BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1Ter/Ter mice (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 15.
Abstract
A homozygous nonsense mutation (Ter) in murine Dnd1 (Dnd1Ter/Ter) results in a significant early loss of primordial germ cells (PGCs) prior to colonization of the gonad in both sexes and all genetic backgrounds tested. The same mutation also leads to testicular teratomas only on the 129Sv/J background. Male mutants on other genetic backgrounds ultimately lose all PGCs with no incidence of teratoma formation. It is not clear how these PGCs are lost or what factors directly control the strain-specific phenotype variation. To determine the mechanism underlying early PGC loss we crossed Dnd1Ter/Ter embryos to a _Bax_-null background and found that germ cells were partially rescued. Surprisingly, on a mixed genetic background, rescued male germ cells also generated fully developed teratomas at a high rate. Double-mutant females on a mixed background did not develop teratomas, but were fertile and produced viable off-spring. However, when Dnd1Ter/Ter XX germ cells developed in a testicular environment they gave rise to the same neoplastic clusters as mutant XY germ cells in a testis. We conclude that BAX-mediated apoptosis plays a role in early germ cell loss and protects from testicular teratoma formation on a mixed genetic background.
Keywords: Dnd1, Ter, testicular teratoma, teratocarcinoma, testicular germ cell tumor
Introduction
Testicular teratomas arise as benign, non-seminomatous germ cell tumors characterized by the differentiation of a diverse array of cell and tissue types within the tumor, including cartilage, muscle, hair, and glandular tissue. Although spontaneous testicular teratomas are virtually non-existent in mice, Leroy Stevens established a substrain of mice in the 1950s (129Sv/J) in which the incidence of testicular teratomas was approximately 1% (Stevens and Little, 1954; Stevens 1959). In 1973, Stevens discovered a spontaneous mutation on the 129Sv/J genetic background (called Ter) that raised the incidence of teratomas to 17% in heterozygotes and 94% in homozygous mutants, but never induced ovarian teratomas (Noguchi and Noguchi, 1985; Stevens, 1973). Despite the higher incidence on the 129Sv/J background, when Ter was crossed onto other inbred strains, such as C57BL/6J, LTXBJ, and C3H/HeJ, testicular teratomas were not found (Noguchi et al., 1996; Stevens, 1981). The variable penetrance of the tumor phenotype in Ter mice of different genetic backgrounds faithfully mimics the etiology of germ cell tumors in humans. Germ cell tumors are the most common form of cancer in men between the ages of 15 and 34, and are highly correlated with ethnicity and other complex genetic influences (Brown et al., 1987; Brown et al., 1986; Hussain et al., 2008; Linger et al., 2007).
The identity of the Ter locus was recently mapped to a gene called Dead-end 1 (Dnd1), which is expressed in primordial germ cells (PGCs) (Youngren et al., 2005). Although teratomas in 129Sv/J Ter (Dnd1Ter/Ter) mice are male-specific, both sexes show a severe, but incomplete, loss of PGCs early in development on all genetic backgrounds. It is not known how PGCs are lost in Dnd1Ter/Ter mutants, or how strain-specific differences contribute to teratoma formation. In zebrafish, where the Dnd phenotype was originally characterized, PGCs are specified but never exhibit motile characteristics, and thus fail to migrate to the site of the developing gonad and are lost completely (Weidinger et al., 2003). No tumor formation has been reported in zebrafish Dnd morphants. In mice, although the normal number of PGCs is specified, there is an immediate decline in germ cell population size in mutant embryos compared to wild-type, beginning at the time of migration (around E8.5) (Sakurai et al., 1995). However, unlike the zebrafish phenotype, no migration defect was detected. By the time germ cells have arrived in the gonad (E11.5), wild-type germ cells number in the thousands while only a few dozen (at most) persist in the Dnd1Ter/Ter gonad. On most genetic backgrounds, adult Dnd1Ter/Ter males are completely sterile, with no germ cells remaining and no evidence of teratomas after birth. By contrast, in the 129Sv/J testis, germ cells are either lost or give rise to teratomas in ~95% of homozygous mutants during fetal development (Stevens, 1973). Female Dnd1Ter/Ter mice on the 129Sv/J background (and all other backgrounds tested) are subfertile but show no incidence of teratoma formation indicating that either the XX germ cell or the ovarian environment is not susceptible to this pathway of teratoma formation.
Elegant genetic studies have identified several loci on particular 129 chromosomes that act as modifiers of the tumor phenotype (Hammond et al., 2007; Heaney et al., 2008; Heaney and Nadeau, 2008; Lam et al., 2007; Lam and Nadeau, 2003). The mechanism of how these loci interact with Dnd1 on a molecular level has yet to be determined. Because teratoma formation likely involves both cell autonomous and paracrine signaling, it is important to determine whether Dnd1 expression is restricted to germ cells in the developing gonad, or also extends to the somatic cells that surround germ cells and regulate their development. In situ expression studies reveal Dnd1 transcripts in the genital ridge of developing male and female gonads. In E12.5–E14.5 XY gonads, the expression pattern is limited to testis cords, which contain both Sertoli cells and germ cells (Youngren et al., 2005). One group has shown that the transformed Sertoli cell lines TM4, 15P-1, and MSC1 do not express DND1 (Bhattacharya et al., 2007). However, a conflicting report demonstrated that Dnd1Ter/Ter mutant gonadal somatic cells failed to support cultures of wild-type or mutant germ cells, suggesting a secreted _Ter_-dependent factor necessary for germ cell survival (Takabayashi et al., 2001).
To resolve these issues and investigate strain- and sex-specific phenotypes, we have taken genetic and cell biology approaches. We demonstrate that Dnd1 is expressed only in germ cells in the developing testis between E12.5 and E15.5. We found that XX germ cells in the context of a testis can also initiate teratoma formation. By genetically blocking BAX-mediated apoptosis we show that early germ cell loss in mutants is at least partially due to apoptosis. Blocking cell death in mutants on a mixed genetic background is also associated with an increased incidence of testicular teratomas. Our data support a model where BAX-mediated cell death plays a role in the initial loss of mutant germ cells and suggest that the rate of transformation and perhaps the efficiency of cell death pathways differ between 129Sv/J and other strains.
Materials and Methods
Mice, timed matings, and genotyping
Dnd1Ter/+ mice were provided by Dr. Joseph Nadeau at Case Western Reserve University and maintained on the 129Sv/J background. Bax+/− mice (1-Baxtm1Sjk/J B6.129) were imported from Jackson Laboratories and maintained on a mixed genetic background (129Sv/J;C57BL/6J). Dnd1Ter/+ mice were crossed to Bax+/− mice and the F1 offspring were intercrossed to maintain the line and obtain double heterozygous animals for timed matings. Sox9-ECFP mice were maintained as a homozygous line on a C57BL/6 background and outcrossed to CD-1 for timed matings. XY SryMYC mice were maintained on a 129Sv/J strain, crossed to Dnd1Ter/+ mice to obtain heterozygotes, and the progeny were mated with Dnd1Ter/+ females. For timed matings, males and females were setup in the afternoon and the next morning females were checked for plugs and counted as day E0.5 if positive. Tail DNA was extracted using standard methods and genotyped using the following primer sets: TerF 5′-GTA GTT CAG GAA CTC CAC TTG TG-3′, TerR 5′-GCT CAA GTT CAG TAC GCA C-3′, Bax ex5F 5′-GAG CTG ATC AGA ACC ATC ATG-3′, Bax In5R 5′-GTT GAC CAG AGT GGC GTA GG-3′, Bax NeoR 5′-CCG CTT CCA TTG CTC AGC GG-3′, MycF 5′-AAT CAT AGC AAG GGG GAG TGT TG-3′, MycR 5′-ATG GAG AGC TTG GGC GAC CTC-3′. Bax mice were genotyped using the protocol found on the Jax website. SryMYC mice were genotyped by PCR using an annealing temperature of 60°C and running the 130bp product on a 2% agarose gel. Dnd1Ter mice were genotyped by PCR using an annealing temperature of 62°C. The PCR product (145bp) was digested overnight at 37°C with the restriction enzyme DdeI and run on a 4% agarose gel or 6% acrylamide gel. DdeI digestion of DNA from mice with the Dnd1Ter mutation produces 123bp and 22bp products.
Flow cytometry and RT-PCR
E12.5–E15.5 Sox9-ECFP gonads were dissected and separated from the mesonephros, collected on ice in PBS, and trypsinized at 37°C for 15 minutes. The trypsin was removed and 500uL of Dulbecco’s Modified Eagle Medium was added to stop the reaction. The tissue was drawn through a 27 ½ gauge needle to break up cells, then put over a cell strainer and spun down briefly to collect the flow through. The suspension was subjected to fluorescence activated cell sorting (FACS) at a core facility. ECFP-positive and negative cell fractions were obtained in PBS and centrifuged to pellet the cells. The PBS was aspirated and a total RNA extraction was performed using TRIzol® (Invitrogen) and chlorophorm with a 100% isopropanol precipitation and 75% ethanol wash. cDNA was generated from RNA using the iScript™ cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s protocol. PCR was performed on each cDNA fraction using primers for Dnd1, Sox9, Oct4, and HPRT using an annealing temperature of 62°C for 29 cycles. Each primer set was designed to span an intron and one primer from each set to overlap an exon-exon boundary by at least 7-nucleotides on each side. Primers used for each are as follows: Dnd1F 5′-GCC CTG GTA GAA GGT CAG TCA C-3′, Dnd1R 5′-GCC CTG TTC CTA AAC ACT TGG TC-3′, Sox9F 5′-GCG GAG CTC AGC AAG ACT CTG-3′, Sox9R 5′-ATC GGG GTG GTC TTT CTT GTG-3′, Oct4F 5′-GGA GGA AGC CGA CAA CAA TGA-3′, Oct4R 5′-TCC ACC TCA CAC GGT TCT CAA-3′, HprtF 5′-TGG ACT GAT TAT GGA CAG GAC TGA A-3′, HprtR 5′-TCC AGC AGG TCA GCA AAG AAC T-3′. The products were run on a 2% agarose gel and imaged using a Bio-Rad gel-doc and Quantity One software imaging system.
In situ hybridization
Gonads were dissected, fixed in 4% paraformadlehyde (PFA) overnight at 4°C, washed in 0.1% Tween-20/PBS, dehydrated in 100% methanol and stored at −20°C until used. Full-length Dnd1, cloned into pGEM®-T Easy vector (Promega) was used as a probe. In situ hybridization on gonads was performed as described previously (Henrique et al., 1995; Kim et al., 2006). A digoxigenin labeled RNA probe for Dnd1 was detected using an alkaline phosphatase-conjugated anti-digoxigenin antibody.
Busulfan treatment
CD-1 pregnant females were given a 150 mg/kg (~4.5 mg/mouse) dose of Busulfan. 30 mg Busulfan was dissolved in 500uL 95°C DMSO and combined with an equal volume of 95°C water, then cooled to 37°C before intraperitoneal injection at E10.5. Mice were dissected at E14.5 and germ cell depletion was confirmed by alkaline phosphatase staining of one pair of gonads from the litter. The remainder were processed for in situ hybridization.
Immunofluorescence and histology
Fluorescent immunocytochemistry was performed on whole mount or cryosectioned gonads which were fixed overnight at 4°C in 4% PFA, washed in PBS for whole mount antibody staining, or put through a sucrose gradient (10%, 15%, 20%, 20%:OCT 1:1 overnight at 4°C) before embedding in OCT for sectioning. Antibodies (Rat anti-GCNA1 (kindly provided by George Enders 1:50), Rabbit anti-MVH (Abcam cat. #ab13840 1:500), Rat anti-E-cadherin (Zymed Laboratories cat. #13–1900 1:500)) were added to the blocking solution (PBS and 0.1% Triton X-100, 3% BSA, and 10% heat-inactivated goat serum) and incubated rocking overnight at 4°C (or stationary for sections). Samples were washed four times over a 30 minute period in washing solution (PBS and 0.1% Triton X-100, 3% BSA, and 1% heat-inactivated goats serum), and then incubated 1 hour at room temperature in blocking solution with Cy2-, Cy3-, or Cy5-conjugated secondary antibodies (1:500; Jackson Immunoresearch). Samples were then washed four times for 30 minutes total in washing solution, then mounted in DABCO and imaged on a Zeiss LSM510 confocal microscope. For histology, ovaries were dissected and fixed in Bouin’s solution overnight, then washed thoroughly in 70% ethanol. After embedding in paraffin, ovaries were sectioned (5um), stained with haematoxylin and eosin, and mounted in DABCO.
Results
Dnd1 is expressed only in germ cells of the developing testis, not in Sertoli cells, between E12.5 and E15.5
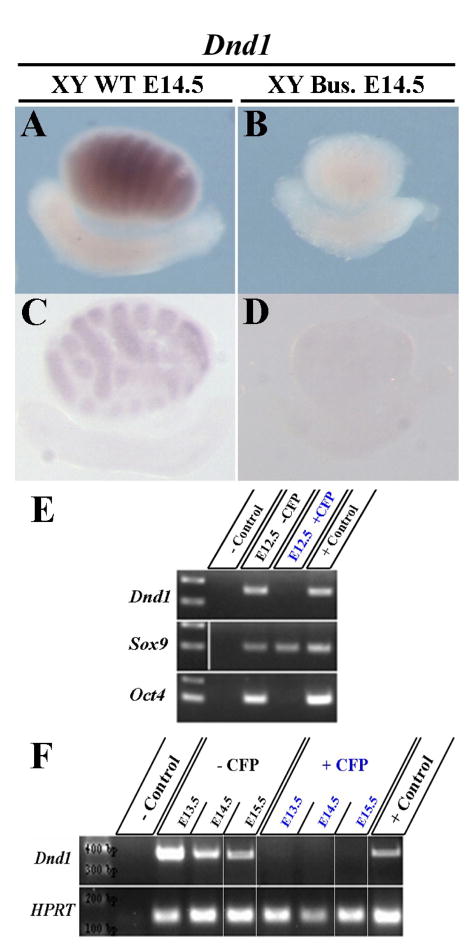
Previous experiments indicated that somatic cells may be altered in Dnd1Ter/Ter testes (Takabayashi et al., 2001). To determine whether Dnd1 expression is restricted to germ cells or is also characteristic of Sertoli cells, we injected a chemotherapeutic agent, busulfan, into pregnant females to eliminate germ cells from the developing embryonic gonads by E14.5. We then compared the Dnd1 expression pattern to that of uninjected E14.5 testes (Fig. 1A,C). After busulfan treatment, expression of Dnd1 was eliminated in the testis (Fig. 1B,D), suggesting that Dnd1 is either not expressed in Sertoli cells or requires the presence of germ cells.
Figure 1. Expression of Dnd1 is restricted to germ cells of the developing testis.
Dnd1 in situ expression in a whole mount (A,B) and sectioned (C,D) E14.5 WT testis is restricted to testis cords, and disappears in a Busulfan treated testis (B,D). Busulfan is a chemical that eliminates germ cells. (E,F) RT-PCR on Sox9-ECFP positive and negative cells, sorted from E12.5–E15.5 gonads. Dnd1 expression is only detected in the ECFP-negative fraction (containing the Oct4 positive germ cell population) and is excluded from the pure Sertoli cell fractions (exclusively Sox9 positive).
To investigate this question further we made use of the transgenic reporter line, Sox9-ECFP. The Sox9-ECFP line contains an ECFP reporter driven by a Sox9 enhancer element (Sekido and Lovell-Badge, 2008), and is specific to Sertoli cells in the gonad. RT-PCR analysis of sorted cells between stages E12.5 and E15.5 revealed Dnd1 expression in the ECFP-negative pool that contains Oct4 expressing cells (germ cells), but not in the ECFP-positive (Sertoli cell only) pool (Fig. 1E,F). Note that cell sorting did not eliminate all _Sox9_-positive cells from the negative pool (e.g., _Sox9_-positive cells remain in the _Sox9-ECFP_-negative pool). However, the _Sox9-ECFP_-positive pool is pure and contains no _Oct4_-expressing cells (Fig. 1E). These experiments indicate that Dnd1 transcript is located within germ cells and not in Sertoli cells.
Early germ cell loss in mutants is partially due to BAX-mediated apoptosis
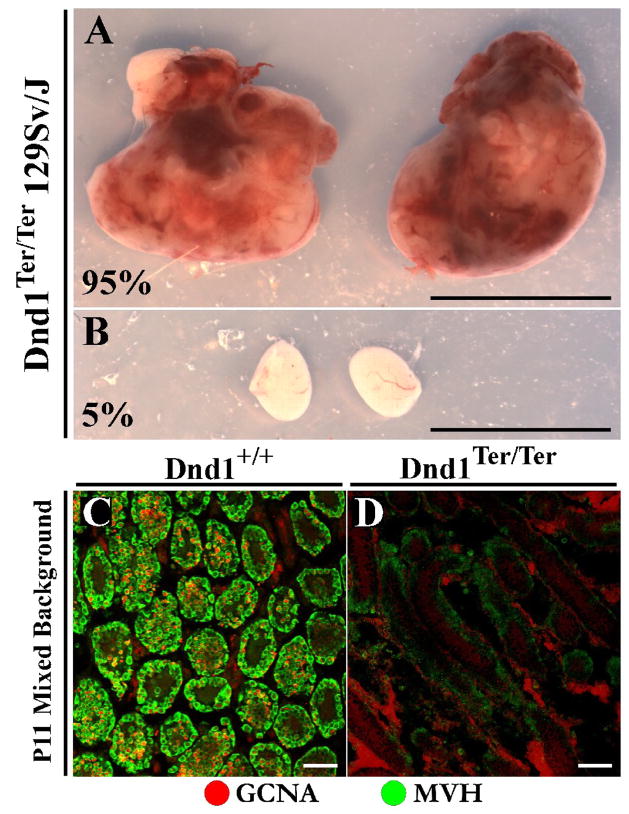
Dnd1Ter/Ter embryos suffer a significant reduction in the number of germ cells arriving in the gonads of both sexes on all backgrounds tested (Sakurai et al., 1995). However, the fate of male and female mutant germ cells diverges after colonization of the gonad. The few mutant XX germ cells that successfully migrate to the gonad are maintained in the ovary and give rise to mature oocytes, albeit in greatly reduced numbers. Adult mutant females have small ovaries and are sub-fertile, but can produce offspring at low rates when crossed with wild-type males (Noguchi and Noguchi, 1985). By contrast, mutant XY germ cells colonize the testis but transform on the 129Sv/J background, giving rise to testicular teratomas at a rate of approximately 95% (Fig. 2A,B). On other backgrounds, XY germ cells are lost completely by perinatal stages. Examination of a mixed background Dnd1Ter/Ter testis revealed that by postnatal day eleven (P11), no germ cells could be detected by analysis with antibodies against mouse Vasa homolog (MVH) or germ cell nuclear antigen 1 (GCNA1) (Fig. 2C–D).
Figure 2. Teratomas form in mutants on the 129Sv/J background but germ cells are lost completely on other genetic backgrounds.
**(A)**Large bilateral testicular teratomas from a P17 129Sv/J Dnd1Ter/Ter mutant compared to (B) a mutant from the same litter that did not develop teratomas. On the 129Sv/J background testes develop teratomas at a rate of ~95% while the remainder simply lose their germ cells. Scale bars represent 1cm. (C) P11 wildtype testis section in which germ cells within testis cords are immunostained with antibodies against GCNA (red) and MVH (green). (D) P11 Dnd1Ter/Ter testis section on a mixed genetic background contains no germ cells that stain positive for either GCNA or MVH within testis cords by this stage. Scale bars represent 50 μm.
It is unknown why this drastic phenotypic variation occurs between strain backgrounds. We initially hypothesized that the tumor susceptibility phenotype could be positively correlated with the number of germ cells colonizing the gonads in Dnd1Ter/Ter embryos. Previously, alkaline phosphatase stains had been carried out on mutant C57BL/6J gonads to identify and count PGC numbers (Sakurai et al., 1995). However, upon examination of PGC numbers in 129Sv/J mutants it was obvious that there was no significant difference in germ cell population size in mutant gonads between these different backgrounds (data not shown). This indicates that the mechanism underlying early loss of germ cells is similar to both backgrounds and suggests that the strain-specific tumor phenotype is not affected by the number of colonizing germ cells, but rather by other strain-specific properties.
The early PGC loss common to mutants of all backgrounds is not well-understood. Although the correct number of PGCs is specified in Dnd1Ter/Ter mice, as early as E8.5, a difference can be noted in the number of migrating germ cells entering the hindgut between mutants and wild-type, based on alkaline phosphatase staining (Sakurai et al., 1995). Germ cell loss is exacerbated during migration, as only a few dozen germ cells reach the Dnd1Ter/Ter gonad (compare Fig. 3A and 3C). However, reports have been inconclusive in determining whether differences in mutant germ cell number are due to active cell death, lack of proliferation, or differentiation during migration. We investigated whether cell death plays a role in early germ cell loss by genetically blocking one apoptotic pathway.
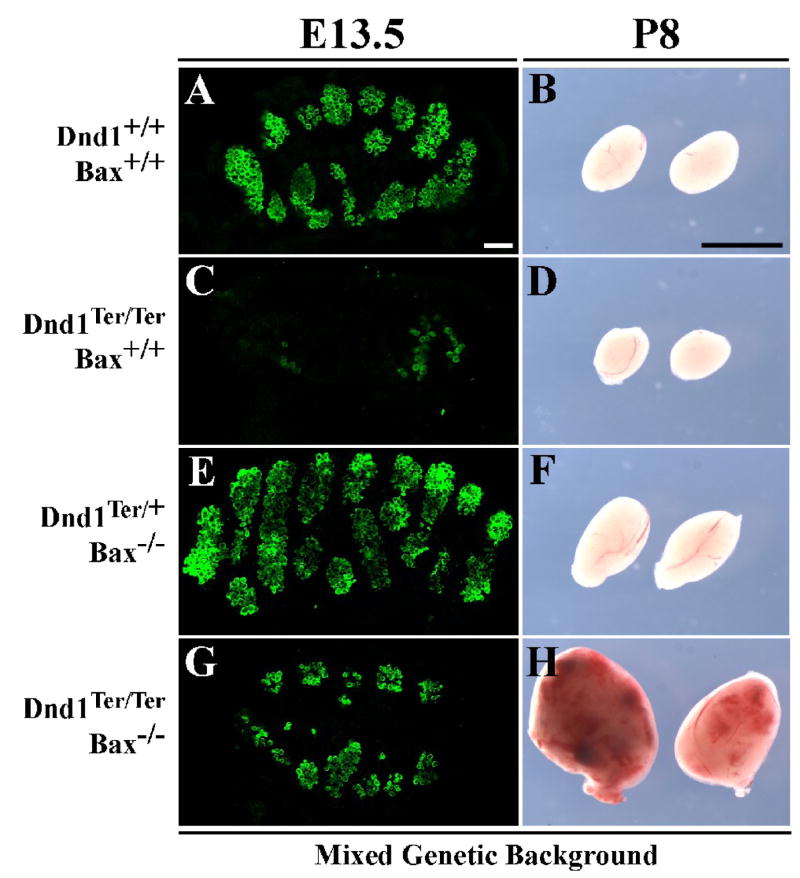
Figure 3. On a Bax mutant background, germ cells are partially rescued in Dnd1Ter/Ter embryos, and these double-mutants develop testicular teratomas on a mixed genetic background.
(A) Germ cells (immunostained with MVH in green) are grouped inside testis cords in a wildtype testis at E13.5. Germ cell numbers are greatly reduced in a Dnd1Ter/Ter testis (C), and somewhat increased in a Dnd1Ter/+ mouse carrying two loss of function alleles of Bax (E). (G) A Dnd1Ter/Ter;Bax−/− double-mutant testis shows a significant increase in germ cell number compared to C. Scale bars represent 50μm. (B) P8 wildtype testes. On a mixed genetic background, Dnd1Ter/Ter mutant testes are reduced in size as a result of germ cell loss (D as compared to B). Loss of Bax on a Dnd1+/+ background leads to an increase in testis size (F), whereas loss of Bax on a Dnd1Ter/Ter background results in teratoma formation (H). Scale bars represent 0.5cm.
Based on data showing that blocking BAX-mediated apoptosis rescues PGCs in other germ cell-deficient mutants (Stallock et al., 2003; Suzuki et al., 2008), we crossed the Bax mutation (1-Baxtm1Sjk/J B6.129) onto Dnd1Ter mice. To this end, Bax heterozygotes on a mixed genetic background (129Sv/J. C57BL/6J. CD1) were mated to Dnd1 heterozygotes of the 129Sv/J background to obtain double heterozygotes. These offspring were intercrossed to produce compound double-mutants on a mixed background. _Dnd1Ter/Ter;Bax_−/− double-mutants showed a significant germ cell rescue (~50%) by stage E13.5 on a mixed background and in both sexes (Fig. 3G and data not shown), indicating that Bax-mediated apoptosis is an important mechanism of initial germ cell loss in Dnd1Ter/Ter mutants. However, the rescued phenotype is not complete indicating that there may be other BAX-independent cell death pathways involved, defects in proliferation, or aberrant differentiation contributing to the loss of mutant germ cells.
Dnd1Ter/Ter;Bax−/− males develop testicular teratomas on a mixed genetic background
In Dnd1Ter/Ter males, few germ cells colonize the gonad, but those that do either transform on a 129Sv/J genetic background or undergo cell death resulting in testes completely devoid of germ cells by birth on other genetic backgrounds. We investigated whether rescuing early germ cell loss on a Bax mutant background would lead to the formation of teratomas in a non-129Sv/J testis with increased numbers of mutant germ cells. Surprisingly, on a mixed genetic background, we found that double-mutant male samples exhibited typical testicular teratomas (Fig. 3H) at a high rate, as compared to single Dnd1Ter/Ter mutant testes (Fig. 3D) on a similar background, which are normally devoid of germ cells by the time of birth. However, the incidence of teratoma formation in mice on a mixed background (Table 1) was slightly lower than that reported for a pure 129Sv/J background, possibly due to a smaller sample size: 91% (20/22) of mixed background double-mutants developed teratomas, compared to ~95% on the 129Sv/J background. The double-mutants that did not develop teratomas lost their germ cells entirely. Moreover, 52% (12/23) of Dnd1Ter/Ter;Bax+/− mice on a mixed background developed teratomas, although smaller in size and typically unilateral. As a control, Dnd1Ter/Ter;Bax+/+ samples on this mixed background were examined and none (0/10) developed teratomas by P20. At this stage, all XY gonads from this group were completely devoid of germ cells.
Table 1.
Teratoma penetrance in double-mutant gonads on a mixed genetic background.
| Genotype | Frequency | Characterization |
|---|---|---|
| _Dnd1Ter/Ter Bax_−/− | 91% (20/22) | large, bilateral |
| Dnd1Ter/Ter Bax+/− | 52% (12/23) | small, unilateral |
| Dnd1Ter/Ter Bax+/+ | 0% (0/10) | ----- |
These experiments suggest that while mutant germ cells on the 129Sv/J background efficiently generate teratomas, mutant germ cells on other backgrounds also have this capacity, but are less efficient at undergoing the transformation event, or are normally cleared more efficiently by competing mechanisms of cell death.
Dnd1Ter/Ter;Bax−/− females do not form teratomas and are fertile, but XX germ cells in a testis can transition into neoplastic clusters
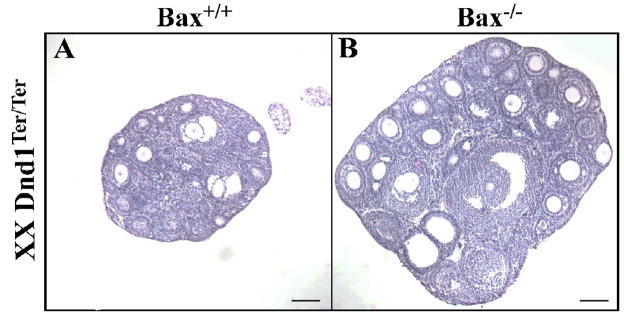
In Dnd1Ter/Ter mutants, the significant early loss of germ cells results in sub-fertile females (Noguchi and Noguchi, 1985). The few germ cells that colonize the gonad survive throughout ovarian development. However, the ovaries are small and contain very few oocytes (Fig. 4A). Rescuing the early germ cell loss on a Bax mutant background resulted in double-mutant females that maintained many more oocytes than Dnd1Ter/Ter single mutants alone, as determined by histological analysis (Fig. 4A–B). Additionally, these double-mutant females were fertile and produced viable, fertile offspring when out-crossed to CD-1 mice (data not shown). As reported previously, there was no incidence of ovarian teratoma in mutant females (Noguchi et al., 1996).
Figure 4. _Dnd1Ter/Ter;Bax_−/− ovaries have more oocytes than Dnd1Ter/Ter ovaries.
Representative sections from the thickest part of (A) a P16 Dnd1Ter/Ter ovary and (B) a P18 _Dnd1Ter/Ter;Bax_−/− ovary stained with hematoxylin and eosin. Scale bars represent 100 μm.
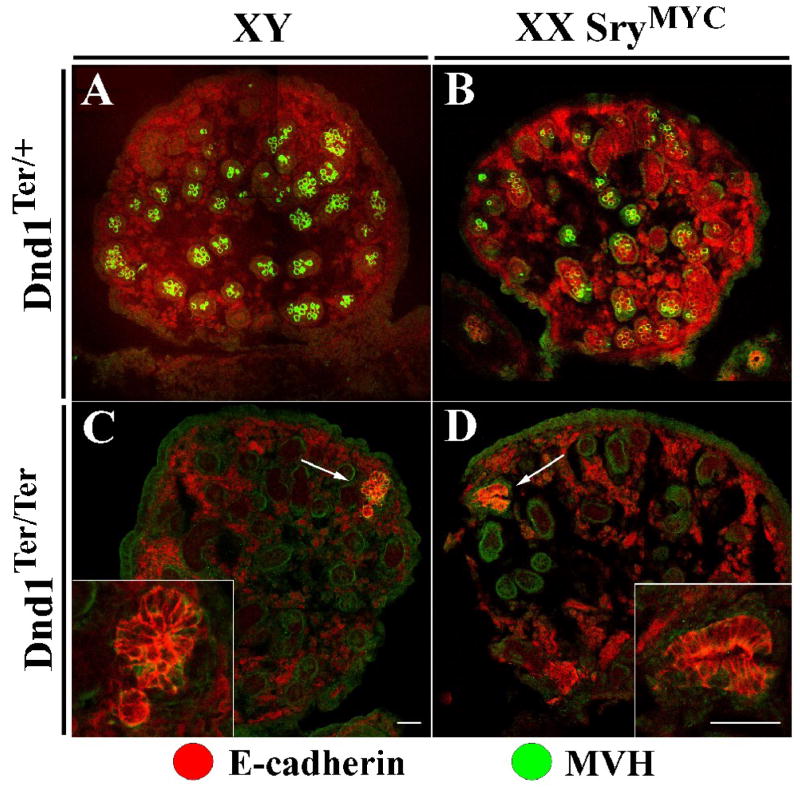
The fact that testes and not ovaries form teratomas in Dnd1Ter/Ter embryos indicates that there is something specific either to XY germ cells or to the male pathway of development that predisposes mutant germ cells to transformation. To distinguish between these possibilities, we crossed 129Sv/J XY SryMYC males to 129Sv/J Dnd1Ter/+ heterozygous females. SryMYC mice contain an autosomal MYC-tagged SRY protein that causes female-to-male sex reversal. Intercrosses between XX Dnd1Ter/+ heterozygotes and XY SryMYC; Dnd1Ter/+ heterozygotes produced sex-reversed XX Dnd1Ter/Ter testes. Normally, mutant XY germ cells on a 129Sv/J background give rise to transforming germ cell clusters during embryonic development. These clusters can be identified histologically as early neoplasias that ultimately give rise to the nascent teratomas (Cook et al., in preparation; Rivers and Hamilton, 1986). We are able to detect these clusters based on morphology and immunofluorescent staining for E-cadherin by E18.5 (Fig. 5C arrow and inset). Examination of mutant XX germ cells in a sex-reversed testis at this stage also revealed similar neoplasias (Fig. 5D arrow and inset). This indicates that the initiation of teratoma formation is dependent upon a male developmental pathway and not the sex chromosomes of the PGCs. Despite the presence of XX neoplasias (n=2/3 at E18.5) that resembled the early stage of teratoma formation in XY testicular germ cells, we never saw mature teratomas after birth in XX SryMYC; Dnd1Ter/Ter mice (n=0/10 at P14 and later). Few germ cells persist in sex-reversed controls compared to normal testis controls at later developmental stages and after birth (data not shown). As XX germ cells are usually lost perinatally in XX sex-reversed males (McLaren, 1984), it is possible that germ cell loss and other testis cord defects reported for XX sex-reversed testes (Ishii et al., 2007) prevent formation of fully-developed teratomas even though early neoplasias can be identified.
Figure 5. XX germ cells in an XX SryMYC testis form neoplastic clusters like XY germ cells in a testis.
XY germ cells are MVH positive (green) in an E18.5 control XY testis (A), and in an XX SryMYC (sex-reversed) testis (B) although their numbers are somewhat reduced. (C) At E18.5, the XY Dnd1Ter/Ter testis lacks MVH-positive cells, but an E-cadherin-positive (red) neoplastic cluster is detected. These clusters typically give rise to nascent teratomas (Cook et al., in preparation). (D) At E18.5 the XX SryMYC Dnd1Ter/Ter testis also lacks MVH-positive germ cells but contains a similar E-cadherin positive neoplastic cluster. All testis samples are on a 129Sv/J background. Scale bars represent 50 μm.
Despite a role in germ cell death, loss of Dnd1 does not affect embryonic viability
While only germ cells express Dnd1 in the developing gonad, in situ analysis detects transcript in other tissues of the developing embryo (Youngren et al., 2005) and ESTs have been identified in different organs of the adult including the heart and brain. Since loss of Dnd1 in PGCs can lead to BAX-mediated apoptosis, it is possible that loss of expression in other tissues could also lead to cell death. Corroborating this, a previous report observed a role for DND1 in embryonic viability (Bhattacharya et al., 2007). It was shown that loss of Dnd1 on a 129Sv/J background, but not on a C57BL/6J background, leads to embryonic lethality.
We examined five hundred progeny from Dnd1Ter/+ 129Sv/J crosses and calculated the ratios of wild-type, heterozygous, and homozygous mutants (Table 2). We found that the proportions fell within Mendelian ratios, with a P-value greater than 0.975. These data indicate that, in our colony on a 129Sv/J genetic background, the Dnd1Ter mutation does not affect embryonic viability.
Table 2.
Comparison of genotypes from 129Sv/J-Ter derived progeny.
| Genotype | Total no. examined | X2 | P | |||
|---|---|---|---|---|---|---|
| +/+ | Ter/+ | Ter/Ter | ||||
| Observed | 124 | 249 | 127 | 500 | 0.044 | 0.978 |
| Expected | 125 | 250 | 125 |
Discussion
Dnd1 expression
Testicular teratomas in Dnd1Ter/Ter mice are believed to arise from fetal germ cells that are misregulated in the developing gonad. We have shown here that Dnd1 is expressed in germ cells and not in Sertoli cells between E12.5–15.5, the time period when teratoma formation begins in Dnd1Ter/Ter mutants (Rivers and Hamilton, 1986). This expression pattern indicates that the direct effects of the Dnd1Ter mutation occur in germ cells, and not in the somatic supporting cell lineage. Teratomas arise from defects in the intrinsic cellular program in germ cells, within the context of the strain-specific background. It is not yet clear whether the genetic background affects the frequency or degree of misregulation of the intrinsic germ cell program, or whether it affects the somatic environment and its ability to regulate or clear mutant germ cells.
While expression of Dnd1 is important for germ cell development, little is known about its function in other tissues. ESTs and other expression analyses indicate that Dnd1 is expressed in various organs such as heart and brain, suggesting other possible roles for Dnd1 in development and adult life. Here we investigated five hundred adult mice from heterozygous crosses in our colony and found no evidence for a negative impact of the Dnd1Ter mutation on embryonic viability on a 129Sv/J genetic background. The difference between our finding and a previous report demonstrating a role for Dnd1 in embryonic viability on the 129Sv/J background (Bhattacharya et al., 2007) could be due to sub-strain differences that have arisen since separation of the two colonies.
Early cell death
Dnd1Ter/Ter mice display two very distinct phenotypes: (1) initial loss of germ cells common to both sexes on all genetic backgrounds and (2) male-specific teratoma formation only on the 129Sv/J genetic background. The germ cell loss phenotype is similar to the Nanos3 null phenotype (Suzuki et al., 2008). Like DND1, NANOS3 is a conserved RNA binding protein expressed in PGCs after specification. A null mutation in Nanos3 leads to the loss of a high proportion of germ cells prior to their arrival in the gonad, similar to Dnd1 mutants. Also like the Nanos3 mutants, blocking BAX-mediated apoptosis only partially rescues the germ cell loss. This suggests that other mechanisms may contribute to germ cell loss in both mutants, including BAX-independent cell death, lack of proliferation, or even trans-differentiation of germ cells during migration. While it was found that the Nanos3 null germ cells did not trans-differentiate or have proliferation defects during migration (Suzuki et al., 2008), this has not been investigated in Dnd1 mutants.
Zebrafish DND binds the 3′UTR of Nanos in primordial germ cells to promote translation by protecting the transcript from miRNA-mediated repression (Kedde et al., 2007). Based on the high conservation of Nanos in organisms from C. elegans to H. sapiens, it is possible that a similar mechanism of early misregulation of the murine Nanos3 transcript (functionally homologous to zebrafish Nanos) leads to the early germ cell loss phenotype of Dnd1Ter/Ter mutants (Kedde and Agami, 2008; Saga, 2008).
Testicular teratoma formation and the role of BAX
Bax rescue significantly increases the number of oocytes in adult females, which makes them more fertile than Dnd1Ter/Ter single mutant ovaries. These females produce viable, fertile offspring, indicating that PGCs rescued in Bax mutants are not significantly impaired, at least with respect to the female developmental pathway. This suggests that teratomas arise during the male developmental program as the result of a secondary function of DND1 within germ cells in the fetal testis. This model is further supported by our results, which demonstrate that the genotypic sex of the germ cells is irrelevant to tumor susceptibility. Rather, the testis environment is crucial, as XX Dnd1Ter/Ter germ cells developing within a testis are also susceptible to transformation.
The 129Sv/J specific phenotype of testicular teratoma formation in Dnd1Ter/Ter mice has been particularly enigmatic since the early 1970s. Very few germ cells colonize the gonad, sometimes none, but those that arrive frequently transition into teratomas, or are lost by birth. Previously we hypothesized that the number of colonizing germ cells might be higher in 129Sv/J mice and could be directly related to the incidence of teratomas. However, there is virtually no difference in initial colonizing germ cell numbers between strain backgrounds. Therefore this does not explain the inherent strain difference in susceptibility.
Nonetheless, we found that if the number of mutant germ cells in the Dnd1Ter/Ter testis is increased by blocking BAX-mediated apoptosis, teratomas arise on a mixed genetic background at a rate of 91% and at a rate of 52% in Bax heterozygotes, even though heterozygotes do not show an increase in germ cell number. Two explanations for these results seem plausible: (1) an increased number of germ cells due to specific loss of the Bax pathway may compromise the capacity of the non-129Sv/J fetal testis to clear misregulated germ cells; and/or (2) the transition of mutant germ cells to teratoma occurs at a lower frequency in backgrounds other than 129Sv/J, and inhibiting apoptosis increases the number of germ cells and uncovers this lower frequency event.
The rate of tumor formation on the mixed _Dnd1Ter/Ter;Bax_−/− background is quite high and intriguingly, tumors also arise in Bax heterozygotes (although smaller and typically unilateral). This suggests that BAX may normally eliminate mutant germ cells from the fetal testis prior to their transition into teratomas. However, BAX-mediated apoptosis is obviously not the only mechanism for clearing defective germ cells, as mutant germ cells are still cleared in some double homozygous mutants (2/22 double-mutant samples without teratomas in Table 1). This reasoning might predict that pathways for clearing mutant germ cells, including the BAX pathway, are less efficient in the 129Sv/J strain, which could explain the high incidence of teratomas. To investigate the hypothesis that Bax is expressed at lower levels in 129Sv/J gonads than in other strains we performed quantitative RT-PCR (qRT-PCR) on E13.5 gonads from 129Sv/J and C57BL/6J strains and found that Bax transcript levels between backgrounds are virtually identical (data not shown). These data do not rule out the possibility that Bax activity differs between stains. However, the reported frequency of teratoma formation is slightly higher in 129Sv/J Dnd1Ter/Ter mutants (95%) than in _Dnd1Ter/Ter;Bax_−/− mutants on a mixed genetic background (91%), which may suggest an additional factor that affects the rate of transformation and penetrance of the phenotype. It remains to be seen whether similar tumor susceptibility results will be uncovered for double mutants on a pure non-129Sv/J background (C57BL/6J for example). We are in the process of back-crossing the Bax-null allele to a pure C57BL/6J background for an analysis of germ cell rescue and teratoma formation.
More research is necessary to fully understand the effects of Bax rescue and the strain-specific differences inducing testicular teratomas. To elucidate the underlying cause of transformation in Dnd1Ter/Ter germ cells, it will be essential to perform a full molecular analysis of the transition of mutant germ cells into nascent teratomas. Importantly, the _Dnd1Ter/Ter;Bax_−/− double-mutant is a tool that can be used to compare mutant germ cells undergoing transformation to those that are not. A critical event that occurs during the period of male germ cell development when teratomas arise is mitotic arrest. Mitotic arrest is presumably not occurring in mutant germ cells based on the large size of the tumors that form and previous findings that proliferation is not arrested in Dnd1Ter/Ter mutants (Rivers and Hamilton, 1986). Among targets of DND1 recently identified in human cell lines are several cell cycle regulators that are involved in mitotic arrest in the fetal testis (Kedde et al., 2007; Western et al., 2008). Future studies will determine whether misregulation of mitotic arrest plays a direct role in tumorigenesis or aberrant development of germ cells in Dnd1Ter/Ter mice. Linking the cell cycle to cellular pluripotency in developing germ cells will undoubtedly shed light on pathways involved in tumorigenesis. Investigating these unusual tumors which reflect the close relationship between germ cells, embryonic stem cells, and tumor cells, will reveal important clues about the mechanisms that program these cell fates and regulate the transitions between them.
Acknowledgments
We thank George Enders for the GCNA1 antibody. We also thank Robin Lovell-Badge and Ryo Sekido for providing the SryMYC mice and Fan Wang for providing the Bax mutant mice. This research was supported by a grant from the Lance Armstrong Foundation and by NCI grant CA75056 to JHN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhattacharya C, Aggarwal S, Zhu R, Kumar M, Zhao M, Meistrich ML, Matin A. The mouse dead-end gene isoform alpha is necessary for germ cell and embryonic viability. Biochem Biophys Res Commun. 2007;355:194–9. doi: 10.1016/j.bbrc.2007.01.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Pottern LM, Hoover RN. Testicular Cancer in Young Men - the Search for Causes of the Epidemic Increase in the United-States. Journal of Epidemiology and Community Health. 1987;41:349–354. doi: 10.1136/jech.41.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Pottern LM, Hoover RN, Devesa SS, Aselton P, Flannery JT. Testicular Cancer in the United-States - Trends in Incidence and Mortality. International Journal of Epidemiology. 1986;15:164–170. doi: 10.1093/ije/15.2.164. [DOI] [PubMed] [Google Scholar]
- Hammond S, Zhu R, Youngren KK, Lam J, Anderson P, Matin A. Chromosome X modulates incidence of testicular germ cell tumors in Ter mice. Mamm Genome. 2007;18:832–8. doi: 10.1007/s00335-007-9075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JD, Lam MY, Michelson MV, Nadeau JH. Loss of the transmembrane but not the soluble kit ligand isoform increases testicular germ cell tumor susceptibility in mice. Cancer Res. 2008;68:5193–7. doi: 10.1158/0008-5472.CAN-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JD, Nadeau JH. Testicular germ cell tumors in mice: new ways to study a genetically complex trait. Methods Mol Biol. 2008;450:211–31. doi: 10.1007/978-1-60327-214-8_15. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–90. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Ma YT, Palmer DH, Hutton P, Cullen MH. Biology of testicular germ cell tumors. Expert Rev Anticancer Ther. 2008;8:1659–73. doi: 10.1586/14737140.8.10.1659. [DOI] [PubMed] [Google Scholar]
- Ishii M, Tachiwana T, Hoshino A, Tsunekawa N, Hiramatsu R, Matoba S, Kanai-Azuma M, Kawakami H, Kurohmaru M, Kanai Y. Potency of testicular somatic environment to support spermatogenesis in XX/Sry transgenic male mice. Development. 2007;134:449–54. doi: 10.1242/dev.02751. [DOI] [PubMed] [Google Scholar]
- Kedde M, Agami R. Interplay between microRNAs and RNA-binding proteins determines developmental processes. Cell Cycle. 2008;7:899–903. doi: 10.4161/cc.7.7.5644. [DOI] [PubMed] [Google Scholar]
- Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JA, Slanchev K, le Sage C, Nagel R, Voorhoeve PM, van Duijse J, Orom UA, Lund AH, Perrakis A, Raz E, Agami R. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131:1273–86. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MY, Heaney JD, Youngren KK, Kawasoe JH, Nadeau JH. Trans-generational epistasis between Dnd1Ter and other modifier genes controls susceptibility to testicular germ cell tumors. Hum Mol Genet. 2007;16:2233–40. doi: 10.1093/hmg/ddm175. [DOI] [PubMed] [Google Scholar]
- Lam MY, Nadeau JH. Genetic control of susceptibility to spontaneous testicular germ cell tumors in mice. APMIS. 2003;111:184–90. doi: 10.1034/j.1600-0463.2003.11101221.x. discussion 191. [DOI] [PubMed] [Google Scholar]
- Linger R, Dudakia D, Huddart R, Easton D, Bishop DT, Stratton MR, Rapley EA. A physical analysis of the Y chromosome shows no additional deletions, other than Gr/Gr, associated with testicular germ cell tumour. British Journal of Cancer. 2007;96:357–361. doi: 10.1038/sj.bjc.6603557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- Noguchi M, Watanabe C, Kobayashi T, Kuwashima M, Sakurai T, Katoh H, Moriwaki K. The ter mutation responsible for germ cell deficiency but not testicular nor ovarian teratocarcinogenesis in ter/ter congenic mice. Dev Growth Differ. 1996;38:59–69. doi: 10.1046/j.1440-169X.1996.00008.x. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Noguchi M. A recessive mutation (ter) causing germ cell deficiency and a high incidence of congenital testicular teratomas in 129/Sv-ter mice. J Natl Cancer Inst. 1985;75:385–92. [PubMed] [Google Scholar]
- Rivers EN, Hamilton DW. Morphologic analysis of spontaneous teratocarcinogenesis in developing testes of strain 129/Sv-ter mice. Am J Pathol. 1986;124:263–80. [PMC free article] [PubMed] [Google Scholar]
- Saga Y. Mouse germ cell development during embryogenesis. Curr Opin Genet Dev. 2008;18:337–41. doi: 10.1016/j.gde.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Iguchi T, Moriwaki K, Noguchi M. The ter mutation first causes primordial germ cell deficiency in ter/ter mouse embryos at 8 days of gestation. Dev Growth Differ. 1995;37:293–302. doi: 10.1046/j.1440-169X.1995.t01-2-00007.x. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–4. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Stallock J, Molyneaux K, Schaible K, Knudson CM, Wylie C. The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development. 2003;130:6589–97. doi: 10.1242/dev.00898. [DOI] [PubMed] [Google Scholar]
- Stevens LC. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J-Natl-Cancer-Inst. 1973;50:235–42. doi: 10.1093/jnci/50.1.235. issn: 0027–8874. [DOI] [PubMed] [Google Scholar]
- Stevens LC. Genetic influences on teratocarcinogenesis and parthenogenesis. Prog Clin Biol Res. 1981;45:93–104. [PubMed] [Google Scholar]
- Suzuki H, Tsuda M, Kiso M, Saga Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev Biol. 2008;318:133–42. doi: 10.1016/j.ydbio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Takabayashi S, Sasaoka Y, Yamashita M, Tokumoto T, Ishikawa K, Noguchi M. Novel growth factor supporting survival of murine primordial germ cells: evidence from conditioned medium of ter fetal gonadal somatic cells. Mol Reprod Dev. 2001;60:384–96. doi: 10.1002/mrd.1101. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, Thisse C, Thisse B, Raz E. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–34. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008;26:339–47. doi: 10.1634/stemcells.2007-0622. [DOI] [PubMed] [Google Scholar]
- Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, Lamb BT, Deng JM, Behringer RR, Capel B, Rubin EM, Nadeau JH, Matin A. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–4. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]