Different Fecal Microbiotas and Volatile Organic Compounds in Treated and Untreated Children with Celiac Disease (original) (raw)
Abstract
This study aimed at investigating the fecal microbiotas of children with celiac disease (CD) before (U-CD) and after (T-CD) they were fed a gluten-free diet and of healthy children (HC). Brothers or sisters of T-CD were enrolled as HC. Each group consisted of seven children. PCR-denaturing gradient gel electrophoresis (DGGE) analysis with V3 universal primers revealed a unique profile for each fecal sample. PCR-DGGE analysis with group- or genus-specific 16S rRNA gene primers showed that the Lactobacillus community of U-CD changed significantly, while the diversity of the Lactobacillus community of T-CD was quite comparable to that of HC. Compared to HC, the ratio of cultivable lactic acid bacteria and Bifidobacterium to Bacteroides and enterobacteria was lower in T-CD and even lower in U-CD. The percentages of strains identified as lactobacilli differed as follows: HC (ca. 38%) > T-CD (ca. 17%) > U-CD (ca. 10%). Lactobacillus brevis, Lactobacillus rossiae, and Lactobacillus pentosus were identified only in fecal samples from T-CD and HC. Lactobacillus fermentum, Lactobacillus delbrueckii subsp. bulgaricus, and Lactobacillus gasseri were identified only in several fecal samples from HC. Compared to HC, the composition of Bifidobacterium species of T-CD varied, and it varied even more for U-CD. Forty-seven volatile organic compounds (VOCs) belonging to different chemical classes were identified using gas-chromatography mass spectrometry-solid-phase microextraction analysis. The median concentrations varied markedly for HC, T-CD, and U-CD. Overall, the _r_2 values for VOC data for brothers and sisters were equal to or lower than those for unrelated HC and T-CD. This study shows the effect of CD pathology on the fecal microbiotas of children.
The human gastrointestinal (GI) tract is a complex ecosystem containing up to 1014 total bacteria. These microorganisms belong to more than 500 different bacterial species, even though 99% of the total community consists of only 30 to 40 species. The GI microbiota plays a key role in health and disease (11, 23). The health effects are direct, due to a stable microbiota resistant to incoming potential pathogenic microbes, and/or indirect, due to cross talk with the gut-associated lymphoid tissue (1). The GI microbiota metabolizes nutrients and activates both innate and adaptive immunity (27). Specific strains of the GI microbiota and/or supplied probiotics decrease intestinal inflammation and normalize dysfunction of the GI mucosa (39). The GI microbiota is also involved in the pathogenesis of chronic inflammatory bowel diseases (IBD) and other immunity-related disorders (42). Overall, IBD patients have altered densities of mucosa-associated bacteria compared to healthy subjects. In particular, the numbers of protective Bifidobacterium and Lactobacillus cells are lower, while the numbers of harmful Bacteroides and Escherichia coli cells are higher (42). Recently, microbial infections and especially imbalances in the composition of the GI microbiota were associated with the presentation of celiac disease (CD) (3, 41).
CD is an inflammatory disorder of the small intestine that affects genetically predisposed individuals when they ingest gluten from any Triticum species and similar proteins of barley and rye and their crossbred varieties. CD is associated with maldigestion and malabsorption of nutrients, vitamins, and minerals. Epidemiological studies in Europe and the United States have indicated that the incidence of CD is increasing and that CD affects approximately 1% of the general population (31). The pathogenesis of CD involves interactions between genetic, immunological, and environmental factors. HLA-DQ2/DQ8 molecules of antigen-presenting cells bind and present gluten peptides to the lamina propria of CD4+ T cells, triggering a T helper 1-based immune response, along with the synthesis of gamma interferon. Frequently, gamma interferon enhances the synthesis of tumor necrosis factor alpha and plays a crucial role in damaging the intestinal mucosa (3, 29). Events leading to CD also involve activation of the innate immunity mediated by interleukin-15 and are characterized by expansion of intraepithelial T-cell receptor γ/δ+ and CD8+ T-cell receptor α/β+ lymphocytes. These lymphocytes are cytotoxic and also contribute to tissue damage (10). Currently, the only treatment for CD consists of a life-long gluten-free diet. Complete removal of gluten from the diet of CD patients results in symptomatic, serological, and histological remission in the majority of cases (32). With strict dietary control, antibody levels may revert to normal levels within 6 to 12 months, while complete histological resolution may take up to 2 years (31). Nevertheless, in some pathological conditions, such as lactose malabsorption and refractory sprue, symptoms may persist. Such pathological conditions are also characterized by small intestine bacterial overgrowth (44).
The role of bacteria during development and treatment of CD should be elucidated (29, 38). The inflammatory milieu caused by gluten antigens could lead to imbalances in the GI microbiota of CD patients. Compared to healthy individuals, CD patients seem to be characterized by higher numbers of gram-negative bacteria and lower numbers gram-positive bacteria (3). Overall, gram-negative bacteria could activate proinflammatory pathways, while gram-positive bacteria, such as lactic acid bacteria and bifidobacteria, could inhibit toxic effects induced by other GI species (26) or gluten antigens (5, 19).
This study aimed at investigating the differences in the fecal microbiota between treated children with CD (T-CD), untreated children with CD (U-CD), and healthy children (HC). HC belonging to the same family unit as T-CD were enrolled to avoid interference due to genetic factors and dietary components. The fecal microbiota was characterized by using an integrate approach involving culture-independent and culture-dependent methods and analysis of the fecal volatile organic compounds (VOCs).
MATERIALS AND METHODS
Subjects.
The following three groups of children, who were between 6 and 12 years of age, were included in this study: (i) seven symptom-free CD patients who had been fed a gluten-free diet for at least 2 years (T-CD) (children 1, 4, 9, 10, 13, 14, and 17); (ii) seven CD patients who had been fed a gluten-containing diet, showed clinical symptoms of the disease, and had positive celiac serology markers and signs of severe enteropathy as determined by duodenal biopsy examination (U-CD) (children 5, 6, 7, 18, 19, 20, and 22); and (iii) seven children with no known food intolerance (HC) (children 2, 3, 11, 12, 15, 16, and 21). Pathology was diagnosed using criteria provided by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The HC group was included to examine any impact of host genetics, environment, and/or lifestyle. All children in the HC group were brothers and sisters of T-CD. The pairs of T-CD and HC were children 1 and 2, 4 and 3, 9 and 11, 10 and 12, 13 and 21, 14 and 15, and 17 and 16. Since each of these pairs of children belonged to the same family unit, the major dietary difference was a difference in gluten. Children included in the study were not treated with antibiotics, functional foods (probiotics and/or prebiotics), fermented milk, or yoghurt for at least 1 month before samples were obtained. Children were enrolled in the study after written informed consent was obtained both from the parents and the institutional ethics committee of the Faculty of Medicine and Surgery of the University of Bari.
Collection of fecal samples.
Each child provided three fecal samples over time. After collection, feces (ca. 15 g), in a sterile plastic box, were immediately mixed (1:1, wt/wt) with Amies transport medium (Oxoid Ltd., Basingstoke, Hampshire, England) under anaerobic conditions (AnaeroGen; Oxoid Ltd.). Samples were immediately subjected to analysis for plate counting or frozen at −80°C for DNA extraction.
Extraction of DNA from fecal samples.
An aliquot (about 300 μl) of each fecal slurry sample was diluted in 1 ml of phosphate-buffered saline (PBS) (0.01 M, pH 7.2) containing 0.01 M EDTA (PBS-EDTA). After centrifugation (14,000 × g at 4°C for 5 min), the pellet was washed two times to reduce the content of PCR inhibitors. The resulting pellet was resuspended in 300 μl of PBS-EDTA and used for DNA extraction (2) with a FastDNA Pro Soil-Direct kit (MP Biomedicals, California) and a FastPrep instrument (Bio 101) used according to the manufacturer's instructions. The final product was 100 μl of application-ready DNA (21). The quality and concentration of DNA extracts were determined using 0.7% agarose—0.5× Tris-borate-EDTA gels stained with ethidium bromide and by spectrophotometric measurement at 260, 280, and 230 nm using a NanoDrop ND-1000 spectrophotometer (ThermoFisher Scientific Inc., Milan, Italy).
PCR amplification and denaturing gel electrophoresis (DGGE) analysis.
Eubacterial universal and group-specific 16S rRNA gene primer sets were used for PCR assays with DNA extracted from fecal samples. All primers used in this study are listed in Table 1. The forward or reverse primer in each set was extended with a 40-mer GC clamp at the 5′ end to separate the PCR products in the gradient gel (28). The specificity of each primer pair was experimentally tested by using DNA extracted from the following bacteria: Bacteroides fragilis DSM 2151, Bifidobacterium bifidum DSM 20082, Lactobacillus plantarum ATCC 14917, Weissella confusa DSM 2196, Pediococcus pentosoceus DSM 20336, Leuconostoc lactis DSM 20202, Enterococcus durans DSM 20633, Enterococcus faecium DSM 2918, Clostridium coccoides DSM 935, Staphylococcus aureus DSM 20714, Enterobacter aerogenes DSM 30053, E. coli DSM 30083, and Yersinia enterocolitica DSM 4780. Each primer set gave positive PCR results for the corresponding target bacteria and did not cross-react with any of the nontarget microorganisms.
TABLE 1.
Primers and conditions used for DGGE analysis
| Primer | Sequence (5′-3′)a | Amplicon size (bp) | Annealing temp (°C) | DGGE gradient (%) | Target group | Reference |
|---|---|---|---|---|---|---|
| F357-GC (V3 region) | GC clamp-TACGGGAGGCAGCAG | 217 | 55 | 25-50 | Eubacteria | 40 |
| R518 (V3 region) | ATTACCGCGGCTGCTGG | |||||
| F968-GC (V6-V8 region) | GC clamp-AACGCGAAGAACCT | 489 | 55 | 40-60 | Eubacteria | 24 |
| R1401(V6-V8 region) | CGGTGTGTACAAGACCC | |||||
| Lac1 | AGCAGTAGGGAATCTTCCA | 380 | 61 | 35-50 | Lactobacillus groupb | 33 |
| Lac2GC | GC clamp-ATTYCACCGCTACACATG | |||||
| g-Bifid F | CTCCTGGAAACGGGTGG | 596 | 65 | 45-65 | Bifidobacterium | 18 |
| g-Bifid R-GC | GC clamp-GGTGTTCTTCCCGATATCTACA | |||||
| Ent. 1017F | CCTTTGACCACTCTAGAG | 300 | 62 | 45-55 | Enterococcus | 45 |
| Ent. 1263R-GC | GC clamp- CTTAGCCTCGCGACT | |||||
| g-Ccoc F | AAATGACGGTACCTGACTAA | 440 | 50 | 40-55 | Clostridium | 24 |
| g-Ccoc R | GC clamp-CTTTGAGTTTCATTCTTGCGAA | |||||
| Bact. 596F | TCAGTTGTGAAAGTTTGCG | 287 | 60 | 35-50 | Bacteroides | 45 |
| Bact. 826R-GC | GC clamp-GTRTATCGCMAACAGCGA |
Each PCR mixture (buffered final volume, 50 μl) contained 50 ng of template DNA, 50 pmol of each primer, 10 nmol of each 2′-deoxynucleoside 5′-triphosphate, 3 U of Taq DNA polymerase (EuroTaq; EuroClone, Italy), and 2.5 mM MgCl2. One PCR core program was used for all primer pairs: initial denaturation at 95°C for 3 min; 30 cycles of denaturation at 95°C for 20s, annealing at a primer-specific temperature (Table 1) for 45 s, and extension at 72°C for 1 min; and a final extension at 72°C for 7 min. PCR amplification products were checked by electrophoresis in 1.5% agarose ethidium bromide-stained gels and stored at −20°C.
Amplicons were separated by DGGE using the Bio-Rad DCode universal mutation detection system (Bio-Rad Laboratories, Hercules, CA). Different denaturing gradient conditions were used depending on the amplified target sequence (Table 1). Sybr green I-stained gels were photographed and acquired with the Bio-Rad Gel Doc 2000 documentation system (Bio-Rad Laboratories). In order to compensate for internal distortions occurring during electrophoresis, binding patterns were digitally aligned by comparison with an external reference pattern (low-range DNA ladder; Fermentas International Inc., Canada) using the Bionumerics software (version 4.5; Applied Maths, Belgium). Comparison and clustering of profiles were carried out using the unweighted-pair group method with the arithmetic average (UPGMA) clustering algorithm based on the Pearson product-moment correlation coefficient (r) (34, 45) and resulted in a distance matrix.
Enumeration of cultivable bacteria.
Diluted fecal samples (20 g) were mixed with 80 ml sterilized peptone water and homogenized. Counting of viable bacterial cells was carried out as described by Macfarlane et al. (21, 22). The following selective media were used: MRS agar (lactobacilli), Beerens agar (bifidobacteria), Baird-Parker medium (staphylococci and micrococci), blood azide agar (enterococci), Wilkins-Chalgren agar (total anaerobes), Wilkins-Chalgren agar plus GN selective supplements (Bacteroides, Porphyromonas, and Prevotella), reinforced clostridial medium supplemented with 8 mg liter−1 novobiocin and 8 mg l−1 colistin (Clostridium), MacConkey agar no. 2 (enterobacteria), and nutrient agar (total anaerobes) (13). All media were purchased from Oxoid Ltd. (Hampshire, England).
Isolation of lactic acid bacteria and bifidobacteria.
Fifteen to 20 colonies of presumptive lactic acid bacteria were isolated from the highest plate dilutions on MRS agar and blood azide agar. Gram-positive, catalase-negative, nonmotile rods and cocci were cultivated in MRS or blood azide broth (Oxoid Ltd.) at 30, 37, or 42°C for 24 h and restreaked into the same media containing agar.
Presumptive bifidobacteria were isolated from the highest plate dilutions of Beerens agar medium. Fifteen to 20 colonies from T-CD, U-CD, or HC fecal samples were picked and cultivated in Beerens broth at 37°C for 24 h under anaerobic conditions. To confirm that isolates presumptively belong to the genus Bifidobacterium, cell morphology and Gram staining were used. All isolates considered for further analyses showed the capacity to acidify the liquid culture medium. All cultures were stored at −80°C in 10% (vol/vol) glycerol.
DNA extraction and molecular identification by 16S rRNA, pheS, and recA gene sequencing.
Total DNAs of presumptive isolates of lactic acid bacteria and bifidobacteria were extracted from 2-ml samples of overnight cultures grown at 37°C in MRS, blood azide, or Beerens broth. Total DNAs were obtained as described by De Los Reyes-Gavilán et al. (8). The concentration and purity of DNA were assessed by using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific Inc.). The LpigF/LpigR primer pair (Invitrogen Life Technologies, Milan, Italy) (5′-TACGGGAGGCAGCAGTAG-3′ and 5′-CATGGTGTGACGGGCGGT-3′) (6), corresponding to positions 369 to 386 and 1424 to 1441, respectively, of the 16S rRNA gene sequence of Lactobacillus mucosae (accession number AF126738), was used to amplify the 16S rRNA gene fragment of presumptive lactic acid bacteria. Species- and group-specific primers targeted to 16S rRNA of human intestinal bifidobacteria were used to identify presumptive bifidobacterial isolates as described by Kok et al. (15) and Matsuki et al. (25). Each PCR mixture (50 μl) contained 200 μM of each 2′-deoxynucleoside 5′-triphosphate, 1 μM of both the forward and reverse primers, 2 mM MgCl2, 2 U of Taq DNA polymerase (Invitrogen Life Technologies) in the supplied buffer, and approximately 50 ng of DNA. PCR amplification was performed using a GeneAmp 9700 PCR system thermal cycler (Applied Biosystems, United States). PCR products were separated by electrophoresis on a 1.5% (wt/vol) agarose gel (Gibco BRL, France) stained with ethidium bromide (0.5 μg ml−1). The amplicons were eluted from the gel and purified with a GFX PCR DNA and gel band purification kit (GE Healthcare Life Sciences, Milan, Italy). DNA sequencing reactions were performed by MWG Biotech AG (Ebersberg, Germany) using both forward and reverse primers. Taxonomic identification of strains was performed by comparing the sequences of each isolate with those reported in the basic BLAST database (http://www.ncbi.nlm.nih.gov).
Primers casei and para were used to discriminate between the species Lactobacillus casei, Lactobacillus paracasei, and Lactobacillus rhamnosus (49). Primers pheS-21-F and pheS-23-R were used to identify Enterococcus species (30). Primers designed for the recA gene were also used to discriminate between the species L. plantarum, Lactobacillus pentosus, and Lactobacillus paraplantarum. Part of the recA gene was amplified using the degenerate primers (MWG Biotech AG, Ebersberg, Germany) recALb1F (5′-CRRTBATGCGBATGGGYG-3′) and recALb1R (5′-CGRCCYTGWCCAATSCGRTC-3′) derived from the homologous regions of the recA gene sequences of L. plantarum (accession no. AJ621668).
PCRs and separation, purification, and sequencing of amplicons were carried out as described above for 16S rRNA gene.
Genotypic characterization by RAPD-PCR analysis.
Genomic DNA was extracted from each isolate as described above. Three oligonucleotides, P4 (5′-CCGCAGCGTT-3′), P7 (5′-AGCAGCGTGG-3′), and M13 (5′-GAGGGTGGCGGTTCT-3′) (7), with arbitrarily chosen sequences were used for isolate biotyping. The reaction mixture and PCR conditions used for primers P4, P7, and M13 were those described by De Angelis et al. (7). PCR products (15 μl) were separated by electrophoresis at 100 V for 200 min on a 1.5% (wt/vol) agarose gel, and DNA was detected by UV transillumination after staining with ethidium bromide (0.5 μg ml−1). The molecular sizes of the amplified DNA fragments were estimated by comparison with 1-kb DNA molecular size markers (Invitrogen Life Technologies). Randomly amplified polymorphic DNA-PCR (RAPD-PCR) analysis profiles were acquired by using the Gel Doc EQ system (Bio-Rad Laboratories) and were compared using Fingerprinting II Informatix software (Bio-Rad). The similarity of the electrophoretic profiles was evaluated by determining the Dice coefficients of similarity and by using the UPGMA method.
Gas chromatography-mass spectrometry-solid-phase microextraction analysis of fecal VOCs.
After preconditioning according to the manufacturer's instructions, a carboxen-polydimethylsiloxane-coated fiber (85 μm) and a manual solid-phase microextraction holder (Supelco Inc., Bellefonte, PA) were used. Before headspace sampling, the fiber was exposed to a gas chromatograph inlet for 5 min for thermal desorption at 250°C. Three grams of fecal sample was placed into 10-ml glass vials, and of 10 μl of 4-methyl-2-pentanol (final concentration, 4 mg liter−1) was added as the internal standard. Samples were then equilibrated for 10 min at 45°C. The solid-phase microextraction fiber was exposed to each sample for 40 min. Both equilibration and absorption phases were carried out with stirring. The fiber was then inserted into the injection port of the gas chromatograph for 5 min of sample desorption. Gas chromatography-mass spectrometry analyses were carried out with an Agilent 7890A gas chromatograph (Agilent Technologies, Palo Alto, CA) coupled to an Agilent 5975C mass selective detector operating in electron impact mode (ionization voltage, 70 eV). A Supelcowax 10 capillary column (length, 60 m; inside diameter, 0.32 mm; Supelco, Bellefonte, PA) was used. The temperature program was 50°C for 1 min, followed by an increase at a rate of 4.5°C min−1 to 65°C, an increase at a rate of 10°C min−1 to 230°C, and then 230°C for 25 min. The injector, interface, and ion source temperatures were 250, 250, and 230°C, respectively. The mass-to-charge ratio interval was 30 to 350 Da at a rate of 2.9 scans per s. Injection was carried out in splitless mode, and helium (flow rate, 1 ml min−1) was used as the carrier gas. Molecules were identified based on comparison of their retention times with those of pure compounds (Sigma-Aldrich, Milan, Italy). Identities were confirmed by searching mass spectra in the available databases (NIST, version 2005; Wiley, version 1996) and a previous paper (9). Quantitative data for the compounds identified were obtained by interpolation of the relative areas versus the internal standard area. All data were obtained at least in triplicate. Analysis of variance was carried out with transformed data, followed by separation of means with Tukey's honestly significant difference test using the statistical software Statistica 6.0 per Windows 1998 (StatSoft, Vigonza, Italy). Significantly different groups (P < 0.05) as determined by Tukey's test are indicated below. Principal component analysis (PCA) of median values for VOCs was carried out using Statistica 6.0 per Windows 1998 (StatSoft, Vigonza, Italy).
RESULTS
Molecular analysis of the bacterial community.
Fecal samples were studied by performing a PCR-DGGE analysis, using the 16S rRNA gene as the target molecule. The predominant fecal microbiota was analyzed by using two universal primer sets targeting the V3 and the V6-V8 regions. In addition, a set of group-specific primers was used to obtain a more in-depth view of the following subpopulations: the Bacteroides fragilis subgroup, the genus Bifidobacterium, Lactobacillus spp., and the genus Enterococcus.
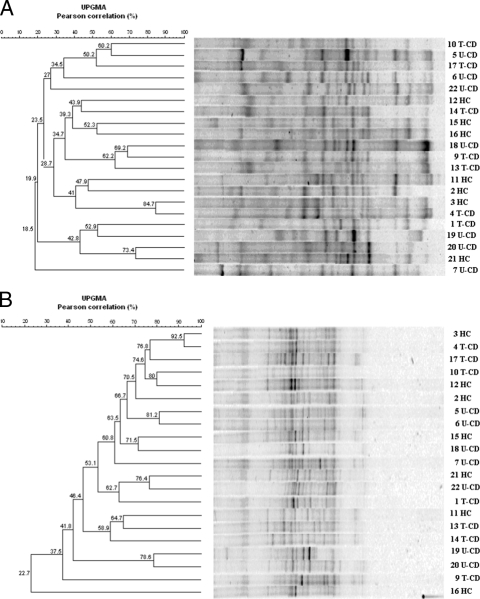
As shown in Fig. 1A, the DGGE profiles obtained with the V3 universal primers were complex and characteristic for each of the 21 children; only some common bands were present in different lanes. Although the fingerprints appeared contain many well-resolved and strong bands, some regions of the gel contained unresolved bands or very weak separate fragments. The uniqueness of the patterns was confirmed by cluster analysis; the Pearson similarity values for dendrograms almost always were low (r < 60%), the mean similarity coefficient was 45.3%, and there was no clear cluster grouping.
FIG. 1.
Clustering of DGGE profiles of fecal samples from 21 children (children 1 to 7 and 9 to 22). The V3 universal primers (A) or V6-V8 universal primers (B) were used. Clustering was carried out using the UPGMA method based on the Pearson correlation coefficient. See Materials and Methods for an explanation of the numbered fecal samples.
Figure 1B shows the DGGE binding patterns obtained with the universal primers for the V6-V8 region. Most of the weak and strong bands were in the middle of the denaturing gel, while in the upper part almost all lanes contained a smear. Visual comparison showed that the variability among samples was less marked than that in the V3 profiles, as clearly indicated by the generally high similarity coefficients (r > 60%) derived from computer-aided analysis, and the mean similarity coefficient was 64%.
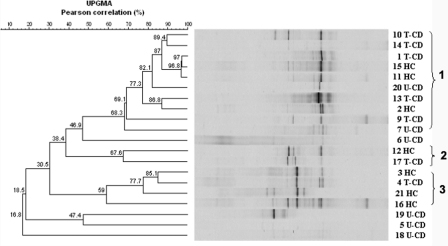
In general, the DGGE profiles of the PCR amplicons obtained with primers Lac1 and Lac2GC (Fig. 2) had few (one to five) strong well-resolved bands, some bands with lower intensity, and a number of poorly resolved fragments resulting in a smear. Analysis of the banding patterns, processed by using the Bionumerics software, revealed Pearson correlation coefficients ranging from 16.8% to 97%; at a similarity level of ca. 54%, three clusters were found. With the exception of samples from U-CD patients 7 and 20, clusters 1, 2, and 3 contained only fecal samples from T-CD and HC, while the U-CD samples showed the lowest Pearson coefficient values and most of them did not group with any cluster.
FIG. 2.
Clustering of DGGE profiles of fecal samples from 21 children (children 1 to 7 and 9 to 22). Primers Lac1 and Lac2GC were used to amplify the Lactobacillus group. Clustering was carried out using the UPGMA method based on the Pearson correlation coefficient. See Materials and Methods for an explanation of the numbered fecal samples.
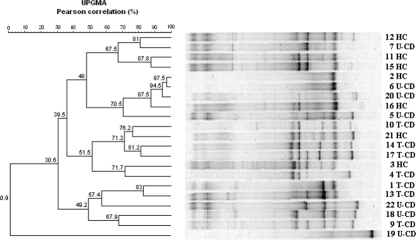
Bifidobacterium sp. isolates were detected by PCR in all fecal samples. The relative DGGE profiles (Fig. 3) showed 3 to 12 strong well-resolved amplicons, as well as faint well-resolved amplicons and regions where the banding pattern was a smear. In some cases the UPGMA analysis clearly grouped HC and U-CD samples with high similarity Pearson coefficients, although two HC profiles were coupled to T-CD and the profile of one U-CD was quite similar to that of a T-CD. The U-CD sample from patient 19 was the only sample that did not group with any cluster.
FIG. 3.
Clustering of DGGE profiles of fecal samples from 21 children (children 1 to 7 and 9 to 22). The g-Bifid primers were used to amplify Bifidobacterium spp. Clustering was carried out using the UPGMA method based on the Pearson correlation coefficient. See Materials and Methods for an explanation of the numbered fecal samples.
DGGE profiles for Enterococcus sp. contained one to five amplicons; in most cases, the unique or dominant amplicon corresponded to E. faecium (data not shown). Clostridium sp. isolates were detected in all fecal samples with only two to four amplicons, one of which always corresponded to C. coccoides. Except for the sample from U-CD patient 19, which clustered separately, all fingerprints were closely related (r > 83%) (data not shown). DGGE profiles for Bacteroides sp. showed 3 to 15 amplicons with similarity values of >66% and no significant grouping of samples related to different variables studied (data not shown).
Enumeration of cultivable bacteria.
Selective media were used to enumerate cultivable cells of the main microbial groups (Table 2). For T-CD and U-CD the median values for presumptive lactic acid bacteria were similar (P = 0.36) (8.09 and 8.02 log CFU g−1, respectively). The median value was highest (8.89 log CFU g−1) for HC. Nevertheless, large differences were found for presumptive lactic acid bacteria within each group. The concentrations varied from ca. 5.0 to 10.19 log CFU g−1 for T-CD and U-CD and from 6.20 to 10.08 log CFU g−1 for HC. The numbers of presumptive Bifidobacteria sp. were significantly (P = 0.03) different for different groups. The median values were 6.83, 5.51, and 7.88 log CFU g−1 for T-CD, U-CD, and HC, respectively. In contrast, significantly (P = 0.045) higher counts of presumptive Bacteroides and Clostridium were obtained for fecal samples from T-CD and U-CD than for HC fecal samples. The lowest numbers of cells of presumptive staphylococci and micrococci were found in fecal samples from U-CD. The median concentrations of presumptive Enterobacteriaceae were in the range from 6.69 to 8.18 log CFU g−1 and were lowest for U-CD. The median concentrations of total anaerobes were not significantly different (P value range, 0.183 to 0.117) for different groups (9.63 to 10.3 log CFU g−1). Based on linear regression analysis, the _r_2 values obtained for the main microbial groups from brothers and sisters were generally equal to and/or lower than those obtained for unrelated HC and T-CD (data not shown).
TABLE 2.
Numbers of cultivable cells of the main microbial groups in fecal samples
| Microbial group | No. of cultivable cells (log CFU g−1)a | |||||
|---|---|---|---|---|---|---|
| T-CD | U-CD | HC | ||||
| Median | Range | Median | Range | Median | Range | |
| Lactic acid bacteria | 8.09 B | 5.00-10.00 | 8.02 B | 5.0-10.19 | 8.89 A | 6.20-10.08 |
| Bifidobacterium | 6.83 B | 3.50-8.80 | 5.51 B | 1.20-8.02 | 7.88 A | 6.41-9.7 |
| Bacteroides | 8.31 A | 3.70-10.04 | 8.69 A | 4.20-9.44 | 7.05 B | 4.10-9.62 |
| Clostridium | 8.07 A | 5.50-9.24 | 8.04 A | 5.62-9.11 | 5.50 B | 4.88-6.40 |
| Staphylococcus/Micrococcus | 7.42 B | 6.85-7.97 | 6.00 C | 4.00-7.50 | 8.05 A | 4.50-9.18 |
| Enterobacteriaceae | 8.18 A | 3.70-10.85 | 6.69 C | 4.00-9.41 | 8.05 B | 4.15-9.62 |
| Total anaerobes | 9.63 A | 9.17-10.18 | 9.87 A | 4.30-10.73 | 10.03 A | 9.61-10.77 |
Identification and typing of lactic acid bacteria.
Colonies of presumptive lactic acid bacteria were randomly isolated from the highest plate dilutions on MRS or blood azide agar and used for further analysis. Gram-positive, catalase-negative, nonmotile cocci and rods able to acidify MRS or blood azide broth (ca. 430 isolates) were identified by sequence analysis of at least 700 bp of the 5′ region of the 16S rRNA gene. Discrimination between Enterococcus faecalis, E. faecium, and E. durans, between L. plantarum, L. pentosus, and L. paraplantarum, or between L. paracasei, L. casei, and L. rhamnosus was possible after partial sequencing of recA or pheS genes. E. faecium was the only species identified in all fecal samples (see Table S1 in the supplemental material). Overall, L. plantarum, L. paracasei, and L. rhamnosus were identified in each group. Lactobacillus brevis, Lactobacillus rossiae, and L. pentosus were identified only in fecal samples from T-CD and HC. In contrast, Lactobacillus fermentum, Lactobacillus delbrueckii subsp. bulgaricus, and Lactobacillus gasseri were identified only in several fecal samples from HC. Except for HC patient 11 and T-CD patient 9, who belong to the same family unit, no pair of siblings harbored the same species of lactic acid bacteria.
To exclude the possibility of clonal relatedness and to compare the diversity of strains of lactic acid bacteria in T-CD, U-CD, and HC, isolates from MRS and blood azide agar media were analyzed by RAPD-PCR using three primers (M13, P4, and P7). The number of strains identified for each fecal sample is shown in Table S1 in the supplemental material. The percentages of strains identified as lactobacilli among all of the strains identified using MRS and blood azide agar media were significantly (P < 0.05) different for different groups, as follows: HC (ca. 38%) > T-CD (ca. 17%) > U-CD (ca. 10%). No pairwise correlation was found for strains of lactic acid bacteria isolated from brothers and sisters since the _r_2 values were generally equal to and/or lower than those obtained for unrelated HC and T-CD.
Identification of bifidobacteria.
Table 3 shows the percentages of samples positive for species of bifidobacteria based on the total number of samples assayed. Bifidobacterium longum was detected in all fecal samples from T-CD and HC and in 85.7% of U-CD fecal samples. Bifidobacterium infantis and Bifidobacterium lactis were found more frequently in T-CD and HC than in U-CD. In contrast, Bifidobacterium dentium was more prevalent in U-CD and HC samples, and B. bifidum was isolated only from T-CD and HC samples. Bifidobacterium animalis subsp. lactis was found only in a sample from U-CD patient 19. The cell density of each Bifidobacterium species was lowest in U-CD, intermediate in T-CD, and the highest in HC (data not shown). Based on cell density, the total compositions of Bifidobacterium species were significantly (P = 0.03) different in HC and T-CD siblings.
TABLE 3.
Bifibobacterium species in fecal samples
| Species or subspecies | Prevalence (%) | ||
|---|---|---|---|
| T-CD | U-CD | HC | |
| B. longum | 100 | 85.7 (5, 6, 7, 18, 20, 22)a | 100 |
| B. infantis | 71.4 (1, 4, 10, 14, 17) | 14.3 (20) | 85.7 (3, 11, 12, 15, 16, 21) |
| B. lactis | 71.4 (4, 9, 13, 14, 17) | 42.8 (5, 7, 22) | 57.1 (3, 11, 12, 15) |
| B. dentium | 71.4 (4, 9, 10, 14, 17) | 57.1 (5, 18, 20, 22) | 42.8 (12, 15, 21) |
| B. bifidum | 57.1 (4, 10, 14, 17) | 0 | 57.1 (3, 11, 12, 21) |
| B. animalis subsp. lactis | 0 | 14.3 (19) | 0 |
VOCs.
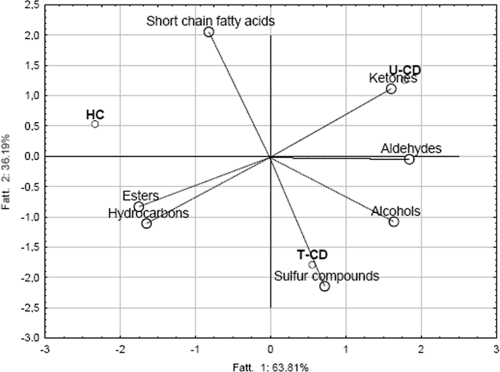
Forty-seven VOCs were identified in fecal samples from T-CD, U-CD, or HC and were grouped according to chemical class (see Table S2 in the supplemental material). The concentrations of VOCs in each child at the three sampling times did not differ (P > 0.05). In contrast, high variability was found within the same group. The total median values for VOCs mainly differentiated U-CD from HC. Data for VOCs were elaborated by using PCA to differentiate fecal samples from T-CD, U-CD, and HC (Fig. 4). The three groups of children were distributed in three different zones. Except for ethyl acetate, ethyl propanoate, and octyl acetate, the largest amount of esters was found for HC (Fig. 4; see Table S2 in the supplemental material). The total median values for ketones did not differ (P > 0.05) for T-CD and U-CD and were lowest for HC. The total median values for alcohols, aldehydes, sulfur compounds, and hydrocarbons were higher for U-CD than for T-CD and HC. The total median values for alcohols and sulfur compounds did not differ (P = 0.21) for T-CD and HC. In particular, the median concentrations of _p_-cresol, 3-methyl-propanol, 2-methyl-butanal, and benzaldehyde for U-CD were at least twice those for HC. The median concentration of total short-chain fatty acids (SCFAs) was significantly (P < 0.05) higher in fecal samples from T-CD and HC than in fecal samples from U-CD. Major differences were found for butyric, isovaleric, and pentanoic acids. Overall, the _r_2 values for VOC data for brothers and sisters were equal to and/or lower than those for VOC data for unrelated HC and T-CD.
FIG. 4.
Plot of the first and second principal components after PCA based on the median data for VOCs of T-CD, U-CD, and HC.
DISCUSSION
Fecal samples from each child showed unique DGGE profiles when the V3 primers were used. In agreement with previous findings (38), it was shown that no amplicon obtained by DGGE analysis with universal primers could be unequivocally correlated with CD and/or siblings. It has been demonstrated (45) that the V6-V8 universal primers are less efficient and usually generate DGGE profiles with higher levels of similarity than the V3 primers, which give more complex profiles. In this study, when the V6-V8 primers were used, the fingerprints were generally similar, with no significant clustering of samples. Therefore, genetic fingerprints of two different regions of the 16S rRNA gene of the whole eubacterial community did not reveal any relevant change in the structure of the fecal microbiota of children affected by CD based on samples obtained 2 years after remission, as well as at the time of diagnosis (T-CD), compared to the results for HC. It is also likely that universal primers are too generic to detect changes that eventually occur in response to the CD pathology. Taking this hypothesis into account, genetic diversity was also investigated at the group or genus level.
Lactobacillus sp., Enterococcus sp., Bifidobacterium sp., Clostridium sp., and Bacteroides sp. have been detected in human feces from healthy individuals as well as from CD patients by using group-specific PCR-DGGE (47, 48) and by fluorescent in situ hybridization (3). Huys et al. (14) recently reviewed the success of sequence-dependent electrophoresis fingerprinting techniques, such as DGGE, and the current state of the art of these approaches to analyze human intestinal microbiota from healthy individuals and from patients with intestinal disorders before and after nutritional intervention. Members of the genera Lactobacillus and Bifidobacterium are considered plausible significant factors in gut health (38). Our data indicated that the Lactobacillus sp. community in patients still receiving a gluten-containing diet (U-CD) changed significantly, while the diversity of the Lactobacillus sp. community of T-CD was quite comparable to that of HC. The conclusion was less evident when the Bifidobacterium community was investigated. Indeed, in some cases the structure of the Bifidobacterium sp. community of HC was similar to that of U-CD, while in other cases it mirrored that of T-CD. Genetic fingerprinting of Enterococcus sp., Clostridium sp., and Bacteroides sp. did not reveal any significant difference in the fecal microbiota between CD patients and HC. By considering the fact that the intestinal tract is an ecosystem with a high level of diversity and that only predominant bacterial species generate clearly detectable DGGE bands, we hypothesized that in some cases changes in microbial community structure may be underestimated. It is also likely that individual variability could overcome changes occurring in response to disease and a gluten-free diet, making the multifactor approach used in this study indispensable for investigating such effects. So far, few data are available for the composition of the intestinal microbiota of children with CD. Kopečný et al. (16, 17) briefly reported that the fecal bacterial population, especially the Bifidobacterium population, was significantly different in different individuals and was influenced by both diet and CD. The compositions of the Lactobacillus sp. and Bifidobacterium populations were shown to be different in CD patients and HC (38). Nevertheless, in both cases there is a strong need for further characterization of the gut microbiota in CD patients.
As determined by conventional culture-dependent techniques, the cell densities of the main fecal microbial groups were significantly (P < 0.05) different in T-CD, U-CD, and HC. Except for presumptive Bacteroides and Clostridium, fecal samples from U-CD harbored the lowest number of the microbial groups. Increased numbers of Bacteroides and Clostridium cells are usually found in fecal samples from children affected by GI inflammatory diseases, including IBD and CD (3, 39). The ratio of lactic acid bacteria and Bifidobacterium to Bacteroides and enterobacteria (including E. coli) was lower in T-CD and especially in U-CD than in HC. The same results were found using duodenal biopsy specimens from CD patients (22). The cell density of Enterobacteriaceae was lowest in fecal samples from U-CD. The decrease in the number of Enterobacteriaceae cells, especially when it was associated with the persistence of virulence biotypes of E. coli, together with an increase in the number of Bacteroides cells, could be an environmental factor involved in CD or the consequence of CD pathogenesis (37).
Lactic acid bacteria were identified and subjected to RAPD-PCR analysis to determine qualitative and quantitative differences among T-CD, U-CD, and HC. The dominance of lactic acid bacteria has functional importance. In particular, lactobacilli positively influence the biochemistry, immunology, and population dynamics of the host GI tract (20, 47). E. faecium was the common and dominant species. L. plantarum, L. paracasei, and L. rhamnosus were found in fecal samples from T-CD, U-CD, and HC. L. brevis, L. rossiae, and L. pentosus were found only in fecal samples from T-CD and HC. The isolation percentage for lactobacilli was lowest for U-CD. In agreement with DGGE analyses, these features supported the similarity of the fecal dominant microbiota in T-CD and HC compared to that in U-CD and HC. The prevalence of the L. casei group (L. casei, L. paracasei, L. rhamnosus, and/or Lactobacillus zeae) in HC was shown previously (38). This study showed the presence of other species, such as L. fermentum, L. delbrueckii subsp. bulgaricus, and L. gasseri, in fecal samples from HC.
The composition of Bifidobacterium species is thought to influence the host immune responses (50). In this study, the dominant bifidobacteria found in fecal samples from T-CD, U-CD, and HC were B. longum, B. infantis, B. lactis, and B. breve. B. longum was the species most commonly found in the fecal microbiota (4, 12). In agreement with a previous study (4), an imbalance in the bifidobacterial composition was found between HC and T-CD and especially between HC and U-CD. B. bifidum was absent in U-CD, but it was found in T-CD. This confirmed the hypothesis that a gluten-free diet partially restores the balance of Bifidobacterium species.
The qualitative and quantitative differences found for the dominant fecal microbiota affected the concentration of VOCs. A few studies have been carried out with VOCs from fecal samples from children (43). Garner et al. (9) identified 297 VOCs in the feces of adults. The concentration of VOCs varied between and within subjects (9). SCFAs, such as butyric, isovaleric, and pentanoic acids, are the main products of the microbial breakdown of carbohydrates and constitute the major anions in the lumen of the large intestine (46). SCFAs provide energy to colonocytes (35) and stimulate Na+ and water absorption from the large intestine, even under diarrheic conditions (46). It was hypothesized that probiotic bacteria (e.g., Lactobacillus and Bifidobacterium) may modify the metabolism in the large intestine by increasing the synthesis of SCFAs (36). The concentration of SCFAs was higher in fecal samples from T-CD and HC than in fecal samples from U-CD.
Compared to HC, CD children had an altered fecal microbiota and VOCs which were partially different in T-CD and U-CD. This study showed that the fecal microbiota and VOCs of T-CD were more similar to those of HC than to those of U-CD. This may indicate that a gluten-free diet partially restores the balance between species of the fecal microbiota of CD patients. Lactobacillus and Bifidobacterium strains isolated from HC could be of interest as probiotic treatments to restore the balance of the GI microbiota in T-CD and especially in U-CD.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank Sonya Siragusa and Daniela Pinto (Department of Plant Protection and Applied Microbiology, University of Bari) for technical assistance.
Footnotes
▿
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Adlerberth, I., M. Cerquetti, I. Poilane, A. Wold, and A. Collignon. 2000. Mechanisms of colonisation and colonisation resistance of the digestive tract. Microb. Ecol. Health Dis. 11**:**223-239. [Google Scholar]
- 2.Bornay-Llinares, F. J., A. J. da Silva, I. N. S. Moura, P. Myjak, H. Pietkiewicz, W. Kruminis-Lozowska, T. K. Graczyk, and N. J. Pieniazek. 1999. Identification of Cryptosporidium felis in a cow by morphologic and molecular methods. Appl. Environ. Microbiol. 65**:**1455-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collado, M. C., M. Calabuig, and Y. Sanz. 2007. Differences between the fecal microbiota of celiac infants and healthy controls. Curr. Issues Intest. Microbiol. 8**:**9-14. [PubMed] [Google Scholar]
- 4.Collado, M. C., E. Donat, C. Ribes-Koninckx, M. Calabuig, and Y. Sanz. 2008. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 8**:**232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Angelis, M., C. G. Rizzello, A. Fasano, M. G. Clemente, C. De Simone, M. Silano, M. De Vincenzi, I. Losito, and M. Gobbetti. 2005. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue. Biochim. Biophys. Acta 1762**:**80-93. [DOI] [PubMed] [Google Scholar]
- 6.De Angelis, M., S. Siragusa, L. Caputo, A. Ragni, R. Burzigotti, and M. Gobbetti. 2007. Survival and persistence of Lactobacillus plantarum 4.1 and Lactobacillus reuteri 3S7 in the gastrointestinal tract of pigs. Vet. Microbiol. 123**:**133-144. [DOI] [PubMed] [Google Scholar]
- 7.De Angelis, M., S. Siragusa, M. Berloco, L. Caputo, L. Settanni, G. Alfonsi, M. Amerio, A. Grandi, A. Ragni, and M. Gobbetti. 2006. Selection of potential probiotic lactobacilli from pig feces to be used as additives in pelleted feeding. Res. Microbiol. 157**:**792-801. [DOI] [PubMed] [Google Scholar]
- 8.De Los Reyes-Gavilán, C. G., G. K. Y. Limsowtin, P. Tailliez, L. Séchaud, and J. P. Accolas. 1992. A _Lactobacillus helveticus_-specific DNA probe detects restriction fragment length polymorphisms in this species. Appl. Environ. Microbiol. 58**:**3429-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garner, E. G., S. Smith, B. L. Costello, P. White, R. Spencer, C. S. J. Probert, and M. N. Ratcliffe. 2007. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 21**:**1675-1688. [DOI] [PubMed] [Google Scholar]
- 10.Gianfrani, C., S. Auricchio, and R. Troncone. 2005. Adaptive and innate immune responses in celiac disease. Immunol. Lett. 99**:**141-145. [DOI] [PubMed] [Google Scholar]
- 11.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125**:**1401-1412. [DOI] [PubMed] [Google Scholar]
- 12.Grönlund, M. M., M. Gueimonde, K. Laitinen, G. Kociubinski, T. Grönroos, S. Salminen, and E. Isolauri. 2007. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergenic disease. Clin. Exp. Allergy 37**:**1764-1772. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins, M. J., R. Sharp, and G. T. Macfarlane. 2001. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48**:**198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huys, G., T. Vanhoutte, and P. Vandamme. 2008. Application of sequence-dependent electrophoresis fingerprinting in exploring biodiversity and population dynamics of human intestinal microbiota: what can be revealed?, article ID 597603. In V. B. Young, R. A. Britton, and T. M. Scmidt (ed.), Hindawi Publishing Corporation interdisciplinary perspectives on infectious diseases, vol. 2008. Hindawi Publishing Corporation, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kok, R. R., A. De Waal, T. Schut, G. W. Welling, G. Weenk, and K. J. Hellingwerf. 1996. Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl. Environ. Microbiol. 62**:**3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopečný, J., J. Mrázek, K. Fliegerová, and T. Kott. 2006. Effect of gluten-free diet on microbes in the colon. Folia Microbiol. 51**:**287-290. [DOI] [PubMed] [Google Scholar]
- 17.Kopečný, J., J. Mrázek, K. Fliegerová, P. Frühauf, and L. Tučková. 2008. The intestinal microflora of childhood patients with indicated celiac disease. Folia Microbiol. 53**:**214-216. [DOI] [PubMed] [Google Scholar]
- 18.Langendijk, P. S., F. Shut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S ribosomal RNA-targeted probes and its application in fecal samples, Appl. Environ. Microbiol. 61**:**3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layton, A., L. McKay, D. Williams, V. Garrett, R. Gentry, and G. Sayler. 2006. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl. Environ. Microbiol. 72**:**4214-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindfors, K., T. Blomqvist, K. Juuti-Uusitalo, and S. Stenman. 2008. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 152**:**552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macfarlane, G. T., J. H. Cummings, and C. Allison. 1986. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 132**:**1647-1656. [DOI] [PubMed] [Google Scholar]
- 22.Macfarlane, S., M. E. Quingley, M. J. Hopkins, D. F. Newton, and G. T. Macfarlane. 1998. Polysaccharide degradation by human intestinal bacteria during growth under multi-substrate limiting conditions in a three-stage continuous culture system. FEMS Microbiol. Ecol. 26**:**231-243. [Google Scholar]
- 23.Mai, V., L. H. Colbert, S. N. Perkins, A. Schatzkin, and S. D. Hursting. 2007. Intestinal microbiota: a potential diet-responsive prevention target in ApcMin mice. Mol. Carcinog. 46**:**42-48. [DOI] [PubMed] [Google Scholar]
- 24.Matsuki, T., K. Watanabe, T. Tanaka, and H. Oyaizu. 1998. Rapid identification of human intestinal bifidobacteria by 16S rRNA-target species- and group-specific primers. FEMS Microbiol. Lett. 167**:**113-121. [DOI] [PubMed] [Google Scholar]
- 25.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu, and R. Tanaka. 2002. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68**:**5445-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina, M., G. De Palma, C. Ribes-Koninckx, M. Calabuig, and Y. Sanz. 2008. Bifidobacterium strains suppress in vitro the pro-inflammatory milieu triggered by the large intestinal microbiota of coeliac patients. J. Inflamm. 5**:**19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau, M. C., and V. Gaboriau-Routhiau. 2001. Influence of resident intestinal microflora on the development and functions of the gut-associated lymphoid tissue. Microb. Ecol. Health Dis. 13**:**65-68. [Google Scholar]
- 28.Muyzer, G., E. C. de Wall, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes encoding for 16S rRNA. Appl. Environ. Microbiol. 59**:**695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadal, I., E. Donant, C. Ribes-Koninckx, M. Calabuig, and Y. Sanz. 2007. Imbalance in the composition of the duodenal microbiota of children with celiac disease. J. Med. Microbiol. 56**:**1669-1674. [DOI] [PubMed] [Google Scholar]
- 30.Naser, S. M., F. Thompson, B. Hoste, D. Gevers, P. Dawyndt, M. Vancanneyt, and J. Swings. 2005. Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology 151**:**2141-2150. [DOI] [PubMed] [Google Scholar]
- 31.Niewinski, M. M. 2008. Advances in celiac disease and gluten-free diet. J. Am. Diet. Assoc. 108**:**661-672. [DOI] [PubMed] [Google Scholar]
- 32.Pietzak, M. M. 2005. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology 128**:**S135-S141. [DOI] [PubMed] [Google Scholar]
- 33.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8**:**151-156. [Google Scholar]
- 34.Rademaker, J. L. W., and F. J. De Bruijn. 2004. Computer-assisted analysis of molecular fingerprint profiles and database construction. Mol. Microb. Ecol. 705**:**1397-1446. [Google Scholar]
- 35.Roediger, R. 1995. The place of short-chain fatty acids in colonocyte metabolism in health and ulcerate colitis: the impaired colonocyte barrier, p. 337-351. In J. Cummings, J. Rombeau, and T. Sakata (ed.), Physiological and clinical aspects of short-chain fatty acids. Cambridge University Press, Cambridge, United Kingdom.
- 36.Sakata, T., T. Kojima, M. Fujieda, M. Takahashi, and T. Michibata. 2003. Influences of probiotic bacteria on organic acid production by pig caecal bacteria in vitro. Proc. Nutr. Soc. 62**:**73-80. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez, E., I. Nadal, E. Donat, C. Ribes-Koninckx, M. Calabuig, and Y. Sanz. 2008. Reduced diversity and increased virulence-gene carriage in intestinal enterobacteria of children with coeliac disease. BMC Gastroenterol. 8**:**50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz, Y., E. Sànchez, M. Marzotto, M. Calabuig, S. Torriani, and F. Dell'aglio. 2007. Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol. Med. Microbiol. 51**:**562-568. [DOI] [PubMed] [Google Scholar]
- 39.Sartor, B. R. 2004. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126**:**1620-1633. [DOI] [PubMed] [Google Scholar]
- 40.Simpson, J. M., V. J. McCracken, H. R. Gaskins, and V. Mackie. 2000. Denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA amplicons to monitor changes in fecal bacterial populations of weaning pigs after introduction of Lactobacillus reuteri strain MM53. Appl. Environ. Microbial. 66**:**4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stene, L. C., M. C. Honeyman, E. J. Hoffenberg, J. E. Haas, R. J. Sokol, L. Emery, I. Taki, J. M. Norris, H. A. Erlich, G. S. Eisenbarth, and M. Rewers. 2006. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am. J. Gastroenterol. 101**:**2333-2340. [DOI] [PubMed] [Google Scholar]
- 42.Swidsinski, A., A. Ladhoff, A. Pernthaler, S. Swidsinski, V. Loening-Baucke, M. Ortner, J. Weber, U. Hoffmann, S. Schreiber, M. Dietel, and H. Lochs. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122**:**44-54. [DOI] [PubMed] [Google Scholar]
- 43.Tjellström, B., L. Stenhammar, L. Högberg, K. Fälth-Magnusson, K.-E. Magnusson, T. Midtvedt, T. Sundqvist, and E. Norin. 2005. Gut microflora associated characteristics in children with celiac disease. Am. J. Gastroenterol. 100**:**2784-2788. [DOI] [PubMed] [Google Scholar]
- 44.Tursi, A., G. Brandimarte, and G. Giorgetti. 2003. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am. J. Gastroenterol. 98**:**839-843. [DOI] [PubMed] [Google Scholar]
- 45.Vanhoutte, T., G. Huys, E. De Brandt, and J. Swings. 2004. Temporal stability analysis of the microbiota in human feces by denaturing gradient gel electrophoresis using universal and group-specific 16S rRNA gene primers. FEMS Microbiol. Ecol. 48**:**437-446. [DOI] [PubMed] [Google Scholar]
- 46.von Engelhardt, W., J. Barteles, S. Kirschberger, H. Meyer Zu Düttingdorf, and R. Busche. 1998. Role of short-chain fatty acids in the hind gut. Vet. Q. 20**:**S52-S59. [PubMed] [Google Scholar]
- 47.Walter, J., G. W. Tannock, A. Tilsala-Timisjarvi, S. Rodtong, D. M. Loac, K. Munro, and T. Alatossava. 2000. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66**:**297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67**:**2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward, L. J. H., and M. J. Timmins. 1999. Differentiation of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus by polymerase chain reaction. Lett. Appl. Microbiol. 29**:**90-92. [DOI] [PubMed] [Google Scholar]
- 50.Young, S. L., M. A. Simon, M. A. Baird, G. W. Tannock, R. Bibiloni, K. Spencely, J. M. Lane, P. Fitzharris, J. Crane, I. Town, E. Addo-Yobo, C. S. Murray, and A. Woodcock. 2004. Bifidobacterial species differentially affect expression of cell surface markers and cytokines of dendritic cells harvested from cord blood. Clin. Diagn. Lab. Immunol. 11**:**686-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]