A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates (original) (raw)
Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates serum LDL cholesterol (LDL-C) by interacting with the LDL receptor (LDLR) and is an attractive therapeutic target for LDL-C lowering. We have generated a neutralizing anti-PCSK9 antibody, mAb1, that binds to an epitope on PCSK9 adjacent to the region required for LDLR interaction. In vitro, mAb1 inhibits PCSK9 binding to the LDLR and attenuates PCSK9-mediated reduction in LDLR protein levels, thereby increasing LDL uptake. A combination of mAb1 with a statin increases LDLR levels in HepG2 cells more than either treatment alone. In wild-type mice, mAb1 increases hepatic LDLR protein levels ≈2-fold and lowers total serum cholesterol by up to 36%: this effect is not observed in LDLR−/− mice. In cynomolgus monkeys, a single injection of mAb1 reduces serum LDL-C by 80%, and a significant decrease is maintained for 10 days. We conclude that anti-PCSK9 antibodies may be effective therapeutics for treating hypercholesterolemia.
Keywords: antibody, LDL-C, LDLR, PCSK9, hypercholesterolemia
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has been implicated as an important regulator of LDL metabolism (1, 2). Human genetic studies provide strong validation for the role of PCSK9 in modulating LDL cholesterol (LDL-C) levels and the incidence of coronary heart disease (CHD) in man. Gain-of-function (GOF) mutations in the PCSK9 gene are associated with elevated serum LDL-C levels (>300 mg/dL) and premature CHD (3), whereas loss-of-function (LOF) mutations are associated with low serum LDL-C (≤100 mg/dL) (4). Strikingly, subjects harboring the heterozygous LOF mutations exhibited an 88% reduction in the incidence of CHD over a 15-year period relative to noncarriers of the mutations (5). Moreover, despite a complete loss of PCSK9 and serum LDL-C of <20 mg/dL, the 2 subjects carrying compound heterozygote LOF mutations appear healthy (6, 7).
PCSK9 belongs to the subtilisin family of serine proteases and consists of a prodomain, catalytic domain, and C-terminal V domain (8). Expressed highly in the liver, PCSK9 is secreted after autocatalytic cleavage of its zymogen form (1). The prodomain remains noncovalently associated with the catalytic domain and seems to inhibit further proteolytic enzyme activity (8, 9). Secreted PCSK9 modulates LDL-C levels by posttranslational downregulation of hepatic LDL receptor (LDLR) protein (1). The precise mechanism is unknown, but a direct interaction between repeat A of the LDLR EGF homology domain and the PCSK9 catalytic domain is required (10, 11). Proteolytic cleavage of the LDLR by PCSK9 does not occur (12, 13); rather, the PCSK9:LDLR complex is endocytosed and directed to the endosome/lysosome compartment for degradation (14, 15). Current understanding of the LDLR pathway asserts that apolipoprotein B (apoB) and E (apoE) containing lipoprotein particles endocytosed with the LDLR are transported to the acidic environment of the endosome, where they dissociate from the receptor and are subsequently catabolized in lysosomes, while the LDLR recycles back to the cell surface (16). By interacting with the LDLR, PCSK9 plays a regulatory role in this cycle and directly affects the maintenance of cellular and whole-body cholesterol balance.
Various approaches for inhibiting PCSK9 have been reported, including strategies based on gene silencing by siRNA or antisense oligonucleotides, and disruption of protein–protein interaction by antibodies and LDLR subfragments (17–22). Here, we report on a neutralizing monoclonal antibody against PCSK9 with cholesterol-lowering efficacy in mice and nonhuman primates. The antibody, termed mAb1, disrupts the interaction of PCSK9 with the LDLR, causing increased hepatic LDLR protein expression and LDL uptake. Statins coordinately regulate expression of hepatic LDLR and PCSK9 (23), and it has been hypothesized that the statin-mediated increase of circulating PCSK9 levels may attenuate their cholesterol-lowering effect (1, 24). We show here that mAb1 functions cooperatively with statins to enhance LDLR regulation in vitro.
Results
Generation of mAb1, a Fully Human Monoclonal Antibody to Human PCSK9.
Mice engineered to express arrays of fully human IgGκ and IgGλ antibodies were immunized with soluble, full-length, mature human PCSK9 (huPCSK9). Enriched B cells from immune animals were fused to nonsecretory myeloma cells to generate hybridomas. Three thousand hybridomas were evaluated for cross-reactivity to mouse PCSK9, for binding to the huPCSK9 GOF mutant D374Y (used in subsequent screening assays), and for their ability to block the binding of PCSK9 to LDLR. We identified 85 hybridoma lines that bound both WT and D374Y PCSK9, blocked the binding of PCSK9 to LDLR, and cross-reacted to varying degrees with murine PCSK9. These lines were ranked using a cell-based LDL uptake assay, and a panel of the most potent lines was subcloned for further characterization, yielding the antibody termed “mAb1.” Both the IgG2 and IgG4 isotypes of mAb1 were used in subsequent studies, with no apparent differences.
mAb1 is a High-Affinity Antagonist of PCSK9 Function.
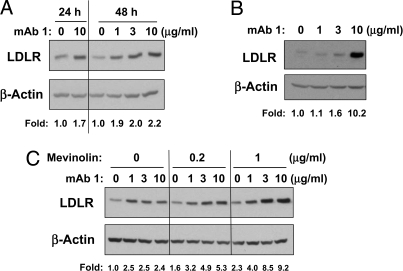
Functional properties of mAb1 were characterized using in vitro assays. Equilibrium dissociation constants (KD) were 4 pM, 4 pM, and 160 pM for human, cynomolgus, and mouse PCSK9, respectively. mAb1 blocked huPCSK9 binding to the LDLR, with an IC50 of 2.08 ± 1.21 nM (n = 3) [supporting information (SI) Fig. S1_A_]. The PCSK9-mediated reduction of LDL uptake in HepG2 cells was reversed by mAb1, with an EC50 of 174.3 ± 11.8 nM (n = 3) (Fig. S1_B_). Incubation of HepG2 cells with 1, 3, or 10 μg/mL of mAb1 increased LDLR protein in cell lysates by 1.7–2.2-fold relative to untreated cells when cultured for 24–48 h (Fig. 1A). The baseline level of secreted PCSK9 in HepG2 cells was 932.1 ± 155.3 ng/mL (n = 3) after 48 h in culture. In HepG2 cells overexpressing huPCSK9, the level of secreted PCSK9 reached 3,595 ± 909 ng/mL after 24 h in culture (n = 3). mAb1 at 10 μg/mL was sufficient to neutralize the high levels of secreted PCSK9 in these cells, and LDLR protein levels were increased 10.2-fold relative to untreated overexpressing cells (Fig. 1B).
Fig. 1.
In vitro effect of mAb1. (A) mAb1 induced LDLR protein in HepG2 cells after 24 and 48 h, as assessed by Western blot analysis. (B) mAb1 increased LDLR protein in a HepG2 stable cell line overexpressing PCSK9. Cells were incubated with mAb1 for 24 h. The fold change in a and b was calculated as the ratio of LDLR in the presence of mAb1 to LDLR in the absence of mAb1, after normalization of LDLR to β-actin in each lane. (C) A combination of mAb1 with mevinolin induces LDLR protein more than either treatment alone. HepG2 cells were incubated with mAb1 (1, 3, or 10 μg/mL) with or without mevinolin (0.2 or 1 μg/mL) for 48 h, followed by Western blot analysis of whole-cell lysates. The fold change in c was calculated as the ratio of LDLR in the presence of mAb1 and/or mevinolin to LDLR in the untreated cells, after normalization of LDLR to β-actin in each lane. All data shown are representative of at least 3 separate studies.
Statins induce both LDLR and PCSK9 expression (23); therefore, combining mAb1 with a statin may be more effective at regulating the LDLR than either treatment alone. To test this hypothesis, we treated HepG2 cells with mevinolin (a statin) and/or mAb1 and assessed levels of LDLR protein in cell lysates and secreted PCSK9 in the medium (Fig. 1C). Mevinolin alone (0.2 or 1 μg/mL) increased LDLR protein levels 1.6- or 2.3-fold after 48 h, and secreted PCSK9 levels increased ≈1.3-fold above those in untreated cells, to 1,176.4 ± 173.0 and 1,239.8 ± 150.7 ng/mL (n = 3), respectively. mAb1 alone (1, 3, or 10 μg/mL) increased LDLR protein levels ≈2.5-fold, irrespective of dose. In the presence of 0.2 or 1 μg/mL mevinolin, mAb1 dose-dependently increased LDLR protein up to 5.3-fold or 9.2-fold relative to untreated cells, respectively. These data indicate that mAb1 has the ability to act at least additively in conjunction with a statin.
mAb1 Sterically Hinders the Binding of PCSK9 to LDLR.
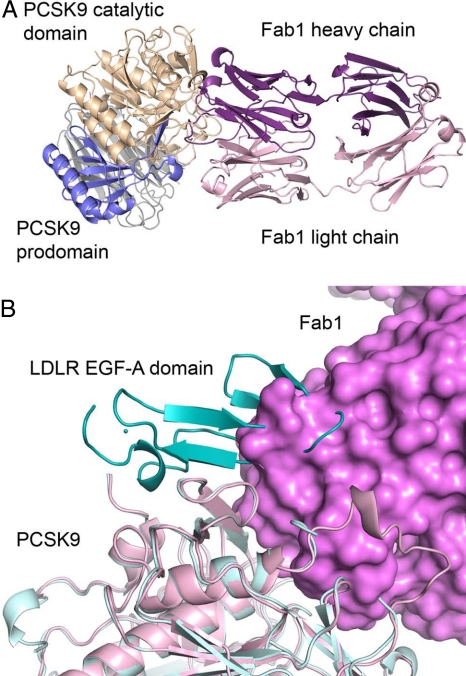
The crystal structure of PCSK9 in complex with a Fab fragment from mAb1 (Fab1) was solved to 2.3 Å resolution. A summary of the crystallography data can be found in Table S1. mAb1 binds to the catalytic domain of PCSK9 (Fig. 2A and Fig. S2). The complementary determining regions of the mAb1 heavy chain fill a concave surface of PCSK9 on top of the catalytic site and form numerous hydrogen-bond and hydrophobic interactions with PCSK9. The light chain of mAb1 is more distant and does not form direct hydrogen-bond interactions with PCSK9. In addition to interacting with a large number of amino acid residues of the catalytic domain, mAb1 interacts with the prodomain, namely amino acid residues of the C terminus that bind within the catalytic site and residues from the N terminus. By comparing the PCSK9:Fab1 complex with the structure of PCSK9 bound to the EGF-AB domain of the LDLR (11), a mechanism for mAb1 action may be postulated. Fab1 and the EGF-A domain of the LDLR bind to adjacent sites on PCSK9 and are sterically hindered from simultaneously binding to the PCSK9 protein (Fig. 2B). Thus, by blocking the interaction of PCSK9 with the LDLR, mAb1 inhibits PCSK9-mediated degradation of the LDLR.
Fig. 2.
Structure of the PCSK9:Fab1 complex. (A) Fab1 binds to PCSK9 at the catalytic site and interacts with residues from both the prodomain and catalytic domain. The binding of Fab1 to PCSK9 buries 2307 Å2 total surface area. (B) Superposition of the PCSK9:Fab1 complex (pink and magenta) and the PCSK9:EGF-AB complex (11) (light cyan and cyan). Fab1 overlaps with the C-terminal side of the EGF-A domain from the LDLR.
mAb1 Increases Hepatic LDLR and Lowers Serum Cholesterol in Mice.
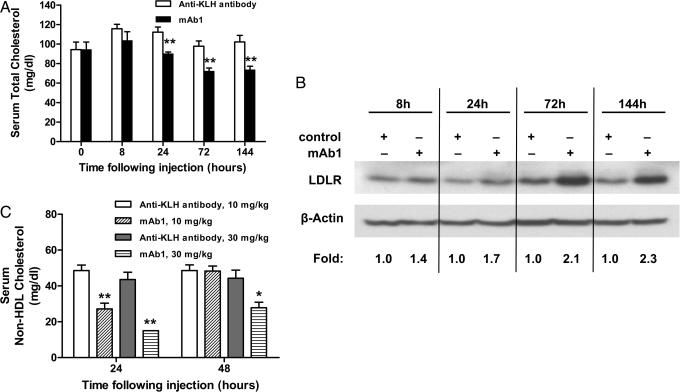
We used C57BL/6 mice to assess whether mAb1 regulates the LDLR and lowers serum cholesterol in vivo. Mice (n = 7 per treatment group) were given a single i.v. injection of mAb1 (10 mg/kg) or a control antibody against keyhole limpet hemocyanin (KLH). mAb1 significantly reduced total cholesterol (TC) levels by 20% (P = 0.002), 26% (P = 0.0014), and 28% (P = 0.0029) at 24, 72, and 144 h after injection, respectively (Fig. 3A). Because mice carry the majority of their circulating cholesterol in HDL particles containing apoE (25), the changes in TC reflected similar lowering in serum HDL cholesterol (HDL-C) (31% at 72 h, P = 0.006; and 24% at 144 h, P = 0.007). mAb1 treatment increased hepatic LDLR protein levels by as much as 2.3-fold relative to control animals, consistent with the observed changes in serum TC and with the hypothesized mechanism of action (Fig. 3B). Serum levels of mAb1 exceeded endogenous steady-state serum PCSK9 levels (≈50 ng/mL) by more than 180-fold throughout the study (Fig. S3).
Fig. 3.
Changes in serum total cholesterol after i.v. administration of mAb1 to C57BL/6 mice. (A) Injection of 10 mg/kg mAb1 caused a statistically significant decrease in TC. Results are expressed as the mean ± SEM, n = 7 per group. (B) mAb1 induced hepatic LDLR protein expression, as assessed by Western blot analysis of pooled liver lysates. The fold change was calculated as the ratio of LDLR in the presence or absence of mAb1 for each time point after normalization of the LDLR to β-actin in each lane. (C) mAb1 dose-dependently lowered serum non-HDL-C in mice expressing huPCSK9 by adeno-associated virus (AAV) (n = 7 per treatment group). Results are expressed as the mean ± SEM. *P < 0.05; **P < 0.01 vs. anti-KLH control antibody at the same time point and dose.
When mice were treated with increasing doses of mAb1, we observed significantly lower serum TC levels 3 days after injection in all groups, calculated as percentage change relative to control animals: 26% (P = 0.0046), 28% (P = 0.0031), and 36% (P = 0.0002) at 3, 6, or 10 mg/kg, respectively (Fig. S4). At postinjection day 9, statistically significant TC lowering was observed only in animals treated with 6 mg/kg mAb1 (20%, P = 0.0068) or 10 mg/kg mAb1 (30%, P = 0.0001). By day 12, TC levels in all treatment groups were similar to those observed in control animals. Thus, TC lowering was reversible, and the duration was dose dependent.
Next, we evaluated mAb1 in LDLR−/− mice, a model characterized by markedly elevated levels of serum LDL-C (26). Using a dose of mAb1 that was efficacious in C57BL/6 mice (10 mg/kg, i.v.), we did not detect a significant effect of mAb1 on LDL-C levels at either 24 h (158 ± 8 mg/dL vs. 172 ± 14 mg/dL in control animals, P = 0.4000), or at 72 h after injection (206 ± 17 mg/dL vs. 208 ± 24 mg/dL, P = 0.9500) (mean ± SEM, n = 6 animals per group). Thus, the presence of the LDLR is requisite for TC lowering by mAb1 in mice.
To assess the effects of mAb1 on huPCSK9 in vivo, we generated huPCSK9-C57BL/6 mice that exhibited a circulating huPCSK9 level of ≈12 μg/mL and markedly elevated serum levels of non-HDL-C (40 ± 4 mg/dL vs. <10 mg/dL in WT mice), HDL-C (161 ± 6 mg/dL vs. 81 ± 6 mg/dL), and TC (201 ± 10 mg/dL vs. 94 ± 8 mg/dL). After a single i.v. injection of either 10 or 30 mg/kg mAb1, a dose-dependent lowering of non-HDL-C was observed 24 h after injection (Fig. 3C). Compared with control animals, mAb1 lowered non-HDL-C by 44% (P = 0.0031) and 66% (P = 0.0094) at 10 and 30 mg/kg, respectively. At 48 h after injection, only animals treated with 30 mg/kg mAb1 maintained a statistically significant lowering of non-HDL-C (37%, P = 0.0438). Similar significant dose-dependent cholesterol lowering was observed for TC (Fig. S5_A_) and HDL-C (Fig. S5_B_).
mAb1 Lowers Serum TC and LDL-C in Cynomolgus Monkeys.
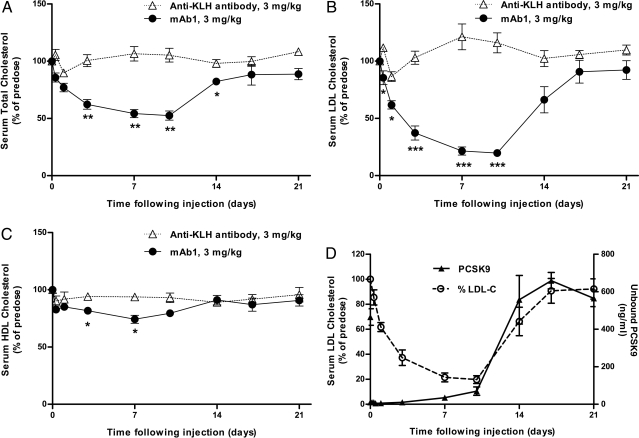
We used nonhuman primates as a model to further characterize in vivo properties of mAb1. Cynomolgus monkeys with an average predose serum LDL-C of ≈72 mg/dL were treated with a single 3-mg/kg i.v. dose of mAb1 or anti-KLH antibody (n = 4 animals per group). mAb1 led to a statistically significant lowering of serum TC as early as 3 days after injection, with a maximal lowering of 48% ± 4% at day 10, compared with an increase of 5% ± 4% in control animals (P = 0.0012) (Fig. 4A). More importantly, mAb1 caused a rapid decrease in circulating levels of LDL-C, reaching significance as early as 8 h after injection and a maximum decrease of 80% ± 3% at day 10, as compared with an increase of 17% ± 9% in control animals at the same time (P = 0.0002) (Fig. 4B). mAb1 treatment was also associated with a minor lowering of HDL-C, reaching statistical significance at day 3 (18% ± 2% vs. 6% ± 2% in control animals) (P = 0.0184) and at day 7 (26% ± 3% vs. 6% ± 3%) (P = 0.0178) (Fig. 4C). Serum triglyceride levels were not altered by mAb1 (Fig. S6_A_). Serum mAb1 concentration was monitored throughout the study, yielding a terminal half-life of 61 ± 9 h (mean ± SD) (Fig. S6_B_). Within 15 min of mAb1 administration, less than 3% of free circulating PCSK9 was detectable. These levels were maintained for 3 days, with a gradual return to predose levels by day 14 (Fig. 4D). Notably, LDL-C and free PCSK9 levels seemed to return to predose levels at a similar rate.
Fig. 4.
Changes in serum LDL-C after i.v. administration of mAb1 to cynomolgus monkeys. A single injection of mAb1 led to (A) a significant lowering of serum TC, observed as early as 8 h after administration of mAb1; (B) a significant lowering in serum LDL-C, with maximal lowering observed at 10 days after injection; and (C) a significant lowering of HDL-C at day 3 and day 7. Results are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 vs. anti-KLH control antibody at the same time point, n = 4 per group. (D) Temporal relationship between free circulating PCSK9 levels and serum LDL-C after administration of mAb1.
Discussion
We describe the identification of a neutralizing antibody against huPCSK9, mAb1, that disrupts the interaction between PCSK9 and LDLR and effectively lowers serum cholesterol in mice and in nonhuman primates. Our findings expand the current knowledge of PCSK9's mechanism of action and demonstrate that blocking secreted, circulating PCSK9 with an antagonistic antibody has clear therapeutic potential for treating hypercholesterolemia.
Using x-ray crystallography, we showed that mAb1 binds to the catalytic domain of PCSK9. Although mAb1 occludes the catalytic site of PCSK9, we believe it is unlikely that mAb1 neutralizes PCSK9 by inhibiting proteolysis because the LDLR-lowering effect of PCSK9 has been shown to be independent of its proteolytic activity (12, 13, 27). Indeed, robust measurements of PCSK9 proteolytic activity have been difficult to demonstrate experimentally, which precludes us from testing this hypothesis in vitro (8, 9). Rather, the mAb1 epitope on PCSK9 is adjacent to the region of PCSK9 required for LDLR interaction, and mAb1 binding to PCSK9 sterically hinders the association between PCSK9 and LDLR. Accordingly, mAb1 efficiently blocked the binding of PCSK9 to LDLR in vitro, resulting in the attenuation of PCSK9-mediated inhibition of LDL uptake in cultured HepG2 cells. Our findings are consistent with a recently published report describing the properties of unrelated polyclonal anti-PCSK9 antibodies in similar cell-based systems (17).
In WT mice, mAb1 led to a ≈2-fold increase in hepatic LDLR protein levels, as expected on the basis of studies of PCSK9 GOF and LOF models (14, 25, 28). This was associated with serum TC lowering of up to 36%. By comparison, PCSK9-specific siRNAs and antisense oligonucleotides have been reported to lower TC in mice by 20–30% and by ≈50%, respectively (18, 19). mAb1 did not lower TC in LDLR−/− mice, consistent with the previously reported lack of TC modulation by PCSK9 overexpression or deletion in LDLR−/− mice (28–30). mAb1 lowered non-HDL-C very effectively in mice expressing huPCSK9, indicating the neutralizing capacity of mAb1 against the human protein in an in vivo setting. Taken together, our studies in mice demonstrate that neutralizing circulating PCSK9 can lead to TC lowering and indicate that the effect on TC is specifically mediated through PCSK9 and via modulation of the LDLR.
Unlike rodents, nonhuman primates carry a significant portion of their serum cholesterol in LDL particles and are therefore more relevant models for studying the effect of LDL-C lowering interventions (31, 32). In cynomolgus monkeys, a single injection of mAb1 led to rapid and significant TC and LDL-C lowering, observed as early as 8 h after injection, with LDL-C reaching a maximum of 80% below predose levels by day 10. mAb1 also caused a rapid depletion of free serum PCSK9 that preceded LDL-C lowering, likely reflecting the time required for the hepatic LDLR protein levels to increase and a new rate of LDL clearance to be established. The temporal correlation between the rise in free PCSK9 and LDL-C as the antibody loses effect is consistent with a direct and rapid posttranslational effect of PCSK9 on the LDLR. The metabolic fate of the immune complex formed between mAb1 and PCSK9 is currently unclear. We have not determined the level of such a complex in the present studies, but the possibility that it accumulates cannot be excluded, which could potentially have pharmacodynamic and toxicologic consequences. This can likely be addressed by monitoring the immune complex in future longer-term, repeated-dose studies.
Inhibition of PCSK9 in cynomolgus monkeys with PCSK9-specific siRNAs has been reported to produce a maximal serum LDL-C decrease of ≈50% within 48 h after infusion, associated with circulating PCSK9 levels of ≈20% relative to vehicle-treated animals (18). The more rapid and pronounced LDL-C-lowering effect achieved with an antibody against PCSK9 may be due in part to a more rapid and sustained depletion of circulating PCSK9 and supports the concept that extracellular, secreted PCSK9 plays a major role in modulating LDLR function. Serum triglycerides remained unaffected by mAb1 treatment in our experiment, but we observed a minor yet statistically significant decrease of HDL-C. We speculate that this effect may be attributed to transient clearance of apoE-containing HDL particles by an upregulated LDLR (33).
If the rapid and robust LDL-C lowering achieved with mAb1 in cynomolgus monkeys is predictive for its efficacy in hypercholesterolemic patients, an antibody modality may represent a novel approach for the treatment of hypercholesterolemia that is superior to currently available therapies, including statins. Results from the recently completed JUPITER trial may support a wider use of cholesterol-lowering agents in the future, as well as lower LDL-C targets for a wide range of CHD patients (29). An antibody approach may also complement statins in situations in which statin therapy alone achieves suboptimal results. Indeed, in vitro studies described here demonstrate that a combination of a statin with an anti-PCSK9 antibody can effectively elevate LDLR protein levels more than either treatment alone. Thus, 2 potential target populations could hypothetically benefit from a PCSK9-based therapy: very-high-risk CHD patients who do not meet target LDL-C goals when treated with the highest possible statin dose, and hypercholesterolemic patients who do not tolerate high doses of statins.
In summary, the data presented here provide evidence that a neutralizing antibody directed against huPCSK9 may represent a viable therapeutic approach for the treatment of hypercholesterolemia. mAb1 will serve as a tool for further elucidating opportunities and challenges associated with the development of such therapeutics.
Methods
Antibody Generation and Screening Strategy.
Fully human antibodies to PCSK9 were generated by immunizing XMG2-KL and XMG4-KL strains of transgenic mice engineered to express diverse repertoires of fully human IgGκ and IgGλ antibodies of the corresponding isotype (XenoMouse mice) (34, 35). Mice were immunized with soluble, full-length, mature huPCSK9 cloned from HepG2 cDNA. Enriched B cells from immune animals were fused to nonsecretory myeloma P3 × 63Ag8.653 cells (American Type Culture Collection) to generate hybridomas using standard techniques (36). Hybridoma panels were then screened for binding to human WT, D374Y, and mouse PCSK9 (reagents generated by Amgen) by ELISA.
To screen for antibodies that block PCSK9 binding to the LDLR, D374Y PCSK9 was biotinylated via NHS chemistry and preincubated with hybridoma exhaust supernatant (1:5 final dilution). The mixture was transferred to an ELISA plate coated with a goat anti-LDLR polyclonal antibody (R&D Systems) at 2 μg/mL and then loaded with 400 ng/mL of soluble human LDLR extracellular domain (R&D Systems). Binding of biotinylated PCSK9 to the ELISA plates was detected using streptavidin–HRP (Pierce) and 3,3′,5,5′-tetramethylbenzidine substrate (Neogen). A relative ranking of the hybridoma lines was performed according to the degree of inhibition of PCSK9 binding to LDLR.
PCSK9 proteins and mAb1 were expressed and purified as described in SI Text.
LDL Uptake Assays.
Recombinant WT huPCSK9 (25 μg/mL) was added to HepG2 cells, and a reduction in uptake of fluorescent BODIPY-LDL (6 μg/mL; Invitrogen) was measured as the cell-associated fluorescence using a Safire plate reader (Tecan Systems) after a 3-h incubation. Preincubation of PCSK9 with antibody at varying concentrations (3–100 μg/mL) restored LDL uptake and permitted the determination of antibody potency in a cell-based assay. The EC50 values were determined using a Graphpad Prism sigmoidal dose–response curve-fitting program (version 4.02; GraphPad Software).
Binding Affinity.
Binding of mAb1 to human and cynomolgus monkey PCSK9 was examined via KinExA 3000 (Sapidyne Instruments). Briefly, Reacti-Gel (6×) (Pierce) was precoated with each PCSK9 protein and blocked with BSA. mAb1 (10 or 100 pM) was incubated with various concentrations of PCSK9 (0.1 pM to 10 nM) at room temperature for 8 h before incubation with the PCSK9-coated beads. The amount of bead-bound mAb1 was quantified by fluorescent Cy5-labeled goat antihuman IgG (H+L) antibody (Jackson ImmunoResearch). The equilibrium dissociation constant (KD) was obtained from nonlinear regression of the competition curves using a one-site homogeneous binding model in KinExA Pro software. Binding of mAb1 to mouse PCSK9 was tested in a BIAcore solution equilibrium binding assay on T100 (GE Healthcare) using methodology described previously (37).
Western Blot Analysis.
Western blot analysis of liver and HepG2 cell lysates was performed using either goat antimouse LDLR antibody (R&D Systems), rabbit antihuman LDLR antibody (Fitzgerald), or anti–β-actin antibody (Sigma and Cell Signaling). Bands were quantified by densitometry using ImageJ (National Institutes of Health).
X-Ray Crystallography.
Full-length PCSK9 with an N533A mutation was expressed and purified as described previously (8). Fab1, a Fab fragment with a variable domain identical to that of mAb1, was expressed in Escherichia coli with a C-terminal His-6 tag on the Fab heavy chain. Fab1 was purified by nickel affinity chromatography, size-exclusion chromatography, and anion-exchange chromatography. The PCSK9:Fab1 complex was generated by incubating a 1.5-molar excess of Fab1 with PCSK9, followed by purification on a size-exclusion chromatography column. The 5-mg/mL PCSK9:Fab1 complex crystallizes in 0.1 M Tris (pH 8.3), 0.2 M sodium acetate, 10–15% PEG 4000, and 3–6% dextran sulfate sodium salt (Mr 5000). Crystals were transferred to a well solution supplemented with glycerol, and datasets were collected at cryogenic temperatures.
An initial dataset for the PCSK9:Fab1 crystal was collected on a Rigaku FR-E x-ray source, processed with denzo/scalepack (38), and was used to solve the structure. A higher-resolution dataset from a crystal of the same crystal form was collected at the Berkeley Advanced Light Source beamline 5.0.2, processed with MOSFLM (39), and was used for refinement. PCSK9:Fab1 crystals grow in the C2 space group with 1 molecule (complex) per asymmetric unit and 76% solvent. The PCSK9:Fab1 structure was solved by molecular replacement with the program MOLREP (40) using the full-length PCSK9 structure (2PMW) as the starting model. Keeping the PCSK9 structure fixed, a search for an antibody variable domain was performed. Keeping the PCSK9:Fab1 variable domain fixed, an antibody constant domain was used as the final search model. The complete structure was improved with multiple rounds of model building with Quanta and refinement with cnx (41).
Studies in Mice.
All mice were group housed, maintained under standard environmental conditions, and given murine chow (Teklad 2918) and water ad libitum. All animal care procedures and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Amgen Inc.
C57BL/6 mice and LDLR−/− mice were obtained from Jackson Laboratories. Mice expressing huPCSK9 by AAV (huPCSK9-C57BL/6) were generated by injection (i.v. at 5 mL/kg) of ≈5 × 1012 pfu of a genetically engineered version of AAV5, which provided expression of the human protein. Animals were then screened for serum non-HDL-C (defined as the sum of LDL-C and very-low-density lipoprotein cholesterol), HDL-C, and TC levels and were assigned to treatment groups with similar average values for each of these parameters.
For each experiment, cohorts of mice were administered a single i.v. dose of either anti-KLH antibody (control) or mAb1 (10 mg/kg in C57BL/6 and LDLR−/− mice, and 10 or 30 mg/kg in huPCSK9-C57BL/6 mice). At various time points after injection, animals were killed, and blood and liver were collected. Designated groups of untreated mice were killed at T = 0 to establish baseline cholesterol and PCSK9 levels.
Studies in Monkeys.
Male cynomolgus macaques were maintained at MPI Research under standard environmental conditions in individual cages. The animals were fed twice daily (Primate Diet 5408; Lab Diet), and water was provided ad libitum. All animal care and experimental procedures were approved by the IACUC at MPI Research. After baseline blood collection, monkeys were administered a single i.v. dose of either 3 mg/kg anti-KLH control antibody or 3 mg/kg mAb1. At specific time points after injection, blood was collected.
Serum Lipid, PCSK9, and mAb1 Analyses.
Mouse and monkey serum was obtained from whole blood by centrifugation. Serum cholesterol analysis was performed using a Hitachi 912 or a Cobas Integra 400 chemistry analyzer. To assess changes in serum cholesterol in LDLR−/− mice, FPLC fractionation of sera was performed using a Superose 6 10/300 GL column (GE Healthcare). The TC content for each lipoprotein particle population was determined by assaying individual FPLC fractions using the TC Infinity kit (Thermo DMA).
Levels of PCSK9 and serum antibody concentrations were determined using a specific sandwich ELISA in each case as described (see SI Text).
Statistical Methods.
Results from in vitro and pharmacokinetic studies were expressed as the mean ± SD. Results from in vivo studies were expressed as the mean ± SEM. All statistical tests were evaluated at a significance level of α = 0.05. Multiple comparison corrections were applied when appropriate. Comparisons were deemed statistically significant if the P value or adjusted P value was <0.05. Data analysis from studies in C57BL/6 mice was performed by one-way ANOVA, comparing the difference between mAb1-treated and control animals at each time point or dose, followed by Dunnett's multiple comparison posttest to adjust for multiplicity. The exact Wilcoxon test was applied for analysis of data from huPCSK9-C57BL/6 mice, because non-HDL-C data were heavily tied. For monkey studies, a one-way ANOVA model was applied, and the P value was adjusted using the Bonferroni correction.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Kenneth Walker for providing anti-KLH antibody; the entire group at Amgen British Columbia for support with antibody generation; and Drs. Jonathan Cohen, Peter Coward, and Alykhan Motani for critically reviewing the manuscript. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02–05CH11231.
Footnotes
Conflict of interest statement: The Sponsor declares a conflict of interest (such as defined by PNAS policy). I am a consultant to Amgen Inc. They do not provide research funds to me or my laboratory, but I am paid an hourly consulting fee in annual visits to the company. The authors declare a conflict of interest (such as defined by PNAS policy). Employees of Amgen Inc.
Data deposition: The atomic coordinates have been deposited in Protein Data Bank, www.pdb.org (PDB ID code 3H42).
See Commentary on page 9546.
References
- 1.Horton JD, Cohen JC, Hobbs HH. PCSK9: A convertase that coordinates LDL catabolism. J Lipid Res. 2008;50:S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidah NG, Prat A. The proprotein convertases are potential targets in the treatment of dyslipidemia. J Mol Med. 2007;85:685–696. doi: 10.1007/s00109-007-0172-7. [DOI] [PubMed] [Google Scholar]
- 3.Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 6.Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007;193:445–448. doi: 10.1016/j.atherosclerosis.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper DE, et al. The crystal structure of PCSK9: A regulator of plasma LDL-cholesterol. Structure. 2007;15:545–552. doi: 10.1016/j.str.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol. 2007;14:413–419. doi: 10.1038/nsmb1235. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DW, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–18612. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]
- 11.Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci USA. 2008;105:1820–1825. doi: 10.1073/pnas.0712064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, et al. Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem J. 2007;406:203–207. doi: 10.1042/BJ20070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNutt MC, Lagace TA, Horton JD. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem. 2007;282:20799–20803. doi: 10.1074/jbc.C700095200. [DOI] [PubMed] [Google Scholar]
- 14.Lagace TA, et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang DW, Garuti R, Tang WJ, Cohen JC, Hobbs HH. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc Natl Acad Sci USA. 2008;105:13045–13050. doi: 10.1073/pnas.0806312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis CG, et al. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 1987;326:760–765. doi: 10.1038/326760a0. [DOI] [PubMed] [Google Scholar]
- 17.Duff CJ, et al. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J. 2009;419:577–584. doi: 10.1042/BJ20082407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank-Kamenetsky M, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham MJ, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48:763–767. doi: 10.1194/jlr.C600025-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.McNutt MC, et al. Antagonism of secreted PCSK9 increases low-density lipoprotein receptor expression in HEPG2 cells. J Biol Chem. 2009;284:10561–10570. doi: 10.1074/jbc.M808802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian YW, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res. 2007;48:1488–1498. doi: 10.1194/jlr.M700071-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Shan L, et al. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm. 2008;375:69–73. doi: 10.1016/j.bbrc.2008.07.106. [DOI] [PubMed] [Google Scholar]
- 23.Dubuc G, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004;24:1454–1459. doi: 10.1161/01.ATV.0000134621.14315.43. [DOI] [PubMed] [Google Scholar]
- 24.Careskey HE, et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–398. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Rashid S, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–D525. doi: 10.2741/fazio. [DOI] [PubMed] [Google Scholar]
- 27.Grefhorst A, McNutt MC, Lagace TA, Horton JD. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J Lipid Res. 2008;49:1303–1311. doi: 10.1194/jlr.M800027-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279:50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaid A, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): Hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–654. doi: 10.1002/hep.22354. [DOI] [PubMed] [Google Scholar]
- 31.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 32.Turley SD, Spady DK, Dietschy JM. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J Lipid Res. 1995;36:67–79. [PubMed] [Google Scholar]
- 33.Zanni EE, Stephan ZF, Zannis VI, Breslow JL, Hayes KC. ApoE distribution among lipoproteins of rhesus monkeys is modulated by dietary fat and cholesterol. J Nutr. 1986;116:1611–1619. doi: 10.1093/jn/116.9.1611. [DOI] [PubMed] [Google Scholar]
- 34.Mendez MJ, et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Genet. 1997;15:146–156. doi: 10.1038/ng0297-146. [DOI] [PubMed] [Google Scholar]
- 35.Kellermann SA, Green LL. Antibody discovery: The use of transgenic mice to generate human monoclonal antibodies for therapeutics. Curr Opin Biotechnol. 2002;13:593–597. doi: 10.1016/s0958-1669(02)00354-3. [DOI] [PubMed] [Google Scholar]
- 36.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 37.Yie J, et al. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 2009;583:19–24. doi: 10.1016/j.febslet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Leslie AGW. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography, No. 26. 1992 [Google Scholar]
- 40.Collaborative Computational Project The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 5):760–763. doi: 10.1107/S0907444994003112. Number 4. [DOI] [PubMed] [Google Scholar]
- 41.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information