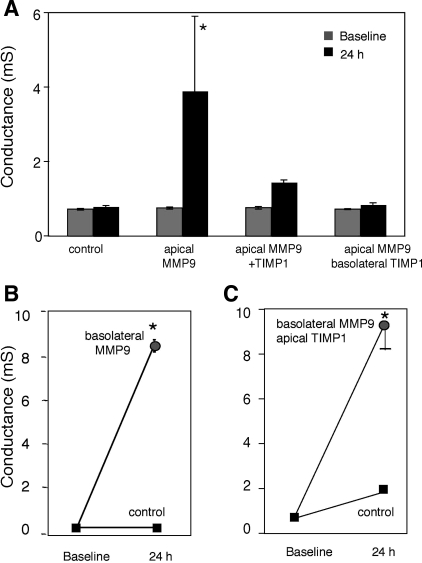
Our data suggested that apical MMP9 alters epithelial permeability and induces anoikis similar to EGTA. This led us to hypothesize that the effect of MMP9 on epithelial integrity is mediated via its effect on the adherens junction. The adherens junction is found just beneath the tight junction and plays an important role in cell-cell adhesion. Disruption of the adherens junction by calcium chelation, soluble cadherin peptide, cadherin antibody, or soluble CAR results in an increased epithelial conductance (32, 37, 45, 47). To investigate whether MMP9 affects the adherens junction, we took advantage of the ability to treat with the MMP9 inhibitor, TIMP1, either from the apical or basolateral surfaces. Figure 9_A_ shows that basolateral treatment with TIMP1 was sufficient to block the effect of apical MMP9, suggesting that inactivation of MMP9 below the tight junction is required to prevent the effect on permeability. Moreover, basolateral treatment with MMP9 resulted in increased permeability, and apical TIMP1 did not block this effect (Fig. 9, B and C). Together, these data suggest that although the cleavage at the tight junction possibly has no direct effect on conductance, it is likely required for the subsequent cleavage of cadherins that, similar to EGTA treatment, results in total collapse of cell junctions.
Fig. 9.
MMP9 treatment alters Adherens junction leading to increased transepithelial conductance. A: untreated control epithelial electrical conductance does not change significantly in 24 h, whereas apical treatment with MMP9 leads to a significant increase. Apical treatment with the complex of active MMP9 with TIMP1 inhibits the effect on conductance. Similarly, treatment with apical MMP9 and simultaneous treatment with basolateral TIMP1 has no effect on conductance. B: electrical conductance increases following basolateral MMP9 treatment, whereas that of controls remains steady over 24 h. C: simultaneous treatment with basolateral MMP9 and apical TIMP1 still results in conductance increase relative to control. *P < 0.05.
DISCUSSION
In this study, we describe a mechanism of epithelial injury in which a proteolytic inflammatory component associated with asthma targets epithelial cell-cell junctions, impairing barrier function. Once barrier function is lost, the airways become vulnerable to inhaled environmental agents (allergens, viruses, and bacteria). Elevated MMP9 in asthmatic BAL suggests that the apical surfaces of inflamed airways bathe in this protease. Our electrical conductance data together with the immunolocalization studies suggest that apical MMP9 has functional and structural consequences on the airway epithelium itself leading to remodeling changes associated with disease and poorly controlled asthmatic inflammation. However, MMP9 could have multiple cellular effects beyond those tested in these studies.
Apical treatment with MMP9 significantly increases transepithelial conductance as well as alters the immunolocalization of the tight junction proteins, claudin-1 and occludin. The ability of TIMP1 to block the effect on conductance demonstrates that proteolysis mediated by MMP9 leads to these effects. For MMP9, the target protein(s) in airway epithelia remain unknown, but clearly the effect on integrity leave the epithelium vulnerable to viral infection. When examined in the context of inflammatory airways disease, increased susceptibility to viral infection has significant physiological impacts.
In addition to altered epithelial integrity, MMP9-treated epithelial cells increased in size relative to control. This altered morphology was associated with decreased cell number and increased caspase-3 staining. Together, the data suggest that MMP9 proteolytic activity leads to decreased cell viability, either directly or indirectly. We tested two potential mechanisms leading to epithelial apoptosis: Fas and PAR receptor activation. Neither treatment with sFasL nor thrombin stimulated apoptosis in airway epithelia, suggesting that the affect of MMP9 on cell death is not mediated indirectly via either of these pathways. Moreover, our finding that apical TIMP1/basolateral MMP9 treatment leads to increased conductance whereas apical MMP9/basolateral TIMP1 does not, suggests that MMP9-mediated proteolysis within cellular junctions may directly activate cell death via anoikis.
Interestingly, apical MMP9 treatment resulted in epithelial changes surprisingly similar to those seen following treatment with EGTA with one key difference: it takes hours for the effects of MMP9 on conductance and anoikis to become evident. In addition, the ability of basolateral TIMP1 to block the effect of apical MMP9 on conductance suggests that the predominant effect of MMP9 is on the adherens junction. How MMP9 in the ASL gains access to the adherens junction requires further study. Some studies have suggested that cellular adhesion complexes are not static structures but rather undergo constant remodeling (28, 38). If so, it is feasible that as the junctions remodel, ASL components slowly leak between cells, gaining access to the adherens junction. Regardless of the mechanism of access, once at the adherens junction, the effects of MMP9 on cell adhesion and survival are clear: MMP9 increases transepithelial conductance and leads to activated caspase-3 staining and cell extrusion suggestive of anoikis.
Anoikis is defined as detachment-induced apoptosis, that is to say, the activation of cell death programs via the loss of cell-matrix and/or cell-cell anchorage (14, 15, 17). Cells undergoing classic, receptor-mediated apoptosis become detached from their neighbors and underlying matrix components as well. Thus it becomes challenging to experimentally differentiate between cell death induced by receptor activation and that which is detachment induced. Therefore, at some level, it is a chicken-and-egg question: did the cell undergo programmed cell death because it detached from its neighbors and matrix, or did the initiation of programmed cell death lead to cellular detachment from its neighbors and matrix? Although our study does not solve this conundrum, the data clearly demonstrate that both EGTA and MMP9 treatment induce a program of cell death associated with disruption of the adherens junction.
In addition, the data demonstrate that, as a consequence of cell death and extrusion, the remaining epithelial cells become larger in size, appearing to stretch and flatten to cover the area vacated by extruded, dead, and dying cells. These phenotypic changes of columnar epithelial cells taking on a flattened appearance are reminiscent of remodeling changes associated with airway disease. Although the current study does not address other components of remodeling (fibrosis, goblet cell hyperplasia, and epithelial thickening), one can imagine a scenario where cell loss triggers basal and/or stem cells in the airways to undergo mitosis in an attempt to repopulate the epithelium. Cellular proliferation, in the context of an inflammatory environment as seen in the asthmatic lung, is unable to recapitulate the normal epithelial complement of cell types but rather may predominantly generate goblet cells (43), perpetuating airway remodeling.
Another hallmark of airway remodeling associated with asthma is subepithelial fibrosis, yet how inhaled allergens and the subsequent induction of an inflammatory response leads to fibrosis is not clearly understood. The significance of the contribution of MMP9 in remodeling of the asthmatic lung is exemplified by the MMP9 knockout mouse. These null mice are fertile and demonstrate no obvious phenotypic defects (44). Lim et al. (26) showed that wild-type mice sensitized and repeatedly challenged with OVA demonstrated a significant increase in fibrosis compared with similarly treated MMP9 knockouts. In addition, total lung collagen was decreased in the challenged MMP9 knockout mice. MMP9 may also play a role in eosinophil infiltration into the BAL since this effect was significantly decreased in the knockout mice compared with controls. Cataldo et al. (11) found less peribronchial inflammation in the MMP9 knockout mice following OVA exposure compared with the wild-type mice.
Given these findings, one could hypothesize that if MMP9 plays a central role in airway remodeling in asthma, blocking MMP9 activity might serve to inhibit these effects. Kumagai et al. (24) showed that allergen challenge leads to cellular changes in the BAL from sensitized mice. Specifically, eosinophils, lymphocytes, macrophages, and neutrophils increased at different time points following OVA challenge, whereas no cellular changes were evident in the BAL from mice challenged with saline. These changes correlated with gelatin zymography and Western blot analysis demonstrating increased MMP9 in BAL following OVA challenge in mice (24). Importantly, intranasal instillation of recombinant human TIMP1, TIMP2, or a synthetic MMP inhibitor (R-94138) reversed these cellular changes, strongly suggesting that inflammatory cell infiltration into the airway lumen is, at least in part, regulated by MMP9 activity.
An important role of MMP inhibition in asthma is further supported by a study demonstrating a TIMP1 polymorphism (C536T) associated with asthma in women. Whether this polymorphism alters TIMP1 activity or ability to recognize and/or bind MMPs remains unclear (27). Whereas a polymorphism in the promoter region of MMP9 (C1562T) increases promoter activity, a small study of a Czechoslovakian population showed no association with asthma (48), however, a link to the development of emphysema has been demonstrated (20, 30). Together, these data support a role for MMP9 and TIMP1 in airway disease and remodeling.
The current study highlights some significant aspects of airway epithelial biology and disease. First, consistent with previous studies (1, 49), the lack of activated caspase-3 staining in control epithelia demonstrates that healthy epithelial cells do not turn over rapidly. The ability of an inflammatory mediator such as MMP9 to trigger cell death within hours of treatment suggests that, in the diseased lung that might be chronically or sporadically (e.g., following allergen exposure) inflamed, cellular turnover is greatly enhanced. The mechanisms of increased cell death and subsequent activation of mitosis may both depend on disruption of epithelial integrity, as this would allow for factors in the ASL to access the basolateral compartment. Some ASL factors that have been previously characterized, including growth factors that activate a family of receptor tyrosine kinases (erbB), can potently stimulate cellular proliferation (42, 46). In the context of disease and inflammation, stimulated cellular proliferation may potentiate remodeling changes associated with disease and decreased lung function. Together, loss of epithelial barrier function, inflammation, and increased cellular turnover may create an environment that fosters structural remodeling changes associated with disease. Understanding the mechanisms regulating these changes identifies targets with potential for therapeutic interventions.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant R01-HL-075276-04.
Supplementary Material
[Supplemental Figures and Videos]
Acknowledgments
We thank the University of Iowa Cells and Models Facility for the generation of primary human airway epithelial cultures.
Present address of P. D. Vermeer: Sanford Research, 1400 West 22nd St., Sioux Falls, SD 57105.
REFERENCES
- 1.Adamson IY Development of lung structure. In: The Lung: Scientific Foundations. New York: Raven, 1991, p. 663–670.
- 2.Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol 248: 261–298, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol 16: 140–145, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens 16: 459–464, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci 120: 921–928, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Balda MS, Matter K. Tight junctions. J Cell Sci 111: 541–547, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Belleguic C, Corbel M, Germain N, Lena H, Boichot E, Delaval PH, Lagente V. Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy 32: 217–223, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Bertorelli G, Bocchino V, Zhuo X, Chetta A, Del Donno M, Foresi A, Testi R, Olivieri D. Heat shock protein 70 upregulation is related to HLA-DR expression in bronchial asthma. Effects of inhaled glucocorticoids. Clin Exp Allergy 28: 551–560, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanovic R, Holgate ST, Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol 27: 179–185, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Cataldo D, Foidart JM, Lau L, Bartsch P, Djukanovic R, Louis R. Induced sputum: comparison between isotonic and hypertonic saline solution inhalation in patients with asthma. Chest 120: 1815–1821, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cataldo DD, Tournoy KG, Vermaelen K, Munaut C, Foidart JM, Louis R, Noel A, Pauwels RA. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am J Pathol 161: 491–498, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 3: 347–353, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol 5: 257–263, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol 9: 701–706, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 13: 555–562, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore AP Anoikis. Cell Death Differ 12, Suppl 2: 1473–1477, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Grossmann J Molecular mechanisms of “detachment-induced apoptosis–Anoikis.” Apoptosis 7: 247–260, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 231: 20–27, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Ilowite JS, Bennett WD, Sheetz MS, Groth ML, Nierman DM. Permeability of the bronchial mucosa to 99mTc-DTPA in asthma. Am Rev Respir Dis 139: 1139–1143, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Joos L, He JQ, Shepherdson MB, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet 11: 569–576, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Karp PH, Moninger T, Weber SP, Nesselhauf TS, Launspach J, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods and evaluation of primary cultures. In: Epithelial Cell Culture Protocols, edited by Wise C. Totowa, NJ: Humana, 2002, p. 115–137. [DOI] [PubMed]
- 22.Kelly EA, Busse WW, Jarjour NN. Increased matrix metalloproteinase-9 in the airway after allergen challenge. Am J Respir Crit Care Med 162: 1157–1161, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Koval M Claudins–key pieces in the tight junction puzzle. Cell Commun Adhes 13: 127–138, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol 162: 4212–4219, 1999. [PubMed] [Google Scholar]
- 25.Landau D Epithelial paracellular proteins in health and disease. Curr Opin Nephrol Hypertens 15: 425–429, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Lim DH, Cho JY, Miller M, McElwain K, McElwain S, Broide DH. Reduced peribronchial fibrosis in allergen-challenged MMP-9-deficient mice. Am J Physiol Lung Cell Mol Physiol 291: L265–L271, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lose F, Thompson PJ, Duffy D, Stewart GA, Kedda MA. A novel tissue inhibitor of metalloproteinase-1 (TIMP-1) polymorphism associated with asthma in Australian women. Thorax 60: 623–628, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci 117: 1247–1257, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Mattos W, Lim S, Russell R, Jatakanon A, Chung KF, Barnes PJ. Matrix metalloproteinase-9 expression in asthma: effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest 122: 1543–1552, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Minematsu N, Nakamura H, Tateno H, Nakajima T, Yamaguchi K. Genetic polymorphism in matrix metalloproteinase-9 and pulmonary emphysema. Biochem Biophys Res Commun 289: 116–119, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H, Smith T, Staddon JM. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neuroscience 147: 664–673, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Noe V, Willems J, Vandekerckhove J, Roy FV, Bruyneel E, Mareel M. Inhibition of adhesion and induction of epithelial cell invasion by HAV-containing E-cadherin-specific peptides. J Cell Sci 112: 127–135, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4: 617–629, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Polosa R, Puddicombe SM, Krishna MT, Tuck AB, Howarth PH, Holgate ST, Davies DE. Expression of c-erbB receptors and ligands in the bronchial epithelium of asthmatic subjects. J Allergy Clin Immunol 109: 75–81, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 9: 1441–1447, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev 24: 425–439, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Rothen-Rutishauser B, Riesen FK, Braun A, Gunthert M, Wunderli-Allenspach H. Dynamics of tight and adherens junctions under EGTA treatment. J Membr Biol 188: 151–162, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci USA 100: 3971–3976, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol 152: 1183–1196, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka H, Miyazaki N, Oashi K, Tanaka S, Ohmichi M, Abe S. Sputum matrix metalloproteinase-9: tissue inhibitor of metalloproteinase-1 ratio in acute asthma. J Allergy Clin Immunol 105: 900–905, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys 408: 155–161, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Vermeer PD, Einwalter LA, Moninger T, Rokhlina T, Kern JA, Zabner J, Welsh M. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422: 322–326, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol 28: 286–295, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93: 411–422, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J Biol Chem 277: 23709–23713, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci 65: 1566–1584, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winter MC, Shasby SS, Ries DR, Shasby DM. PAR2 activation interrupts E-cadherin adhesion and compromises the airway epithelial barrier: protective effect of β-agonists. Am J Physiol Lung Cell Mol Physiol 291: L628–L635, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Henney A, Eriksson P, Hamsten A, Watkins H, Ye S. Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12.2–13.1. Hum Genet 105: 418–423, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Zsengeller ZK, Halbert C, Miller AD, Wert SE, Whitsett JA, Bachurski CJ. Keratinocyte growth factor stimulates transduction of the respiratory epithelium by retroviral vectors. Hum Gene Ther 10: 341–353, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Figures and Videos]