Suppression of Adult Neurogenesis Leads to an Increased HPA Axis Response (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 22.
Abstract
Stress and glucocorticoids are among the strongest inhibitors of adult hippocampal neurogenesis. Despite the known role of the hippocampus in negative feedback regulation of the hypothalamo-pituitary adrenal axis, whether loss of hippocampal neurogenesis affects this inhibition has not been examined. Here we tested whether suppression of adult neurogenesis affected the hypothalamo-pituitary-adrenal axis response. Our results show that suppression of neurogenesis leads to a potentiated hypothalamo-pituitary-adrenal axis response following exposure to a mild stressor. This study suggests that suppressed neurogenesis directly regulates the hypothalamo-pituitary-adrenal axis response.
Keywords: neurogenesis, hippocampus, stress, glucocorticoid, HPA axis, dentate gyrus
Introduction
The hippocampus shows remarkable structural and functional plasticity even in the adult animal. A fundamental component of its structural plasticity is its capacity for adult neurogenesis. New neurons are generated in the brain throughout the life of many animal species including rodents, non-human primates and humans in the subgranular zone of the dentate gyrus in the hippocampus. Recent evidence has linked exposure to stressful life events to altered neurogenesis in the hippocampus [1–3].
Exposure to stressful events results in a series of responses that act to preserve or restore homeostasis. The key neuroendocrine response to stress is activation of the hypothalamo-pituitary-adrenal (HPA) axis, which triggers increased production of glucocorticoids (GC). Stress is a key etiological factor in depressive disorders; up to 50% of affected patients exhibit some form of HPA axis abnormality [4]. GCs are potent factors in the regulation of both proliferation and differentiation of new neurons in the dentate gyrus [5,6]. Adrenal removal results in accelerated cell proliferation in the subgranular zone and increases the number of newly formed, surviving neurons. Conversely, corticosterone administration decreases the proliferation and survival of progenitor cells [7]. Moreover, exposing animals to various forms of stress, a process that activates the adrenal glands and results in increased levels of corticosterone, has similar effects on hippocampal neurogenesis [1–3]. Importantly, it has been shown that this effect is dependent on corticosterone [2].
The hippocampus negatively regulates the HPA axis and this inhibitory feedback is altered by various forms of chronic stress [8,9]. As discussed, it is known that chronic stress results in significantly decreased rates of hippocampal neurogenesis [1]. However, whether loss of neurogenesis itself regulates the HPA axis has not been studied. Thus, we wondered whether loss of neurogenesis in the hippocampus may lead to less efficient inhibitory control of hypothalamic cells that produce glucocorticotrophin-releasing hormone, with a resultant increased HPA-axis response.
Methods
Animals
Adult transgenic mice and control littermates were used for all experiments. hGFAPtk transgenic mice were generated as described below and backcrossed >10 times to a C57Bl/6J background. Animals were housed 4 per cage in a 12h (6am-6pm) light-dark colony room. The procedures described herein were conducted in accordance with the National Institutes of Health guidelines and were approved by the NIMH Institutional Animal Care and Use Committee.
Transgenic mouse production
To generate mice expressing Herpes-Simplex Virus Thymidine Kinase (HSV-tk) under the control of the human GFAP promoter, plasmid pGFA2-TK1 (a generous gift from Dr. Michael Brenner, UAB Department of Neurobiology, Birmingham, AL) was used. Transgenic mice were generated by microinjecting 2picoliters of a solution of plasmid DNA into the male pronucleus of fertilized oocytes from a mixed C57Bl/6J and DBA2 F1 background. Founder mice and subsequent offspring within lines were identified by PCR analysis of DNA extracted from tail snips.
Drugs
Valganciclovir (VGCV, Roche, Indianapolis, IN) – the L-valyl ester of ganciclovir - was administered for 12 weeks through the animals chow at a concentration of 15mg/kg body weight/day. VGCV has a high (approx. 85%) bioavailability and after oral administration is rapidly converted into ganciclovir by intestinal and hepatic esterases. After phosphorylation by HSV-tk ganciclovir is toxic to proliferating cells in S-phase of mitosis. Since control mice do not express HSV-tk, VGCV administration does not suppress proliferation of GFAP-positive cells. To control for any possible effects of the drug, both control as well as hGFAPtk mice received VGCV-containing chow.
Mild stressor
Animals were taken from their home cage and placed into a clean, standard mouse cage containing no bedding or nesting material in a brightly-lit procedure room for 15 minutes.
Corticosterone assay
Mice were quickly decapitated for trunk blood collection. Plasma was isolated and blood levels of corticosterone were quantified using a corticosterone double antibody radio-immunoassay kit (MP Biomedicals) following the manufacturer's protocol.
Immunohistochemistry and Cell Counting
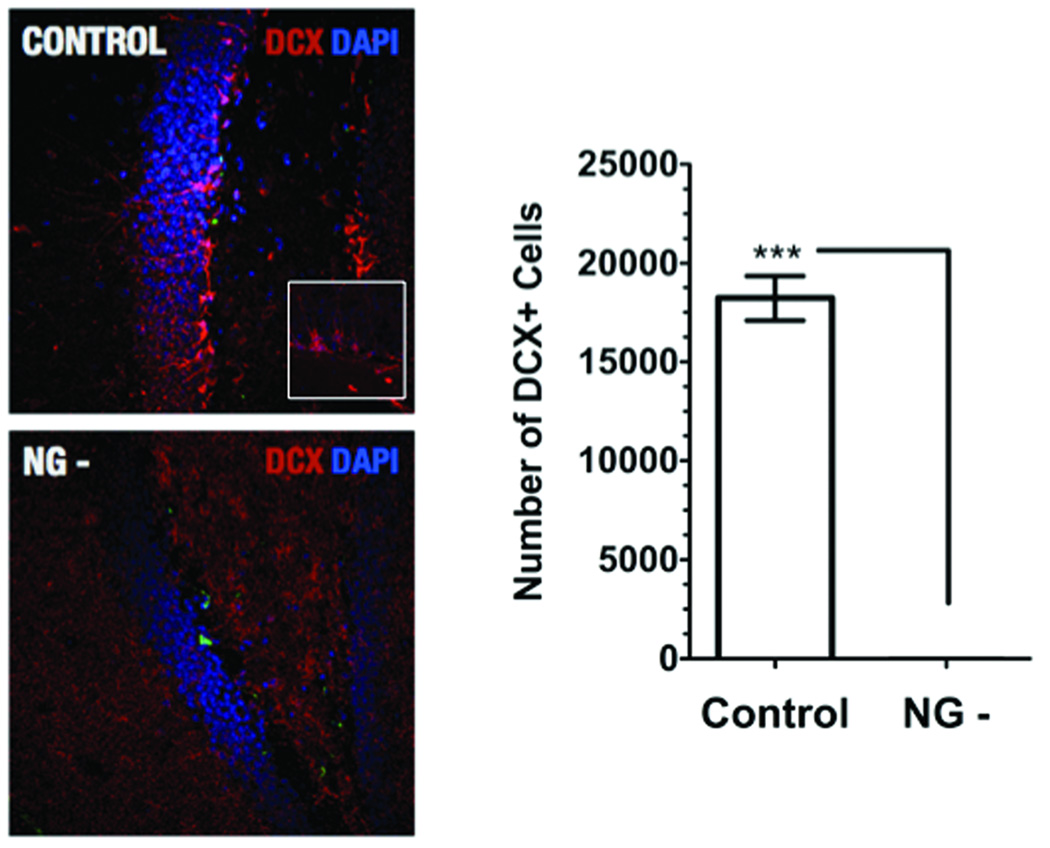
A separate group of animals (Control n=8, NG- n=8) was treated for 12 weeks with VGVC to control for suppression of adult hippocampal neurogenesis by immunohistochemistry against doublecortin. Mice were deeply anesthetized with isofluorane inhalation and transcardially perfused with 4% PFA, pH 7.4. Brains were dissected from their skull and postfixed in the same fixative overnight at 4°C. Brains were transferred to a 30% sucrose solution for cryopreservation and incubated at 4°C for 3 days. Brains were mounted on a freezing stage (Model BFS-MP30, Physitemp Instruments, Inc., Clifton, NJ) set to −25°C and coronal sections (40µm) were cut using a sliding microtome (LEICA, Germany). Sections were systematically sampled 480µm apart into 12 wells of a 24 well plate and stored in PBS, pH 7.4. Sections were immunofluorescently stained for doublecortin using a standard protocol. In brief, sections were washed for 15min in PBS, pH 7.4 containing 0.5% Tween20 and subsequently incubated for 30min at RT in blocking solution (PBS, pH 7.4, 0.5% Tween20, 3% normal donkey serum). Following blocking sections were incubated overnight at 4°C with the primary antibody (doublecortin, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Sections were washed for 15 min followed by incubation for 2h at RT with secondary antibodies (Alexa-555 donkey anti-goat IgG, 1:250, Invitrogen Corporation, Carlsbad, CA). Sections were counterstained using DAPI (Sigma-Aldrich Corporation, St. Louis, MO) and subsequently mounted on glass-slides and coverslipped with aqueous hardening mounting medium (Immu-Mount, Thermo-Fisher Scientific, Inc., Waltham, MA). Fluorescently immunolabed sections were analyzed on a Nikon Eclipse 800 fluorescent microscope (Nikon Instruments, Inc., Melville, NY). In brief, every twelfth section through the hippocampal dentate gyrus was identified and all doublecortin positive cell bodies containing a DAPI positive nucleus were counted. A total cell count was determined by multiplying by 12 to calculate the total number of doublecortin positive cells per dentate gyrus. For photomicrographs showing the dentate gyrus of Control and NG- animals, images were captured on a Zeiss Meta confocal microscope (Model LSM 510; Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Tissue preparation
After collection of trunk blood, brains were immediately extracted on ice and the hippocampus was quickly dissected. Hippocampi were minced and placed directly into RNALater (Ambion) where they were kept at 4°C until RNA extraction.
RNA preparation and real-time quantitative PCR
Total RNA was isolated and extracted from the hippocampus using Trizol (Invitrogen) according to the manufacturer's instructions. RNA was subsequently purified using an RNease mini column (Qiagen) with on-column DNase removal (Qiagen). RNA was quantified using a NanoDrop spectrophotometer (ThermoScientific) and 2ug of total RNA was reverse-transcribed into single-stranded cDNA using Superscipt III (Invitrogen) following the manufacturer's protocol. Quantitative RT-PCR was performed in triplicate using the IQ5 iCycler (Bio-Rad) using iQ SYBR Greener supermix (Invitrogen) with 20ng of synthesized cDNA/reaction. PCR efficiencies of primers were examined by standard curve of serial-diluted cDNA and melting curve functionality. The sequence mRNA levels were normalized for each well to the actin mRNA levels. Sequences of primers used were as follows:
- GR-Exon 17-F: 5′ AGG AGC CAG GGA GAA GAG AA 3′
- GR-Exon 17-R: 5′ TGA AGA CGC AGA AAC CTT GA 3′
- GR-F: 5’ ACA GAC TTT CGG CTT CTG GA 3’
- GR-R: 5’ AAC TCC TTC TCT GTC GGG GT 3’
- Actin-F: 5’ GCC TTC CTT CTT GGG TAT G 3’
- Actin-R: 5’ ACC ACC AGA CAA CAC TGT G 3’
Results
In order to study a potential role for adult neurogenesis in the regulation of HPA axis activity we generated transgenic mice in which we could conditionally suppress adult neurogenesis. In these mice the viral thymidine kinase (HSV-tk) is expressed under control of the mouse GFAP promoter. Expression of HSV-tk allows for a nearly complete reduction of proliferating GFAP-positive neural progenitor cells by administration of the antiviral drug Valganciclovir (VGCV). HSV-tk phosphorylates the prodrug ganciclovir into a toxic compound that selectively kills cells undergoing S-phase of mitosis. Adult progenitors in the subgranular zone express GFAP and thus administration of VGCV to transgenic mice results in the suppression of neurogenesis. Twelve weeks of VGCV treatment results in complete suppression of doublecortin-positive neuroblasts in the dentate gyrus of transgenic mice (Figure 1). The general health of transgenic mice with suppressed neurogenesis (NG- group) does not appear to be compromised by effects of either the transgene alone or prolonged VGCV treatment. No changes in body weight, reflexes or motor behavior were observed after up to 12 weeks of treatment (data not shown).
Figure 1.
Conditional ablation of adult neurogenesis in the dentate gyrus of the hippocampus. Micrographs show immunohistochemical staining of the dentate gyrus of Control and NG- mice: doublecortin positive neuroblast (red), DAPI counterstain (blue). In the NG- group adult hippocampal neurogenesis is completetely ablated (no doublecortin positive neuroblasts can be detected). [Control, 18224 ± 1134 SEM, N=8, white bars; NG-, 12 ± 5.07 SEM, N=8, black bars; t-test, p<0.0001]
In order to study the stress-induced response of the HPA axis in mice without neurogenesis, we measured plasma concentrations of corticosterone in mice in their home cage environment or after exposure to a mild stressor consisting of introduction to a novel environment. As expected, exposure to a novel environment resulted in a significant increase in corticosterone levels. However, comparison of groups by a one-way ANOVA revealed significantly higher corticosterone levels in NG- mice as compared to WT animals (Figure 2).
Figure 2. Stressful experiences lead to an increased HPA axis response in animals lacking adult neurogenesis.
Quantification of plasma levels of corticosterone in both wild-type animals (Ctrl) and animals with suppressed neurogenesis (NG-) in both a non-stressed state and after exposure to a mild stressor consisting of introduction to am empty, novel cage under bright lighting conditions. One-way ANOVA with Tukey-Kramer post-hoc analysis revealed a significant difference between non-stressed and stressed conditions in both groups as well as between the Ctrl and NG- group in the stressed condition [Ctrl (non-stressed): Mean=19.73 +/−3.017 SEM, n=9, white bars-left side; NG- (non-stressed): Mean=42.40 +/−9.228 SEM, n=9, black bars-left side; Ctrl (mild stress): Mean=166.5 +/−7.442 SEM, n=12, white bars-right side; NG- (mild stress): Mean=228.7 +/−22.9 SEM, n=12, black bars-right side] F3,35=40.75, p=<0.0001, Tukey-Kramer post hoc: Ctrl (non-stressed) X NG- (non-stressed), p>0.05; Ctrl (non-stressed) X Ctrl (mild stress), p<0.001; Ctrl (non-stressed) X NG- (mild stress), p<0.001; NG-(non-stressed) X Ctrl (mild stress), p<0.001; NG-(non-stressed) X NG- (mild stress), p<0.001; Ctrl (mild stress) X NG- (mild stress), p<0.05].
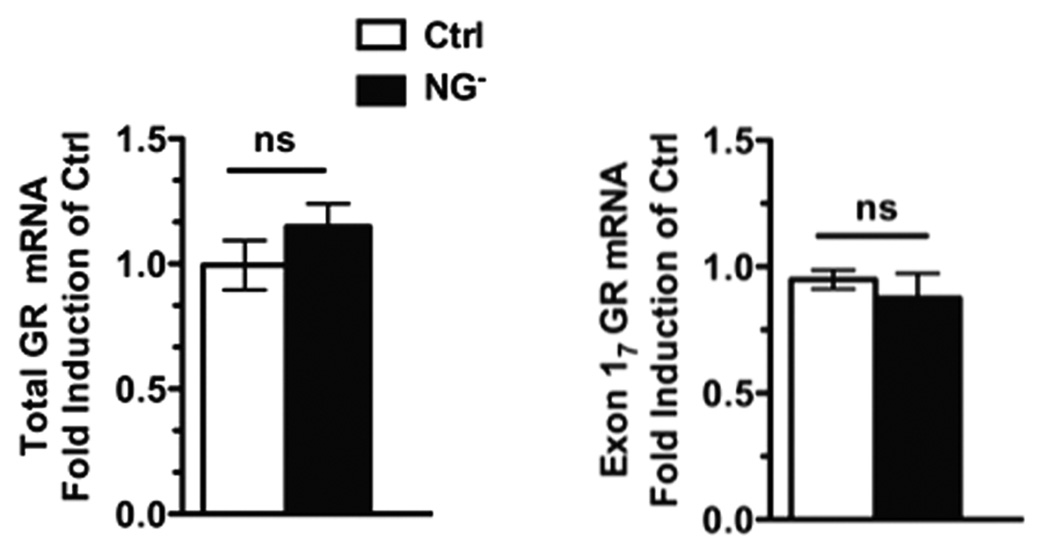
It has been demonstrated that differences in the levels of glucocorticoid receptor (GR) expression in the hippocampus are correlated with the extent of GC feedback sensitivity and the HPA response to stress [10, 11]. These studies have suggested that differences in hippocampal GR expression may serve as a mechanism for the development of individual differences in HPA responses to stress. In particular, it has been demonstrated in rats that expression of GR mRNAs specifically containing exon 17 is decreased in offspring from dams giving poor maternal care as compared to those showing better maternal care [12]. As adults offspring arising out of poorer maternal care also show potentiated HPA-axis responses after exposure to stress [10, 11]. Considering the importance of the hippocampal GR system for negative-feedback regulation of HPA activity, we examined GR mRNA expression in both wild type and NG- mice. The existence of an exon 17 splice variant has been postulated in mice based on sequence homology, but has not been reported definitively in the literature. We confirmed the production of this transcript via sequencing of the cDNA amplified with a forward primer in exon 17 and a reverse primer in exon 2. We observed no difference in the level of total GR receptor expression or in the exon 17 splice variant within the hippocampus (Figure 3), suggesting that loss of new neurons in the hippocampus affects HPA-axis inhibition via an alternative mechanism.
Figure 3. Suppression of neurogenesis does not affect expression levels of GR in the hippocampus.
Quantification using real-time PCR and analysis with two-tailed t-test shows that mRNA levels of neither total GR (left) [Ctrl: Mean=0.9946 +/− 0.09915 SEM and NG−: Mean=1.1148 +/− 0.09180 SEM, N=4 animals X 3 replicates each group, p=0.3005] nor of the exon 17 GR splice variant (right) [Ctrl: Mean= 0.9493 +/−0.03710 SEM and NG−: Mean=0.8761 +/−0.09696 SEM, N=4 animals X 3 replicates each group, p=0.5070] are not significantly changed in controls (white bars) versus animals with suppressed neurogenesis (black bars).
Discussion
We observed that mice with suppressed neurogenesis show an increased HPA-axis response after exposure to a stressful situation, suggesting that newly formed neurons in the dentate gyrus are important for the known inhibitory function of the hippocampus over the HPA axis. It is well accepted that both stress and GCs are amongst the most potent inhibitors of neurogenesis. However, to our knowledge, studies into whether intact neurogenesis may be important for the hippocampus in providing its inhibitory influence over the HPA axis have not yet been reported.
It has been demonstrated in rats that adverse early life experiences, including maternal deprivation, result in decreased rates of hippocampal neurogenesis in the adult animal that do not appear to result directly from increased GCs [13]. Maternally deprived rats also demonstrate impaired negative feedback of the HPA axis in response to stressful situations [14, 15]. It was suggested that the decrease in adult-generated neurons observed with maternal deprivation could also contribute to the altered negative feedback of the HPA axis in the adult [13]. Our data support this notion by showing that newly born neurons in the hippocampus do indeed contribute to the hippocampus' ability to mediate proper inhibitory regulation over the HPA axis.
Antidepressant therapies, including SSRIs, tricyclics, MAO inhibitors, tianeptine and electroconvulsive shock have been shown to stimulate adult neurogenesis. A functional role for this increase in neurogenesis is supported by experiments showing that the success of antidepressant treatment in a rodent model of depression was dependent on intact neurogenesis [16]. Successful antidepressant treatment is associated with resolution of impairments in HPA axis negative feedback [17, 18]. Recent reports have also suggested limited efficacy of SSRIs in patients with abnormal HPA axis function, which has prompted the assertion that concurrent manipulation of the HPA system with antidepressant therapy may improve antidepressant efficacy. Monoaminergic antidepressants do not directly target the HPA axis; however, they may interrupt this circuit by stimulating neurogenesis. In theory, this could compensate for neuronal atrophy in the hippocampus, thus allowing for normal hippocampal functioning, including inhibition of the HPA axis. Support for this idea comes from experiments showing that therapeutic effects can be achieved even after suppression of hippocampal neurogenesis via manipulations that directly target the HPA axis.
Evidence points to changes in hippocampal GR expression in some models as a mediator of HPA axis inhibition efficiency [10, 11]. Previous reports have shown that regulated expression of hippocampal GR mRNA is restricted to the dentate gyrus and the CA1 subfield [19]. Decreased GR mRNA and hence receptor production, would be expected to decrease sensitivity to negative feedback by GCs on the HPA axis. Our data demonstrate that the HPA axis hyperactivity seen in response to stress in animals lacking adult neurogenesis is not a result of altered GR expression in the hippocampus. Thus, we favor the idea that the incorporation of new neurons may alter the electrophysiological properties at CA1 synapses, the only subregion in the hippocampus containing neurons that project to the hypothalamus.
Conclusion
We conclude that newly born neurons in the adult play a role in the functional inhibition of the HPA axis by the hippocampus.
Acknowledgements
This research was supported in whole by the National Institutes of Health, National Institute of Mental Health (Bethesda, MD). We thank Diane Venable for outstanding technical assistance.
Funding was provided by the National Institute of Mental Health.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. The Journal of comparative neurology. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 3.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbons JL. Cortisol Secretion Rate in Depressive Illness. Archives of general psychiatry. 1964;10:572–575. doi: 10.1001/archpsyc.1964.01720240026004. [DOI] [PubMed] [Google Scholar]
- 5.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 6.Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors' survival in the adult hippocampus. The European journal of neuroscience. 2004;20:2491–2498. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science (New York, NY. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 11.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science (New York, NY. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 12.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 13.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature neuroscience. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 14.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. Journal of neuroendocrinology. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 16.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, NY. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 17.Heuser IJ, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, et al. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. The American journal of psychiatry. 1996;153:93–99. doi: 10.1176/ajp.153.1.93. [DOI] [PubMed] [Google Scholar]
- 18.Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, et al. 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. The Journal of clinical endocrinology and metabolism. 1987;65:141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- 19.Meaney MJ, Viau V, Aitken DH, Bhatnagar S. Glucocorticoid receptors in brain and pituitary of the lactating rat. Physiology & behavior. 1989;45:209–212. doi: 10.1016/0031-9384(89)90187-x. [DOI] [PubMed] [Google Scholar]