Studies on free radicals, antioxidants, and co-factors (original) (raw)
Abstract
The interplay between free radicals, antioxidants, and co-factors is important in maintaining health, aging and age-related diseases. Free radicals induce oxidative stress, which is balanced by the body’s endogenous antioxidant systems with an input from co-factors, and by the ingestion of exogenous antioxidants. If the generation of free radicals exceeds the protective effects of antioxidants, and some co-factors, this can cause oxidative damage which accumulates during the life cycle, and has been implicated in aging, and age dependent diseases such as cardiovascular disease, cancer, neurodegenerative disorders, and other chronic conditions. The life expectancy of the world population is increasing, and it is estimated that by 2025, 29% of the world population will be aged ≥60 years, and this will lead to an increase in the number of older people acquiring age-related chronic diseases. This will place greater financial burden on health services and high social cost for individuals and society. In order to acheive healthy aging the older people should be encouraged to acquire healthy life styles which should include diets rich in antioxidants. The aim of this review is to highlight the main themes from studies on free radicals, antioxidants and co-factors, and to propose an evidence-based strategy for healthy aging.
Keywords: free radicals, antioxidants, co-factors, age-related diseases, healthy aging
Introduction
One of the main driving force, which helps to sustain human life, are the biochemical reactions which take place within the organelles and cells of the body. The laws of nature are such that one moves from infancy, to childhood, then into adulthood, and finally one becomes a frail human being eventually leading to death. This aging process is a common feature of the life cycle of virtually all multicellular organisms. The number of people aged 65 and over is predicted to increase by approximately 53% in the United Kingdom by the year 2031 and similar changes are likely to be seen in other developed countries due to low birth rates and increasing life expectancy, which will lead to an increasingly elderly population (Majeed and Aylin 2005). This predicted gain in life expectancy would potentially lead to an increase in the number of older people acquiring age-related chronic diseases of the cardiovascular, brain, and immune systems. This can cause loss of autonomy, dependence and high social costs for individuals and society, and will impose increased workload and financial pressures on healthcare systems worldwide. Due to this there is a major interest in understanding of the biochemistry of aging and providing a database of “anti-aging” medicines, diet and commercial products which can provide safe, effective and practical methods for increasing longevity with a good quality of life during aging, and thus decrease the dependence of elderly people on expensive high-tech medicine (Rahman 2003). The three main areas of research, which are interlinked and can contribute or delay the aging process are; studies involving free radicals, antioxidants, and co-factors. There have been a significant number of studies within these areas and the purpose of this review is to highlight the main themes from these studies which can provide a better insight into the mechanisms of aging, and thus provide an anti-aging strategy.
Free radicals
Free radicals can be defined as reactive chemical species having a single unpaired electron in an outer orbit (Riley 1994). This unstable configuration creates energy which is released through reactions with adjacent molecules, such as proteins, lipids, carbohydrates, and nucleic acids. The majority of free radicals that damage biological systems are oxygen-free radicals, and these are more generally known as “reactive oxygen species” (ROS). These are the main byproducts formed in the cells of aerobic organisms, and can initiate autocatalytic reactions so that molecules to which they react are themselves converted into free radicals to propagate the chain of damage. ROS can be (i) generated during UV light irradiation and by X-rays and gamma rays (ii) produced during metal catalyzed reactions (iii) are present in the atmosphere as pollutants (iv) are produced by neutrophils and macrophages during inflammation, and (iv) are by-products of mitochondrial catalyzed electron transport reactions, and various other mechanisms (Cadenas 1989). The amount of free radical production is determined by the balance of many factors, and ROS are produced both endogenously and exogenously. The endogenous sources of ROS include mitochondria, cytochrome P450 metabolism, peroxisomes, and inflammatory cell activation (Inoue et al 2003). Hydrogen peroxide, although not a radical species is produced in the mitochondria as is its ROS precursor superoxide. It has been proposed that ubisemiquinone is the main reductant of oxygen in mitochondrial membranes and the generation of superoxide within mitochondria is approximately 2–3 nmol/min per mg of protein, the presence of ubiquitous indicates it to be the most important physiological source of this radical in living organisms (Inoue et al 2003). Since mitochondria are the major site of free radical generation, they contain a variety of antioxidants, which are present on both sides of their membranes in order to minimize ROS induced stress (Cadenas and Davies 2000). There are also other cellular sources of superoxide radicals present such as the enzyme xanthine oxidase, which catalyzes the reaction of hypoxanthine to xanthine and xanthine to uric acid. In both steps, molecular oxygen is reduced, forming the superoxide anion followed by the generation of hydrogen peroxide (Valko et al 2004).
Additional endogenous sources of cellular ROS are neutrophils, esinophils and macrophages. On activation, macrophages initiate an increase in oxygen uptake giving rise to a variety of ROS, including superoxide anion, nitric oxide and hydrogen peroxide (Conner and Grisham 1996). Cytochrome P450 has also been proposed as a source of ROS since on its induction, superoxide anion and hydrogen peroxide production takes place following the breakdown or uncoupling of the P450 cycle (Valko et al 2006). In addition, microsomes and peroxisomes are sources of ROS, and microsomes are responsible for the majority of hydrogen peroxide produced in vivo at hyperoxia sites (Gupta et al 1997). ROS can also be produced by a host of exogenous sources such as xenobiotics, chlorinated compounds, environmental agents, metals (redox and nonredox), ions, and radiation (Valko et al 2006).
The generation of free radicals in cells is closely linked with the participation of redox-active metals (Shi et al 2004), which in itself is largely linked to an iron (in part to copper) redox couple, and is maintained within strict physiological limits. The iron under certain circumstances, can participate in the Fenton reaction, generating the highly reactive hydroxyl radical (Leonard et al 2004) which if produced in vivo, can react close to its site of formation hence causing localized damage. Additional radicals derived from oxygen are peroxy radicals, which are high-energy species, and they display biological diversity in their actions. These induce lipid peroxidation whose measurement is the most frequently cited evidence to support the involvement of peroxyl radical in human disease and toxicology (Gutteridge 1995; Cadenas and Sies 1998).
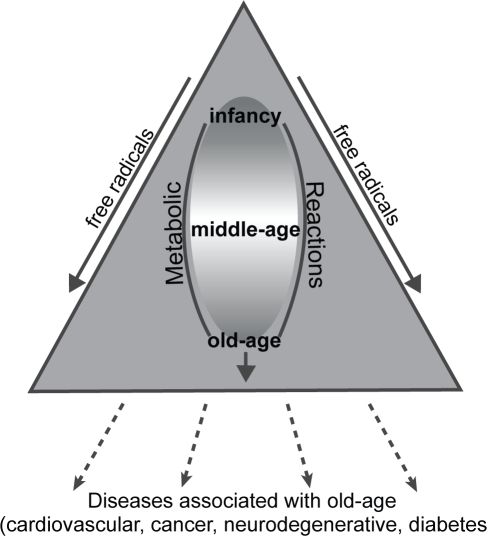
It has been established that ROS can be both harmful and beneficial in biological systems depending on the environment (Lopaczynski and Zeisel 2001; Glade 2003). Beneficial effects of ROS involve, for example, the physiological roles in cellular responses to noxia such as defense against infectious agents, and in the function of a number of cellular signaling systems. In contrast, at high concentrations, ROS can be mediate damage to cell structures, including lipids and membranes, proteins and nucleic acids; this damage is often referred as “oxidative stress” (Poli et al 2004). The harmful effects of ROS are balanced by the action of antioxidants, some of which are enzymes present in the body (Halliwell 1996). Despite the presence of the cell’s antioxidant defense system to counteract oxidative damage from ROS, oxidative damage accumulates during the life cycle and has been implicated in aging and age-dependent diseases such as cardiovascular disease, cancer, neurodegenerative disorders and other chronic conditions (Rahman 2003) (see Figure 1).
Figure 1.
Association of free radicals with age, and age-related diseases.
Free radicals and aging
Aging can generally be defined as a progressive decline in the efficiency of biochemical and physiological processes after the reproduction phase of life. This contribution of the aging process to changes occurring with age are small early in life but rapidly increase with age because of the exponential nature of aging (Figure 1). Many theories have been put forward to explain the phenomenon of aging (Armbrecht 2001; Biesalski 2002; Finkel and Holbrook 2002; Sohal et al 2002; Balaban et al 2005); and all of these have their strengths and weaknesses, and it’s likely that they all contribute to the mechanisms of aging. Among the theories proposed, the “free radical theory of aging” (Harman 1956) has gained universal acceptance and is supported by the fact that production of free radicals and free radical damage increases with age (Sohal and Weindruch 1996). This theory postulates that free radicals in the body cause oxidative damage to cellular components, a process which results in altered cellular function, compromised tissue and organ function, and ultimately death. The body takes molecular oxygen and uses it to produce energy via oxidative phosphorylation in mitochondria, and this, and other metabolic reactions generate free radicals imposing oxidative stress on proteins, DNA and lipids. The free radial theory is supported by the “rate of living” hypothesis, which inversely links metabolic rate with the longevity of the organisms (Ku et al 1993) and it is also well established that oxidative damage to proteins, DNA and lipids increases with age (Sohal and Weindruch 1996). Evidence to support the free radical theory of aging has been mainly obtained in experimental animal models. The restriction of caloric intake in rodents has been shown to increase lifespan, increase free radical defenses, and reduce oxidative damage. The tissues of species which live longer are also less susceptible to oxidative damage then tissue of species which have shorter life spans and this is supported by the fact that conditions which generate increased free radical production such as a high metabolic rate are associated with a shorter lifespan (Agarwal and Sohal 1996). Although evidence exists to support the free radical theory and the decline in physiological function in aging, some questions relating to aging are still unresolved. Free radical mediated oxidative stress increases with age, and thus may overwhelm the natural repair systems in the elderly (Kowald and Kirkwood 2000) and is a major contributor to diseases associated with aging (Ames et al 1993) an outline of which is given below.
Cardiovascular disease
The development of atherosclerosis depends on the balance between proinflammatory; anti-inflammatory, and antioxidative defense mechanisms (Scott 2004). Vascular proliferation and inflammation are closely linked (Dzay et al 2002), and excessive proliferation of vascular cells plays an important role in the pathology of vascular occlusive disease. Free radicals are considered to play a casual role in this process (Schachinger and Zeiher 2002), and ROS lead to the oxidation of low density lipoprotein (OxLDL), and this accumulates within plaques, and contributes to the inflammatory state of atherosclerosis and plays a key role in its pathogenesis (Galle et al 2006). Oxidized-LDL leads to endothelial dysfunction, and can result in either cell growth or apoptotic cell death and can cause vasoconstriction.
Free radicals have also been implicated in congestive heart failure (CHF), the annual incidence of which is one to five per 1000 person, and the relative incidence doubles for each decade of life after the age of 45. Experimental evidence suggests a direct link between free radical production and CHF (Mariani et al 2005) and the presence of ROS in circulating blood is also the key intermediary related to vascular injury and organ dysfunction (Fukai et al 2002; Elahi and Matata 2006).
Stroke
In Western countries stroke is the main cause of disability and mortality among the aging population, and ischemic stroke accounts for about 75% of all cases while hemorrhagic stroke is responsible for almost 15% of all strokes (Mariani et al 2005). There is evidence that stroke is associated with free radicals arising from sources such as xanthine oxidase, cyclooxygenase, inflammatory cells and mitochondria (Piantadosi and Zhang 1996), and these can potentially cause neuronal death (Alexandrova et al 2004). The mitochondrial electron transport chain is altered during ischemia and reperfusion and is also a likely source of free radicals (Simms and Anderson 2002). This can lead to an increased formation of superoxide radical anions as supported by the fact that knockout mice for mitochondrial superoxide dismutase (mSOD) genes display larger brain lesions after focal ischemia (Murakami et al 1998). The accumulation of blood borne inflammatory cells such as neutrophils and monocytes/macrophages, which can occur during reperfusion, can also promote further oxidative stress. Increased levels of oxidative damage to DNA and evidence for lipid peroxidation has also been demonstrated in ischemic stroke patients (Mariani et al 2005). In addition, the increased levels of ROS can make the brain more susceptible to oxidative stress due to a variety of reasons namely: the brain consumes a significant amount of the body oxygen, has a relatively poor antioxidant defense system, is enriched in pro-oxidant molecules and contains high concentration of readily peroxidizable lipids (Cherubini et al 2005a).
Neurodegenerative diseases
Neurodegenerative diseases affect the central nervous system and are characterized by loss of specific neuronal populations and quite often intraneuronal, as well as extracellular accumulation of fibrillary materials. Decrements in motor function and decrements in memory are two main behavioral parameters that are altered in senescence in both humans and animals. Primary degenerative brain disease and diseases related to cerebral vascular disturbances are the leading cause of disability in old age and can cause loss of autonomy, dependence, and high social costs for individuals and the society. There is growing evidence that free radicals are involved in the initiation of cellular injury observed in neurodegenerative diseases (Emerit et al 2004) an outline of which are given below:
Alzheimers’s disease (AD)
This is the most common neurodegenerative disorder and is characterized by loss of neurons and synapses resulting in cognitive impairment and a gradual loss of memory, language skills, and reasoning leading to dementia and finally death (Selkoe 2004). The onset of AD is gradual, with clinical symptoms appearing between 60–70 years of age and is characterized by both synaptic loss and nerve cell loss. It is associated with aging and several studies show logarithmic age-dependent increases in oxidized proteins, lipids and DNA in AD patients (Floyd and Hensley 2002); these observed increases are not accounted for by the decreased activity of the antioxidant protective enzymes. Oxidative damage may also play a role in amyloid deposition in AD, and oxidizing conditions can cause protein cross-linking and aggregation of β-amyloid protein (Dyrks et al 1993), and also contribute to aggregation of tau (Troncoso et al 1993), and other cytoskeletal proteins (Bellomo and Mirabelli 1992). The β-amyloid protein is also reported to cause the oxidation of the nonsaturated carbohydrate side chains of membrane lipids, which leads to the disintegration of the neural membrane thus resulting in cell lysis (Behl et al 1994). Lipid peroxidation has also been quantitatively assessed in AD brains and increased brain levels of 4-hydroxy-2-noneanal glutathione conjugates have been recently reported (Völkel et al 2006). There is also an increase in the DNA damage of lymphocytes obtained from AD donors (Mecocci et al 1998) whilst oxidative modification of proteins in the frontal cortex of AD brain has also been reported (Korolainen et al 2006).
Huntington’s disease (HD)
This is an inherited, autosomal dominant neurodegenerative disease, which causes uncontrollable movements and restlessness as well as irritability and depression (Margolis and Ross 2003). Direct evidence for a defect in oxidative phosphorylation in HD patients is supported by the discovery of a threefold increase in lactate concentrations in the occipital cortex and in the basal ganglia (Jenkins et al 1993). There is further evidence to support the involvement of free radicals in the pathogenesis of HD in that increased levels of F2-isoprostanes have been detected in the cerebro-spinal fluid of HD patients compared to the control group (Montine et al 1999).
Parkinson’s disease (PD)
This is a progressive neurodegenerative movement disorder and is the most common form of motor system degeneration affecting approximately 1% of the population over the age of 65 (Moore et al 2005). Clinical symptoms include bradykinesia, rigidity, postural instability, and resting tremor. Experimental evidence supports the involvement of free radicals in the pathogenesis of PD. It has been observed that that oxidation of dopamine yields potentially toxic semiquinones and that the accelerated metabolism of dopamine by monoamine-oxidase-B may induce an excessive formation of hydrogen peroxide, superoxide anions, and hydroxyl radicals. Further evidence of the involvement of free radicals comes from the fact that oxidative stress is responsible for the initiation of nigral dopamine neuron loss. The substantia nigra has a high metabolic rate combined with both a high content of oxidizable species, including dopamine and dopamine-derived ROS, neuromelanin, polyunsaturated fatty acids, iron, and a low content of antioxidants. Thus oxidative stress can dominate and result in the production of ROS, which serve both to maintain the oxidative stress level, and to initiate/propagate apoptosis of the dopaminergic neurons (Wersinger and Sidhu 2002; Hald and Lotharius 2005). PD has also been found to be associated with increased oxidative damage to DNA (Migliore et al 2002) proteins (Choi et al 2006) and lipids (Agil et al 2006), and further signs of oxidative damage in PD patients is supported by the finding that elevated levels of the pro-oxidant iron are present in the brains of PD patients (Fasano et al 2006).
Cancer
Carcinogenesis is a complicated, multi-stage process in which healthy cells are transformed into abnormal cells as a result of a series of mutations and changes in the patterns of gene expression. Factors predisposing to malignancy include, inherited traits, environmental agents, diet, and the risk of cancer increases with age. Cancer development can be described by three stages: initiation, promotion and progression, and ROS can act in all these stages of carcinogenesis (Klaunig and Kamendulis 2004). It is also well established that free radicals are known to react with all components of DNA, thus damaging its bases and the deoxyribose backbone (Dizdaroglu et al 2002) causing mutations in crucial genes, which ultimately may lead to cancer (Ames and Shigenaga 1992).
The permanent modification of genetic material induced by free radicals represents the first step involved in mutagenesis, carcinogenesis, and aging. In support of this free radical-mediated damage to DNA has been found in various cancer tissues, and there is also a direct link between the size of benign tumors and the amount of DNA oxidized product, 8-hydroxyguanine (8-OH-G) adduct formation; indicating that the level of 8-OH-G may be important in the transformation of benign to malignant tumor (Loft and Poulsen 1996). This damage to the DNA can result either in arrest or induction of transcription, induction of signal transduction pathways, replication errors, and genomic instability, all of which are associated with carcinogenesis (Marnett 2000; Cooke et al 2003). A high level of oxidative stress can induce apoptosis or even necrosis; however, a low level of oxidative stress can stimulate cell division and thus promote tumor growth (Dreher and Junod 1996). ROS probably enhance the final irreversible stage of carcinogenesis, which is characterized by accumulation of additional genetic damage, leading to the transition of the cell from benign to malignant.
Diabetes
There is increasing evidence that free radical induced damage also plays a significant part in the development of insulin resistance, β-cell dysfunction, impaired glucose tolerance, and type 2 diabetes mellitus (Jay et al 2006; Wright et al 2006). Hyperglycemia can induce oxidative stress, which increases with age, via several mechanisms including glucose auto oxidation, the formation of advanced glycation end-products (AGE), and activation of the polyol pathway. Other circulating factors that are elevated in diabetics such as free fatty acids and leptin also contribute to increased ROS (Jay et al 2006). There is a significant increase in protein glycation (AGE) with age (Poggioli et al 2002), which is also increased in diabetics (Wautier and Schimdt 2004). The accumulation of AGE leads to an increase in the micro vascular lesions, which are present in diabetic retinopathy, and is also responsible for cardiovascular complications, which are seen in diabetic patients (Wautier and Schmidt 2004; Jay et al 2006). The damage caused by ROS has also been implicated in primary open angle glaucoma (POGA), which is the leading cause of irreversible blindness and the second most common cause of all blindness after cataracts. The incidence of POAG is linked to old age, thus advanced age represents a major risk factor for this disease (Izzotti et al 2006).
The biochemical and physiological damage induced due to free radical mediated oxidative stress can be counteracted by antioxidants, which are discussed below.
Antioxidants
The term “antioxidant” refers to any molecule capable of stabilizing or deactivating free radicals before they attack cells. Humans have evolved highly complex antioxidant systems (enzymic and nonenzymic), which work synergistically, and in combination with each other to protect the cells and organ systems of the body against free radical damage. The antioxidants can be endogenous or obtained exogenously eg, as a part of a diet or as dietary supplements. Some dietary compounds that do not neutralize free radicals, but enhance endogenous activity may also be classified as antioxidants.
An ideal antioxidant should be readily absorbed and quench free radicals, and chelate redox metals at physiologically relevant levels. It should also work in both aqueous and/or membrane domains and effect gene expression in a positive way. Endogenous antioxidants play a crucial role in maintaining optimal cellular functions and thus systemic health and well-being. However, under conditions, which promote oxidative stress, endogenous antioxidants may not be sufficient and dietary antioxidants may be required to maintain optimal cellular functions. The most efficient enzymatic antioxidants involve glutathione peroxidase, catalase and superoxide dismutase (Mates et al 1999). Nonenzymatic antioxidants include Vitamin E and C, thiol antioxidants (glutathione, thioredoxin and lipoic acid), melatonin, carotenoids, natural flavonoids, and other compounds (McCall and Frei 1999). Some antioxidants can interact with other antioxidants regenerating their original properties; this mechanism is often referred to as the “antioxidant network” (Sies et al 2005). There is growing evidence to support a link between increased levels of ROS and disturbed activities of enzymatic and nonenzymatic antioxidants in diseases associated with aging.
Enzymatic antioxidants
Glutathione peroxidase
There are two forms of this enzyme, one which is selenium-dependent (GPx, EC1.11.1.19) and the other, which is selenium-independent (glutathione-S-transferase, GST, EC2.5.1.18) (Mates et al 1999). The differences are due to the number of subunits, catalytic mechanism, and the bonding of selenium at the active centre, and glutathione metabolism is one of the most important antioxidative defense mechanisms present in the cells. There are four different Se-dependent glutathione peroxidases present in humans (Chaudière and Ferrari-Iliou 1999), and these are known to add two electrons to reduce peroxides by forming selenoles (Se-OH) and the antioxidant properties of these seleno-enzymes allow them to eliminate peroxides as potential substrates for the Fenton reaction. Selenium-dependent glutathione peroxidase acts in association with tripeptide glutathione (GSH), which is present in high concentrations in cells and catalyzes the conversion of hydrogen peroxide or organic peroxide to water or alcohol while simultaneously oxidizing GSH. It also competes with catalase for hydrogen peroxide as a substrate and is the major source of protection against low levels of oxidative stress (Chaudière and Ferrari-Iliou 1999).
Catalase (EC1.11.1.6)
This enzyme is present in the peroxisome of aerobic cells and is very efficient in promoting the conversion of hydrogen peroxide to water and molecular oxygen. Catalase has one of the highest turnover rates for all enzymes: one molecule of catalase can convert approximately 6 million molecules of hydrogen peroxide to water and oxygen each minute (Mates et al 1999).
Superoxide dismutase (SOD), (EC 1.15.1.1)
This is one of the most effective intracellular enzymatic antioxidants and it catalyzes the conversion of superoxide anions to dioxygen and hydrogen peroxide. Superoxide dismutase exists in several isoforms, which differ in the nature of active metal centre, amino acid composition, co-factors and other features. There are three forms of SOD present in humans: cytosolic Cu, Zn-SOD, mitochondrial Mn-SOD, and extra cellular-SOD (Landis and Tower 2005). Superoxide dismutase neutralizes superoxide ions by going through successive oxidative and reductive cycles of transition metal ions at its active site (Chaudière and Ferrari-Iliou 1999). Cu, Zn-SOD has two identical subunits with a molecular weight of 32 kDa (Mates et al 1999) and each of the subunit contains as the active site, a dinulcear metal cluster constituted by copper and zinc ions, and it specifically catalyzes the dismutation of the superoxide anion to oxygen and water. The mitochondrial Mn-SOD is a homotetramer with a molecular weight of 96 kDa and contains one manganese atom per subunit (Mates et al 1999), and it cycles from Mn(III) to Mn(II), and back to Mn(III) during the two-step dismutation of superoxide. Extra cellular superoxide dismutase contains copper and zinc, and is a tetrameric secretary glycoprotein having a high affinity for certain glycosaminoglycans such as heparin and heparin sulphate (Mates et al 1999), however, its regulation in mammalian tissues occurs primarily in a manner coordinated by cytokines, rather than as a response to oxidative stress.
Nonenzymatic antioxidants
Vitamin E
This is a fat-soluble vitamin existing in eight different forms. In humans, α-tocopherol is the most active form, and is the major powerful membrane bound antioxidant employed by the cell (Hensley et al 2004). The main function of Vitamin E is to protect against lipid peroxidation (Pryor 2000), and there is also evidence to suggest that α-tocopherol and ascorbic acid function together in a cyclic-type of process. During the antioxidant reaction, α-tocopherol is converted to an α-tocopherol radical by the donation of a labile hydrogen to a lipid or lipid peroxyl radical, and the α-tocopherol radical can therefore be reduced to the original α-tocopherol form by ascorbic acid (Kojo 2004).
Vitamin C (ascorbic acid)
This is an important and powerful water-soluble antioxidant and thus works in aqueous environments of the body. Its primary antioxidant partners are Vitamin E and the carotenoids as well as working along with the antioxidant enzymes. Vitamin C cooperates with Vitamin E to regenerate α-tocopherol from α-tocopherol radicals in membranes and lipoproteins (Carr and Frei 1999; Kojo 2004), and also raises intracellular glutathione levels thus playing an important role in protein thiol group protection against oxidation (Naziroglu and Butterworth 2005).
Thiol antioxidants
The major thiol antioxidant is the tripeptide glutathione (GSH), which is a multifunctional intracellular antioxidant and is considered to be the major thiol-disulphide redox buffer of the cell (Masella et al 2005). It is abundant in cytosol, nuclei, and mitochondria, and is the major soluble antioxidant in these cell compartments (Masella et al 2005). Glutathione has also been shown to play a role in cell senescence since studies involving human fibroblasts have shown that the intracellular glutathione level has a strong influence on the induction of a post-mitotic phenotype, and that by implication depletion of glutathione may play a significant role in the cellular aging in human skin (Alaluf et al 2000). The reduced form of glutathione is GSH, glutathione, whilst the oxidized form is GSSG, glutathione disulphide. The antioxidant capacity of thiol compounds is due to the sulphur atom, which can easily accommodate the loss of a single electron (Karoui et al 1996). Oxidized glutathione (GSSG) is accumulated inside the cells and the ratio of GSH/GSSG is a good measure of oxidative stress of an organism (Dröge 2002). The main protective roles of glutathione against oxidative stress are that it can act as a co-factor for several detoxifying enzymes, participate in amino acid transport across plasma membrane, scavenge hydroxyl radical and singlet oxygen directly, and regenerate Vitamins C and E back to their active forms (Masella et al 2005).
Another thiol antioxidant is the thioredoxin (TRX) system; these are proteins with oxidoreductase activity and are ubiquitous in both mammalian and prokaryotic cells (Holmgren 1985). It also contains a disulphide and possesses two redox-active cysteins within a conserved active site (Cys-Gly-Pro-Cys) (Nakamura et al 1997). Thioredoxin contains two adjacent –SH groups in its reduced form that are converted to a disulphide unit in oxidized TRX when it undergoes redox reactions with multiple proteins.
Thioredoxin levels are much less than GSH, however, TRX and GSH may have overlapping as well as compartmentalized functions in the activation and regulation of transcription factors (Valko et al 2006).
The third important thiol antioxidant is the natural compound α-Lipoic acid (ALA), which is a disulphide derivative of octanoic acid and is sometimes referred to as thiothic acid. It is both water and fat-soluble, and therefore, is widely distributed in both cellular membranes and the cytosol of eukaryotic and prokaryotic cells. α-Lipoic acid is readily absorbed from the diet and is converted rapidly to its reduced form, dihydrolipoic acid (DHLA) (Smith et al 2004). Both ALA and DHLA are powerful antioxidants and they exert their effects by scavenging free radicals, metal ion chelation and antioxidant recycling, and repairing protein damage due to oxidative stress either in the cytosol or hydrophobic domains (Navari-Izzo et al 2002). Dihydrolipoic acid is a stronger antioxidant than lipoic acid and can act synergistically with other antioxidants such as glutathione, ascorbate and tocopherol. However, it can also exert pro-oxidant properties both by its iron-reducing ability and by its ability to generate sulfur-containing radicals that can damage proteins (Navari-Izzo et al 2002).
Melatonin (N-acetyl-5-methoxytryptamine)
This is an indoleamine neurohormone that is synthesized mainly in the pineal gland and has many effects on a wide range of physiopathological functions. One major function of melatonin is to scavenge free radicals in oxygen metabolism, thereby potentially protecting against free radical-induced damage to DNA, proteins and membranes, thus it has the potential to play an important role in the reduction of free radical mediated diseases (Rahimi et al 2005).
Carotenoids
These are mainly colored pigments present in plants and microorganisms and epidemiological studies have revealed that an increased consumption of a diet rich in carotenoids is correlated with a lower risk of age-related diseases. Carotenoids contain conjugated double bonds and their antioxidant activity arises due to the ability of these to delocalize unpaired electrons (Mortensen et al 2001). This is also responsible for the ability of carotenoids to physically quench singlet oxygen without degradation and for the chemical reactivity of carotenoids with free radicals. The efficacy of carotenoids for physical quenching is related to the number of conjugated double bonds present in the molecule, which determines their lowest triplet energy level. They can also scavenge peroxy radical thus preventing damage in lipophilic compartments (Stahl and Sies 2003), however, the carotenoid β-carotene can also act as a pro-oxidant causing an increase in lipid peroxidation (Polozza et al 2003). The concentrations of carotenoids and the partial pressure of oxygen are also important factors in their effectiveness as antioxidants. Carotenoids, in particular β-carotene exhibit antioxidant properties at low oxygen partial pressure but become pro-oxidants at high pressures of oxygen and similarly, at high carotenoid concentrations, pro-oxidant behavior is displayed (Rice-Evans et al 1997; Stahl and Sies 2003).
Flavonoids
These are a broad class of low molecular ubiquitous groups of plant metabolites and are an integral part of the human diet (Rice-Evans 2001). Flavonoids are benzo-γ-pyrone derivatives consisting of phenolic and pyrane rings and during metabolism hydroxyl groups are added, methylated, sulfated or glucuronidated.
There is intense interest in flavonoids due to their anti-oxidant and chelating properties and their possible role in the prevention of chronic and age-related diseases (Schroeter et al 2002).
Flavonoids are present in food mainly as glycosides and polymers (Hammerstone et al 2000) and these comprise a substantial fraction of dietary flavonoids (Santos-Buelga and Scalbert 2000). The biological properties of flavonoids are determined by the extent, nature, and position of the substituents and the number of hydroxyl groups (Schroeter et al 2002). These factors also determine whether a flavonoid will act as an antioxidant or as a modulator of enzyme activity, or whether it possesses antimutagenic or cytotoxic properties. The most reported activity of flavonoids is their protection against oxidative stress (Rice-Evans 2001). Thus flavonoids can scavenge peroxyl radicals, and are effective inhibitors of lipid peroxidation, and can chelate redox-active metals, and thus prevent catalytic breakdown of hydrogen peroxide (Fenton chemistry). However, under certain conditions, flavonoids can also display pro-oxidant activity and this is thought to be directly proportional to the total number of hydroxyl groups (Cao et al 1997), and they have also been reported to modulate cell signaling (Schroeter et al 2002).
Antioxidants and age-related diseases
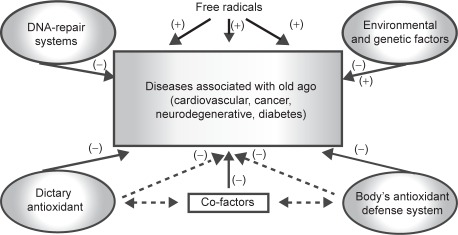
The human body has a host of mechanisms such as the DNA-repair systems to deal with free radical induced damage and depending on the circumstances, environmental and genetic factors can either increase or decrease the incidence of diseases associated with old age (Figure 2). Epidemiological studies have demonstrated that diet plays a crucial role in the prevention of age-related chronic diseases especially if combined with regular physical activity and abstaining from smoking (Willett 2006). Free radicals and oxidative stress are recognized as important factors in the biology of aging and of many age-related diseases. One mechanism to slow down the aging process and the decline in the vital body functions is to modulate oxidative stress by calorie restriction, however, this is difficult to achieve. Hence, dietary components with antioxidant activity have received particular attention because of their potential role in modulating oxidative stress associated with aging and chronic conditions. Several studies have indicated potential roles for dietary antioxidants in the reduction of age-related diseases (Meydani et al 2001). This is supported by the fact that in elderly subjects a higher daily intake of fruits and vegetables is associated with an improved antioxidant status compared to subjects consuming diets poor in fruits and vegetables (Anlasik et al 2005). Therefore, the use of antioxidants by this group may lower the prevalence of diseases associated with old age; evidence supporting this is outlined below.
Figure 2.
Summary of mechanisms involved in the prevention of diseases associated with old age.
Notes: (–) Reduction of diseases; (+) Induction of diseases. There is interaction between body’s antioxidant defense system, dietary antioxidants, and co-factors in the reduction of diseases associated with old age.
Mediterranean diet, which is rich in fruits and vegetables, has been shown to reduce the incidence of cardiovascular disease (Serra-Majem et al 2006; Willett 2006). Evidence is now emerging that some dietary antioxidants besides displaying traditional antioxidant potential can influence signaling pathways and gene expression relevant in atherosclerosis by mechanisms other than antioxidative ones. Vitamin C has been shown to inhibit LDL oxidation in vitro (Alul et al 2003) primarily by scavenging free radicals and other ROS, thereby preventing them from interacting with LDL. The observational data in humans suggest that vitamin C ingestion is associated with reduced cardiovascular disease, however, the results of randomized controlled trials have been mainly disappointing (Cherubini et al 2005b). A randomized double-blind crossover trial has shown a positive correlation of plasma Vitamin C with resistance to LDL to oxidation (Samman et al 2003), however, in contrast to this, in another recent study no correlation between Vitamin C and LDL resistance to oxidation has also been reported (Kaliora et al 2006). Vitamin E has also been shown to inhibit LDL oxidation in vitro (Andrikopoulos et al. 2002; Tucker and Townsend 2005), and also increases LDL oxidative resistance (Dieber-Rotheneder et al 1991; Heitzer et al 1999, Palomaki et al 1999; Hodis et al 2002), decreases agonist-induced platelet aggregation (Munteanu et al 2004), and preserves agonist-induced vasodilation (Keaney et al 1993; Munteanu et al 2004) ex vivo. Long term supplementation with Vitamin E in hypercholesterolemic patients and/or chronic smokers has shown to increase levels of autoantibodies against oxidized LDL (Heitzer et al 1999) and it has recently been shown to prevent ischemic heart disease (Chattopadhay and Bandyopadhyay 2006) however, evidence from other clinical trials is controversial and confusing (Kaliora et al 2006) since some studies have failed to show a link between dietary supplementation of ∝-tocopherol and LDL resistance to oxidation. This may be due to the fact that vitamin E not only acts as an antioxidant but can also interact with enzymes and modulate genes involved in atherosclerosis (Munteanu et al 2004).
A protective role flavonoids in the diet of humans has been indicated in some large prospective studies. In vitro inhibition of LDL oxidation by flavonoids has also been demonstrated (Andrikopoulos et al 2002; Vitseva et al 2005) whilst total antioxidant capacity is increased and LDL oxidizability is reduced after consumption of several natural products that are rich in flavonoids (Rahman 2003; Ruel et al 2005). A high flavonoid intake is also associated with a lower mortality rate from coronary heart disease and lower incidence of myocardial infarction in older men (Hertog et al 1993), and a reduced risk of coronary heart disease in post-menopausal women has been observed (Yochum et al 1999). The Zutphen Elderly study also demonstrated an inverse relationship between consumption of catechin, a predominant flavonoid in tea and ischemic heart disease mortality in a cohort of 806 men (Arts et al 2001). In support of this, black tea consumption has shown a decrease in markers of oxidative stress and inflammation in patients with coronary artery disease (Widlansky et al 2005). Carotenoids have shown to increase LDL oxidative resistance in ex vivo studies (Levy et al 2000; Upritchard et al 2000), however, in another study involving elderly healthy subjects supplementation with a carotene mixture or lycopene had no effect on oxidative modification of LDL in vitro, despite significant increase in plasma and LDL concentrations of lycopene, α-carotene and β-carotene (Carroll et al 2000). In contrast, in another clinical trial a significant decrease in serum LDL cholesterol was observed which was in parallel with an increase in serum lycopene (Agarwal and Rao 1998). In addition, in patients with diabetes mellitus increased susceptibility to LDL oxidation was normalized by natural β-carotene or lycopene dietary supplements (Levy et al 2000; Omoni and Aluko 2005).
The effect of antioxidants has also been investigated on the vascular endothelium since it plays a key role in the regulation of vascular tone and its dysfunction correlates with cardiovascular disease. Garlic, which is high in antioxidants, inhibits the ability of platelets to aggregate, increases anti-oxidants levels and also inhibits LDL oxidation (Rahman 2003). It also increases intracellular glutathione (GSH) levels in vascular endothelial cells by modulation of the GSH redox cycle specifically increasing glutathione disulfide (GSSG) reductase activity and superoxide dismutase (SOD) activity (Geng and Lau 1997). The role of antioxidants in preventing platelet aggregation is still a matter of controversy and contradictory results have been obtained with the antioxidant Vitamins C and E (Kaliora et al 2006). Although, the intake of dietary flavonids is inversely correlated with the risk of mortality from coronary artery disease (Gelijnse et al 1999; Omoni and Aluko 2005) its role in platelet function has provided contrasting evidence. For example, cocoa supplementation in healthy subjects significantly increased flavonoids levels and decreased platelet aggregation (Murphy et al 2003), in support of this in another study chocolate consumption also decreased platelet aggregation (Innes et al 2003). In contrast, some studies have shown no effect of flavonoids on platelet aggregation despite an increase in its plasma concentration (Gooderham et al 1996; Conquer et al 1998). There is currently little data on the effectiveness of carotenoids on platelet function.
Vitamin C supplementation in healthy humans has shown recently that its intake results in significant reduction of oxidative stress and inflammation as shown by a reduction in the concentration of F2-isoprostanes, prostaglandin E2, and monocyte chemotactic protein-1 (Sánchez-Moreno et al 2006). The relationship between antioxidants and gene expression has also been investigated and evidence is emerging that antioxidants may prevent cardiovascular disease influencing gene expression directly or via gene promoters, via control of regulatory signals, and via post-transcriptional pathways (Kaliora et al 2006). The role of phytochemicals in the inhibition of cancer and inflammation has also been extensively studied and it is now clear that these exert their action by modulating phase I and phase II enzymes and by modulating the cell signaling pathways involved in inflammation (Issa et al 2006). In future the role of proteomics and nutrigenomics will be important in determining the diet-gene relationship.
Since free radicals are implicated in the pathogenesis of neurodegenerative diseases (Emerit et al 2004), the role of antioxidants in their prevention has been gaining popularity. It has been reported that the concentration of antioxidant varies within the different regions of the brain and some enzymatic antioxidants such as catalase are found in lower concentrations in the brain when compared to other tissues (Gilgun-Sherki et al 2001). A variety of antioxidants have been investigated for the reduction of oxidative stress associated with AD. It has also been reported that the concentrations of Vitamins A, C, E and β-carotene in plasma, serum or cerebrospinal fluid are lower in AD patients than in controls (Schippling et al 2000; Bourdel-Marchasson et al 2001) and supplementation with these vitamins is useful in the prevention of AD (Frank and Gupta 2005). Vitamin E is also reported to slow the rate of motor dysfunction in HD (Peyser et al 1995; Butterfield et al 2002). In contrast, no effects of these antioxidants on AD have also been reported (Luchsinger et al 2003). It has been suggested that flavonoids may have neuroprotective effects both in vitro and in vivo possibly by their abilities to scavenge ROS (Sutherland et al 2006). This is supported by the fact that polyphenols found in blueberry have been shown to reverse age-related declines in neuronal signal transduction as well as cognitive and motor deficits and increase hippocampal plasticity (Lau et al 2005). In addition, Concord grape juice reverses the course of neuronal and behavioral aging possibly through a multiplicity of direct and indirect effects that can affect a variety of neuronal parameters (Shukitt-Hale et al 2006) and curcumin, a powerful antioxidant from the curry spice turmeric reduces oxidative damage and amyloid pathology associated with AD (Calabrese et al 2003). Garlic, a strong antioxidant is also reported to protect against age-related maculopathy and cataract formation in the elderly (Cumming et al 2000). The role of garlic in preventing cerebral aging and dementia is also supported by other studies which indicate that phytochemicals displaying antioxidant properties can improve neurological dysfunctions (Youdim and Joseph 2001; Deschamps et al 2001).
Ginkgo extract has also been investigated in the prevention of neurodegenerative diseases and has a beneficial effect in the treatment of AD patients (Oken et al 1998; Frank and Gupta 2005). In contrast no efficacy of Gingko extract on AD subjects has also been noted (Van Dongen et al 2000; Schneider et al 2005). Hence, larger studies are needed to clarify the therapeutics effects of Gingko extract in AD subjects.
Melatonin is a potent free radical scavenger and its levels decline with age and patients with neurodegenerative diseases have significant reductions of this substance (Liu et al 1999; Hardeland et al 2006). It also displays neuroprotective and antioxidant properties against amyloid β-protein mediated oxidative damage (Frank and Gupta 2005; Hardeland et al 2006), and displays immunomodulatory properties, and thus can play a role in healthy aging (Karasek 2004).
An early biochemical change in PD patients is a reduction in total glutathione levels (Bharath and Anderson 2005). Infusion of GSH in PD patients has been demonstrated to improve the symptoms but the therapeutic effects only lasted between 2–4 months after GSH treatment was stopped (Sechi et al 1996). However, the role of induction of endogenous antioxidants in the prevention of neurodegenerative diseases needs further investigation.
Free radicals can induce DNA damage, which can lead to mutations in crucial genes thus ultimately leading to cancer (Ames and Shigenaga 1992). There is evidence to indicate that ROS are involved in cancer initiation and promotion and malondialdehyde (MDA) concentration is increased in patients with neoplasms (Yeh et al 2005). Consumption of potent dietary antioxidants can lower the effects of oxidative DNA damage in the aged besides lowering the overall risk of cancer (Block 1991; Donaldson 2004; Serra-Majem et al 2006). A recent study has indicated that a combination of antioxidants is a powerful adjunctive preventive treatment for cancer (Eli and Fasciano 2006) since the total activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase is reduced in certain types of cancers (Oberley 1998; Mates et al 1999). However, the invasive potential of cancer cells is also increased in the presence of abnormally high levels of Mn-SOD (Valko et al 2006). The antioxidant vitamins C and E also have the potential to reduce certain types of cancers. Many studies have shown that vitamin C protects against cell death triggered by various stimuli and this protection is associated with its antioxidant property. Vitamin C supplementation studies have shown a reduction in markers of oxidative DNA, lipid and protein damage, and in support, vitamin C has been shown to regulate factors that can influence gene expression, apoptosis, and other cellular functions (You et al 200). Intervention with vitamin E supplementation has shown a reduction in the risk of colorectal adenomas and prostate cancer (Borek 2005; Tucker and Townsend 2005). However, controversy surrounds the effectiveness of vitamins in reducing cancer and negative effects of vitamin C and E have also been reported. A study by Miller and colleagues (2005) has revealed that vitamin E at doses of 400IU or more can actually increase the risk of death, however, no risk was reported when vitamin E was used at 200IU or less. There is also an association between cancer incidence and various disorders of GSH-related enzyme functions especially the alterations of glutathione S-transferases (GSTs) (Valko et al 2006).
Carotenoids also display antiproliferative properties when tested in various cancer cell lines. Increased intake of lycopene has been reported to attenuate alcohol-induced apoptosis in 2E1 cells, and reduces the risk of prostate, lung and digestive cancers. This cancer preventative property of lycopene is associated with its antioxidant property and its ability to induce and stimulate intercellular communication via gap junctions which are known to play a role in the regulation of cell growth, differentiation, and apoptosis (Tapiero et al 2004). In this context the redox state of the cell is also important, as there is evidence to show that redox balance is impaired in cancer cells compared with normal cells, which may be related to oncogenic stimulation. Antioxidants may prevent cancer by inducing phase II detoxifying enzymes and activating transcription factors and endogenous antioxidant enzymes such as glutathione peroxidase and catalase (Frei and Higdon 2003). It is known that altered levels of antioxidant enzymes and nonenzymatic antioxidants as well as changes in the related signal pathways are evident in many human cancers (McEligot et al 2005). Evidence is also emerging that flavonoids such as garlic, green tea, silibinin, and curcumin have cancer preventive properties (Rahman 2003; Mandel et al 2005). In support of this garlic is known to enhance scavenging systems in the cells such as glutathione, SOD, catalase, and glutathione peroxidase (Wei and Lau 1998). Many of these dietary compounds appear to act on multiple target signaling pathways which include down-regulation of cyclooxygenase-2 (COX-2) and down- regulation of the transcription activators, AP-1 and NF-κB known to be extremely important in tumor promoter-induced cell transformation and tumor promotion, and both are influenced differentially by the MAPK pathways (Huang et al 2002; Sarkar and Li 2004). Further support for the role of flavonoids in preventing cancer has come from grape seed proanthocyanidins which have been reported to inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes (Mantena and Katiyar 2006). Dietary Antioxidants may also prevent cancer by potentially suppressing angiogenesis by inhibiting interleukin-8 production and the cell junction molecule VE-cadherin (Meydani 2001). These studies concur with the epidemiologic, clinical and animal studies that consumption of antioxidants is associated with a reduced risk of cancer among the elderly.
Oxidative stress is increased with aging and is a contributing factor for the initiation and progression of complications in diabetes mellitus such as lens cataracts, nephropathy and neuropathy (Atli et al 2004, Osawa and Kato et al 2005). The use of antioxidants in preventing and treating diabetes has been investigated over the last decade. Dietary supplementation with a combination of antioxidants, and vitamins C and E has reported a reduction in oxidative stress markers in patients with type 2 diabetes (Farvid et al 2004; Neri et al 2005). In contrast, clinical trials involving Vitamin E supplementation on diabetic complications have shown conflicting data (Ble-Castillo et al 2005; Guerrero-Romero and Rodríguez-Morán 2005), however, Vitamin E has a greater effect in protecting LDL oxidation in type 2 diabetics who are at a greater risk of cardiovascular disease (Anderson et al 1999). Similarly, conflicting data has also been obtained with vitamin C supplementation such that decreased fasting plasma insulin levels and improved insulin action (Penckofer et al 2002) has been reported, whilst another study has reported no effect (Darko et al 2002). Further conflicting data has been observed in diabetic postmenopausal women who display a higher level of oxidative stress (Varma et al 2005) and supplementation with high Vitamin C in this group has been reported to have an increased risk of mortality from cardiovascular disease (Lee et al 2004).
Flavonoids also have a role to play in the treatment of diabetes (Rahman 2003; Rahimi et al 2005) as these have shown to protect against hyperglycemic and alloxan-induced oxidative stress in experimental animal models (Hedge et al 2005). In support, in clinical trials flavonoids have shown to offer protection against type 2 diabetes in a large cohort of women (Song et al 2005). Melatonin has been shown to reduce diabetic nephropathy and neuropathy in experimental animal models (Baydas et al 2003; Cam et al 2003) but more work is required to assess its efficacy in humans.
Finally, there is evidence to support the use of lipoic acid in treating type 2 diabetes as lipoic acid displays strong antioxidant properties and increases glucose uptake through recruitment of the glucose transporter-4 to plasma membranes, a mechanism that is shared with insulin-stimulated glucose uptake (Packer et al 2001). Lipoic acid is also reported to improve neural blood flow, endoneural glucose uptake, and metabolism and nerve conduction (Ruhnau et al 1999; Smith et al 2004). It probably exerts its effect in diabetic patients by reducing lipid accumulation in adipose and nonadipose tissue (Song et al 2004), by increasing glucose uptake and by activating pyruvate dehydrogenase complex, which is known to play a major role in the oxidation of glucose-derived pyruvate (Korotchkina et al 2004). It’s clear that more human clinical trials are required in order to establish the role of antioxidants in the prevention and treatment of diabetes.
Antioxidants may also have a role to play in the treatment of symptoms and pathological processes associated with menopause (Miquel et al 2006) especially in women who suffer high levels of oxidative stress, do not consume a healthy diet and are seeking alternative treatments for the symptoms of menopause.
Co-factors
The biochemical definition of a co-factor is that it is an ion or a molecule that binds to the catalytic site of an apoenzyme rendering it active. Many enzymes have a requirement for metal ions for their activity and these metal ions are also referred to as co-factors. The major antioxidant enzymes possess transition metals or selenium at the catalytic site and the availability of cofactors can determine the activity of such enzymes. Some of the co-factors involved in the oxidant/antioxidant mechanisms have already been outlined, the rest are discussed below.
Different essential metals play an important role in controlling oxidative reactions in biological tissues. For example copper (Cu) is an essential cofactor in a number of critical enzymes including cytochrome C oxidase and copper, zinc-superoxide dismutase (Cu, ZN-SOD) (Arredondo and Núñez 2005). Although unregulated Cu is also a well known pro-oxidant it can through the action of transporter proteins such as metallothineins and ceruplasmin exert its antioxidative effects (Leung 1998). A Cu deficiency-induced decrease in the activity of CuZn-SOD in humans and animals has been reported (Turnlund et al 1997; Uriu-Adams et al 2005). Copper deficiency also decreases the activity of ceruloplasmin, which requires Cu for its ferroxidase function (Hellman and Gitilin 2002), and it can also lead to a reduction in enzymes of the oxidant defense system such as selenium-dependent glutathione peroxidase (Se-GPX) and catalase (Chen et al 1994). Further more a deficiency in Cu can also alter other ROS scavengers including metallothionein (a Cu and Zn containing protein) (Tapia et al 2004) and the nonprotein thiol, glutathione (Uriu-Adams and Keen 2005). Copper and zinc are also essential co-factors for enzymes involved in the synthesis of various bone matrix constituents and could be important in the elderly since they may play an important role in reducing bone loss in osteoporosis (Lowe et al 2002).
Iron (Fe) is an essential constituent of catalase enzymes, hemoglobin and myoglobin, but is also a prooxidant (via Fenton reactions) when it is present in excess (Gutteridge 1995; Leung 1998; Puntarulo 2005). In the presence of lipids iron creates oxidative stress and it has been suggested that subjects with high levels of lipids and serum iron are at an increased risk of cancer (Mainous et al 2005). Thus, iron chelators such as albumin, haptoglobin, lactoferrin, transferring and urate also have an important role to play in preventing oxidative stress-related diseases (Gutteridge 1995).
Selenium (Se) is another important co-factor and epidemiological findings have linked a lowered Se status to neurodegenerative and cardiovascular diseases as well as to an increased risk of cancer (Brenneisen et al 2005). There is an association between Se reduction and DNA damage, and oxidative stress, and some evidence that Se may affect not only cancer risk but also progression and metastasis (Rayman 2005). Selenium intervention in subjects with a lower Se status has shown some benefits in reducing the incidence and mortality in all cancers but more specifically in liver, prostate, colo-rectal and lung cancers. Its protective effects appear to be associated with its presence in the multiform of glutathione peroxidases, which are known to protect DNA and other cellular damage from oxidative stress (Schrauzer 2000; Trueba et al 2004).
The element manganese (Mn) is another co-factor involved in antioxidant defense mechanisms and is a vital component of Mn-SOD enzyme, which plays a crucial role in protecting mitochondria from free radical attack (Leung 1998). Zinc (Zn) another component of SOD is also involved in antioxidant defense systems and protects the vascular and immunological systems from the damaging affects of free radical species (Kuppusamy et al 2005), and it is also a key constituent or co-factor of over 300 mammalian proteins which may have a role in the prevention of initiation and progression of cancer. Evidence supports the fact that Zn deficiency can impair the host protective mechanisms designed to protect against DNA damage, thus increasing the risk of cancer (Ho 2004), and it also plays an important role as an antioxidant and/or as a co-factor in keeping the skin healthy (Rostan et al 2002), thus it can play an important role in healthy aging.
Coenzyme Q10 (Co Q10) (Ubiquinone) is fat-soluble quinine that transfers electrons from complexes I and II to complex III within the mitochondria, this process being coupled to ATP production. In its reduced form, Co Q10 also inhibits lipid peroxidation and can protect mitochondrial inner-membrane proteins and DNA from oxidative damage, and is the most widely used co-factor supplement in the treatment of mitochondrial disorders (Turunen et al 2004). CoQ is commonly used for treatment of cardiomyopathy, (Langsjoen and Langsjoen 1999) and neurological disorders such as Parkinson’s disease and diabetes, and can thus prevent age-related mitochondrial dysfunction (Littarru and Tiano 2005). Most promising results have been obtained in the treatment of neurological disorders whilst its use in the treatment of cardiovascular disease and diabetes has produced contradictory data (Bonakdar and Guarneri 2005).
Riboflavin (Vitamin B2) is another co-factor, which is converted to flavin dinucleotide, which serves as a coenzyme for glutathione reductase and other enzymes (Manthey et al 2006). Low intakes of Riboflavin have been associated with different diseases including cancer and cardiovascular diseases and there is some evidence that treatment with riboflavin can provide some benefit against diseases associated with oxidative stress (Bonnefont-Rousselot 2004; Manthey et al 2006).
Another co-factor Thiamine (Vitamin B1) has been investigated for its role in the treatment of oxidative stress-related diseases. Thiamine diphosphate is the active form of thiamine and it serves as a co-factor for several enzymes, which are important in the biosynthesis of reducing equivalents used in oxidant stress defenses (Singleton and Martin 2001). Thiamine deficiency has been linked to the promotion of neurodegeneration and an increase in oxidative stress (Gibson and Zhang 2002); however, its effectiveness in treating diseases associated with free radicals is still unclear (Bonnefont-Rousselot 2004; Nascimento et al 2006). Nicotinamide, the amide form of niacin or nicotinic acid is a precursor for both nicotinamide adenine dinucleotide (NAD/NADH), and nicotinamide adenine dinucleotide phosphate (NADP). It plays an important role in energy metabolism, signal transduction, cellular injury, aging (Aksoy et al 2006), and shows significant inhibition of oxidative damage induced by ROS (Kamat and Devasagayam 1999; Feng et al 2006), however, its mechanism of action is unknown.
Carnitine, which transfers long-chain fatty acids across the mitochondrial membrane, has also been investigated for its property to scavenge free radicals. It has been shown to protect mitochondrial membrane damage during the aging process in an experimental model (Savitha and Panneerselvam 2006), and to display antioxidant properties in the prevention of acetic acid- induced colitis (Cetinkaya et al 2006). It has also been reported to inhibit hepatocarcinogeneis via improvement of mitochondrial dysfunction in an experimental model (Chang et al 2005), and it has been reported to improve fatigue symptoms in cancer patients (Gramignano et al 2006).
The biochemical interaction between free radicals, antioxidants, and co-factors needs to be considered further and results from long-term trials are needed to evaluate the safety and beneficial role of these in the prevention and treatment of diseases associated with free radicals. A summary of mechanisms involved in the prevention of diseases associated with aging is represented in Figure 2.
Conclusions
There is now universal agreement that free radicals are involved in the physical, biochemical, and pathological changes associated with aging. Oxidative damage to proteins, lipids, and DNA accumulates and increases with age, and is associated with age-related diseases such as cardiovascular disease, neurodegenerative diseases, cancer, and diabetes (Rahman 2003). The human body deals with the pathological effects of ROS by utilizing the endogenous antioxidant system (eg, enzymes such as superoxide dismutase), and by the ingestion of exogenous antioxidants in the diet (eg, flavonoids). The presence of co-factors is also important for the antioxidants to exert optimum effects. If the oxidative stress exceeds the protection afforded by antioxidants the aging process and some of the diseases associated with it can accelerate.
According to the World Health Organization 20% of the current world population are aged ≥60 years and life expectancy is continuing to increase throughout the world, and it is estimated that by 2025 this number will have grown to 29% (WHO 2002).
This change in the world population is been accompanied by rise in living standards leading to lifestyle and behavior changes that are having an adverse impact on population health. This increase in older people is likely to place greater financial burden on the health services and high social costs for individuals and society if not managed properly.
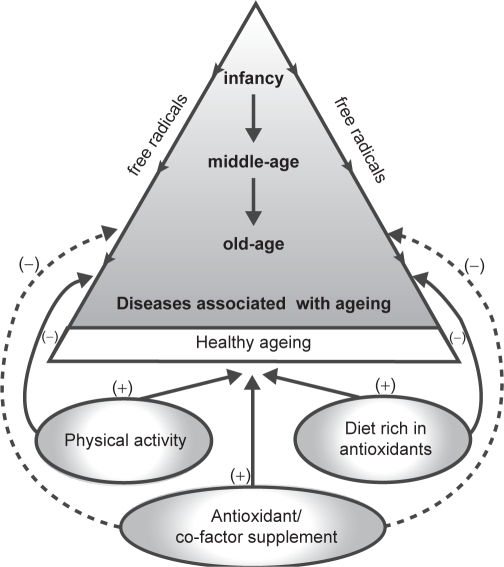
Healthy aging involves the interaction between genes, the environment and life styles, and in order for the elderly to live independently and relatively disease and disability free requires that healthy life styles are promoted throughout life. The most modifiable lifestyle factors are physical activity and diet, and the elderly population should be encouraged to take up physical activity since it has a positive effect on decreasing the risk of many diseases associated with old age (Peel et al 2005). The elderly should also be encouraged to consume a diet rich in antioxidants as there is evidence that such a diet especially in combination with a healthy life style can lower the rate of all-causes and cause-specific mortality by more than 50% in the 70–90 years old (Knoops et al 2004), (see Figure 3). Although, some of the evidence that certain dietary antioxidants and some co-factors can reduce free radical mediated damage and promote healthy aging is controversial, the elderly should be encouraged to take exogenous antioxidants and co-factors, which have shown efficacy in scientific studies. However, more controlled studies are needed in order to investigate the efficacy and safety of antioxidants and co-factors, and their mode of action especially in the elderly. The scientific community has a moral responsibility to ensure the healthy aging of the world population.
Figure 3.
Summary of factors involved in healthy aging.
Notes: (+) Promotes healthy aging; (–) Reduces free radical induced diseases.
References
- Agarwal RS, Sohal RS. Relationship between susceptibility to protein oxidation, aging, and maximum life span potential of different species. Exp Gerontol. 1996;31:365–72. doi: 10.1016/0531-5565(95)02039-x. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Rao AV. Tomato lycopene and low density lipoprotein oxidation; a human dietary intervention study. Lipids. 1998;33:981–84. doi: 10.1007/s11745-998-0295-6. [DOI] [PubMed] [Google Scholar]
- Agil A, Durản R, Barrero F, et al. Plasma lipid peroxidation in sporadic Parkinson’s: Role of the L-dopa. J Neuro Scien. 2006;240:31–6. doi: 10.1016/j.jns.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Aksoy P, White TA, Thompson M, et al. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem Biophys Res Comm. 2006;345:1386–92. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Alaluf S, Muir-Howie H, Hu HL, et al. Atmospheric oxygen accelerates the induction of a post-mitotic phenotype in human dermal fibroblasts: the key protective role of glutathione. Differentiation Res Biol Diversity. 2000;66:147–55. doi: 10.1046/j.1432-0436.2000.660209.x. [DOI] [PubMed] [Google Scholar]
- Alul RH, Wood M, Longo J, et al. Vitamin C protects low-density lipoprotein from homocysteine-mediated oxidation. Free Rad Bio Med. 2003;34:881–91. doi: 10.1016/s0891-5849(03)00028-5. [DOI] [PubMed] [Google Scholar]
- Alexandrova M, Bochev P, Markova V, et al. Dynamics of free radical processes in acute ischemic stroke: influence on neurological status and outcome. J Clin Neurosci. 2004;11:501–6. doi: 10.1016/j.jocn.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and degenerative diseases of aging. Proc Natl Acad Sci. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Ann NY Acad Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Gowri MS, Turner J, et al. Antioxidant supplementation effects on low-density lipoprotein oxidation for individuals with type 2 diabetes mellitus. J Am Coll Nutr. 1999;18:451–61. doi: 10.1080/07315724.1999.10718883. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos NK, Kaliora AC, Assimopoulou AN, et al. Inhibitory activity of minor polyphenolic and nonpolyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J Med Food. 2002;5:1–7. doi: 10.1089/109662002753723160. [DOI] [PubMed] [Google Scholar]
- Anlasik T, Sies H, Griffiths HR, et al. Dietary habits are major determinants of the plasma antioxidant status in healthy elderly subjects. Brit J Nutr. 2005;94:639–42. doi: 10.1079/bjn20051574. [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ. The biology of ageing. J Lab Clin Med. 2001;138:220–5. doi: 10.1067/mlc.2001.118090. [DOI] [PubMed] [Google Scholar]
- Arredondo M, Núñez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26:313–27. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Arts IC, Hollman PC, Feskens EJ. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–32. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- Atli T, Keven K, Avci A, et al. Oxidative stress and antioxidant status in elderly diabetes mellitus and glucose intolerance patients. Arch Geront Geriatr. 2004;39:269–75. doi: 10.1016/j.archger.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, Oxidants, and Aging. Cell Vol. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Baydas G, Reiter RJ, Yaser A, et al. Melatonin produces glial reactivity in the hippocampus, cortex, and cerebellum of streptozocin-induced diabetic rats. Free Rad Bio Med. 2003;35:797–804. doi: 10.1016/s0891-5849(03)00408-8. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, et al. Hydrogen peroxide mediates amyloid protein activity. Cell. 1994;77:817–27. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Bellomo G, Mirabelli F. Oxidative stress and cytoskeletal alterations. Ann NY Acad Sci. 1992;663:97–109. doi: 10.1111/j.1749-6632.1992.tb38653.x. [DOI] [PubMed] [Google Scholar]
- Bharath S, Anderson JK. Glutathione depletion in a midbrain-derived immortalized dopaminergic cell line results in limited tyrosine nitration of mitochondrial complex I subunits: implications for Parkinson’s disease. Antioxid Redox Signal. 2005;7:900–10. doi: 10.1089/ars.2005.7.900. [DOI] [PubMed] [Google Scholar]
- Biesalski HK. Free radical theory of ageing. Curr Opin Clin Nutr Metab Care. 2002;5:5–10. doi: 10.1097/00075197-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Ble-Castillo JL, Carmona-Díaz E, Méndez JD, et al. Effect of α-tocopherol on the metabolic control and oxidative stress in female type 2 diabetics. Biomed Pharmacother. 2005;59:290–5. doi: 10.1016/j.biopha.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Block G. Vitamin C and cancer prevention; the epidemiological evidence. Am J Clin Nutr. 1991;53:270S–82S. doi: 10.1093/ajcn/53.1.270S. [DOI] [PubMed] [Google Scholar]
- Bonakdar RA, Guarneri E. Coenzyme Q10. Am Fam Physician. 2005;72:1065–70. [PubMed] [Google Scholar]
- Bonnefont-Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol. 2004;3:41–52. doi: 10.2165/00024677-200403010-00005. [DOI] [PubMed] [Google Scholar]
- Borek C. Antioxidants and the prevention of hormonally regulated cancer. The J Mens Health Gender. 2005;2:346–52. [Google Scholar]
- Bourd-Marchasson I, Delmas-Beauvieux MC, Peuchant E, et al. Antioxidant defenses and oxidative markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2001;30:235–41. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Steinbrenner H, Helmut Sies. Selenium, oxidative stress, and health aspects. Mol Aspects Med. 2005;26:256–67. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Drake J, et al. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci. 2002;5:229–39. doi: 10.1080/10284150290028954. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Rad Biol Med. 2000;29:222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Sies H. The lag phase. Free Rad Res. 1998;28:601–9. doi: 10.3109/10715769809065816. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Ann Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Colombrita C, et al. Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids. 2003;25:437–44. doi: 10.1007/s00726-003-0048-2. [DOI] [PubMed] [Google Scholar]
- Cam M, Yavuz O, Guven A, et al. Protective effects of chronic melatonin treatment against renal injury in strptozocin-induced diabetic rats. J Pineal Res. 2003;35:212–20. doi: 10.1034/j.1600-079x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior RL, et al. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Rad Biol Med. 1997;22:749–60. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Carr A, Frei B. Does Vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–24. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- Carroll YL, Corridan BM, Morrissey PA. Lipoprotein carotenoid profiles and the susceptibility of low density lipoprotein to oxidative modification in healthy elderly volunteers. Eur J Clin Nutr. 2000;54:500–7. doi: 10.1038/sj.ejcn.1601046. [DOI] [PubMed] [Google Scholar]
- Cetinkaya A, Bulbuloglu E, Kantarceken B, et al. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–94. doi: 10.1007/s10620-006-3160-9. [DOI] [PubMed] [Google Scholar]
- Chang B, Nishikawa M, Nishihuchi S, et al. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int J Cancer. 2005;113:719–29. doi: 10.1002/ijc.20636. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Bandyopadhyay D. Vitamin E in the prevention of ischemic heart disease. Pharmacol Rep. 2006;58:179–87. [PubMed] [Google Scholar]
- Chaudière J, Ferrari-Iliou R. Intracellular Antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–62. doi: 10.1016/s0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Saari JT, Kang YJ. Weak antioxidant defenses make the heart a target for damage in copper-deficient rats. Free Rad Bio Med. 1994;17:529–36. doi: 10.1016/0891-5849(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Ruggiero C, Cristina M, et al. Potential markers of oxidative stress in stroke. Free Rad Biol Med. 2005a;39:841–52. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Vigna GB, Zuliani G, et al. Role of antioxidants an atherosclerosis: epidemiological and clinical update. Curr Pharm Des. 2005b;11:2017–32. doi: 10.2174/1381612054065783. [DOI] [PubMed] [Google Scholar]
- Choi J, Sullards MC, Olzmann JA, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–24. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–7. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- Conquer JA, Maiani G, Azzini E, et al. Supplementation with quercetin markedly increases quercetin concentrations without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–7. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, et al. DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Mitchell P, Wayne S, et al. Diet and cataract. The blue mountains eye study. Ophthalmology. 2000;107:450–6. doi: 10.1016/s0161-6420(99)00024-x. [DOI] [PubMed] [Google Scholar]
- Darko D, Dornhorst A, Kelly FJ, et al. Lack of effect of vitamin C on blood pressure, oxidative stress and endothelial function in Type II diabetes. Clin Sci. 2002;103:339–44. doi: 10.1042/cs1030339. [DOI] [PubMed] [Google Scholar]
- Deschamps V, Barbereger-Gateau P, Peuchant E. Nutritional factors in cerebral aging and dementia: epidemiological arguments for a role of oxidative stress. Neuroepidemolog. 2001;20:7–15. doi: 10.1159/000054752. [DOI] [PubMed] [Google Scholar]
- Dieber-Rotheneder M, Puhl H, Waeg G, et al. Effect of oral supplementation with D-∝-tocopherol on the Vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991;32:1325–32. [PubMed] [Google Scholar]
- Dizdaroglu M, Jaruga P, Birincioglu M, et al. Free-radical-induced damage to DNA: mechanisms and measurement. Free Rad Bio Med. 2002;32:1102–15. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004;3:19–40. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32A:30–8. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- Dröge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exper Geront. 2002;37:1333–45. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Dyrks T, Dyrks E, Masters CL, et al. Amyloidogenicity of rodent and human beta A4 sequences. FEBS Lett. 1993;324:231–6. doi: 10.1016/0014-5793(93)81399-k. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Ned. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- Elahi MM, Matata BM. Free radicals in blood: Evolving concepts in the mechanism of ischemic heart disease. Arch Biochem Biophys. 2006;450:78–88. doi: 10.1016/j.abb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Eli R, Fasciano JA. An adjunctive preventive treatment for cancer: Ultraviolet light and ginkgo biloba, together with other antioxidants, are a safe and powerful, but largely ignored, treatment option for the prevention of cancer. Medical Hypothesis. 2006;66:1152–6. doi: 10.1016/j.mehy.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Farvid MS, Siassi F, Jalali M, et al. The impact of vitamin and/or mineral supplementation on lipid profiles in type 2 diabetes. J Am Coll Nutr. 2004;23:272–9. doi: 10.1080/07315724.2004.10719370. [DOI] [PubMed] [Google Scholar]
- Fasano M, Bergamasco B, Lopiano L. Modifications of the iron-neuromelanin in Parkinson’s disease. J Neurochem. 2006;96:909–16. doi: 10.1111/j.1471-4159.2005.03638.x. [DOI] [PubMed] [Google Scholar]
- Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006;69:117–22. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Frank B, Gupta S. A review of antioxidants and Alzheimer’s disease. Ann Clin Psychiatry. 2005;17:269–86. doi: 10.1080/10401230500296428. [DOI] [PubMed] [Google Scholar]
- Frei B, Higdon JV. Antioxidant activity of tea phenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Fukai T, Folz RJ, Landmesser U, et al. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–49. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185:219–26. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Launer LJ, Hofman A, et al. Tea flavonoids may protect against atherosclerosis. The Rotterdam study. Arch Intern Med. 1999;159:2170–4. doi: 10.1001/archinte.159.18.2170. [DOI] [PubMed] [Google Scholar]
- Geng Z, Lau BHS. Aged garlic extract modulates redox cycle and superoxide dismutase activity in vascular endothelial cells. Phytother Res. 1997;11:54–6. [Google Scholar]
- Gibson GE, Zhang H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Intern. 2002;40:493–504. doi: 10.1016/s0197-0186(01)00120-6. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–75. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Glade MJ. The role of reactive oxygen species in Health and Disease Northeast Regional Environmental Public Health Center University of Massachusetts. Amerst Nutrition. 2003;19:401–3. [Google Scholar]
- Gooderham MH, Adlercreutz H, Ojala ST, et al. A soy protein isolate rich in genistein and daidzein and its effects on plasma isoflavone concentrations, platelet aggregation, blood lipids and fatty acid composition of plasma phospholipids in normal men. J Nutr. 1996;126:2000–6. doi: 10.1093/jn/126.8.2000. [DOI] [PubMed] [Google Scholar]
- Gramignano G, Lusso MR, Madeddu C, et al. Efficacy of L-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006;22:136–45. doi: 10.1016/j.nut.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Guerrero-Romero F, Rodríguez-Morán M. Complementary therapies for diabetes: The case for chromium, magnesium, and antioxidants. Arch Med Res. 2005;36:250–7. doi: 10.1016/j.arcmed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Gupta M, Dobashi K, Greene JK, et al. Studies on hepatic injury and antioxidant enzyme activities in rat sub-cellular organelles following in vivo ischemia and reperfusion. Mol Cell Biochem. 1997;176:337–47. [PubMed] [Google Scholar]
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a casual link? Exp Neurol. 2005;193:279–90. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidants in human health and disease. Ann Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- Hammerstone JF, Lazarus SA, Schmitz HH. Procyanidin content and variation in some commonly consumed foods. J Nutr. 2000;130:2086S–92S. doi: 10.1093/jn/130.8.2086S. [DOI] [PubMed] [Google Scholar]
- Hardland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38:313–16. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Harman D. Ageing: a theory based on free radical and radiation chemistry. J Gerontol. 1956;2:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hedge PS, Rajasekaran NS, Chandra TS. Effects of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced rats. Nutr Res. 2205;25:1109–20. [Google Scholar]
- Heitzer T, Yla Herttuala S, Wild E, et al. Effect of Vitamin E on endothelial vasodilator function in patients with hypercholesterolemia, chronic smoking or both. J Am Coll Cardiol. 1999;33:499–505. doi: 10.1016/s0735-1097(98)00584-1. [DOI] [PubMed] [Google Scholar]
- Hellman NE, Gitilin JD. Ceruloplasmin metabolism and function. Ann Rev Nutr. 2002;22:439–58. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- Hensley K, Benakass EJ, Bolli R, et al. New perspectives on vitamin E: γ-tocopherol and carboxyethylhydroxychroman metabolites in biology and medicine. Free Rad Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Feskens EJM, Hollman PCH, et al. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zupthen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–8. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, LaBree L, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–9. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Ann Rev Biochem. 1985;54:237–71. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Huang C, Huang Y, Li J, et al. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappaB by black raspberry extracts. Cancer Res. 2002;62:6857–63. [PubMed] [Google Scholar]
- Innes AJ, Kennedy G, McLaren M, et al. Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets. 2003;14:325–7. doi: 10.1080/0953710031000123681. [DOI] [PubMed] [Google Scholar]
- Inoue M, Sato EF, Nishikawa M, et al. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495–505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- Issa AY, Volate SR, Wargovich MJ, et al. The role of phytochemicals in inhibition of cancer and inflammation: New directions and perspectives. J Food Comp Analysis. 2006;19:405–19. [Google Scholar]
- Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutation Res. 2006;612:105–14. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Rad Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Koroshetz WJ, Beal MF, et al. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–95. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- Kaliora AC, Dedoussis GVZ, Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Kamat JP, Devasagayam TP. Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep. 1999;4:179–88. doi: 10.1179/135100099101534882. [DOI] [PubMed] [Google Scholar]
- Karasek M. Melatonin, human aging, and age-related diseases. Exp Geront. 2004;39:1723–9. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Karoui H, Hogg N, Frejaville C, et al. Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite-ESR-SPIN trapping and oxygen uptake studies. J Biol Chem. 1996;271:6000–9. doi: 10.1074/jbc.271.11.6000. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Guo Y, Cunningham D, et al. Dietary antioxidants preserve endothelium-dependent vessel relaxation in cholesterol-fed rabbits. Proc Natl Acad Sci USA. 1993;90:11880–4. doi: 10.1073/pnas.90.24.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Ann Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Knoops KTB, Groot LCPGM de, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. J Amer Med Asso. 2004;292:1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem. 2004;11:1041–64. doi: 10.2174/0929867043455567. [DOI] [PubMed] [Google Scholar]
- Korolainen MA, Goldsteins G, Tuula A, et al. Oxidative modification of proteins in the frontal cortex of Alzheimer’s disease brain. Neuro Biol Aging. 2006;27:42–53. doi: 10.1016/j.neurobiolaging.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Korotchkina LG, Sidhu S, Patel MS, et al. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Rad Res. 2004;38:1083–92. doi: 10.1080/10715760400004168. [DOI] [PubMed] [Google Scholar]
- Kowald A, Kirkwood TB. Accumulation of defective mitochondria through delayed degradation of damaged organelles and its possible role in ageing of post-mitotic and dividing cells. J Theor Biol. 2000;202:145–60. doi: 10.1006/jtbi.1999.1046. [DOI] [PubMed] [Google Scholar]
- Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Rad Biol Med. 1993;15:621–7. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- Kuppusamy UR, Dharmani M, Kanthimathi MS, et al. Antioxidant enzyme activities of human peripheral blood mononuclear cells exposed to trace elements. Biol Trace Element Res. 2005;106:29–39. doi: 10.1385/BTER:106:1:029. [DOI] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126:365–79. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9:273–84. doi: 10.1002/biof.5520090224. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA, et al. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26:128–32. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee DH, Folsom AR, Harnack L, et al. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr. 2004;80:1194–2000. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Harris GK, Shi XL. Metal-induced oxidative stress and signal transduction. Free Rad Biol Med. 2004;37:1921–42. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Leung FY. Trace elements that act as antioxidants in parenteral micronutrition. J Nutr Biochem. 1998;9:304–7. [Google Scholar]
- Levy Y, Zaltsberg H, Ben-Amotz A, et al. Dietary supplementation of a natural isomer mixture of β-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann Nutr Metab. 2000;44:54–60. doi: 10.1159/000012821. [DOI] [PubMed] [Google Scholar]
- Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Curr Opin Clin Metab Care. 2005;8:641–6. doi: 10.1097/01.mco.0000171123.60665.16. [DOI] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, J van Heerikhuize MA, et al. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s’ disease, and apolipoprotein E-epsilon4/4 genotype. J Clin Endocrinol Metab. 1999;84:323–7. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- Lopaczynski W, Zeisel SH. Antioxidants, programmed cell death, and cancer. Nutr Res. 2001;21:295–307. [Google Scholar]
- Lowe NM, Frase WD, Jackson MJ, et al. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc Nutr Soc. 2002;61:181–5. doi: 10.1079/PNS2002154. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, et al. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–8. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- Mainous AG, Wells BJ, Koopman RJ, et al. Iron, lipids, and risk of cancer in the Framingham Offspring cohort. Am J Epidemiol. 2005;161:1115–22. doi: 10.1093/aje/kwi131. [DOI] [PubMed] [Google Scholar]
- Majeed A, Aylin P. The ageing population of the United Kingdom and cardiovascular disease. BMJ. 2005;331:1362. doi: 10.1136/bmj.331.7529.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S, Packer L, Moussa BH, et al. Proceedings from the “Third International Conference on mechanism of Action of Nutraceuticals”. J Nutr Biochem. 2005;16:513–20. doi: 10.1016/j.jnutbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Rad Biol Med. 2006;40:1603–14. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Manthey KC, Rodriguez-Melendez R, Hoi JT, et al. Riboflavin deficiency causes protein and DNA damage in HepG2 cells, triggering arrest in G1 phase of the cell cycle. J Nutr Biochem. 2006;17:250–6. doi: 10.1016/j.jnutbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RL, Ross CA. Diagnosis of Huntington’s disease. Clin Chem. 2003;49:1726–32. doi: 10.1373/49.10.1726. [DOI] [PubMed] [Google Scholar]
- Mariani E, Polidori MC, Cherubini A, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J Chromat B. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- Masella R, Di Benedetto R, Vari R, et al. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–86. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Mates JM, Perez-Gomez C, De Castro IN. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Rad Biol Med. 1999;26:1034–53. doi: 10.1016/s0891-5849(98)00302-5. [DOI] [PubMed] [Google Scholar]
- McEligot AJ, Yang S, Meyskens FL, et al. Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Ann Rev Nutr. 2005;25:261–95. doi: 10.1146/annurev.nutr.25.050304.092633. [DOI] [PubMed] [Google Scholar]
- Meococci P, Polidori MC, Ingegni T, et al. Oxidative damage to DNA in lymphocytes from AD patients. Neurology. 1998;51:1014–17. doi: 10.1212/wnl.51.4.1014. [DOI] [PubMed] [Google Scholar]
- Meydani M. Nutrition interventions in aging and age-associated diseases. Ann NY Acad Sci. 2001;928:226–35. doi: 10.1111/j.1749-6632.2001.tb05652.x. [DOI] [PubMed] [Google Scholar]
- Migliore L, Petrozzi L, Lucetti C, et al. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology. 2002;58:1809–15. doi: 10.1212/wnl.58.12.1809. [DOI] [PubMed] [Google Scholar]
- Miller ER, Pastor-Barriuso D, Dalal RA, et al. Meta-analysis: high-dosage Vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Miquel J, Ramirez-Bosca A, Ramirez-Bosca JV, et al. Menopause: a review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch Gerontol Geriatr. 2006;42:289–306. doi: 10.1016/j.archger.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Beal MF, Robertson D, et al. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology. 1999;52:1104–5. doi: 10.1212/wnl.52.5.1104. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, et al. Molecular pathology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mortensen A, Skibsted LH, Truscott TG. The interaction of dietary carotenoids with radical species. Arch Biochem Biophs. 2001;385:13–19. doi: 10.1006/abbi.2000.2172. [DOI] [PubMed] [Google Scholar]
- Munteanu A, Zingg JM, Azzi A. Anti-atherosclerotic effects of vitamin E-myth or reality? J Cell Mol Med. 2004;8:59–76. doi: 10.1111/j.1582-4934.2004.tb00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawasw Y, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–13. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Chronopoulos AK, Singh I, et al. Dietary flavonoids and procyanindin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77:1466–73. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Ann Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- Nascimento MM, Suliman ME, Murayama Y, et al. Effect of high-dose thiamine and pyridoxine on advanced glycation end products and other oxidative stress markers in hemodialysis patients: a randomized placebo-controlled study. J Ren Nutr. 2006;16:119–24. doi: 10.1053/j.jrn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Navari-Izzo F, Quartacci MF, Sgherri C. Lipoic acid: a unique anti-oxidant in the detoxification of activated oxygen species. Plant Physiol Biochem. 2002;40:463–70. [Google Scholar]
- Naziroglu M, Butterworth P. Protective effects of moderate exercise with dietary vitamin C and E on blood antioxidative defense mechanism in rats with streptozotocin-induced diabetes. Can J Appl Physiol. 2005;30:172–85. doi: 10.1139/h05-113. [DOI] [PubMed] [Google Scholar]
- Neri S, Signorelli SS, Torrisi B, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Therapeut. 2005;27:1764–73. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Oberley LW. Role of antioxidant enzymes in cell immortalization and transformation. Mol Cell Biochem. 1998;84:147–53. doi: 10.1007/BF00421049. [DOI] [PubMed] [Google Scholar]
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol. 1998;55:1409–15. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- Omoni AO, Aluko RE. The anti-carcinogenic and anti-atherogenic effects of lycopene: a review. Trends Food Sci Tech. 2005;16:344–50. [Google Scholar]
- Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann NY Acad Sci. 2005;1043:440–51. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888–95. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Palomaki A, Malminiemi K, Malminiemi O, et al. Effects of lovastatin therapy on susceptibility of LDL to oxidation during ∝-tocopherol supplementation. Aterioscler Thromb Vasc Biol. 1999;19:1541–8. doi: 10.1161/01.atv.19.6.1541. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Di Nicuolo F, et al. Prooxidant effects of β-carotene in cultured cells. Mol Aspect Med. 2003;24:353–62. doi: 10.1016/s0098-2997(03)00031-1. [DOI] [PubMed] [Google Scholar]
- Peel NM, McClure RJ, Bartlett HP, et al. Behavioral determinants of healthy aging. Am J Prevent Med. 2005;28:298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Penckofer S, Schwertz D, Florczak K, et al. Oxidative stress and cardiovascular disease in type 2 diabetes: the role of antioxidants and prooxidants. J Cardiovasc Nurs. 2002;16:68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Peyser CE, Folstein M, Chase GA, et al. Trial of α-tocopherol in Huntington’s disease. Am J Psych. 1995;152:1771–5. doi: 10.1176/ajp.152.12.1771. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–32. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- Poggioli S, Hilaire B, Friguet B. Age-related increase of proteins glycation in peripheral blood lymphocytes is restricted to preferential target proteins. Exp Geront. 2002;37:1207–15. doi: 10.1016/s0531-5565(02)00145-6. [DOI] [PubMed] [Google Scholar]
- Poli G, Leonarduzzi G, Biasi F, et al. Oxidative stress and cell signaling. Curr Med Chem. 2004;11:1163–82. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trials. Free Rad Biol Med. 2000;28:141–64. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- Puntarulo S. Iron, oxidative stress and human health. Mol Aspects Med. 2005;26:299–312. doi: 10.1016/j.mam.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rahimi R, Nikfar S, Larijani B, et al. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacothe. 2005;59:365–73. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rahman K. Garlic and aging: new insights into an old remedy. Ageing Res Rev. 2003;2:39–56. doi: 10.1016/s1568-1637(02)00049-1. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–42. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8:797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Sampson J, Bramley PM, et al. Why do we expect carotenoids to be antioxidants in vivo? Free Rad Res. 1997;26:381–98. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- Riley PA. Free radicals in biology: oxidative stress and effects of ionizing radiation. Int J Rad Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- Rostan EF, DeBuys HV, Madey DL, et al. Evidence supporting zinc as an important antioxidant for skin. Intern J Dermatol. 2002;41:606–11. doi: 10.1046/j.1365-4362.2002.01567.x. [DOI] [PubMed] [Google Scholar]
- Ruel G, Pomerleau S, Couture P, et al. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term cranberry juice consumption. Metabolism. 2005;54:856–61. doi: 10.1016/j.metabol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Ruhnau KJ, Meissner HP, Finn JR, et al. Effects of a 3-week oral treatment with the antioxidant thiotic acid (a-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet Med. 1999;16:1040–3. doi: 10.1046/j.1464-5491.1999.00190.x. [DOI] [PubMed] [Google Scholar]
- Samman S, Sivarajah G, Man JC, et al. A mixed fruit and vegetable concentrate increases plasma antioxidant vitamins and folate and lowers plasma homocysteine in men. J Nutr. 2003;133:2188–93. doi: 10.1093/jn/133.7.2188. [DOI] [PubMed] [Google Scholar]
- Sánchez-Moreno C, Cano MP, Begoña de A, et al. Mediterranean vegetable soup consumption increases vitamin C and decreases F2-isoprostanes, prostaglandin E2, and monocyte chemotactic protein-1 in healthy humans. J Nutr Biochem. 2006;17:183–9. doi: 10.1016/j.jnutbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds in human nutrition. J Food Sci Agr. 2000;80:1094–117. [Google Scholar]
- Sarkar FH, Li Y. Cell signaling pathways altered by natural chemo-preventative agents. Mutat Res. 2004;555:53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Savitha S, Panneerselvam C. Mitochondrial membrane damage during aging process in rat heart: potential efficacy of L-carnitine and DL alpha lipoic acid. Mech Ageing Dev. 2006;127:349–55. doi: 10.1016/j.mad.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Zeiher AM. Atherogenesis-recent insights into basic mechanisms and their clinical impact. Nephrol Dial Transplant. 2002;17:2055–64. doi: 10.1093/ndt/17.12.2055. [DOI] [PubMed] [Google Scholar]
- Schippling S, Kontush A, Arlt S, et al. Increased lipoprotein oxidation in Alzheimer’s disease. Free Rad Biol Med. 2000;28:351–60. doi: 10.1016/s0891-5849(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Schneider LS, DeKosky ST, Farlow MR, et al. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr Alzheimer Res. 2005;2:541–51. doi: 10.2174/156720505774932287. [DOI] [PubMed] [Google Scholar]
- Schrauzer GN. Anticarcinogenic effects of selenium. Cell Mol Life Sci. 2000;57:1864–73. doi: 10.1007/PL00000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H, Boyd C, Spencer JPE, et al. MAPK signaling in neurodegeneration: influences of flavonoids and of nitric acid. Neurobiol Aging. 2002;23:861–80. doi: 10.1016/s0197-4580(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Scott J. Pathophysiology and biochemistry of cardiovascular disease. Curr Opion Genet Develop. 2004;14:271–9. doi: 10.1016/j.gde.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Sechi G, Deeden MG, Bua G, et al. Reduced intravenous glutathione in the treatment of early Patkinson’s disease. Progress Neuropsychopharmacol Biol Psych. 1996;20:1159–70. doi: 10.1016/s0278-5846(96)00103-0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–38. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. 2004. Free Rad Biol Med. 2004;37:582–93. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey A, Laura SBA, et al. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W, Sevanian A. Nutritional, dietary and post-prandial oxidative stress. J Nutr. 2005;135:969–72. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- Simms NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–26. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001;1:197–207. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- Smith AR, Shenvi SV, Widlansky M, et al. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–46. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of Aging: An appraisal of the oxidative stress hypothesis. Free Rad Biol Med. 2002;33:575–86. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and ageing. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Lee WJ, Koh J, et al. α-Lipoic acid prevents diabetes mellitus in diabetes-prone obese rats. Biochem Biophys Res Comm. 2004;326:197–202. doi: 10.1016/j.bbrc.2004.10.213. [DOI] [PubMed] [Google Scholar]
- Song YQ, Manson JE, Buring JE, et al. Association of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24:376–84. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspect Med. 2003;24:345–51. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Rahman RMA, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Tapia L, Gonzalez-Aguero M, Cisternas MF, et al. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem. 2004;378:617–24. doi: 10.1042/BJ20031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–10. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso JC, Costello A, Watson AL, et al. In vitro polymerization of oxidized tau into filaments. Brain Res. 1993;613:313–6. doi: 10.1016/0006-8993(93)90918-d. [DOI] [PubMed] [Google Scholar]
- Trueba GP, Sanchez GM, Giuliani A. Oxygen free radical and antioxidant defense mechanism in cancer. Front Biosci. 2004;9:2029–44. doi: 10.2741/1335. [DOI] [PubMed] [Google Scholar]
- Tucker JM, Townsend DM. Alpha-tocopherol: roles in prevention and therapy of human disease. Biomed Pharmacother. 2005;59:380–7. doi: 10.1016/j.biopha.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnlund JR, Scott KC, Peiffer GL, et al. Copper status of young men consuming a low-copper diet. Am J Clin Nutr. 1997;65:72–8. doi: 10.1093/ajcn/65.1.72. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–99. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Upritchard JE, Sutherland WH, Mann JL, et al. Effect of supplementation with tomato juice, Vitamins E and C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diab Care. 2000;23:733–8. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26:268–98. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Uriu-Adams JY, Rucker RB, Commisso JF, et al. Diabetes and dietary copper alter Cu metabolism and oxidant defense in the rat. J Nutr Biochem. 2005;16:312–20. doi: 10.1016/j.jnutbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Valko M, Izakovic M, Mazur M, et al. Role of oxygen radicals in DNA damage and cancer incidence. Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronnin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biol Inter. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Van Dongen M, Van Rossum E, Kessels A, et al. The efficacy of gingko for elderly people with dementia and age-associated memory impairment: new results of a randomized clinical trial. J Am Geriatr Soc. 2000;48:1183–94. doi: 10.1111/j.1532-5415.2000.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Varma M, Paneri S, Badi P, et al. Status of MDA and antioxidant enzymes in hyperglycemic postmenopausal women. Flora and Fauna (Jhansi) 2005;11:101–5. [Google Scholar]
- Vitseva O, Varghese S, Chakrabarti S, et al. Grape seed and skin inhibit platelet function and release of reactive oxygen intermediates. J Cardiovasc Pharmacol. 2005;46:445–51. doi: 10.1097/01.fjc.0000176727.67066.1c. [DOI] [PubMed] [Google Scholar]
- Völkel W, Sicilia T, Pähler A, et al. Increased brain levels of 4-hydroxy-2-nonenal glutathione conjugates in severe Alzheimer’s disease. Neurochem Intern. 2006;48:679–686. doi: 10.1016/j.neuint.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Wautier JL, Schmidt AM. Protein glycation. Circulation Res. 2004;95:233–8. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- Wei Z, Lau BHS. Garlic inhibits free radical generation and augments antioxidant enzyme activity in vascular endothelial cells. Nutr Res. 1998;18:61–70. [Google Scholar]
- Wersinger C, Sidhu A. Inflammation and Parkinson’s disease. Curr Drug Targets Inflamm Allergy. 2002;1:221–42. doi: 10.2174/1568010023344580. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Duffy SJ, Hamburg NM, et al. Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease. Free Rad Biol Med. 2005;38:499–506. doi: 10.1016/j.freeradbiomed.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Willett WC. The Mediterranean diet: science and practice. Public Health Nutr. 2006;9:105–10. doi: 10.1079/phn2005931. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Geneva, Switzerland: WHO; 2002. Keeping fit for life: meeting the nutritional needs of older persons. [Google Scholar]
- Wright E, jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycemia. Int J Clin Prac. 2006;60:308–14. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C-C, Hou M-F, Tsai S-M, et al. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clin Chim Acta. 2005;361:104–11. doi: 10.1016/j.cccn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Yochum L, Kushi LH, Meyer K, et al. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149:943–9. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- You WC, Zhang L, Gail MH, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum Vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607–12. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Rad Biol Med. 2001;30:583–94. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]