The role of histamine H4 receptor in immune and inflammatory disorders (original) (raw)
Abstract
Since its discovery at the beginning of the 20th century, histamine has been established to play a pathophysiological regulatory role in cellular events through binding to four types of G-protein-coupled histamine receptors that are differentially expressed in various cell types. The discovery, at the turn of the millennium, that the histamine H4 receptor is largely expressed in haemopoietic cells as well as its chemotactic properties designate its regulatory role in the immune system. H4 receptors modulate eosinophil migration and selective recruitment of mast cells leading to amplification of histamine-mediated immune responses and eventually to chronic inflammation. H4 receptor involvement in dendritic cell activation and T cell differentiation documents its immunomodulatory function. The characterization of the H4 as the immune system histamine receptor directed growing attention towards its therapeutic exploitation in inflammatory disorders, such as allergy, asthma, chronic pruritus and autoimmune diseases. The efficacy of a number of H4 receptor ligands has been evaluated in in vivo and in vitro animal models of disease and in human biological samples. However, before reaching decisive conclusions on H4 receptor pathophysiological functions and therapeutic exploitation, identification of genetic polymorphisms and interspecies differences in its relative actions and pharmacological profile need to be addressed and taken into consideration. Despite certain variations in the reported findings, the available data strongly point to the H4 receptor as a novel target for the pharmacological modulation of histamine-transferred immune signals and offer an optimistic perspective for the therapeutic exploitation of this promising new drug target in inflammatory disorders.
Keywords: allergy, asthma, H4 receptor ligands, histamine, histamine H4 receptor, immune system, immunomodulation, inflammation, pruritus, rheumatoid arthritis
Introduction
Histamine [2-(4-imidazolyl)-ethylamine] is an endogenous short-acting biogenic amine synthesized from the basic amino acid histidine through the catalytic activity of the rate-limiting enzyme histidine decarboxylase and widely distributed throughout the body (Dy and Schneider, 2004). The pharmacological study of histamine started concurrently with its discovery by Sir Henry H Dale at the beginning of the 20th century (Barger and Dale, 1910; Dale and Laidlaw, 1910). One of the first described functions was its ability to mimic anaphylaxis and has since been demonstrated to play a major role in inflammatory processes. Following the recognition of its ‘somewhat complicated action’ by Dale and Laidlaw (1910), histamine has been one of the most studied substances in medicine for nearly a century, possessing a wide spectrum of activities (Figure 1) including its potent mediator role in immediate hypersensitivity reactions (Daugherty, 2004).
Figure 1.
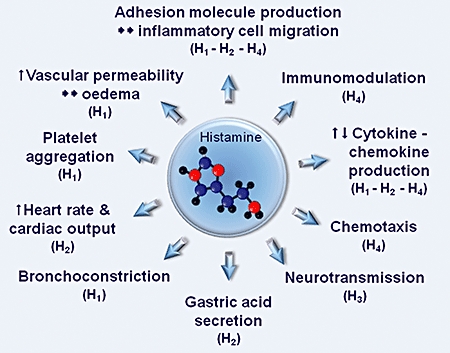
Indicative major effects exerted by histamine. The predominant histamine receptors, designated H1, H2, H3 and H4, eliciting the effects are shown in parentheses.
Undoubtedly, the fundamental pleiotropic regulatory character of histamine in cellular events is attributed to its binding to four subtypes of G-protein-coupled receptors (GPCR), designated H1, H2, H3 and H4 (Figure 1) that are differentially expressed in various cell types (Dy and Schneider, 2004; Akdis and Simons, 2006; Chazot and Tiligada, 2008). Histamine receptor diversity is supported by pharmacological evidence and by the low protein sequence homology, which is suggestive of their evolution from different ancestral genes (Leurs et al., 2000). The H4 receptor shows little homology to the classical pro-inflammatory H1 receptor or the H2 receptor and about 35% homology with the H3 receptor. H3 and H4 receptors are most closely related to each other, and they have a closer phylogenetic relationship with peptide ligand GPCRs, while they are remotely related to other biogenic amine receptors, including H1 and H2 receptors (Nakayama et al., 2004).
Histamine is synthesized in several cell types of peripheral and central tissues, while its release from repository cells is modulated by a variety of stimuli. The classical source of histamine is the pluripotent heterogeneous mast cell (MC) (Riley and West, 1952), where it is stored in cytosolic granules and released by exocytosis to exert its immunomodulatory role in response to various immunological and non-immunological stimuli, including allergens, drugs, mechanical stimulation, cold, ultra violet rays and endogenous polypeptides such as substance P and bradykinin (Kakavas et al., 2006; Krishnaswamy et al., 2006). Non-MC histamine is derived from numerous sources, indicative examples being gastric enterochromaffin-like cells (Prinz et al., 2003), various types of blood cells, such as basophils (Falcone et al., 2006), macrophages, lymphocytes (Zwadlo-Klarwasser et al., 1994) and platelets (Masini et al., 1998), neurons (Arrang et al., 1983; Haas et al., 2008), chondrocytes (Maslinska et al., 2004) and tumours (Falus et al., 2001). In the gastric mucosa, enterochromaffin-like cell-derived histamine acts as a paracrine stimulant to control acid secretion in response to hormonal and neural stimuli (Prinz et al., 2003; Grandi et al., 2008). In the brain, histamine is synthesized exclusively in histaminergic neurons of the tuberomamillary nucleus of the posterior hypothalamus that project all over the central nervous system (Haas and Panula, 2003). In a mutual interaction network with other transmitter systems, brain histamine is implicated in basic homeostatic and higher brain functions, including sleep–wake regulation, circadian and feeding rhythms, immunity, learning and memory (Haas et al., 2008). Thus, in addition to the predominately H1 receptor-mediated actions on smooth muscle, vascular permeability and modulation of allergic responses, the main functions of histamine (Figure 1) include gastric acid secretion basically via H2 receptors (Black et al., 1972), neurotransmission in the central nervous system largely via H3 receptor signalling (Arrang et al., 1983; Haas et al., 2008) and modulation of immune system processes through the H1 receptor and the recently identified H4 receptor (Oda et al., 2000; Liu et al., 2001a; de Esch et al., 2005).
The histaminergic system has proved to be a rich source of drugs over the last five decades with a number reaching blockbuster status. H1 (commonly referred to as antihistamines) and H2 receptor antagonists are widely used in the treatment of allergy and gastrointestinal disorders, respectively, while H3 receptor antagonists may have therapeutic value in dementias, psychotic and sleep disorders as well as obesity (Hill et al., 1997). Most importantly, growing attention is directed towards the therapeutic exploitation of the H4 receptor in inflammation and cancer (Venable and Thurmond, 2006), following its cloning by several groups independently at the turn of the millennium (Nakamura et al., 2000; Oda et al., 2000; Cogé_et al._, 2001; Liu et al., 2001a; Morse et al., 2001; Nguyen et al., 2001; Zhu et al., 2001) and its subsequent characterization as the immune system histamine receptor.
Over the years, studies on the effect of histamine during the immune response have produced a number of apparently conflicting data. These inconsistencies might arise from the differential expression of histamine receptors that may vary with the experimental setup. The contribution of histamine receptors in immune responses is exemplified by the frequently stimulatory effects through H1 receptor and inhibitory actions through H2 receptor signalling, as well as by the regulatory role of these receptors in the balance between type I and II helper T (TH) cells (Dy and Schneider, 2004; Akdis and Simons, 2006). The localization of H4 receptors largely in haemopoietic cells, their distinct pharmacological profile and their role in chemotaxis and mediator release in various cell types (Nakamura et al., 2000; Oda et al., 2000; Liu et al., 2001a) led to the re-evaluation of many biological actions of histamine, resolved some of the inconsistencies regarding the pharmacology of histamine receptor ligands and differentiated its functional roles in physiology and pathophysiology (de Esch et al., 2005; Thurmond et al., 2008).
H4 receptor: the immune system histamine receptor
Histamine H4 receptor is a pertussis-toxin-sensitive GPCR predominantly expressed on cells of the immune system, including MCs, monocytes, eosinophils, dendritic cells (DCs), T cells and natural killer cells (Table 1); in peripheral tissues such as spleen, thymus, colon, blood leukocytes and bone marrow, its expression being induced or altered in response to inflammatory stimuli (Oda et al., 2000; Cogé_et al._, 2001; Liu et al., 2001a; Morse et al., 2001; Lippert et al., 2004; Gutzmer et al., 2005; Damaj et al., 2007; Dijkstra et al., 2007; 2008). The H4 receptor appears to have higher affinity for histamine compared with the H1 receptor, activation leading to leukocyte chemotaxis to sites of inflammation via Gαi/o proteins and increases in intracellular Ca2+ concentration (Hofstra et al., 2003; de Esch et al., 2005; Table 1). In addition to histamine, liver-expressed chemokine LEC/CCL16 has been reported to be a non-histamine endogenous H4 receptor agonist, demonstrating additive effects with histamine and involved in eosinophil trafficking (Nakayama et al., 2004).
Table 1.
Expression and functional characteristics of the histamine H4 receptor in cells associated with inflammatory and immune disorders
| Cell type | H4 receptor expression, characteristics and function | References |
|---|---|---|
| B-lymphocytes | Very low expression level (human blood) | Zhu et al. (2001) |
| Dendritic cells | mRNA expression; chemotaxis with Ca2+ fluxes (human blood) | Zhu et al. (2001); Ling et al. (2004); Damaj et al. (2007) |
| Migration (guinea pig and mouse BM) | Bäumer et al. (2008) | |
| Up-regulation by IFN-γ; down-regulation of TH2-linked chemokine CCL2 and TH1 cytokine IL-12 (human IDECs) | Dijkstra et al. (2008) | |
| mRNA up-regulation during differentiation; suppression of IL-12p70 production; F-actin polymerization → migration (human MoDC) | Gutzmer et al. (2005) | |
| Eosinophils | mRNA expression; intracellular Ca2+ mobilisation, actin polymerization, shape change, up-regulation of CD11b/CD18 and CD54 expression; migration into inflamed tissue (human blood) | Oda et al. (2000); Morse et al. (2001); Buckland et al. (2003); Ling et al. (2004); Barnard et al. (2008) |
| Ca2+ mobilization, chemotaxis (mouse) | O'Reilly et al. (2002) | |
| Fibroblasts | mRNA up-regulation by LPS and indomethacin, protein levels increased by dexamethasone (human, dermal) | Ikawa et al. (2008) |
| HL60.15 cell line | IL-5 induced differentiation → increased H4 receptor expression | Ling et al. (2004) |
| Mast cells | H4 receptor mRNA and protein expression (human skin) | Lippert et al. (2004) |
| Intracellular Ca2+ mobilization; chemotaxis without degranulation (mouse) | O'Reilly et al. (2002); Hofstra et al. (2003); Thurmond et al. (2004) | |
| Enhancement of CXCL12 chemotactic activity on mast cell precursors (human umbilical cord blood) | Godot et al. (2007) | |
| Local mast cell regulation, redistribution in ovalbumin-challenged oesophageal mucosal epithelium → infiltration of eosinophils (guinea pig) | Yu et al. (2008) | |
| Monocytes | Higher expression in resting than in activated cells; up-regulation by IFN-γ; Ca2+ influx, down-regulation of CCL2 synthesis and release → reduced monocyte recruitment (human blood) | Oda et al. (2000); Morse et al. (2001); Zhu et al. (2001); Dijkstra et al. (2007) |
| Natural killer cells | Chemotaxis with no induction of Ca2+ mobilization (human blood) | Damaj et al. (2007) |
| Neutrophils | Expression (human blood) | Oda et al. (2000); Morse et al. (2001) |
| T-lymphocytes | Higher expression in resting than in activated CD4+ and CD8+ cells; increased release of IL-16 from CD8+ cells (human, blood) | Morse et al. (2001); Zhu et al. (2001); Gantner et al. (2002); Ling et al. (2004) |
| Suppression of STAT1α formation, phosphorylation and DNA binding (human non-atopic PBMCs); H4 receptor blockade → inhibition of STAT6 DNA binding (human atopic PBMCs) | Horr et al. (2006); Michel et al. (2008) |
The expression of the H4 receptor in several types of human immune cells and its chemotactic properties denote its role in immunomodulation (Figure 2, Table 1). Despite the interspecies differences in amino acid sequence, expression levels, ligand binding and receptor activation, the comparable tissue distribution suggests similar physiological roles for this receptor across the species (Liu et al., 2001b; Oda et al., 2005; Jiang et al., 2008).
Figure 2.
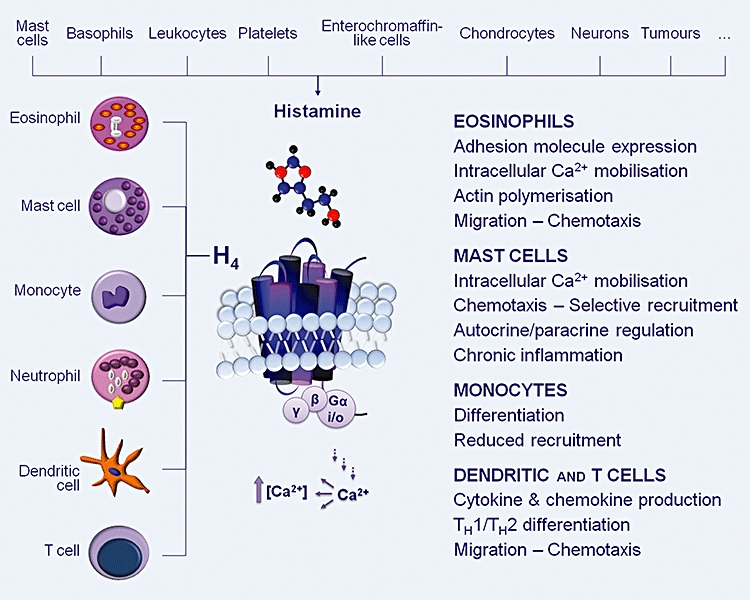
Indicative immunomodulatory actions of histamine that are mediated through histamine H4 receptors (H4) predominately expressed in immune cells. Gαi/o, G-protein; TH, helper T cell.
Eosinophil chemotaxis
Histamine was first described as a selective chemoattractant for eosinophils more than 30 years ago (Clark et al., 1975). In a retrospective literature evaluation, the reported histamine effects on eosinophil chemotaxis can now be attributed to the H4 receptor. The existence of a histamine receptor on the surface of eosinophils that was distinct from H1, H2 or H3 receptors and demonstrated low-affinity binding for _R_-(α)-methylhistamine antagonized by the H3/H4 antagonist thioperamide was hypothesized in 1994 (Raible et al., 1994). Concrete evidence that H4 receptors control leukocyte trafficking and pro-inflammatory responses was derived from the H4 receptor-mediated histamine-induced activation of eosinophils (Table 1), increased expression of adhesion molecules like CD11b/CD18(Mac1) and CD54(ICAM-1) and rearrangement of the actin cytoskeleton leading to eosinophil migration from the bloodstream into the sites of inflammation (O'Reilly et al., 2002; Buckland et al., 2003; Ling et al., 2004; Barnard et al., 2008).
Mast cell chemotaxis and chronic inflammation
Interestingly, human MCs constitutively express H4 receptors that govern autocrine and paracrine histamine-induced processes (Lippert et al., 2004). H4 receptor activation mediates chemotaxis and intracellular Ca2+ mobilization in murine MCs, without affecting degranulation, thus providing a mechanism for the selective recruitment of these effector cells into the tissues and the amplification of the histamine-mediated reaction eventually leading to chronic allergic inflammation (Hofstra et al., 2003). Supportive evidence for an autoregulatory function of the MC-expressed H4 receptor comes from its critical role in zymosan-induced recruitment of neutrophils in vivo, possibly via regulation of leukotriene B4 release from MCs (Takeshita et al., 2003; Thurmond et al., 2004), without however excluding the possibility of additional effects of other immune cells in the response (Liu et al., 2001b; Dunford et al., 2007).
Dentritic cell activation and T cell differentiation
The H4 receptor expressed on DCs, CD4+ and CD8+ T cells appears to control cytokine and chemokine production (Table 1). In general, histamine can enhance TH1 responses through H1 receptor activation and negatively regulate both TH1 and TH2 responses by acting on H2 receptors (Jutel et al., 2001). However, data concerning the differential expression of histamine receptor subtypes that mediate DC activation and TH1/TH2 differentiation have been somewhat contradictory (Dy and Schneider, 2004). The interest was revived after the identification of the H4 receptor, which alongside H1 and H2 receptors, modulates cytokine secretion during the integration of TH1/TH2 differentiation (Jutel et al., 2001). Cytokines mediate their effects via the signal transduction and activators of transcription (STAT), with STAT6 activation causing a shift towards the TH2 response implicated in allergic state development and STAT1 and STAT4 playing a role in the pathogenesis of asthma with distinct responses existing in non-atopic and atopic states. Histamine acting on the H4 receptor has been reported to suppress ex vivo the mitogen-induced STAT1 phosphorylation and its specific interaction with DNA in peripheral blood mononuclear cells derived from non-atopic individuals (Horr et al., 2006), while the H4 receptor antagonist JNJ7777120 inhibited STAT6 DNA binding in cells derived from atopic subjects (Michel et al., 2008). However, in mouse splenocytes, the H4 receptor exerted no effect on STAT4 (Liu et al., 2006) or STAT6 (Kharmate et al., 2007) phosphorylation.
Additional evidence for the immunomodulatory function of the H4 receptor is provided by its involvement in the release of the CD4+ cell chemoattractant interleukin (IL)-16 from human CD8+ T-lymphocytes in vitro (Gantner et al., 2002), the influence on mouse CD4+ T cell activation possibly via signalling in DCs (Dunford et al., 2006), as well as by its up-regulation during monocyte differentiation, suppression of IL-12p70 production and chemoattraction of human monocyte-derived DCs (Gutzmer et al., 2005). A reciprocal crosstalk between histamine and cytokines or chemokines involving the H4 receptor seems to be in operation. Interferon (IFN)-γ up-regulates H4 receptor expression in human peripheral blood monocytes/CD14+ (Dijkstra et al., 2007) and in inflammatory DCs from atopic dermatitis skin (Dijkstra et al., 2008). The H4 receptor-mediated induction of Ca2+ mobilization and down-regulation of synthesis and release of the TH2-linked chemokine CCL2 from monocytes is indicative of a negative feedback mechanism that would avoid the TH2 environment in case of high histamine levels in allergic inflammation and contribute to the shift to TH1 that is observed in the transition from acute to chronic allergic inflammation (Dijkstra et al., 2007). Comparably, H4 receptor stimulation down-regulates the production of the TH1 cytokine IL-12 and that of CCL2 in human monocyte-derived inflammatory dendritic epidermal cells, the latter leading to decreased monocyte migration (Dijkstra et al., 2008).
H4 receptor-mediated effects in inflammatory disorders
Histamine has long been known to mediate inflammatory, and allergic responses acting predominately through H1 receptors and H1 receptor antagonists have been used to treat allergies for many years (Hill et al., 1997). Accumulating evidence derived from diverse in vivo and in vitro studies using animal models of disease and human biological samples (Table 2) substantiates the fundamental role of the H4 receptor in histamine-induced chemotaxis of MCs, eosinophils and other immune cells (Thurmond et al., 2004; Ikawa et al., 2005; Dunford et al., 2006). In addition, the presence of H4 receptors mostly in immune system organs and their immunomodulatory role in cytokine production (Cogé_et al._, 2001; Gantner et al., 2002; Gutzmer et al., 2005) argue for the pathophysiological significance of H4 receptors in inflammatory conditions that are characterized by increases in immune cell numbers, such as asthma, allergic disorders and autoimmune diseases (Figure 3) and imply their contribution not only in the histamine-mediated initial inflammatory signal but also in the maintenance of inflammation (Thurmond et al., 2004; Kakavas et al., 2006; Dunford et al., 2007; Zhang et al., 2007; Table 2).
Table 2.
Evidence for the contribution of histamine H4 receptor to inflammatory disorders
| Condition | Function of the H4 receptor | References |
|---|---|---|
| Airway inflammation | Decreased lung inflammation, lung eosinophil/lymphocyte infiltration and TH2 responses in H4R−/− and JNJ7777120-treated mice; decreased IL-4, IL-5, IL-13, IL-6, IL-17 levels upon ex vivo re-stimulation of mouse T cells → disrupted T cell functions; blockade of DC H4 receptors _in vitro_→ decreased cytokine and chemokine production → limited ability of DC to induce TH2 responses in murine T cells | Dunford et al. (2006) |
| Intratracheal administration of H4 receptor agonist 4-MH before Ag challenge in a murine model of allergic asthma → reduced airway hyperreactivity and inflammation, increased IL-10 and IFN-γ and decreased IL-13 in the bronchoalveolar lavage fluid; accumulation of FoxP3+ T cells | Morgan et al. (2007) | |
| In vitro stimulation of human T cells with H4 receptor agonist 4-MH → increased T cell migration skewed towards CD25- and intracellular FoxP3-expressing CD4+ cells; suppressed proliferation of autologous T cells dependent on IL-10 production | Morgan et al. (2007) | |
| Increased H4 receptor expression in human nasal turbinate mucosa and nasal polyp tissue, tendency for correlation between H4 receptor expression and eosinophil cationic protein | Jókúti et al. (2007) | |
| Suppression of STAT1α formation and phosphorylation by H4 receptor agonist clobenpropit and enhancement of STAT1α levels, phosphorylation and DNA binding by JNJ7777120 in non-atopic human stimulated PBMCs | Horr et al. (2006) | |
| Inhibition of STAT6 DNA binding by JNJ7777120 in atopic human PBMCs | Michel et al. (2008) | |
| Inhibition of MC and eosinophil migration into oesophageal mucosal epithelium of sensitized guinea pigs by the H3/H4 receptor antagonist thioperamide | Yu et al. (2008) | |
| Pruritus & dermatitis | Reduction of H4 receptor agonist clobenpropit-induced scratching by the H3/H4 receptor antagonist thioperamide in female Balb C mice | Bell et al. (2004) |
| No pruritic response with 4-MH in H4R−/− mice; attenuation of MC- or other haematopoietic cell-independent scratching by JNJ7777120 in mice | Dunford et al. (2007) | |
| Strain differences between NMRI and Balb C mice in H4 receptor-mediated scratching, not associated with H4 receptor expression and protein levels in murine BM-derived DCs; clobenpropit-induced DC migration blocked by JNJ7777120 | Bäumer et al. (2008) | |
| H4 receptor expression in skin IDECs, up-regulation by IFN-γ in Mo-IDECs; clobenpropit and 4-MH-induced down-regulation of CCL2 and IL-12 production in Mo-IDECs blocked by JNJ7777120 in human atopic dermatitis | Dijkstra et al. (2008) | |
| Suppression of late-phase swelling and eosinophil infiltration by H3/H4 receptor antagonist thioperamide in Ag-non-specific dermatitis in mice | Hirasawa et al. (2008) | |
| Arthritis | Variable mRNA expression in synovial cells (superficial layer membrane, villi and vascular wall cells, fibroblast- and macrophage-like cells) from rheumatoid arthritis and osteoarthritis patients | Ikawa et al. (2005); Grzybowska-Kowalczyk et al. (2007; 2008); Ohki et al., 2007 |
| Increased cartilage histamine content without arthritis signs in JNJ7777120-treated rats with adjuvant arthritis | Zampeli et al. (2008) | |
| Acute/subchronic inflammation | Reduction of early-phase paw oedema and thermal hyperalgesia by JNJ7777120 and VUF6002 in carrageenan-induced inflammation in rats | Coruzzi et al. (2007) |
| Reduced neutrophil release from BM and MC-dependent neutrophil recruitment by the H3/H4 receptor antagonist thioperamide in zymosan-induced peritonitis in mice | Takeshita et al. (2003; 2004); Thurmond et al. (2004) | |
| Reduction in macroscopic damage, mucosal and submucosal thickness and neutrophil infiltration and inhibition of colonic myeloperoxidase and TNF-α elevation by JNJ10191584 and JNJ7777120 in trinitrobenzene sulphonic acid-induced subchronic colitis in rats | Varga et al. (2005) | |
| Increased conjunctival histamine content upon topical JNJ7777120 instillation in experimental conjunctivitis in rats | Zampeli et al. (2009) | |
| Ischaemia/reperfusion injury | H4 receptor stimulation → prevention of reperfusion injury development in the rat liver | Adachi et al. (2006) |
Figure 3.
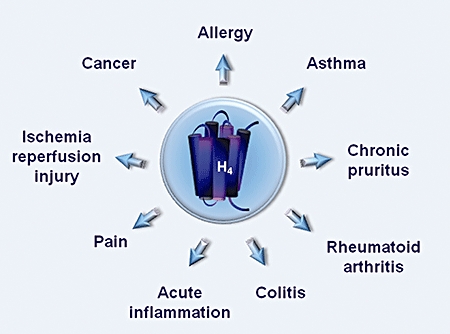
Involvement of the histamine H4 receptor in a number of inflammatory disorders.
Consequently, the H4 receptor is currently an attractive target for the pharmacological modulation of histamine-transferred signals in inflammatory conditions (Buckland et al., 2003; Hofstra et al., 2003; de Esch et al., 2005; Bäumer et al., 2008) and for the development of beneficial therapeutic strategies for these conditions (Kiss et al., 2008; Thurmond et al., 2008). Furthermore, the use of H4 receptor-targeting agents in related studies, including JNJ7777120, the first highly selective H4 receptor antagonist developed (Jablonowski et al., 2003; Thurmond et al., 2004) and the potent selective agonist 4-methylhistamine (Lim et al., 2005), has provided useful information on the physiological role of histamine receptors in inflammatory conditions.
Airway inflammation and allergy
In inflammatory lung disorders, histamine acts as a mediator of both acute and chronic phases. Accumulating data support the function of histamine in cellular immunity through control of cytokine and chemokine production and migration of inflammatory cells, beyond its traditional role in mediating immediate airway hyper-responsiveness (Barnes et al., 1998). Although H1 receptor antagonists offer symptomatic relief in atopic nasal, conjunctival and skin disease, they are not optimally effective in asthma, where the contribution of each type of histamine receptor in histamine-mediated effects is currently subject to intense research. The H4 receptor is present in low amounts in the lung, where its expression in bronchial epithelial and smooth muscle cells and microvascular endothelial cells (Gantner et al., 2002) may contribute to the airway disease phenotypes in various ways.
The H4 receptor mediates redistribution and recruitment of MCs in the mucosal epithelium in response to allergens, thus amplifying allergic symptoms and maintaining chronic inflammation (Thurmond et al., 2004). Supportive evidence is derived from the H4 receptor-mediated synergistic sequential action of histamine and CXCL12, the chemokine that is constitutively expressed in skin and airway epithelium and plays a key role in allergic airway disorders, to induce migration of MC precursors in vitro (Godot et al., 2007). Moreover, the H4 receptor seems to regulate only locally MC redistribution in the oesophageal mucosal epithelium followed by infiltration of eosinophils in ovalbumin-challenged guinea pigs (Yu et al., 2008).
The reduced lung inflammation and the decreases in TH2 responses observed in H4 receptor-deficient (H4R−/−) mice and upon oral gavage administration of selective antagonists in a murine model of allergic airway inflammation documented the role of the H4 receptor in modulating TH2 allergic responses, by influencing CD4+ T cell activation attributed to decreased cytokine and chemokine production by DCs (Dunford et al., 2006). In another study, inhibition of airway resistance and inflammation, mediated through the recruitment of CD25+FoxP3+ T regulatory (Treg) cells was observed by using the selective H4 receptor agonist 4-methylhistamine administered intratracheally into the lungs of asthmatic mice (Morgan et al., 2007). The beneficial actions of both H4 agonists and antagonists observed in asthmatic mice were attributed to the local versus systemic administration of the compounds, respectively, and to the resulting concentration gradient within the lung in the former case that would allow the migration of the immune response suppressive Treg cells (Morgan et al., 2007).
Considering the role of the H4 receptor in the modulation of the asthmatic response revealed by these studies and the reported inhibitory effect of H4 receptor agonists on antigen-specific responses in human peripheral blood mononuclear cells and T cell lines, which, however, was not reversed by the H3/H4 receptor antagonist thioperamide (Sugata et al., 2007), additional data are a prerequisite imperative of providing conclusive evidence concerning the optimal therapeutic exploitation of H4 receptor ligands in chronic airway disease (Daugherty, 2004). Furthermore, species and strain differences should be considered carefully before data interpretation as murine DC chemotactic response needs very high histamine concentrations to be observed (Bäumer et al., 2008) and different cytokine secretion patterns are observed in H4R−/− mice and murine DCs (Dunford et al., 2006).
Chronic pruritus
Chronic pruritus is a common clinical condition associated with cutaneous or systemic disease and although histamine, MCs and basophils are increased in patients, classical H1 receptor antagonists are of limited use due to lack of effectiveness (O'Donoghue and Tharp, 2005). Histamine has been shown to induce scratching in Balb C mice via H4 receptors (Bell et al., 2004). Treatment of mice with JNJ7777120 attenuated the pruritic response to histamine, IgE and compound 48/80, the inhibitory effect of the H4 receptor antagonist being greater than that observed with H1 receptor antagonists (Dunford et al., 2007). Even so, H1 receptor-mediated actions seem to complement the H4 receptor-mediated enhanced DC migration through skin, and therefore H1 combined with H4 receptor antagonists could be a beneficial approach (Bäumer et al., 2008; Roßbach et al., 2009). In addition, although H4 receptor antagonism strongly attenuated the pruritic response to the classical contact allergen 2,4-dinitrochlorobenzene and to the respiratory chemical allergen toluene-2,4-diisocyanate, which mediate TH1- and TH2-dominated inflammation, respectively, JNJ7777120 failed to reduce the allergic inflammatory response associated with allergic dermatitis (Roßbach et al., 2009).
Although H4 receptors are expressed in human skin MCs (Lippert et al., 2004), inflammatory dendritic epidermal cells (Dijkstra et al., 2008) and dermal fibroblasts (Ikawa et al., 2008), the H4 receptor-mediated pruritus was suggested to be MC-independent and possibly coupled to actions on the peripheral terminals of sensory neurons rather than to effects on haemopoietic cells (Dunford et al., 2007). However, before extrapolating these results to human pathophysiology that may lead to misinterpretation of the complex regulatory MC-DC interactions, one should consider the pharmacological differences in agonist affinity between human and mouse H4 receptors. Results from functional studies in experimental animals need cautious interpretation as species differences certainly occur. Indeed, detailed site-directed mutagenesis data reported recently demonstrated Phe169 in the second extracellular loop as the single amino acid responsible for the differences in agonist affinity between the human and mouse H4 receptors (Lim et al., 2008). Moreover, strain differences in the mouse response to the H4 receptor agonist/H3 receptor antagonist clobenpropit were recently reported, NMRI mice being more susceptible to H4 receptor-induced itching compared with Balb C mice (Bäumer et al., 2008).
Autoimmune disorders
Histamine has been recognized to play a role in autoimmune diseases, including rheumatoid arthritis (RA) that is characterized mainly by synovial tissue inflammation leading to erosion and destruction of articular cartilage with subsequent joint deformity (Woolley and Tetlow, 1997). Although a recent report argued for the anti-inflammatory properties of histamine in RA (Adlesic et al., 2007), the amine has been largely regarded as a pro-inflammatory mediator in arthritic disease (Maslinska et al., 2004; Grzybowska-Kowalczyk et al., 2007; 2008; Ohki et al., 2007). Besides the presence of H1 and H2 receptors, the considerable variations in H4 receptor expression in human synovial cells could be related to RA severity and duration (Ikawa et al., 2005). The localization of H4 receptors in synovial and vascular wall cells of patients with RA (Grzybowska-Kowalczyk et al., 2007) and osteoarthritis (Grzybowska-Kowalczyk et al., 2008) and the identification of H4 receptors in fibroblast- and macrophage-like cells from RA synovial tissues (Ohki et al., 2007) further support the contribution of the receptor in the pathophysiology of the disease. Moreover, evidence for the systemic contribution of histamine in the arthritic phenotype was obtained by using a rat model of adjuvant arthritis, and a functional role of the H4 receptor in the normal cartilage has been suggested in the rat, raising attractive questions regarding the H4 receptor-mediated mechanisms in this tissue (Zampeli et al., 2008).
Other inflammatory conditions
The association of the H4 receptor with immune cell function motivated the intense investigation of its implication in virtually every condition that comprises an inflammatory component (Figure 3), mostly using either the mixed H3/H4 receptor antagonist thioperamide or selective H4 receptor agonists and antagonists (Zhang et al., 2007). Thus, H4 receptor agonists prevented the development of reperfusion injury following ischaemia-induced liver damage (Adachi et al., 2006). Beneficial actions of the H4 receptor antagonists have been reported in a rat model of subchronic colitis (Varga et al., 2005) and in MC-dependent mouse models of zymosan-induced pleurisy or peritonitis (Takeshita et al., 2003; Thurmond et al., 2004), used as an experimental model of acute inflammation involving neutrophil recruitment from the bone marrow (Takeshita et al., 2004).
On the other hand, H4 receptor antagonists exerted an inhibitory effect on airway inflammation (Dunford et al., 2006) and pruritic responses (Dunford et al., 2007) in MC-deficient mice but they were not effective in MC-independent mouse models of acute inflammation, such as histamine-induced paw oedema, thioglycollate-induced peritonitis (Thurmond et al., 2004) and carrageenan-induced neutrophilia (Takeshita et al., 2003). In a rat model of carrageenan-induced acute inflammation and pain, H4 receptor antagonists reduced the early phase development of oedema but not the late inflammatory responses and attenuated the hyperalgesic response to thermal stimuli, possibly acting through peripheral nociceptive pathways (Coruzzi et al., 2007).
In addition to the localization of H4 receptors in synovial specimens from RA patients (Ikawa et al., 2005; Grzybowska-Kowalczyk et al., 2007; 2008; Ohki et al., 2007), a limited number of studies have attempted to identify H4 receptor alterations in human samples derived from inflamed tissues. For instance, the increased levels of H4 receptors in normal nasal turbinate mucosa and in nasal polyp tissue and the tendency for correlation between the expression of H4 receptors and eosinophil cationic protein led to the speculation that the receptor may have a role in mediating histamine effects and eosinophil accumulation and activation in nasal and paranasal sinus mucosa inflammatory diseases (Jókúti et al., 2007).
These results certainly reflect the complex cellular and biochemical networks orchestrating inflammation. However, species differences in H4 receptor-mediated responses and/or ligand efficacy need to be elucidated before decisive conclusions are drawn regarding the use of H4 receptor ligands in the treatment of inflammatory human disease. In addition, yet unidentified receptor polymorphisms must also be taken in consideration. The first two alternatively spliced H4 receptor isoforms, which have a dominant negative effect on the full-length H4 receptor functionality by retaining it intracellularly and inactivating it presumably via hetero-oligomerization, have been cloned in CD34+ cord blood-cell-derived eosinophils and MCs (van Rijn et al., 2008).
Future perspectives
Although histamine has not been ignored by immunopharmacologists for nearly a century, the discovery of the H4 receptor offered to the amine a new perspective beyond its traditional pharmacological properties. The complexity of leukocyte immune surveillance, trafficking and recruitment and the plethora of different effects exerted by histamine through a repertoire of four receptor subtypes make hard to predict the overall effect of H4 receptors in inflammatory conditions at present. H4 receptor antagonists may be effective candidates in treating diseases associated with chronic pruritus and asthma, without disregarding the potential clinical application of H4 receptor ligands in autoimmune diseases like RA. A number of candidate drugs targeting the H4 receptor are in preclinical assessment for a range of inflammatory disorders; some showing promise for potential entry into clinical trials and even though the available data are variable in quality, they are adequate in quantity to justify an optimistic perspective for this new drug target.
Glossary
Abbreviations:
4-MH
4-methylhistamine
Ag
antigen
BM
bone marrow
DC
dendritic cell
GPCR
G-protein-coupled receptor
H4R−/−
H4 receptor-deficient mice
HL60.15
eosinophilic precursor cell line
IDEC
inflammatory dendritic epidermal cells
IFN
interferon
IL
interleukin
JNJ10191584
selective H4 receptor antagonist
JNJ7777120
selective H4 receptor antagonist
LPS
lipopolysaccharide
MC
mast cell
Mo-IDEC
monocyte-derived IDEC
PBMC
peripheral blood mononuclear cells
RA
rheumatoid arthritis
STAT
signal transduction and activator of transcription
TH
helper T cell
TNF-α
tumour necrosis factor-alpha
Treg
T regulatory cell
VUF6002
selective H4 receptor antagonist
Conflict of interest
The authors state no conflict of interest.
References
- Adachi N, Liu K, Motoki A, Nishibori M, Arai T. Suppression of ischemia/reperfusion liver injury by histamine H4 receptor stimulation in rats. Eur J Pharmacol. 2006;544:181–187. doi: 10.1016/j.ejphar.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Adlesic M, Verdrengh M, Bokarewa M, Dahlberg L, Foster SJ, Tarkowski A. Histamine in rheumatoid arthritis. Scand J Immunol. 2007;65:530–537. doi: 10.1111/j.1365-3083.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Autoinhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature (Lond) 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Barger G, Dale HH. The presence in ergot and physiological activity of B-iminazoylethylamine. J Physiol. 1910;40:38–40. [Google Scholar]
- Barnard R, Barnard A, Salmon G, Liu W, Sreckovic S. Histamine-induced actin polymerization in human eosinophils: an imaging approach for histamine H4 receptor. Cytometry A. 2008;73:299–304. doi: 10.1002/cyto.a.20514. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- Bäumer W, Wendorff S, Gutzmer R, Werfel T, Dijkstra D, Chazot P, et al. Histamine H4 receptors modulate dendritic cell migration through skin – immunomodulatory role of histamine. Allergy. 2008;63:1387–1394. doi: 10.1111/j.1398-9995.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in BalbC mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Duncan WAM, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H2-receptors. Nature (Lond) 1972;236:385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Buckland KF, Williams TJ, Conroy DM. Histamine induces cytoskeletal changes in human eosinophils via the H4 receptor. Br J Pharmacol. 2003;140:1117–1127. doi: 10.1038/sj.bjp.0705530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot PL, Tiligada E. The European Histamine Research Society (EHRS) symposium for EPHAR 2008. Inflamm Res. 2008;57:S05–S06. [Google Scholar]
- Clark RAF, Gallin JI, Kaplan AP. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975;142:1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogé F, Guénin SP, Rique H, Boutin JA, Galizzi JP. Structure and expression of the human histamine H4-receptor gene. Biochem Biophys Res Commun. 2001;284:301–309. doi: 10.1006/bbrc.2001.4976. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Adami M, Guaita E, de Esch IJ, Leurs R. Antiinflammatory and antinociceptive effects of the selective histamine H4-receptor antagonists JNJ7777120 and VUF6002 in a rat model of carrageenan-induced acute inflammation. Eur J Pharmacol. 2007;563:240–244. doi: 10.1016/j.ejphar.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Dale HH, Laidlaw PP. The physiological action of β-imidazolylethylamine. J Physiol. 1910;41:318–341. doi: 10.1113/jphysiol.1910.sp001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj BB, Becerra CB, Esber HJ, Wen Y, Maghazachi AA. Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. J Immunol. 2007;179:7907–7915. doi: 10.4049/jimmunol.179.11.7907. [DOI] [PubMed] [Google Scholar]
- Daugherty BL. Histamine H4 antagonism: a therapy for chronic allergy? Br J Pharmacol. 2004;142:5–7. doi: 10.1038/sj.bjp.0705730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra D, Leurs R, Chazot P, Shenton FC, Stark H, Werfel T, et al. Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J Allergy Clin Immunol. 2007;120:300–307. doi: 10.1016/j.jaci.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Dijkstra D, Stark H, Chazot PL, Shenton FC, Leurs R, Werfel T, et al. Human inflammatory dendritic epidermal cells express a functional histamine H4 receptor. J Invest Dermatol. 2008;128:1696–1703. doi: 10.1038/sj.jid.5701250. [DOI] [PubMed] [Google Scholar]
- Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 2004;15:393–410. doi: 10.1016/j.cytogfr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- de Esch IJP, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci. 2005;26:462–469. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol. 2006;15:855–864. doi: 10.1111/j.1600-0625.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Falus A, Hegyesi H, Lazar-Molnar E, Pos Z, Laszlo V, Darvas Z. Paracrine and autocrine interactions in melanoma: histamine is a relevant player in local regulation. Trends Immunol. 2001;22:648–652. doi: 10.1016/s1471-4906(01)02050-6. [DOI] [PubMed] [Google Scholar]
- Gantner F, Sakai K, Tusche MW, Cruikshank WW, Center DM, Bacon KB. Histamine H4 and H2 receptors control histamine-induced interleukin-16 release from human CD8+ T cells. J Pharmacol Exp Ther. 2002;303:300–307. doi: 10.1124/jpet.102.036939. [DOI] [PubMed] [Google Scholar]
- Godot V, Arock M, Garcia G, Capel F, Flys C, Dy M, et al. H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. J Allergy Clin Immunol. 2007;120:827–834. doi: 10.1016/j.jaci.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Grandi D, Shenton FC, Chazot PL, Morini G. Immunolocalization of histamine H3 receptors on endocrine cells in the rat gastrointestinal tract. Histol Histopathol. 2008;23:789–798. doi: 10.14670/HH-23.789. [DOI] [PubMed] [Google Scholar]
- Grzybowska-Kowalczyk A, Wojtecka-Lukasik E, Maslinska D, Gujski M, Maslinski S. Distribution pattern of histamine H4 receptor in human synovial tissue from patients with rheumatoid arthritis. Inflamm Res. 2007;56(Suppl.)(1):S59–S60. doi: 10.1007/s00011-006-0529-3. [DOI] [PubMed] [Google Scholar]
- Grzybowska-Kowalczyk A, Maslinska D, Wojciechowska M, Szukiewicz D, Wojtecka-Lukasik E, Paradowska A, et al. Expression of histamine H4 receptor in human osteoarthritic synovial tissue. Inflamm Res. 2008;57(Suppl.)(1):S63–S64. doi: 10.1007/s00011-007-0631-1. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittman M, et al. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- Hirasawa N, Ohsawa Y, Katoh G, Shibata K, Ishihara K, Seyama T, et al. Modification of the picryl chloride-induced allergic dermatitis model in mouse ear lobes by 12-o-tetradecanoylphorbol 13-acetate, and analysis of the role of histamine in the modified model. Int Arch Allergy Immunol. 2008;148:279–288. doi: 10.1159/000170381. [DOI] [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- Horr B, Borck H, Thurmond R, Grösch S, Diel F. STAT1 phosphorylation and cleavage is regulated by the histamine H4 receptor in human atopic and non-atopic lymphocytes. Int Immunopharmacol. 2006;6:1577–1585. doi: 10.1016/j.intimp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Ikawa Y, Suzuki M, Shiono S, Ohki E, Moriya H, Negishi E, et al. Histamine H4 receptor expression in human synovial cells obtained from patients suffering from rheumatoid arthritis. Biol Pharm Bull. 2005;28:2016–2018. doi: 10.1248/bpb.28.2016. [DOI] [PubMed] [Google Scholar]
- Ikawa Y, Shiba K, Ohki E, Mutoh N, Suzuki M, Sato H, et al. Comparative study of histamine H4 receptor expression in human dermal fibroblasts. J Toxicol Sci. 2008;33:503–508. doi: 10.2131/jts.33.503. [DOI] [PubMed] [Google Scholar]
- Jablonowski JA, Grice CA, Chai W, Dvorak CA, Venable JD, Kwok AK, et al. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- Jiang W, Lim HD, Zhang M, Desai P, Dai H, Colling PM, et al. Cloning and pharmacological characterization of the dog histamine H4 receptor. Eur J Pharmacol. 2008;592:26–32. doi: 10.1016/j.ejphar.2008.06.095. [DOI] [PubMed] [Google Scholar]
- Jókúti A, Hellinger E, Hellinger A, Darvas Z, Falus A, Thurmond RL, et al. Histamine H4 receptor expression is elevated in human nasal polyp tissue. Cell Biol Int. 2007;31:1367–1370. doi: 10.1016/j.cellbi.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, et al. Histamine regulated T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- Kakavas S, Zampeli E, Papamichael K, Delitheos B, Tiligada E. The mast cell pathway to inflammation and homeostasis: pharmacological insights. Anti-Inflamm Anti-Allergy Agents Med Chem. 2006;5:323–334. [Google Scholar]
- Kharmate G, Liu Z, Patterson E, Khan MM. Histamine affects STAT6 phosphorylation via its effects on IL-4 secretion: role of H1 receptors in the regulation of IL-4 production. Int Immunopharmacol. 2007;7:277–286. doi: 10.1016/j.intimp.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss R, Kiss B, Könczöl A, Szalai F, Jelinek I, László V, et al. Discovery of novel human histamine H4 receptor ligands by large-scale structure-based virtual screening. J Med Chem. 2008;51:3145–3153. doi: 10.1021/jm7014777. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G, Ajitawi O, Chi DS. The human mast cell: an overview. Methods Mol Biol. 2006;315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- Leurs R, Hoffmann M, Wieland K, Timmerman H. H3 receptor gene is cloned at last. Trends Pharmacol Sci. 2000;21:11–12. doi: 10.1016/s0165-6147(99)01411-x. [DOI] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, et al. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Lim HD, Jongejan A, Bakker RA, Haaksma E, de Esch IJ, Leurs R. Phenylalanine 169 in the second extracellular loop of the human histamine H4 receptor is responsible for the difference in agonist binding between human and mouse H4 receptors. J Pharmacol Exp Ther. 2008;327:88–96. doi: 10.1124/jpet.108.140343. [DOI] [PubMed] [Google Scholar]
- Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004;142:161–171. doi: 10.1038/sj.bjp.0705729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert U, Artuc M, Grutzkau A, Babina M, Guhl S, Haase I, et al. Human skin mast cells express H2 and H4, but not H3 receptors. J Invest Dermatol. 2004;23:116–123. doi: 10.1111/j.0022-202X.2004.22721.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Ma XJ, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, et al. Cloning and pharmalogical characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol. 2001a;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- Liu C, Wilson SJ, Kuel C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001b;299:121–130. [PubMed] [Google Scholar]
- Liu Z, Kharmate G, Patterson E, Khan MM. Role of H1 receptors in histamine-mediated up-regulation of STAT4 phosphorylation. Int Immunopharmacol. 2006;6:485–493. doi: 10.1016/j.intimp.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Masini E, Di Bello MG, Raspanti S, Fomusi Ndisang J, Baronti R, Cappugi P, et al. The role of histamine in platelet aggregation by physiological and immunological stimuli. Inflamm Res. 1998;47:211–220. doi: 10.1007/s000110050319. [DOI] [PubMed] [Google Scholar]
- Maslinska D, Gujski M, Laure-Kamionowska M, Szukiewicz D, Wojtecka-Lukasik E. Subcellular localization of histamine in articular cartilage chondrocytes of rheumatoid arthritis patients. Inflamm Res. 2004;53:S35–36. doi: 10.1007/s00011-003-0316-3. [DOI] [PubMed] [Google Scholar]
- Michel I, Borck H, McElligott S, Krieg C, Diel F. Histamine receptor H4R-selective ligands influence the STAT6 Transcription Activation Domain (TAD) and the DNA-binding. Inflamm Res. 2008;57:S47–S48. doi: 10.1007/s00011-007-0623-1. [DOI] [PubMed] [Google Scholar]
- Morgan RK, McAllister B, Cross L, Green DS, Kornfeld H, Center DM, et al. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol. 2007;178:8081–8089. doi: 10.4049/jimmunol.178.12.8081. [DOI] [PubMed] [Google Scholar]
- Morse KL, Behan J, Laz TM, West RE, Jr, Greenfeder SA, Anthes JC, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther. 2001;296:1058–1066. [PubMed] [Google Scholar]
- Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279:615–620. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Kato Y, Hieshima K, Nagakubo D, Kunori Y, Fujisawa T, et al. Liver-expressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. J Immunol. 2004;173:2078–2083. doi: 10.4049/jimmunol.173.3.2078. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, et al. Discovery of a novel member of the histamine receptor family. Mol Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- O'Donoghue M, Tharp MD. Antihistamines and their role as antipruritics. Dermatol Ther. 2005;18:333–340. doi: 10.1111/j.1529-8019.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- O'Reilly M, Alpert R, Jenkinson S, Gladue RP, Foo S, Trim S, et al. Identification of a histamine H4 receptor on human eosinophils-role in eosinophil chemotaxis. J Recept Signal Transduct Res. 2002;22:431–448. doi: 10.1081/rrs-120014612. [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Oda T, Matsumoto S, Matsumoto M, Takasaki J, Kamohara M, Soga T, et al. Molecular cloning of monkey histamine H4 receptor. J Pharmacol Sci. 2005;98:319–322. doi: 10.1254/jphs.sc0050033. [DOI] [PubMed] [Google Scholar]
- Ohki E, Suzuki M, Aoe T, Ikawa Y, Negishi E, Ueno K. Expression of histamine H4 receptor in synovial cells from rheumatoid arthritic patients. Biol Pharm Bull. 2007;30:2217–2220. doi: 10.1248/bpb.30.2217. [DOI] [PubMed] [Google Scholar]
- Prinz C, Zanner R, Gratzl M. Physiology of gastric enterochromaffin-like cells. Annu Rev Physiol. 2003;65:371–382. doi: 10.1146/annurev.physiol.65.092101.142205. [DOI] [PubMed] [Google Scholar]
- Raible DG, Lenahan T, Fayvilevich Y, Kosinski R, Schulman ES. Pharmacologic characterization of a novel histamine receptor on human eosinophils. Am J Respir Crit Care Med. 1994;149:1506–1511. doi: 10.1164/ajrccm.149.6.8004306. [DOI] [PubMed] [Google Scholar]
- Riley JF, West GB. Histamine in tissue mast cells. J Physiol. 1952;117:72–73. [PubMed] [Google Scholar]
- van Rijn RM, van Marle A, Chazot PL, Langemeijer E, Qin Y, Shenton FC, et al. Cloning and characterization of dominant negative splice variants of the human histamine H4 receptor. Biochem J. 2008;414:121–131. doi: 10.1042/BJ20071583. [DOI] [PubMed] [Google Scholar]
- Roßbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T, et al. Histamine H4 receptor antagonism reduces hapten-induced scratching behaviour but not inflammation. Exp Dermatol. 2009;18:57–63. doi: 10.1111/j.1600-0625.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- Sugata Y, Okano M, Fujiwara T, Matsumoto R, Hattori H, Yamamoto M, et al. Histamine H4 receptor agonists have more activities than H4 agonism in antigen-specific human T-cell responses. Immunology. 2007;121:266–275. doi: 10.1111/j.1365-2567.2007.02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Sakai K, Bacon KB, Gantner F. Critical role of histamine H4 receptor in leukotriene B4 production and mast cell dependent neutrophil recruitment induced by zymosan in vivo. J Pharmacol Exp Ther. 2003;307:1072–1078. doi: 10.1124/jpet.103.057489. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Bacon KB, Gantner F. Critical role of L-selectin and histamine H4 receptor in zymosan-induced neutrophil recruitment from the bone marrow: comparison with carrageenan. J Pharmacol Exp Ther. 2004;310:272–280. doi: 10.1124/jpet.103.063776. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung WP, Hofstra CL, Jiang W, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;1:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- Varga C, Horvath K, Berko A, Thurmond RL, Dunford PJ, Whittle BJ. Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat. Eur J Pharmacol. 2005;522:130–138. doi: 10.1016/j.ejphar.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Venable JD, Thurmond RL. Development and chemistry of histamine H4 receptor ligands as potential modulators of inflammatory and allergic responses. Anti-Inflamm Anti-Allergy Agents Med Chem. 2006;5:307–322. [Google Scholar]
- Woolley DE, Tetlow LC. Observations on the microenvironmental nature of cartilage destruction in rheumatoid arthritis. Ann Rheum Dis. 1997;56:151–161. doi: 10.1136/ard.56.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Stahl E, Li Q, Ouyang A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sci. 2008;82:324–330. doi: 10.1016/j.lfs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Zampeli E, Thurmond RL, Tiligada E. Effect of the H4R antagonist JNJ7777120 on the cartilage histamine content in rats with adjuvant arthritis. Fund Clin Pharmacol. 2008;22(Suppl.)(2):10. [Google Scholar]
- Zampeli E, Thurmond RL, Tiligada E. The histamine H4 receptor antagonist JNJ7777120 induces increases in the histamine content of the rat conjunctiva. Inflamm Res. 2009;58:1–7. doi: 10.1007/s00011-009-8245-4. [DOI] [PubMed] [Google Scholar]
- Zhang M, Thurmond RL, Dunford PJ. The histamine H4 receptor: a novel modulator of inflammatory and immune disorders. Pharmacol Ther. 2007;113:594–606. doi: 10.1016/j.pharmthera.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Michalovich D, Wu H, Tan KB, Dytko GM, Mannan IJ, et al. Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol Pharmacol. 2001;59:434–441. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]
- Zwadlo-Klarwasser G, Braam U, Mühl-Zürbes P, Schmutzler W. Macrophages and lymphocytes: alternative sources of histamine. Agents Actions. 1994;41:C99–C100. doi: 10.1007/BF02007785. [DOI] [PubMed] [Google Scholar]