High antigen levels are the cause of T cell exhaustion during chronic viral infection (original) (raw)
Abstract
Many persistent viral infections induce dysfunctional T cell responses. Although a negative correlation exists between viral load and T cell responses during chronic infection, it is not known whether high antigen levels are the cause or just the consequence of T cell exhaustion. Furthermore, it is unclear what role antigen presentation by bone-marrow (BM) derived versus infected parenchymal cells has on T cell exhaustion. To address these issues, we examined the influence of antigen presentation by different cell types on CD8+ T cell responses during persistent infection of mice with lymphocytic choriomeningitis virus (LCMV) clone 13. We generated BM chimeric mice, in which non-BM derived cells were MHC class I deficient. Virus-specific CD8+ T cells in lymphoid and nonlymphoid tissues were increased in both number and ability to produce cytokines in these mice soon after infection. However, viral clearance from infected MHC I−/− parenchyma was significantly impaired, despite increased populations of cytokine producing CTL. The CD8+ T cell response was overwhelmed by sustained antigen persistence, becoming increasingly exhausted within 4–6 weeks. Thus, we find that (i) sustained antigen presentation directly drives T cell exhaustion during a chronic viral infection, (ii) CTL require direct antigen-MHC interactions to clear virus-infected cells, and (iii) persistent interactions with antigen presented on both hematopoietic and nonhematopoietic cells negatively impacts virus-specific T cell responses during chronic infection.
Keywords: antigen presentation, immune exhaustion, T cell dysfunction
Many chronic infections generate functionally impaired antigen-specific T cell populations. An understanding of the factors responsible for the generation of these T cell populations is important for the development of therapeutic treatments. Functional exhaustion and deletion of CD8+ T cells has been documented during chronic human infections such as HIV (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) and human T-lymphotropic virus (HTLV) (1–3). The exact mechanisms by which infections either resolve in the acute phase or become chronic are largely unknown, although viral load, tissue tropism and the immune response appear intimately involved.
During chronic infection there is an hierarchical loss of T cell function, from mild exhaustion to deletion of the responding cells. Cytotoxic T cells (CTL) lose the ability to produce IL-2, followed by TNF-α and finally IFN-γ as they become more exhausted (4, 5). Alterations to the immunodominance hierarchy and tissue distribution of virus-specific T cells also occurs. We recently identified a major inhibitory pathway, consisting of the inhibitory receptor programmed death 1 (PD-1), which is important for T cell exhaustion during chronic LCMV infection (6). Studies have also demonstrated that the maintenance or survival of CTL during a chronic viral infection requires antigen and extensive cell division, in contrast to normal memory cell maintenance, which is antigen independent (7, 8). Despite these studies, the role of antigen in influencing functional exhaustion has yet to be defined.
Viruses that induce a latent infection (such as HSV), followed by periods of reactivation, induce functional T cell memory. Infections characterized by low levels of viremia (such as CMV), may induce partial functional impairment of virus-specific CTL. In contrast, high level persisting viremia induces more severe T cell dysfunction (such as HIV, HBV, and HCV in humans; SIV in primates; and LCMV in mice). A correlation between viral load and CTL function has been suggested in human chronic infections such as HIV (9, 10). However, this remains poorly defined and a recent study suggested that proliferative capacity, rather than function, correlated inversely with viral load (11). Numerous factors may be influenced by pathogen load, including available levels of different epitopes, levels of inflammatory cytokines and chemokines and tissue accumulation of different immune cells. A number of studies have also shown that CD4-help is important for sustaining CTL responses during persistent infection (5, 12). Thus, it remains unclear whether antigen levels directly drive T cell exhaustion during chronic infection or whether reduced T cell function results from other mechanisms.
Here, we examined the role of antigen presentation after chronic infection of mice with LCMV CL-13. LCMV infects cells by binding to alpha-dystroglycan (α-DG) (13), which is expressed in association with extracellular matrix (ECM) components on stromal and epithelial cells in many tissues, and on dendritic cells (14, 15). We have recently shown that LCMV CL-13 preferentially infects stromal fibroblastic reticular cells (FRC) in the lymphoid tissues (16), and presumably also targets nonhematopoietic cells in other tissues. Using BM chimeric mice lacking MHC class I (MHC I−/−) on radio-resistant non-BM cells, we show here that T cell function was directly influenced by antigen presentation. In the absence of nonhematopoietic antigen presentation CD8+ T cell responses in both lymphoid and nonlymphoid tissues were initially larger and more functional, despite a 2- to 4-fold higher viral load. However, enhanced cytokine production by responding CTL was not sufficient for clearance of virus from infected MHC I−/− tissues, demonstrating a requirement for antigen-MHC interactions to clear virus-infected cells. Viral loads in blood and tissues were 10-fold higher in the MHC I−/− chimeric mice within 4–6 weeks, and resulted in a subsequent decline in T cell numbers and function. These results demonstrate that the amount of direct antigen presentation, from both hematopoietic and nonhematopoietic cells, controls T cell exhaustion during chronic infection.
Results and Discussion
Antigen Presentation by Nonhematopoietic Cells Influences T Cell Function During Chronic Infection.
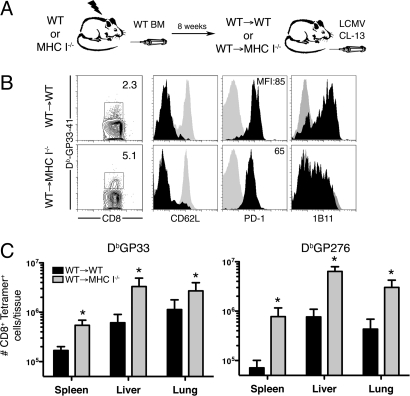
Many organs, including the spleen, lymph nodes, brain, kidney, lung and liver, gut, and pancreas sustain high levels of infected cells during CL-13 infection (4). The expression of α-DG on fibroblasts and epithelial cells in these tissues (14) suggests that these cells may be an important target of CL-13 infection. We have shown that ER-TR7+ FRC are infected by LCMV CL-13 in the spleen and lymph nodes (16). We also observed LCMV antigen colocalization with ER-TR7+ fibroblast-like cells in the brain, liver and kidney after CL-13 infection (Fig. S1). To examine the role of antigen presentation by infected non-BM derived cells on the size and function of the responding virus-specific CD8+ T cell pool we generated BM chimeras by lethally irradiating WT or MHC class I−/− mice, and reconstituting them with WT BM (Fig. 1A). The resulting chimeras either lacked MHC class I on nonhematopoietic cells (WT → MHC I−/−) or had normal expression (WT → WT). To reconstitute the mature CD8+ T cell population in WT → MHC I−/− mice, which lack the ability to select a naïve MHC class I-restricted CD8+ T cell pool in the thymus, mice were given 5 × 107 splenocytes from normal mice before infection. The chimeras displayed similar populations of MHC class I+ CD4 and CD8 T cells before infection (Fig. S2).
Fig. 1.
Virus-specific CD8+ T cell responses in MHC I−/− BM chimeras. (A) MHC I−/− or wild-type (WT) mice were reconstituted with WT BM (WT → MHC Ι−/− or WT → WT) and infected with LCMV CL-13. (B) DbGP33-specific CD8+ T cells were analyzed in the spleen 15 days later. Expression of the indicated markers on Tetramer-specific CD8+ T cells (black histograms). Control staining of naïve CD8+ T cells from the mice is shown (gray histograms). (C) Quantitation of DbGP33- and DbGP276-specific T cells in the spleen, liver and lung 15 days after infection. *, P < 0.05; error bars represent SEM. One representative experiment of 3 is shown.
To assess the role of antigen presentation by nonhematopoietic cells during infection, the chimeric mice were infected with LCMV CL-13 and antigen-specific CD8+ T cells examined by MHC class I tetramer staining after 15 days. Mice lacking MHC I on non-BM cells generated increased numbers of gp33- and gp276-specific CD8+ T cells in the spleen after LCMV CL-13 infection, in comparison with mice with class I-sufficient parenchyma (Fig. 1 B and C). Responding antigen-specific cells were MHC class I restricted and expressed low levels of CD62L and high levels of inhibitory PD-1 in both groups of mice (Fig. 1B and Fig. S2). Interestingly, the level of PD-1 expression in WT → MHC I−/− mice [mean fluorescence intensity (MFI): 65 ± 5] was lower than that in WT → WT (MFI: 85 ± 5.5) mice, which may be indicative of reduced interaction with antigen (17, 18). CTL from MHC I−/− mice also expressed lower levels of CD43 (1B11) (Fig. 1B), although other cell surface markers associated with activation were similar (Fig. S3). These data suggested that the responding CTL were “seeing” less antigen because of an inability to interact with infected MHC I−/− nonhematopoietic cells. Indeed, in agreement with a previous report (19), we observed reduced responses to acute LCMV Armstrong infection of WT → MHC I−/− mice, further demonstrating that nonhematopoietic cells represented a significant source of antigen presentation during LCMV infection.
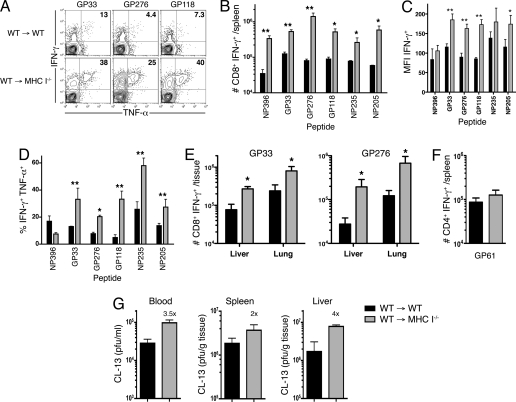
We next examined the function of virus-specific CTL generated in the MHC class I−/− BM chimeric mice after CL-13 infection. A higher proportion and number of virus-specific T cells were capable of producing IFN-γ in the spleen of MHC class I−/− chimeric mice compared with control WT chimeric mice (Fig. 2 A and B). This was observed in CTL responding to 6 different LCMV epitopes (NP396, GP33, GP276, GP118, NP235, and NP205). Moreover, the cells produced greater amounts of IFN-γ, as demonstrated by the MFI of cytokine staining after peptide stimulation (Fig. 2C). We also observed significantly increased TNF-α production in T cells responding to 5 of the 6 epitopes (Fig. 2 A and D). This enhanced functionality indicated that these cells were less exhausted than those generated in mice with MHC I-sufficient parenchyma. However, IL-2 production was unchanged in these mice (data not shown), demonstrating that the cells remained partially dysfunctional (4). As we expected, antigen-specific CD4+ T cell responses in these mice were unchanged (Fig. 2F), suggesting that the increased CTL responses were not due to altered T cell help.
Fig. 2.
Enhanced cytokine production by virus-specific CD8+ T cells in MHC I−/− BM chimeras. (A) IFN-γ and TNF-α production by GP33-, GP276-, and GP118-specific CD8+ T cells from spleens of chimeric mice 15 days after LCMV CL-13 infection. Numbers represent the percentage of IFN-γ+ CD8+ T cells that are TNF-α+. (B) The number of splenic CD8+ T cells producing IFN-γ upon stimulation with either of 6 LCMV peptides as indicated. (C) Mean fluorescence intensity (MFI) of IFN-γ expression in the 6 antigen-specific CD8+ T cell populations after CL-13 infection. (D) TNF-α production by IFN-γ+ CD8+ T cells upon stimulation with the indicated peptides. (E) The number of gp33- or gp276-specific CD8+ T cells from the liver or lung producing IFN-γ upon stimulation. (F) Equivalent GP61-specific CD4+ T cell responses in the spleen after infection, as measured by production of IFN-γ by antigen-specific cells. (G) Viral titers in the blood, spleen and liver in chimeric mice 15 days after LCMV CL-13 infection. Numbers above WT → MHC I−/− bars represent fold increase in viral titer over that in WT → WT mice. *, P = <0.05; **, P = <0.01; error bars represent SEM. One representative experiment of 2–3 is shown.
During LCMV CL-13 infection large numbers of CTL can be found within infected nonlymphoid organs and tissues (4). We compared antigen-specific responses in the liver and lungs from both groups of chimeric mice 15 days after infection. Increased numbers of DbGP33- and DbGP276-specific cells were found in the liver and lungs of mice lacking MHC I on non-BM cells (Fig. 1C). As we had observed in the spleen, there were higher numbers of GP33- and GP276-specific cells producing IFN-γ in these mice (Fig. 2E). Antigen-specific cells from WT → MHC I−/− mice produced more IFN-γ, and of these, a greater proportion were capable of producing TNF-α upon peptide stimulation (data not shown). Surprisingly, despite enhanced CTL function, viral titers in the blood and tissues were increased 2- to 4-fold in mice lacking MHC I on non-BM cells (Fig. 2G). Together, these data demonstrate that antigen presentation by nonhematopoietic cells influenced CTL number and function in both lymphoid and nonlymphoid tissues during chronic LCMV CL-13 infection. Moreover, these data suggest that antigen-MHC interactions directly influenced viral clearance and the development of functional T cell exhaustion after infection.
T Cell Mediated Bystander Affects Are Insufficient to Mediate Viral Clearance from Cells Lacking MHC I.
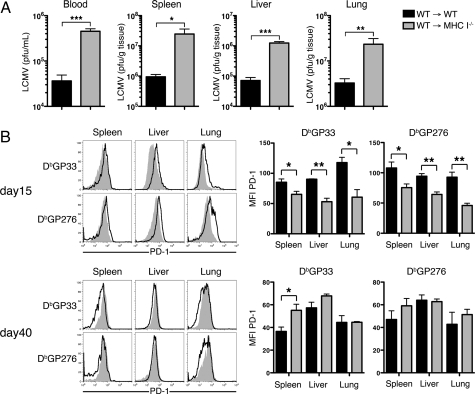
The above experiments demonstrated that MHC class I−/− BM chimeric mice generated increased populations of functional CD8+ T cells soon after LCMV CL-13 infection, yet viral titers were higher in the blood and tissues of these mice. Because these cells showed an enhanced capability of producing cytokines, it remained possible that viral clearance may be improved in these mice over time. Such clearance would have required bystander effects by cytokines produced by CTL in the tissues, in the absence of direct antigen-specific TCR-MHC interactions with infected MHC I−/− cells. However, within 4 weeks after infection mice lacking MHC I on non-BM cells demonstrated a 10- to 25-fold increase in viral titers in the blood and tissues compared with WT mice (Fig. 3A). This was indicative of a reduced ability to clear virus from infected class I negative non-BM cells. Moreover, expression of PD-1 on antigen-specific CD8+ T cells in WT → MHC I−/− mice, although significantly lower than that in WT → WT at day 15, remained high on both GP33- and GP276-specific cells 41 days after infection (Fig. 3B). This was indicative of the persistence of high levels of antigen, because PD-1 expression correlates with levels of virus in the tissues during chronic LCMV infection (17). Early control of virus during Cl-13 infection depends on CD8+ T cells (20), and perforin (21, 22) and IFN-γ (23). Although it is generally thought that CTL mediate antiviral clearance in vivo by killing of target cells (24), clearance of viral infections, including LCMV, can involve antiviral cytokines (TNF-α and IFN-γ) without cell lysis (25). However, it has remained unclear whether direct antigen-specific recognition via MHC is required for CTL to mediate clearance in vivo during infection. In regards to this, our data show that viral clearance during chronic infection required direct antigen-specific recognition of infected cells by CTL.
Fig. 3.
Reduced viral clearance in mice lacking MHC I on nonhematopoietic cells. (A) Viral titers were measured in the serum, spleen, liver and lungs 30 days after LCMV CL-13 infection. (B) Changes in PD-1 expression on antigen-specific CD8+ T cells 2 and 6 weeks after infection. Representative expression of PD-1 on DbGP33- and DbGP276-specific CD8+ T cells in the indicated tissues from WT → WT mice (open histograms) and WT → MHC I−/− mice (filled histograms) is shown at Left. Graphs depict the mean fluorescence intensity (MFI) of PD-1 expression on the tetramer positive cells. *, P = <0.05; **, P = <0.01; error bars represent SEM. All histograms are representative of 2–3 independent experiments and summarized results are pooled from 2 experiments (n = 4–10 mice per group).
Persistence of Antigen-MHC Complexes Drives Functional Exhaustion in T Cells.
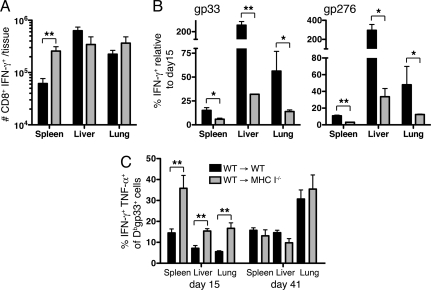
The experiments above demonstrated that antigen-MHC complexes drive T cell exhaustion, yet were required for clearance of virus from the tissues. Because viral titers continued to rise in mice lacking MHC class I (Fig. S4), and thus presumably increased the burden of infected MHC I+ BM-derived cells and MHC I− non-BM cells, we examined what effect this persistent high dose antigen had on the responding T cells over time. Numbers of tetramer positive T cells and functional IFN-γ producing antigen-specific CD8+ T cells remained increased in the spleens of MHC I deficient chimeras 6 weeks after infection when compared with WT chimeras (Fig. 4A and Fig. S4). However, numbers of responding cells were similar or slightly lower in the liver and lungs of MHC I−/− mice compared with WT mice. We examined this in more detail by comparing numbers of GP33- or GP276-specific tetramer-positive cells or IFN-γ producing CD8+ T cells in the spleen, liver, and lungs 41 days after infection with the populations detected at day 15. By day 41 postinfection, the populations of responding GP33- and GP276-specific cells in the spleens of WT mice was 5- to 10-fold lower than at day 15 (Fig. 4B and Fig. S4). A 2- to 5-fold reduction was observed in the lungs, whereas responses in the liver of WT mice were up to 3-fold higher than those at day 15, similar to that observed previously (4). Remarkably, responding CD8+ T cell numbers in WT → MHC I−/− mice were 5- to 30-fold lower in the spleen and up to 20-fold lower in the liver and lungs at day 41 when compared with that at day 15 (Fig. 4B and Fig. S4). This greater reduction in numbers of antigen-specific cells in MHC I−/− chimeras was specific for the CD8+ T cells, because similar numbers of GP61-specific CD4+ T cells were detected in both WT and MHC I−/− mice at this time (Fig. S4).
Fig. 4.
Persistent high levels of antigen drive functional exhaustion of T cells. (A) Total number of IFN-γ producing CD8+ T cells responding to 6 LCMV epitopes (NP396, GP33, GP276, GP118, NP235, and NP205) in the spleen, liver and lungs 41 days after CL-13 infection. (B) The frequency of CD8+ T cells producing IFN-γ after GP33 or GP276 peptide stimulation at day 41 postinfection, relative to that at day 15. (C) The proportion of DbGP33-specific CD8+ T cells producing IFN-γ and TNF-α in the indicated tissues, 15 or 41 days after CL-13 infection. *, P < 0.05; **, P < 0.01; error bars represent SEM. Data are representative of 2 independent experiments of 4–10 mice per group.
The presence of highly functional CD8+ T cells, represented by the proportion of tetramer-positive cells that produced both IFN-γ and TNF-α after peptide stimulation, was significantly increased 15 days after infection of MHC class I-deficient chimeric mice in comparison with WT mice (Fig. 4C). In contrast, 6 weeks after infection, a similar proportion of tetramer-positive cells produced both IFN-γ and TNF-α in WT or MHC I−/− BM chimeras. Together, these data suggest that sustained high levels of virus in the WT → MHC I−/− mice resulted in increased functional exhaustion of the responding CD8+ T cells. Although reducing the availability antigen-MHC class I molecules on nonhematopoietic cells initially improved T cell function, an inability to clear virus from infected parenchymal cells resulted in increased systemic viral load, and presumably the number of infected BM-derived cells. This demonstrates that both BM- and non-BM-derived cells can influence functional exhaustion of T cells in an antigen-dependent manner. Thus, the amount of antigen that T cells see in the context of MHC class I influences their functional state, and inversely, their ability to control the infection. The inflammatory mileau may be increased because of sustained high viral titers, and contribute to exhaustion in these mice. Further, the duration of antigen presentation may also influence exhaustion, suggesting that cumulative signals received after infection may contribute to T cell health and function. Finally, the lack of thymic output throughout infection (26, 27) in the MHC I−/− BM chimeric mice, may have also contributed to the decreasing responses later after infection in these mice because of a lack of continuous recruitment of naïve cells.
Viral tropism, specifically the number and type of cells infected, will likely have a significant impact on the total antigenic burden seen by responding T cells. LCMV CL-13 has a broader tropism and higher degree of infectivity than LCMV Armstrong (28), due at least in part to a higher affinity for α-DG (15). The relative contributions of different cell types, for example infected stromal cells versus infected antigen-presenting cells, may also influence the function of T cells in different tissues. It is likely that different signals, including inhibitory signals [such as PD-1 ligand 1 (PD-L1) or IL-10 (6, 29)] or lack of costimulatory molecules, and local concentrations of antigen-MHC complexes will influence T cell responses. Indeed, concurrent interaction with inhibitory ligands such as PD-L1 and antigen-MHC molecules on nonhematopoietic cells may further enhance T cell exhaustion (30).
It is well established that persistent infections both in humans and in animal models induce T cell responses that display progressive dysfunction, which is thought to contribute to the persistence of the pathogen in the host. It has been unclear whether antigen is the cause or simply the consequence of such T cell dysfunction, despite a correlation between pathogen load and T cell numbers or function in a number of infections, such as HIV and LCMV. A recent report suggested that persistent antigen may be the cause of reduced T cell responses during chronic HIV infection, because reduction in pathogen or antigen levels improved T cell responses (31). By restricting antigen presentation to BM cells in WT → MHC I−/− mice we demonstrate that antigen-MHC complexes directly influenced T cell function during a chronic viral infection. Use of WT → MHC I−/− chimeras meant that thymic selection of CD8+ T cells was impaired. We corrected for this by transferring WT splenocytes. This resulted in a smaller CD8+ T cell pool before infection (3.6% versus 7.9% CD8+ T cells in WT → WT and WT → MHC I−/− mice, respectively). Despite starting at a lower frequency, T cells in the WT → MHC I−/− mice expanded to a higher level than that in WT → WT mice (see Figs. 1–3). This further supports a role for MHC I interactions in influencing exhaustion after infection.
In conclusion, our system allowed direct measurement of the contribution of antigen-MHC complexes to T cell exhaustion during a chronic infection, in the presence of similar inflammatory and helper T cell environments. Functional exhaustion of CD8 T cells was influenced by antigen presentation by both hematopoietic and nonhematopoietic cells. Finally, our data also suggest that direct interaction with antigen-MHC complexes by CTL may be required for viral clearance during chronic viral infection. These observations may have implications for understanding the balance between viral load and immune function during persistent infections and the design of relevant therapies.
Materials and Methods
Mice, Chimeras, and Infections.
Six-week-old female C57BL/6 mice were purchased from Jackson Laboratory. C57BL/6 β2m−H-2Kb−H-2Db− (MHC I−/−) mice were bred and maintained in our animal facility. Bone-marrow chimeras were produced by irradiation of recipient mice with 2 doses of 550 cGr, 3 h apart, followed by reconstitution with 5 × 106 bone-marrow cells from C57BL/6 mice. Mice were allowed to reconstitute for 8 weeks. One day before infection of MHC I−/− chimeras, 50 × 106 C57BL/6 splenocytes were injected per mouse to provide a pool of naïve mature T cells, which are not selected on MHC I deficient thymic stroma. Similar responses were observed in WT mice with or without transfer of splenocytes. For infection, mice received 2 × 106 PFU of Cl-13 or 2 × 105 PFU of Armstrong intravenously. Titers of virus from serum or homogenized tissue samples were determined by plaque assay on Vero cells as described in ref. 32.
Antibodies and Flow Cytometry.
Single cell suspensions were stained with anti-CD8α (53–6.7), -CD62L (MEL-14), -PD-1 (J43), -IFN-γ (XMG1.2), -TNF-α (MP6-XT22), or -IL-2 (JES6–5H4) antibodies (BD Biosciences). Intracellular staining for IFN-γ, TNF-α or IL-2 after 5 h in vitro stimulation with 0.1 μg/mL GP33, GP276, NP396, NP235, NP205, or GP118 peptide was performed using the Cytofix/Cytoperm kit according to the manufacturers instructions (BD PharMingen). MHC class I tetramers were made and used as described in ref. 33. Samples were analyzed using a Becton Dickinson FACScaliber .
Immunofluorescence.
Organs were removed from mice, frozen in OCT (TissueTek) and 20-μm cryostat sections fixed in ice-cold acetone for 10 min. Sections were stained with ER-TR7 to detect FRC (Biogenesis) and polyclonal anti-LCMV guinea-pig serum. Stains were visualized with Alexa Fluor-488 goat anti-rat and Alexa Fluor-568 goat anti-guinea-pig Ig (Molecular Probes) and analyzed by confocal microscopy (LSM510; Zeiss). Images were prepared using ImageJ (National Institutes of Health) and Adobe Photoshop.
Statistical Analysis.
Statistical analysis was performed with 2-tailed unpaired t tests using Graphpad Prism.
Supplementary Material
Supporting Information
Acknowledgments.
This work was supported by National Institutes of Health Grants AI56299, AI30048, and AI04464409 (to R.A.) and a Gates Foundation Grand Challenges in Global Health Grant (to R.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Goepfert PA, et al. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruener NH, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klenerman P, Hill A. T cells and viral persistence: Lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 4.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 7.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogg GS, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 10.Edwards BH, et al. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CL, et al. Proliferative capacity of epitope-specific CD8 T-cell responses is inversely related to viral load in chronic human immunodeficiency virus type 1 infection. J Virol. 2007;81:434–438. doi: 10.1128/JVI.01754-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao W, et al. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 14.Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- 15.Sevilla N, et al. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller SN, et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0702579104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 19.Thomas S, Kolumam GA, Murali-Krishna K. Antigen presentation by nonhemopoietic cells amplifies clonal expansion of effector CD8 T cells in a pathogen-specific manner. J Immunol. 2007;178:5802–5811. doi: 10.4049/jimmunol.178.9.5802. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson BD, Butler LD, Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987;61:3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagi D, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 22.Walsh CM, et al. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 24.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borrow P. Mechanisms of viral clearance and persistence. J Viral Hepat. 1997;4(Suppl 2):16–24. doi: 10.1111/j.1365-2893.1997.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller NE, Bonczyk JR, Nakayama Y, Suresh M. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J Virol. 2005;79:9419–9429. doi: 10.1128/JVI.79.15.9419-9429.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vezys V, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: Importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 31.Streeck H, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: A reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information