Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies (original) (raw)
Abstract
Phyto-oestrogens are plant compounds structurally similar to oestradiol, which have been proposed to have protective effects against breast cancer. The main class of phyto-oestrogens in the Western diet is lignans. Literature reports on the effect of lignans in breast cancer risk have been conflicting. We performed three separate meta-analyses to examine the relationships between (i) plant lignan intake, (ii) enterolignan exposure and (iii) blood enterolactone levels and breast cancer risk. Medline, BIOSIS and EMBASE databases were searched for publications up to 30 September 2008, and 23 studies were included in the random effects meta-analyses. Overall, there was little association between high plant lignan intake and breast cancer risk (11 studies, combined odds ratio (OR): 0.93, 95% confidence interval (95% CI): 0.83–1.03, _P_=0.15), but this association was subjected to marked heterogeneity (_I_2=44%). Restricting the analysis to post-menopausal women, high levels of plant lignan intake were associated with reduced breast cancer risk (7 studies, combined OR: 0.85, 95% CI: 0.78, 0.93, P<0.001) and heterogeneity was markedly reduced (_I_2=0%). High enterolignan exposure was also associated with breast cancer (5 studies, combined OR: 0.73, 95% CI: 0.57, 0.92, _P_=0.009) but, again, there was marked heterogeneity (_I_2=63%). No association was found with blood enterolactone levels (combined OR: 0.82, 95% CI: 0.59–1.14, _P_=0.24). In conclusion, plant lignans may be associated with a small reduction in post-menopausal breast cancer risk, but further studies are required to confirm these results.
Keywords: plant lignans, enterolignans, breast cancer risk
High levels of endogenous circulating oestrogens (Hankinson and Eliassen, 2007) and use of exogenous oestrogens (Beral, 2003) have both been associated with increased breast cancer risk. Isoflavones and lignans are plant compounds structurally similar to 17_β_-oestrodiol known as phyto-oestrogens, capable of oestrogen receptor binding (Kuiper et al, 1998; Mueller et al, 2004). Isoflavones are mostly found in soybean products, which are a staple of the Asian diet, whereas lignans are the principal group of phyto-oestrogens in Western diets. Lignans are more widespread in foods than isoflavones and are present in grain cereals, vegetables, seeds, tea and coffee (Mazur, 1998a; Mazur et al, 1998b). Microflora in the colon (Setchell et al, 1981) convert plant lignans into enterolignans, which are detectable in blood and urine. Their levels have been correlated with the amount of plant lignans ingested (Nesbitt et al, 1999).
In a recent meta-analysis, an inverse dose-response relationship was shown between breast cancer risk and soy-food intake in Asian, but not in Western women (Wu et al, 2008). Lignans have been shown to exhibit anti-carcinogenic properties (Wang et al, 1994; Prasad 2000; Bergman Jungeström et al, 2007), and it is hypothesised that exposure to high levels may be associated with a reduction in breast cancer risk. However, results from a number of studies in Western populations have been variable. The aim of our systematic review was to establish whether an association exists between lignan exposure and breast cancer risk, and to quantify the association through meta-analyses to inform evidence-based dietary guidelines.
Materials and methods
A systematic search of Ovid Medline (US National Library of Medicine, Bethesda, MD, USA), BIOSIS (Thompson Reuters, NY, USA) and EMBASE (Reed Elsevier PLC, Amsterdam, The Netherlands) databases for relevant studies published up to and including the date, 30 September 2008 was carried out. Relevant studies included at least one keyword or Medical Subject Heading from each of the following; (i) plant lignans (matairesinol, secoisolarisiresinol, pinoresinol and lariciresinol), (ii) enterolignans (enterolactone and enterodiol) and (iii) breast cancer. The search strategy excluded reviews, animal and cell culture studies but did not impose any language restrictions.
Abstracts and full texts, where required, were independently screened by two investigators to establish the suitability for inclusion. Studies had to be of case–control or cohort design, evaluating the risk of invasive breast cancer in relation to lignan exposure and reporting odds ratios (ORs) or relative risks, as well as 95% confidence intervals (95% CIs). Cited references were also reviewed for any studies that may have been missed in the database searches.
Eligible publications were then assessed independently by three reviewers. A structured form was used to extract information about the study, subjects’ characteristics including menopausal status, confounding factors and results. Wherever multiple publications of the same study were available, the paper with the most complete set of data was chosen.
Studies were then categorised as those: (i) assessing total plant lignan intake or intake of individual plant lignans if the total was not measured; (ii) investigating exposure to enterolignans (enterolactone and enterodiol) by using values produced from food by in vitro fermentation models; and (iii) examining enterolactone levels in the blood (either plasma or serum). The blood levels of enterodiol were measured in a small number of studies (Piller et al, 2006b; Verheus et al, 2007; Ward et al, 2008) and were, therefore, not considered for analysis.
Separate meta-analyses were performed for each group of studies described in the Methods section using adjusted ORs or relative risks for the highest vs the lowest categories of exposure. If different levels of adjustment had been carried out, the results from the most fully adjusted model were used.
Random effects models were used to calculate pooled estimates, as we anticipated heterogeneity between observational studies (DerSimonian and Laird, 1986). Study-specific weights in the random effects model were calculated and scaled to percentages. The _I_2-statistic was used to test for heterogeneity (Higgins et al, 2003). Publication or selection bias was investigated by checking for asymmetry in funnel plots (Egger et al, 1997).
Analysis was repeated and sub-divided by menopausal status (pre- and post-menopausal). Statistical analyses were performed using the STATA version 9.2 software (Stata Corporation 2005, College Station, TX, USA).
Results
Following screening of abstracts and full texts and grouping into categories, 27 of the 33 articles identified were selected for data extraction. Multiple publications were identified for a number of studies. Four articles (Grace et al, 2004; McCann et al, 2006; Thanos et al, 2006; Piller et al, 2006b) were excluded, as they were based on smaller subgroup analysis of their respective larger studies. The format of certain results prevented their use, but were provided by the authors in a suitable form and therefore included in this study. Overall, 23 publications were used, providing data for 6 cohort, 6 nested case–control and 10 case–control studies. Each article contributed data to one or more meta-analyses resulting in 12 articles on plant lignan intake (see Table 1), 5 on enterolignan exposure (see Table 2) and 9 on blood enterolactone levels (see Table 3). Details of the adjustments made in each study (the most fully adjusted model was used in the meta-analysis) are shown in Tables 1, 2, 3.
Table 1. Characteristics of studies included in the review of plant lignans and breast cancer risk.
| First author/ (year)/ country | Parent study | Study design (follow-up) | Cases | Controls/ cohort size | Menopausal status | Lignans measured | Dietary assessment | Adjusted confounders |
|---|---|---|---|---|---|---|---|---|
| Horn-Ross et al (2002) United States | California Teachers Study | Prospective cohort (222 249 person-years; 2 years**) | 711 | 111 526 | Pre-M and Post-M | M, S | Self-reported 113-item FFQ | Age at 1st birth and menarche, BMI, daily caloric intake, ethnicity, family history, menopausal status, nulliparity, physical activity |
| Touillaud et al (2006) France | E3N Study | Prospective cohort (117 652 person-years; 4.2 years*) | 402 | 26 868 | Pre-M | M, S, P, L | Self-reported 208-item FFQ | Age at 1st birth and at menarche, alcohol, BBD, BMI, education, family history, energy, geographic area, height, OC, parity |
| Touillaud et al (2007) France | E3N Study | Prospective cohort (383 425 person-years; 7.7 years*) | 1469 | 58 049 | Post-M | M, S, P, L | Self-reported 208-item FFQ | Age at 1st birth, at menarche menopause, alcohol, BBD, BMI, energy, family history, height, HRT, OC, parity, smoking |
| Hedelin et al (2008) Sweden | SWLH cohort | Prospective cohort (13 years) | 1014 | 1014 | Pre-M and Post-M | M, S, P, L, Sy, Med | Self-reported 80-item FFQ | Age at menarche and 1st pregnancy, alcohol, BMI, energy, family history, OC, parity, saturated fat |
| Suzuki et al (2008) Sweden | SMC Study | Prospective cohort (430 339 person-years; 8.3 years**) | 1284 | 51 823 | Post-M | M, S, P, L | Self-reported 67-item FFQ (1987), 93-item FFQ (1997) | Age at 1st birth, menarche and menopause, alcohol, BBD, BMI, education, energy, family history, height, HRT, OC, parity |
| Horn-Ross et al (2001) United States | Bay Area Breast Cancer Study | Population-based case–control | 1272 | 1610 | Pre-M and Post-M | M, S | Self-reported 94-item FFQ | Age, age at menarche, BBD, BMI, daily caloric intake, education, family history, HRT, lactation, menopausal status, parity, race |
| dos Santos Silva et al (2004) United Kingdom | Case–control (GP's patient lists) | 240 | 477 | Pre-M and Post-M | M, S | Interviewed 207-item FFQ | Age at 1st birth and at menarche, education, family history, lactation, menopausal status, parity | |
| Linseisen et al (2004) Germany | Population-based case–control | 278 | 666 | Pre-M | M, S | Self-reported 176-item FFQ | Alcohol, BMI, education, energy, family history, lactation, parity | |
| McCann et al (2004) United States | WEB Study | Population-based case–control | 1122 | 2036 | Pre-M and Post-M | M, S | Self-reported 98-item FFQ | Age, age 1st birth, at menarche and menopause, BBD, BMI, education, energy, age at menopause, parity, race, smoking |
| Fink et al (2007) United States | LIBCSP Study | Population-based case–control | 1434 | 1404 | Pre-M and Post-M | M, S | Self-reported 94-item FFQ | Age and energy |
| Cotterchio et al (2008) Canada | Ontario Women's Diet and Health Study | Population-based case–control | 3063 | 3370 | Pre-M and Post-M | M, S, P, L | Self-reported 178-item FFQ | Age, age at 1st live birth, BBD, dietary fibre intake, family history, HRT |
| Torres-Sanchez et al (2008) Mexico | Hospital based case–control | 141 | 141 | Pre-M and Post-M | M, S, P, L | Interviewed 100-item FFQ | Age, energy, lifetime lactation, menopause status |
Table 2. Characteristics of studies included in the review of mammalian enterolignans (enterolactone and enterodiol) and breast cancer risk.
| First author/ (year)/ country | Parent study | Study design (median follow-up) | Cases | Controls/ cohort size | Menopausal status | Diet assessment | Adjusted confounders |
|---|---|---|---|---|---|---|---|
| Keinan-Boker et al (2004) The Netherlands | Prospect- EPIC | Prospective Cohort (5.2 years) | 280 | 80 215 | Pre-M, Peri-M and Post-M combined | Self-reported 178-item FFQ | Age at 1st birth and study entry, education, energy, height, HRT, marital status, OC, parity, physical activity, weight |
| Touillaud et al (2006) France | E3N Study | Prospective Cohort (4.2 years) | 402 | 117 652 | Pre-M | Self-reported 208-item FFQ | Age at 1st birth and menarche, alcohol, BBD, BMI, education, energy, family history, geographic area, height, OC, parity |
| Touillaud et al (2007) France | E3N Study | Prospective cohort (7.7 years) | 1469 | 383 425 | Post-M | Self-reported 208-item FFQ | Age at 1st birth, at menarche and menopause, alcohol, BBD, BMI, energy, family history, geographic area, height, HRT, OC, parity, smoking |
| McCann et al (2002) United States | WEB Study | Population-based case–control | 301 439 | 316 494 | Pre-M Post-M | FFQ | Age at menarche, BBD, BMI, education, energy, family history, parity; further adjusted for age at menopause |
| Linseisen et al (2004) Germany | Population-based case–control | 278 | 666 | Pre-M | Self-reported 176-item FFQ | Alcohol, BMI, breast-feeding, education, energy, family history, parity; controls matched by exact age to cases |
Table 3. Characteristics of studies included in the review of enterolactone exposure as measured in blood and breast cancer risk.
| First author/ (year)/ country | Parent study | Design (follow-up) | Cases | Controls/ cohort size | Method | Menopausal status | Mean ENL cases (nmol/l) | Mean ENL controls/cohort (nmol/l) | Adjusted confounders |
|---|---|---|---|---|---|---|---|---|---|
| Boccardo et al (2003) Italy | Prospective cohort (6.5 years after cyst aspiration) | 18 | 383 | TR-FIA | Pre-M and Post-M | 14.7 | 19.6 | Age, cyst type and family history | |
| Hultén et al (2002) Sweden | VIP, MONIKA and MSP studies | Nested case–referent | 248 | 492 | TR-FIA | Pre-M and Post-M | 26.8 VIP and MONIKA 19.3 MSP | 22.9 VIP and MONIKA 20.4 MSP | BMI, menopausal status, smoking |
| Kilkkinen et al (2004) Finland | Cross-sectional population surveys | Nested case–control | 206 | 215 | TR-FIA | Pre-M and Post-M | 25.2 | 24.0 | None |
| Olsen et al (2004) Denmark | Diet, Cancer and Health Study | Nested case–control | 381 | 381 | TR-FIA | Post-M | Not provided | Not provided | Age, HRT (through matching of controls) |
| Zeleniuch-Jacquotte et al (2004) United States | NYU Women's Health Study | Nested case–control | 417 | 417 | TR-FIA | Pre-M Post-M | 18.3 18.6 | 15.1 18.9 | Age at 1st live birth and menarche, ln(BMI), family history, ln(height), nulliparity |
| Verheus et al (2007) The Netherlands | Prospect-EPIC | Nested case–control | 383 | 383 | LC/MS | Pre-M/Peri-M Post-M | 2.98 (ng/ml) 2.71 (ng/ml) | 2.66(ng/ml) 2.65 (ng/ml) | Age at menarche and family history (Pre-M) Crude OR (Post-M). |
| Ward et al (2008) United Kingdom | EPIC-Norfolk | Nested case–control (9.5 years; 11 261 person-years) | 219 | 891 | LC/MS | All | 5.83 (ng/ml)* | 5.00 (ng/ml)* | Age, age at menarche, breast-feeding, energy, family history, fat, HRT, OC, menopausal status, parity, social class, weight |
| Pietinen et al (2001) Finland | Kuopio Breast Cancer Study | Population-based case–control | 194 | 208 | TR-FIA | Pre-M Post-M | 16.6 21.2 | 20.7 28.9 | Age at 1st birth and at menarche, alcohol, area, BBD, BMI, education, family history, HRT, OC, physical activity, smoking, waist to hip ratio |
| Piller et al (2006a) Germany | Population-based case–control | 192 | 231 | TR-FIA | Pre-M | 11.6 | 12.2 | Age at menarche, alcohol, BMI, breast-feeding, day of analysis, education, family history, OC, parity, time difference between surgery and blood sampling day |
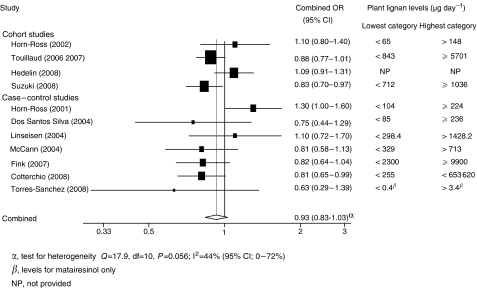
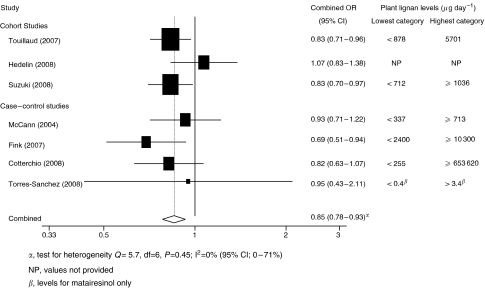
There was no association between plant lignan intake and risk when 11 studies were combined, although there was a slight protective effect. The risk in the highest intake group was 0.93 times (95% CI: 0.83–1.03, _P_=0.15) that of the lowest intake group (see Figure 1). When studies were analysed by menopausal status, a statistically significant reduction in risk was seen with the highest intake category of plant lignans vs the lowest intake in post-menopausal women (7 studies, combined OR: 0.85, 95% CI: 0.78, 0.93, P<0.001), with little sign of between-study heterogeneity (_I_2=0%, 95% CI: 0, 71, _P_=0.46) (see Figure 2). The same effect was not observed in pre-menopausal women (7 studies, combined OR: 0.97, 95% CI: 0.82, 1.15, _P_=0.73). The funnel plot of studies examining plant lignan intake and overall breast cancer risk showed symmetry, suggesting a lack of publication bias.
Figure 1.
Forest plot of highest vs lowest plant lignan intake and breast cancer risk.
Figure 2.
Forest plot of highest vs lowest plant lignan intake and breast cancer risk in post-menopausal women.
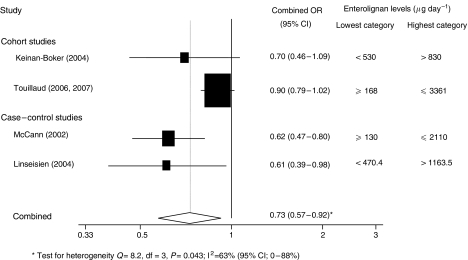
There was a statistically significant inverse association between enterolignan exposure and overall risk (combined OR: 0.73, 95% CI: 0.57, 0.92, _P_=0.009) (Figure 3), although there was marked heterogeneity (_I_2=63%, 95% CI: 0.0, 88, _P_=0.04), but there was no association between exposure and risk by menopausal status (pre-menopausal breast cancer risk: 3 studies, combined OR: 0.67, 95% CI: 0.44–1.02, _P_=0.06; post-menopausal: 2 studies, combined OR: 0.85, 95% CI: 0.72–1.01, _P_=0.06).
Figure 3.
Forest plot of highest vs lowest level of enterolignan exposure and breast cancer risk.
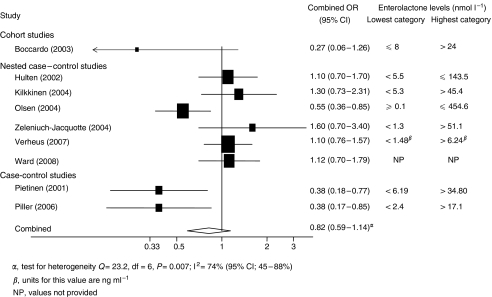
There was no association between blood enterolactone and breast cancer risk (combined OR: 0.82, 95% CI: 0.59–1.14, _P_=0.24) (Figure 4). Results of analysis by menopausal status were similar for both pre-menopausal women (5 studies, combined OR: 0.85, 95% CI: 0.45–1.59, _P_=0.61) and post-menopausal women (6 studies, combined OR: 0.86, 95% CI: 0.66, 1.14, _P_=0.28).
Figure 4.
Forest plot of highest vs lowest enterolactone levels in blood and breast cancer risk.
Discussion
This is the first systematic review and meta-analysis of exposure to lignans and breast cancer risk based on studies using dietary assessments and serum measurements. Although exposure can be assessed by urine analysis, few studies have used this methodology and therefore, these were not included (Ingram et al, 1997; den Tonkelaar et al, 2001; Dai et al, 2002). The results show that there was no association between plant lignan intake and overall risk, and this association was subjected to marked heterogeneity. However in post-menopausal women, there is a small but significant reduction in risk and a reduction in heterogeneity. A significantly decreased risk with increasing enterolignan exposure was also found. However, there was significant heterogeneity between studies making it difficult to draw clear conclusions, and the effect did not persist when analyses were stratified by menopausal status, although the number of studies included in these stratified analyses was very small. Finally, there was no association between enterolactone concentrations in blood and overall risk, or when analysis was stratified by menopausal status.
The protective action of plant lignans against breast cancer in post-menopausal, but not in pre-menopausal women, would suggest that lignan activity has a physiologic effect only at low oestradiol levels. One of the mechanisms of action may be greater sex hormone-binding globulin production and binding of free oestradiol (Adlercreutz et al, 1989, 1992; Zeleniuch-Jacquotte et al, 2004; Low et al, 2007). Binding of type II nuclear oestrogen receptor (Adlercreutz et al, 1992; Adlercreutz, 2007) and altering oestrogen synthesis within the breast cells and extragonadal sites, such as the adipose tissue, are other possible mechanisms (Adlercreutz et al, 1993; Saarinen et al, 2007). Enterolactone has been shown to decrease local oestrogen production by inhibiting 17-hydroxysteroid dehydrogenase type I and aromatase (Wang et al, 1994; Brooks and Thompson, 2005).
The apparent protective effect of dietary plant lignans in post-menopausal women is not supported by the findings from the meta-analysis of studies that measured the enterolactone levels in their blood. It would be expected that women consuming larger amounts of plant lignans would have a higher circulating concentration of enterolactone. There are a number of possible reasons for this disparity. Dietary intake of plant lignans was assessed on the basis of the subjects’ self-reported dietary intake ranging from 6 months before study entry (Hedelin et al, 2008) to 3 years before breast cancer diagnosis, (dos Santos Silva et al, 2004) and thus, it reflects long-term intake. Enterolactone concentration that is measured in a single blood sample may be more indicative of recent dietary habits. There may also be a significant intra-individual variation in serum response to dietary intake of plant lignans (Hausner et al, 2004). For example, blood levels of enterolactone can be modulated by age, smoking, frequency of defecation, weight–obesity–body mass index and regular alcohol intake (Kilkkinen et al, 2001, 2002; Horner et al, 2002; Milder et al, 2007), and these factors could potentially differ by menopausal status (in particular, age and body mass index). As bacterial enzymes are involved in lignan metabolism, the use of antibiotics has also been shown to affect enterolactone serum concentration (Kilkkinen et al, 2002); antibiotic use was generally not controlled for in these studies.
It is also possible that the protective effect is caused directly by the plant lignans or chemicals within the metabolic pathway other than enterolactone, or even by a synergistic effect between plant lignans and enterolignans. However, other food constituents found to be associated with plant lignans may exert the effect. For example, _α_-linoleic acid, which is also thought to have anti-cancer effects (Thompson, 2003; Bougnoux and Chajes, 2003, p. 232), is found in very high levels in flaxseed, the richest source of plant lignans (Thompson et al, 1991).
Determining plant lignan intake has various limitations, which could lead to an over- or under-estimation of food content. Some food composition databases are incomplete in terms of not containing values for the more recently discovered plant lignans (e.g., medioresinol) or for the whole range of foods consumed by the study population. In addition, there are various analytical methods for determining food values ; hence, databases compiled from published values determined by different methodologies may contain inherent errors. It has also been shown that the amount of lignans in food can differ according to crop variety, location, year of harvest and processing (Thompson et al, 1997; Kuijsten et al, 2005). Dietary measurement error associated with FFQs (food frequency questionnaires) is also possible. FFQs that were used varied in length, ranging from 67 to 208 items. Only one study validated its FFQ specifically for plant lignan assessment (Torres-Sanchez et al, 2008), although a UK study used the combination of an FFQ and 24-h recalls to group participants into quartiles of intake (dos Santos Silva et al, 2004). In addition, the possibility of residual confounding cannot be ruled out.
Consumption of soy food, rich in isoflavones, has been shown to reduce breast cancer risk in Asian women but not in Western women (Wu et al, 2008), suggesting that ethnicity may play a role in this effect. It is not known whether there are differential physiologic effects of lignans in people of different races, although there is some evidence of variation in the urinary excretion of lignans between white, African American and Latino women (Horn-Ross et al, 1997). Of the 23 articles used for the meta-analyses, only 3 American studies provided complete data with regard to ethnicity (Horn-Ross et al, 2001, 2002; McCann et al, 2004); hence, it was impossible perform sub-analyses for examining this.
In summary, the meta-analyses presented in this study, indicate that plant lignans and enterolignans are unlikely to significantly protect all women against breast cancer development. However, our results suggest that high plant lignan intake is associated with a 15% decreased risk in post-menopausal women, which is a small reduction that could be due to residual confounding. If real, the reason for the selective effect is not clear. Additional studies of the effect of lignan exposure on post-menopausal breast cancer risk are needed to confirm these findings before reassessing the current dietary guidelines.
Acknowledgments
Part of this work was supported by funding from the Against Breast Cancer charity (Registered Charity No. 1121258).
References
- Adlercreutz H (2007) Lignans and human health. Crit Rev Clin Lab Sci 44: 483–525 [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Bannwart C, Wähälä K, Mäkelä T, Brunow G, Hase T, Arosemena PJ, Kellis Jr JT, Vickery LE (1993) Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol 44: 147–153 [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Hämäläinen E, Gorbach SL, Goldin BR, Woods MN, Dwyer JT (1989) Diet and plasma androgens in postmenopausal vegetarian and omnivorous women and postmenopausal women with breast cancer. Am J Clin Nutr 49: 433–442 [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Mousavi Y, Clark J, Höckerstedt K, Hämäläinen E, Wähälä K, Mäkelä T, Hase T (1992) Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol 41: 331–337 [DOI] [PubMed] [Google Scholar]
- Beral V, Million Women Study Collaborators (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362: 419–427 [DOI] [PubMed] [Google Scholar]
- Bergman Jungeström M, Thompson LU, Dabrosin C (2007) Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res 13: 1061–1067 [DOI] [PubMed] [Google Scholar]
- Boccardo F, Lunardi G, Guglielmini P, Parodi M, Murialdo R, Schettini G, Rubagotti A (2003) Serum enterolactone levels and the risk of breast cancer in women with palpable cysts. Eur J Cancer 40: 84–89 [DOI] [PubMed] [Google Scholar]
- Bougnoux P, Chajes V (2003) _α_-Linoleic acid and cancer. In: Flaxseed in Human Nutrition Thompson LU, Cunnane SC (eds), pp 232–244. AOCS Press: Illinois, USA [Google Scholar]
- Brooks JD, Thompson LU (2005) Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol 94: 461–467 [DOI] [PubMed] [Google Scholar]
- Cotterchio M, Boucher BA, Kreiger N, Mills CA, Thompson LU (2008) Dietary phytoestrogen intake–lignans and isoflavones–and breast cancer risk (Canada). Cancer Causes Control 19: 259–272 [DOI] [PubMed] [Google Scholar]
- Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W (2002) Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev 11: 815–821 [PubMed] [Google Scholar]
- den Tonkelaar I, Keinan-Boker L, Veer PV, Arts CJ, Adlercreutz H, Thijssen JH, Peeters PH (2001) Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 10: 223–228 [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188 [DOI] [PubMed] [Google Scholar]
- dos Santos Silva I, Mangtani P, McCormack V, Bhakta D, McMichael AJ, Sevak L (2004) Phyto-oestrogen intake and breast cancer risk in South Asian women in England: findings from a population-based case-control study. Cancer Causes Control 15: 805–818 [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD (2007) Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol 165: 514–523 [DOI] [PubMed] [Google Scholar]
- Grace PB, Taylor JI, Low YL, Luben RN, Mulligan AA, Botting NP, Dowsett M, Welch AA, Khaw KT, Wareham NJ, Day NE, Bingham SA (2004) Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev 13: 698–708 [PubMed] [Google Scholar]
- Hankinson SE, Eliassen AH (2007) Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol 106: 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner H, Johnsen NF, Hallund J, Tetens I (2004) A single measurement is inadequate to estimate enterolactone levels in Danish postmenopausal women due to large intraindividual variation. J Nutr 134: 1197–1200 [DOI] [PubMed] [Google Scholar]
- Hedelin M, Löf M, Olsson M, Adlercreutz H, Sandin S, Weiderpass E (2008) Dietary phytoestrogens are not associated with risk of overall breast cancer but diets rich in coumestrol are inversely associated with risk of estrogen receptor and progesterone receptor negative breast tumors in Swedish women. J Nutr 138: 938–945 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner NK, Kristal AR, Prunty J, Skor HE, Potter JD, Lampe JW (2002) Dietary determinants of plasma enterolactone. Cancer Epidemiol Biomarkers Prev 11: 121–126 [PubMed] [Google Scholar]
- Horn-Ross PL, Barnes S, Kirk M, Coward L, Parsonnet J, Hiatt RA (1997) Urinary phytoestrogen levels in young women from a multiethnic population. Cancer Epidemiol Biomarkers Prev 6: 339–345 [PubMed] [Google Scholar]
- Horn-Ross PL, Hoggatt KJ, West DW, Krone MR, Stewart SL, Anton H, Bernstei CL, Deapen D, Peel D, Pinder R, Reynolds P, Ross RK, Wright W, Ziogas A (2002) Recent diet and breast cancer risk: the California Teachers Study (USA). Cancer Causes Control 13: 407–415 [DOI] [PubMed] [Google Scholar]
- Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ (2001) Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol 154: 434–441 [DOI] [PubMed] [Google Scholar]
- Hultén K, Winkvist A, Lenner P, Johansson R, Adlercreutz H, Hallmans G (2002) An incident case-referent study on plasma enterolactone and breast cancer risk. Eur J Nutr 41: 168–176 [DOI] [PubMed] [Google Scholar]
- Ingram D, Sanders K, Kolybaba M, Lopez D (1997) Case-control study of phyto-oestrogens and breast cancer. Lancet 350: 990–994 [DOI] [PubMed] [Google Scholar]
- Keinan-Boker L, van Der Schouw YT, Grobbee DE, Peeters PH (2004) Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr 79: 282–288 [DOI] [PubMed] [Google Scholar]
- Kilkkinen A, Pietinen P, Klaukka T, Virtamo J, Korhonen P, Adlercreutz H (2002) Use of oral antimicrobials decreases serum enterolactone concentration. Am J Epidemiol 155: 472–477 [DOI] [PubMed] [Google Scholar]
- Kilkkinen A, Stumpf K, Pietinen P, Valsta LM, Tapanainen H, Adlercreutz H (2001) Determinants of serum enterolactone concentration. Am J Clin Nutr 73: 1094–1100 [DOI] [PubMed] [Google Scholar]
- Kilkkinen A, Virtamo J, Vartiainen E, Sankila R, Virtanen MJ, Adlercreutz H, Pietinen P (2004) Serum enterolactone concentration is not associated with breast cancer risk in a nested case-control study. Int J Cancer 108: 277–280 [DOI] [PubMed] [Google Scholar]
- Kuijsten A, Arts IC, van’t Veer P, Hollman PC (2005) The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J Nutr 135: 2812–2816 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139: 4252–4263 [DOI] [PubMed] [Google Scholar]
- Linseisen J, Piller R, Hermann S, Chang-Claude J (2004) Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int J Cancer 110: 284–290 [DOI] [PubMed] [Google Scholar]
- Low YL, Dunning AM, Dowsett M, Folkerd E, Doody D, Taylor J, Bhaniani A, Luben R, Khaw KT, Wareham NJ, Bingham SA (2007) Phytoestrogen exposure is associated with circulating sex hormone levels in postmenopausal women and interact with ESR1 and NR1I2 gene variants. Cancer Epidemiol Biomarkers Prev 16: 1009–1016 [DOI] [PubMed] [Google Scholar]
- Mazur W (1998a) Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab 12: 729–742 [DOI] [PubMed] [Google Scholar]
- Mazur WM, Wähälä K, Rasku S, Salakka A, Hase T, Adlercreutz H (1998b) Lignan and isoflavonoid concentrations in tea and coffee. Br J Nutr 79: 37–45 [DOI] [PubMed] [Google Scholar]
- McCann SE, Kulkarni S, Trevisan M, Vito D, Nie J, Edge SB, Muti P, Freudenheim JL (2006) Dietary lignan intakes and risk of breast cancer by tumor estrogen receptor status. Breast Cancer Res Treat 99: 309–311 [DOI] [PubMed] [Google Scholar]
- McCann SE, Moysich KB, Freudenheim JL, Ambrosone CB, Shields PG (2002) The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J Nutr 132: 3036–3041 [DOI] [PubMed] [Google Scholar]
- McCann SE, Muti P, Vito D, Edge SB, Trevisan M, Freudenheim JL (2004) Dietary lignan intakes and risk of pre- and postmenopausal breast cancer. Int J Cancer 111: 440–443 [DOI] [PubMed] [Google Scholar]
- Milder IE, Kuijsten A, Arts IC, Feskens EJ, Kampman E, Hollman PC, Van’t Veer P (2007) Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J Nutr 137: 1266–1271 [DOI] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS (2004) Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci 80: 14–25 [DOI] [PubMed] [Google Scholar]
- Nesbitt PD, Lam Y, Thompson LU (1999) Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr 69: 549–555 [DOI] [PubMed] [Google Scholar]
- Olsen A, Knudsen KE, Thomsen BL, Loft S, Stripp C, Overvad K, Møller S, Tjønneland A (2004) Plasma enterolactone and breast cancer incidence by estrogen receptor status. Cancer Epidemiol Biomarkers Prev 13: 2084–2089 [PubMed] [Google Scholar]
- Pietinen P, Stumpf K, Männistö S, Kataja V, Uusitupa M, Adlercreutz H (2001) Serum enterolactone and risk of breast cancer: a case-control study in eastern Finland. Cancer Epidemiol Biomarkers Prev 10: 339–344 [PubMed] [Google Scholar]
- Piller R, Chang-Claude J, Linseisen J (2006a) Plasma enterolactone and genistein and the risk of premenopausal breast cancer. Eur J Cancer Prev 15: 225–232 [DOI] [PubMed] [Google Scholar]
- Piller R, Verla-Tebit E, Wang-Gohrke S, Linseisen J, Chang-Claude J (2006b) CYP17 genotype modifies the association between lignan supply and premenopausal breast cancer risk in humans. J Nutr 136: 1596–1603 [DOI] [PubMed] [Google Scholar]
- Prasad K (2000) Antioxidant Activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol 9: 220–225 [DOI] [PubMed] [Google Scholar]
- Saarinen NM, Wärri A, Airio M, Smeds A, Mäkelä S (2007) Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res 51: 857–866 [DOI] [PubMed] [Google Scholar]
- Setchell KD, Lawson AM, Borriello SP, Harkness R, Gordon H, Morgan DM, Kirk DN, Adlercreatz H, Anderson LC, Axelson M (1981) Lignan formation in man–microbial involvement and possible roles in relation to cancer. Lancet 2: 4–7 [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rylander-Rudqvist T, Saji S, Bergkvist L, Adlercreutz H, Wolk A (2008) Dietary lignans and postmenopausal breast cancer risk by oestrogen receptor status: a prospective cohort study of Swedish women. Br J Cancer 98: 636–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU (2006) Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control 17: 1253–1261 [DOI] [PubMed] [Google Scholar]
- Thompson LU (2003) Flaxseed, lignans and cancer. In Flaxseed in Human Nutrition, Thompson LU, Cunnane SC (eds), pp 194–222. AOCS Press: Illinois, USA [Google Scholar]
- Thompson LU, Rickard SW, Cheung F, Kenaschuk EO, Obermeyer WR (1997) Variability in anticancer lignan levels in flaxseed. Nutr Cancer 27: 26–30 [DOI] [PubMed] [Google Scholar]
- Thompson LU, Robb P, Serraino M, Cheung F (1991) Mammalian lignan production from various foods. Nutr Cancer 16: 43–52 [DOI] [PubMed] [Google Scholar]
- Torres-Sanchez L, Galvan-Portillo M, Wolff MS, Lopez-Carrillo L (2008) Dietary consumption of phytochemicals and breast cancer risk in Mexican women. Public Health Nutr 23: 1–7 [DOI] [PubMed] [Google Scholar]
- Touillaud MS, Thiébaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F (2007) Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst 99: 475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touillaud MS, Thiébaut AC, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F (2006) No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Cancer Epidemiol Biomarkers Prev 15: 2574–2576 [DOI] [PubMed] [Google Scholar]
- Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH (2007) Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol 25: 648–655 [DOI] [PubMed] [Google Scholar]
- Wang C, Mäkelä T, Hase T, Adlercreutz H, Kurzer MS (1994) Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J Steroid Biochem Mol Biol 50: 205–212 [DOI] [PubMed] [Google Scholar]
- Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S, European Prospective into Cancer-Norfolk cohort (2008) Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res 10: R32. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Yu MC, Tseng CC, Pike MC (2008) Epidemiology of soy exposures and breast cancer risk. Br J Cancer 98: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Adlercreutz H, Shore RE, Koenig KL, Kato I, Arslan AA, Toniolo P (2004) Circulating enterolactone and risk of breast cancer: a prospective study in New York. Br J Cancer 91: 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]