Free Fatty Acids Link Metabolism and Regulation of the Insulin-Sensitizing Fibroblast Growth Factor-21 (original) (raw)
Abstract
OBJECTIVE
Fibroblast growth factor (FGF)-21 improves insulin sensitivity and lipid metabolism in obese or diabetic animal models, while human studies revealed increased FGF-21 levels in obesity and type 2 diabetes. Given that FGF-21 has been suggested to be a peroxisome proliferator–activator receptor (PPAR) α–dependent regulator of fasting metabolism, we hypothesized that free fatty acids (FFAs), natural agonists of PPARα, might modify FGF-21 levels.
RESEARCH DESIGN AND METHODS
The effect of fatty acids on FGF-21 was investigated in vitro in HepG2 cells. Within a randomized controlled trial, the effects of elevated FFAs were studied in 21 healthy subjects (13 women and 8 men). Within a clinical trial including 17 individuals, the effect of insulin was analyzed using an hyperinsulinemic-euglycemic clamp and the effect of PPARγ activation was studied subsequently in a rosiglitazone treatment trial over 8 weeks.
RESULTS
Oleate and linoleate increased FGF-21 expression and secretion in a PPARα-dependent fashion, as demonstrated by small-interfering RNA–induced PPARα knockdown, while palmitate had no effect. In vivo, lipid infusion induced an increase of circulating FGF-21 in humans, and a strong correlation between the change in FGF-21 levels and the change in FFAs was observed. An artificial hyperinsulinemia, which was induced to delineate the potential interaction between elevated FFAs and hyperinsulinemia, revealed that hyperinsulinemia also increased FGF-21 levels in vivo, while rosiglitazone treatment had no effect.
CONCLUSIONS
The results presented here offer a mechanism explaining the induction of the metabolic regulator FGF-21 in the fasting situation but also in type 2 diabetes and obesity.
Fibroblast growth factor (FGF)-21 is a recently discovered metabolic regulator of fasting metabolism. FGF-21 activates glucose uptake in adipocytes, protects animals from diet-induced obesity when overexpressed in transgenic mice, and lowers blood glucose and triglyceride levels when administered to diabetic rodents (1–3). Comparably, glucose- and triglyceride-lowering effects were found in diabetic rhesus monkeys during chronic FGF-21 treatment over a period of 6 weeks (4). Therefore, FGF-21 was assumed to be a novel target with potential antidiabetic properties, which might be useful in the treatment of hyperglycemia, insulin resistance, and hyperlipidemia.
However, human data did not directly support these assumptions, since increased serum FGF-21 levels were found in obese individuals, patients with type 2 diabetes, and subjects with metabolic syndrome (5,6). In addition FGF-21 levels correlated positively not only with adiposity but also with fasting insulin and triacylglycerols (5). A recent study (3) reported a significant increase of FGF-21 levels during prolonged fasting. This process appeared to be peroxisome proliferator–activator receptor (PPAR) α–dependent (2,3), although exact mechanisms leading to fasting-induced FGF-21 elevations are yet unknown.
Various data support that metabolic parameters themselves can modulate the circulating levels of hormones and adipokines (7–10). Thus, free fatty acids (FFAs) activate PPARs and were found to be elevated in fasting conditions. We therefore speculated that FFAs themselves might regulate FGF-21. This hypothesis is supported by recent data demonstrating that FGF-21 levels are positively associated with FFAs (11) and triacylglycerols (3) in a cross-sectional study population of nondiabetic individuals. Although the direction of that relation is unclear in a cross-sectional study, it offers a potential mechanism linking starvation to the increased levels of FGF-21. We therefore investigated the effect of fatty acids on FGF-21 secretion in HepG2 cells in vitro. Based on these in vitro data, we performed a randomized controlled trial to explore whether an increase in circulating FFAs and triacylglycerols modifies FGF-21 levels in humans. Since an increase of FFAs is known to induce insulin resistance and hyperinsulinemia, the effect of insulin on FGF-21 was additionally investigated by performing a hyperinsulinemic-euglycemic clamp at baseline of a noncontrolled, rosiglitazone intervention trial. As some recent animal data (12) also suggested a PPARγ-dependent regulation of FGF-21, we finally evaluated the effect of the PPARγ agonist, rosiglitazone, in that human trial.
RESEARCH DESIGN AND METHODS
A total of 13 women and 8 men participated in the first study (lipid study). Briefly, the individuals were normal weight (BMI 23.7 ± 0.8 kg/m2), were aged 26.4 ± 0.8 years, had a waist-to-hip ratio (WHR) of 0.83 ± 0.02, and had basal lipid parameters in the normal range (total cholesterol 4.53 ± 0.17 mmol/l, HDL cholesterol 1.44 ± 0.03 mol/l, LDL cholesterol 2.64 ± 0.14 mmol/l, triacylglycerols 1.01 ± 0.08 mmol/l, and FFAs 0.68 ± 0.12 mmol/l). None of the participants had taken any medication for at least 3 months before the study. All participants were initially screened for any systemic disease or biochemical evidence of impaired hepatic or renal function. Subjects with a history of hypertension, type 2 diabetes, renal or liver disease, dyslipidemia, heart failure, or a family history of diabetes or any other endocrine disorder were excluded from this study. Body weight of the participants was stable for at least 2 months before the study. The 13 healthy young women had regular menstrual cycles. Informed written consent was obtained from each participant.
For the second human trial (PPARγ study) 7 male and 10 female volunteers with impaired glucose tolerance were investigated in a prospective trial. The individuals were overweight (BMI 32.8 ± 2.2 kg/m2), were aged 59.1 ± 1.6 years, and had a WHR of 0.88 ± 0.03. Body weights were stable for at least 2 months before the study. All participants were initially screened for any systemic disease or biochemical evidence of impaired hepatic or renal function. Treatment with insulin, orally taken antidiabetes medications, or glucocorticoids was not allowed. Patients with heart failure, impaired hepatic or renal function, anemia, disturbed coagulation, or any other endocrine disorder were excluded. Informed written consent was obtained from all subjects after explanation of the nature, purpose, and potential risks of the studies. The patients were instructed not to modify their way of living (diet and exercise) during the treatment period. All study protocols were approved by the institutional review board of the Charité Medical School, Campus Benjamin Franklin.
Cell culture.
HepG2 cells were grown in Eagle's minimum essential medium with 10% FCS and 1% NEA; 24 h before analysis, HepG2 cells were incubated with basal serum-free media. To analyze the effect of FFAs, palmitic, oleic, or linoleic acid (Sigma-Aldrich, Seelze, Germany) or a mixture of these fatty acids (one-third of each) were dissolved in water with 125 μmol/l NaOH and 10 μmol/l fatty acid–free bovine serum albumin (BSA) (Calbiochem, Germany) in a concentration of 160 μmol/l (Ratio 16:1) and cells were incubated within that medium for 1, 2, 4, 8, and 24 h. Time course and concentration-dependent effects of FFAs on FGF-21 secretion and expression were analyzed. Finally, FGF-21 mRNA expression was also studied in primary human myoblasts. Therefore, primary human myoblasts were prepared from human skeletal muscle of healthy individuals und cultured in skeletal muscle cell growth medium (PromoCell, Heidelberg, Germany) containing 10% fetal bovine serum and 1.5% glutamine and gentamicin. After the first passage, myoblasts were separated from fibroblasts by neural cell adhesion molecule–labeled magnetic beads (Miltenyi Biotech, Bergisch-Gladbach, Germany).
To analyze the effect of lecithin and glycerol on FGF-21 secretion, HepG2 cells were incubated for 4 h using a concentration of 2.5 mg/ml lecithin (Fluka; Sigma-Aldrich) and 1 mmol/l glycerol (Sigma-Aldrich). The FGF-21 levels were measured in the supernatant and normalized to total protein levels.
Small-interfering RNA (siRNA) experiments were performed to investigate whether those effects depend on PPARα. Therefore, siRNA for PPARα (nos. D-003434-01 and D-003434-02) and nontargeting siRNA (no. D-001210-01) as control was purchased from Dharmacon (Thermo Scientific). A total of 100 pmol/well of each siRNA and electroporation with Amaxa nucleofector II (Lonza Cologne, Cologne, Germany) was used for transfection. FFA stimulations were performed 48 h after transfection. All in vitro experiments were performed using at least five replicates per treatment.
The FGF-21 levels were measured in the supernatant by radioimmunoassay (Phoenix Europe, Karlsruhe, Germany) using 125 I–labeled FGF-21 as a tracer and normalized on total protein levels. Protein quantification was performed using the Roti-Nanoquant (Karl-Roth, Karlsruhe, Germany). For RT-PCR 20 ng of total RNA per well were used and FGF-21 RNA was analyzed according to the manufacturer's the instructions of the iScript SYBR green RT-PCR Kit (Biorad, Hercules, CA). Real-time quantitative PCR was performed using an ABI PRISM 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Primer sequences will be sent upon request. The ddCt-method (change in dCt [= Ct of the target gene minus Ct of the housekeeping gene]) was used for relative quantification. Fold changes in expression were calculated according to the transformation: fold increase = 2− difference in dCt.
Human trials
Lipid study.
Eligible participants were randomly assigned to receive either lipid/heparin infusion (LHI) or saline/heparin infusion (SHI) in a cross-over design for the lipid study. A randomization list was created using block randomization (13). In women, the study was performed during the early follicular phase of two subsequent menstrual cycles (days 4–6). To avoid interactions between the study procedures, the study was performed at intervals of at least 2 days in men. Following a 10-h overnight fast, a short polyethylene catheter was inserted into an antecubital vein for infusion of test substances at 0800 h. Another catheter was placed into the contralateral forearm vein for blood sampling. A 0.9% saline infusion plus heparin (0.4 units · kg−1 · min−1) or a lipid infusion (Abbolipid 20% [contents in 1.000 ml: 100 g soybean oil, 100 g safflower oil, 25.0 g glycerol, and 12.0 g egg phospholipids], Abbott, Wiesbaden, Germany; or Lipovenös 20% [contents in 1.000 ml: 200 g soybean oil, 25.0 g glycerol, 12.0 g egg phospholipids, and 0.3 goleate], Fresenius Kabi, Bad Homburg, Germany) plus heparin (0.4 units · kg−1 · min−1) was infused at a constant rate of 1.5 ml/min for 330 min. Four hours after the start of the saline/heparin infusion a hyperinsulinemic-euglycemic clamp (14) was started in six subjects. This procedure was described previously (15).
PPARγ study.
Each subject was studied before and after an 8-week rosiglitazone treatment (2 × 8 mg/day; GlaxoSmithKline, München, Germany) using an oral glucose tolerance test and a hyperinsulinemic-euglycemic clamp as described previously (16). In brief, 40 mIU/m2 per min human insulin (Actrapid; Novo Nordisk, Bagsvaard, Denmark) and a variable infusion of 10% glucose (Serag Wiessner, Naila, Germany) was used, and capillary glucose concentration was monitored every 5 min and was maintained between 4.0 and 4.9 mmol/l via variation of the glucose infusion rate. Insulin sensitivity was assessed as M value and was calculated by dividing the average glucose infusion rate (mg glucose/min) during the steady state of the clamp by the body weight.
Laboratory tests.
Plasma FGF-21 levels were measured before and during LHI SHI, and steady state at the end of the hyperinsulinemic-euglycemic clamp. Metabolic parameters, including FFAs, triacylglycerols, total cholesterol, LDL cholesterol, HDL cholesterol, glucose, and insulin were determined as described previously (7). Serum FGF-21 levels were determined by radioimmunoassay (Phoenix Europe) using 125 I–labeled FGF-21 as tracer. The linear range of the assay was 0.5–8.5 ng/ml, and the standard range was 0.234–30 ng/ml. The interassay and intra-assay coefficients of variation were 5.0 and 14%, respectively.
Statistical analysis.
All statistical procedures were performed using SPSS version 15.0 (SPSS, Chicago, IL). Profiles of FFAs, insulin, glucose, triacylglycerols, and FGF-21 were compared by repeated-measures ANOVA with treatment and time as within-subject factors and the Huynh-Feldt-ε procedure as correction factor, which corrects for a positive biased F test for within-subject factors. The area under the concentration time curve (AUC) was calculated by using the trapezoidal integration. The change in FGF-21, FFAs, glucose, triacylglycerols and insulin between baseline and after LHI and SHI was calculated by subtracting the baseline value from the value 4 h after each infusion procedure. Data were compared by paired Student's t test for normally distributed data and Wilcoxon test for skewed data. The correlations between the values were estimated by Pearson's correlation test and adjusted for age, sex BMI, and change in insulin. The unpaired t test or Mann-Whitney test was used for group comparison, as appropriate. A general linear model analysis was used to control for potential confounders. Results were considered to be significant if the two-sided α was <0.05. Data are presented as means ± SE, unless otherwise indicated.
RESULTS
Fatty acids stimulate release of FGF-21 in HepG2 cells in a PPARα-dependent fashion.
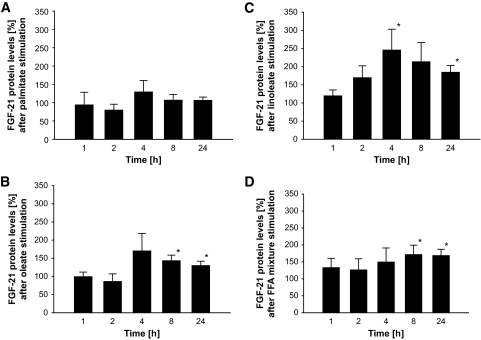
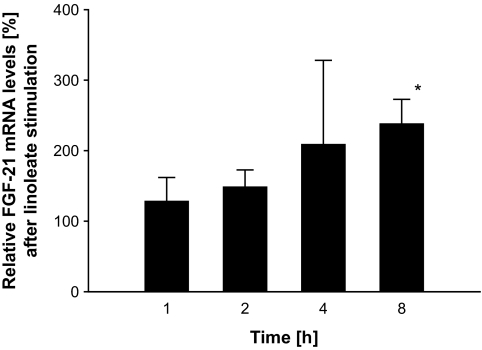
Incubation of HepG2 cells with a mixture of palmitic, linoleic, or oleic acid induced an increase in FGF expression and secretion compared with the BSA control. The analysis of the individual fatty acids revealed that this increase was not detectable for palmitic acid, while stimulation with oleic or linoleic acid resulted in a significant elevation of FGF-21 secretion. Within those experiments, linoleic acid had the strongest effect at ∼4–8 h (Fig. 1A–D), and we analyzed time course–dependent changes in mRNA expression (Fig. 2). The maximum of those effects was observed at 2–4 h for FGF-21 expression and 4–8 h for protein secretion, a time course in line with our in vivo experiments. Furthermore, the dose-response experiments using different oleate-to-BSA ratios (control vs. 1:1, 2:1, 4:1, 8:1, and 16:1) suggested an increase in FGF-21 secretion in a dose-dependent manner (6.61 ± 0.81 vs. 6.93 ± 1.25, 7.36 ± 1.18, 8.98 ± 1.18, 9.66 ± 1.65, and 10.47 ± 1.10 ng/ml; P < 0.05 for control vs. 4:1, 8:1, and 16:1; P < 0.05 for 1:1 vs. 8:1 and 16:1; and P < 0.05 for 2:1 vs. 16:1).
FIG. 1.
Protein levels of FGF-21 in the supernatant of HepG2 cells after stimulation with palmitate (A), oleate (B), linoleate (C), and an FFA mixture (D) for 1, 2, 4, 8, and 24 h. Results are expressed as means ± SE. *P < 0.05 vs. BSA at the same time point, P = 0.072 for FGF-21 protein levels during linoleate stimulation at 2 h vs. BSA, P = 0.069 for FGF-21 protein levels during linoleate stimulation at 8 h vs. BSA, respectively.
FIG. 2.
mRNA Expression of FGF-21 in HepG2 cells after stimulation with linoleate for 1, 2, 4, and 8 h. Results are expressed as means ± SE. *P < 0.05 vs. BSA at the same time point.
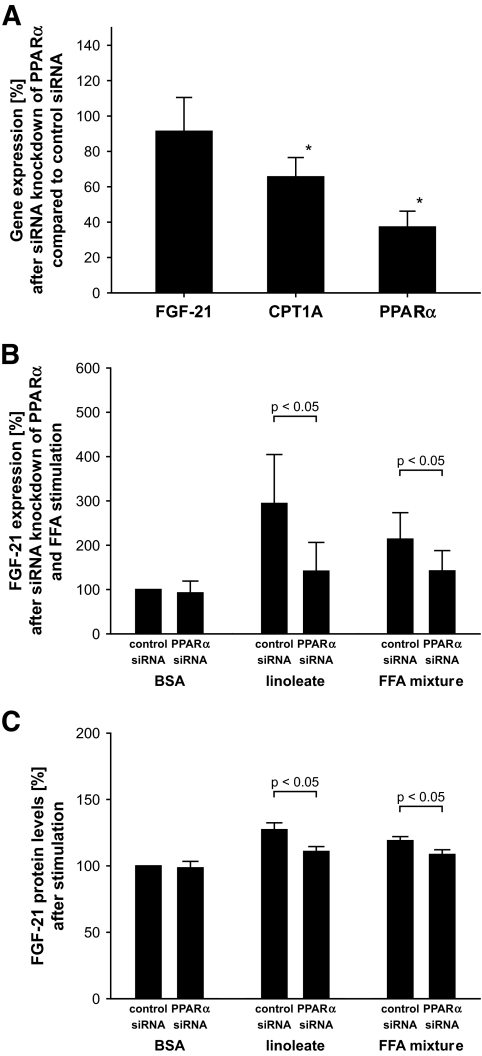
To evaluate whether these effects were mediated via PPARα, we performed siRNA experiments. The PPARα-specific siRNA induced a knockdown of PPARα of ∼60%, although the expression of the PPARα target gene CPT1A was reduced by only ∼35%. Despite the incomplete knockdown of PPARα, the increase in FGF-21 secretion and mRNA expression during stimulation with linoleate and FFA mixture was not further observed. Thus, results at mRNA and protein levels indicate that the FFA-induced effects on FGF-21 depend on activation of PPARα (Fig. 3).
FIG. 3.
A: Gene expression of FGF-21, CPT1A, and PPARα in HepG2 cells in BSA medium after siRNA knockdown of PPARα compared with control siRNA (100%). Results are expressed as means ± SE. *P < 0.05 vs. expression during control siRNA, respectively. B: FGF-21 mRNA expression in HepG2 cells during linoleate or FFA mixture stimulation after siRNA knockdown of PPARα (compared with control siRNA and BSA stimulation [100%]). Results are expressed as means ± SE. *P < 0.05 vs. expression during control siRNA, respectively. C: Concentration of FGF-21 in the supernatant of HepG2 cells after siRNA knockdown of PPARα and subsequent stimulation by linoleate or FFA mixture compared with control siRNA and BSA medium. Results are expressed as means ± SE. *P < 0.05 vs. control siRNA and BSA medium.
As lipid infusion contains a mixture of different fatty acids, glycerol, and lecithin, we analyzed the effect of lecithin and glycerol on FGF-21 secretion in HepG2 cells. Compared with controls, no significant effect of those substances was detectable in HepG2 cells. To evaluate the regulation of FGF-21 expression in human muscle, we aimed to measure FGF-21 expression in primary human myoblasts. Actually, no FGF-21 expression was detectable in these experiments, despite accurate positive controls in HepG2 cells. Those data suggest that the primary human myoblasts used here do not express FGF-21; therefore, we did not analyze the effects of FFAs in those cells.
Lipid infusion induces elevated FGF-21 levels in vivo in humans in a randomized controlled trial.
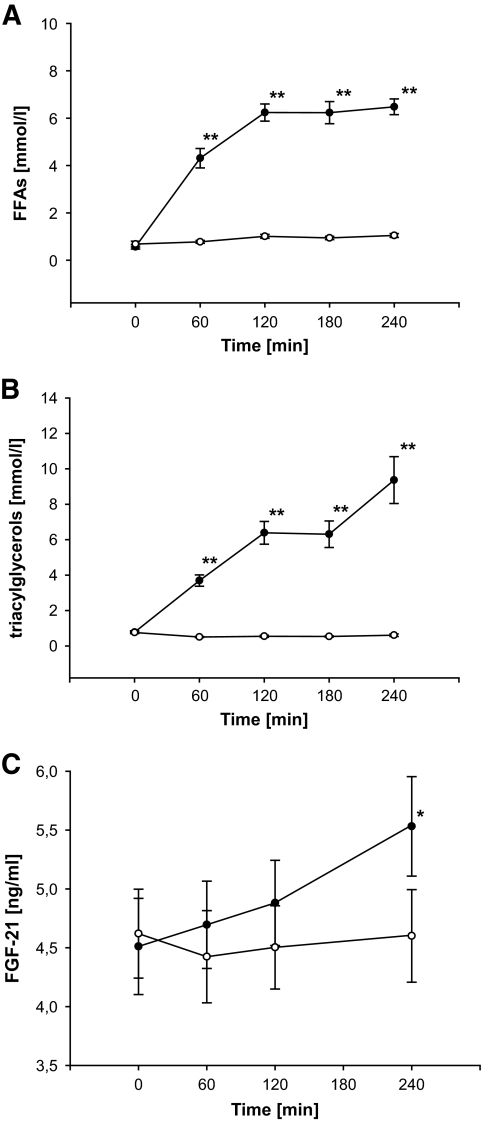
There was no baseline difference of FFAs before LHI and SHI (P = NS). As expected, FFA levels increased during LHI, whereas only a small increase was seen during SHI, resulting in significantly higher FFA levels during LHI compared with SHI (AUC LHI vs. SHI: 1,183.5 ± 66.7 vs. 228.8 ± 17.9 nmol · l−1 · min−1, P < 0.001; treatment-vs.-time interaction: P < 0.001) (Fig. 4A). Accordingly, triglyceride levels rose during LHI (P < 0.05), while a moderate decrease was observed during SHI (P < 0.05), finally also resulting in significantly higher triglyceride levels during LHI (AUC LHI vs. SHI: 1,360.6 ± 172.0 vs. 134.0 ± 12.5 nmol · l−1 · min−1, P < 0.05, P < 0.01; treatment-vs.-time interaction: P < 0.001) (Fig. 4B).
FIG. 4.
Concentrations of FFAs (A), triacylglycerols (B), and FGF-21 (C) during SHI (○) and LHI (●). y-Axis in Fig. 2C does not start at 0. Results are expressed as means ± SE. **P < 0.01; *P < 0.05.
Glucose levels were unchanged during both LHI and SHI (baseline: 4.91 ± 0.80 vs. 4.84 ± 0.54 mmol/l, treatment-vs.-time interaction: P = NS; AUC LHI vs. SHI: 1,130.0 ± 24.9 vs. 1,110.1 ± 17.6 mmol · l−1 · min−1; P = NS). No change of insulin levels was observed during LHI compared with baseline (baseline: 5.17 ± 0.53 vs. 4 h: 4.97 ± 0.62 mU/l; P = NS), while a small, but progressive, decline in insulin concentration was detected during SHI (baseline: 5.80 ± 0.76 vs. 4 h: 2.98 ± 0.32 mU/l; P < 0.05). This decline resulted in lower insulin values during SHI compared with LHI (AUC LHI vs. SHI: 1,384.2 ± 132.5 vs. 1,021.8 ± 109.8 mU · l−1 · min−1, P < 0.001; treatment-vs.-time interaction: P < 0.001).
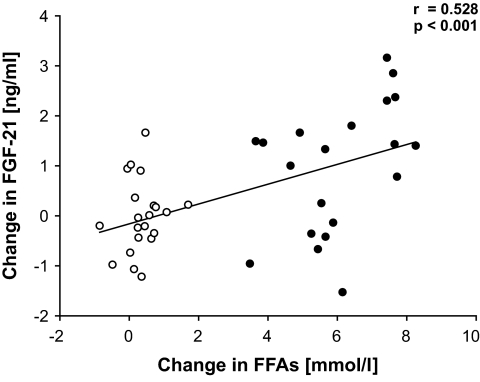
While no change of FGF-21 levels was detected during SHI (P = NS), an increase in FGF-21 levels was found during LHI (P < 0.05). Thus, FGF-21 levels were significantly higher during LHI compared with SHI (treatment-vs.-time interaction: P < 0.05.; AUC: 1,191 ± 91 vs. 1,090 ± 86 ng · ml−1 · min−1; P < 0.05) (Fig. 4C). In six individuals, an hyperinsulinemic-euglycemic clamp was started at 240 min. Notably, we found at 300 min a progressive and highly significant increase of FGF-21 in the remaining 15 participants, who had an isolated lipid infusion over a total of 300 min (P < 0.001; data not shown). Indeed, the circulating FGF-21 levels were nearly doubled at that time point. Interestingly, the change of FGF-21 levels compared with baseline was positively correlated to the change of FFA levels during both LHI and SHI (r = 0.528, P < 0.001) (Fig. 5). Given the circadian changes in the control group, we also included the saline group in the correlation analysis between changes of FFAs and FGF-21. Most importantly, no correlation was found between the change of FGF-21 levels and the change of insulin levels during LHI and SHI, while the correlation between FFAs and FGF-21 was robust, even after adjustment for additional confounders (age, sex BMI, and Δinsulin) (r = 0.474, P < 0.005).
FIG. 5.
Correlation between changes of FGF-21 and FFAs during LHI and SHI. The saline group (○) and the lipid group (●) were included in the correlation analysis. Correlation after adjustment for sex, age, BMI, and change in insulin levels (r = 0.474, P < 0.005).
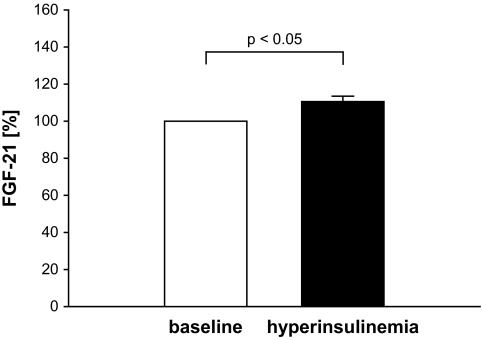
As in some participants, the lipid infusion induced a reduction in FGF-21 concentrations, and we separated those participants according to the change in FGF-21 levels during LHI. However, no significant difference was found between both groups concerning their sex, age BMI WHR, baseline levels of blood glucose, fasting FGF-21, fasting FFAs, and homeostasis model assessment values. To delineate a potential interaction of lipid- and insulin-induced effects on FGF-21, we additionally analyzed hyperinsulinemic-euglycemic clamps, which were performed at baseline in a rosiglitazone treatment trial in 17 subjects with impaired glucose tolerance. Steady-state blood samples were taken not less than 3 h after start of insulin infusion. An ∼700% increase of insulin levels was observed during this protocol (P < 0.005). Under those conditions, a small but significant increase in FGF-21 levels during hyperinsulinemia was observed in these subjects (Fig. 6).
FIG. 6.
Effect of insulin on FGF-21 levels in subjects with impaired glucose tolerance. Results are expressed as means ± SE.
PPARγ stimulation and FGF-21.
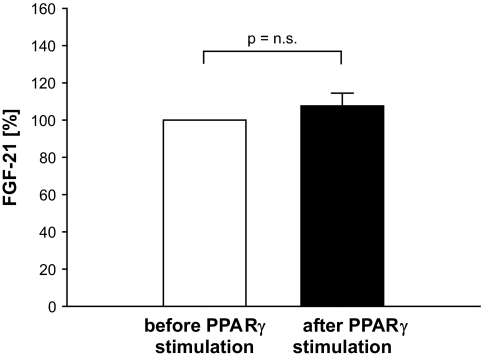
As expected and described in numerous studies, the glucose response during the oral glucose tolerance test and insulin sensitivity were improved after 8 weeks of rosiglitazone treatment. However, no change in serum FGF-21 levels was observed (Fig. 7).
FIG. 7.
Effect of PPARγ stimulation by rosiglitazone treatment on FGF-21 levels in subjects with impaired glucose tolerance. Results are expressed as means ± SE.
The accuracy of the FGF-21 assay was investigated by adding various amounts of lipid solution (Lipovenös 20%; Fresenius Kabi, Bad Homburg, Germany) (0, 0.5, 1.5, and 2.5% vol/vol) to human plasma samples. This ex vivo addition of lipid infusion resulted in measured triglyceride concentrations of 1.28, 3.56, 8.09, and 12.69 mmol/l. FGF-21 values did not differ significantly between those samples. We next added 6 mmol/l oleic acid or linoleic acid to these 0, 0.5, 1.5, and 2.5% vol/vol triglyceride dilutions, resulting in FFA concentrations comparable with those in plasma samples during lipid infusion (4.57–7.31 mmol/l). The subsequently measured FGF-21 values were not significantly different, neither for oleic acid nor for linoleic acid. Taken together, neither triglyceride nor FFA concentrations similar to those observed in vivo in the present study interfered with the measured concentrations of FGF-21.
DISCUSSION
Various recent cross-sectional studies (5,6,11) described a positive correlation between FGF-21 levels and parameters of lipid metabolism, specifically FFAs and triacylglycerols. However, existing experimental data regarding FGF-21 did not yet elucidate the direction of that relationship. Both, the experimental data presented here and the results of the controlled, randomized cross-over human trial strongly suggest a direct regulation of circulating FGF-21 levels in humans by FFAs. Interestingly, insulin appears to have an independent effect on circulating FGF-21, although our data, respective of that effect, are not based on a controlled trial and future studies are required to further strengthen that finding.
In terms of physiology, the FFA-induced increase of FGF-21 might contribute to the adaption of the organism to starvation. In response to fasting, FGF-21 is known to be stimulated by activation of the PPARα, a nuclear receptor, which is itself activated by fatty acids (2,17). FFAs usually increase during fasting, suggesting that a stimulation of FGF-21 secretion may be induced by increased FFAs directly via PPARα activation, an assumption that is strongly underlined by our data. The relevance of PPARα in the regulation of FGF-21 was supported by recent data (3) showing increased FGF-21 levels after pharmacological PPARα activation using fibrates. FGF-21 increased insulin sensitivity in animal experiments and attenuated the hormone-stimulated lipolysis in human adipocytes probably due to reduced expression of perilipin, a phosphoprotein that is thought to recruit several lipases to the surface of the lipid droplets for subsequent triglyceride hydrolysis (18). These biological properties suggest that FGF-21 may contribute to the anabolic switch of the organism under conditions of starvation and the mechanism presented here might explain the previously described elevated FGF-21 levels in patients with prevalent lipid disorders, obesity, or diabetes.
The insulin-sensitizing effect of FGF-21 has been convincingly demonstrated within animal experiments. As current data suggest differential effects of saturated and unsaturated fatty acids, and especially polyunsaturated fatty acids on glucose metabolism (19), it is tempting to speculate that some of these effects might be mediated by FGF-21. Indeed unsaturated fatty acids, and specifically the polyunsaturated linoleic acid, stimulated FGF-21 secretion, while the saturated fatty acid palmitic had no effect on FGF-21 secretion in HepG2 cells. Various studies suggested that the FFA-induced activation of PPARα depends on the degree of saturation of those FFAs (20). Therefore, the different effects on FGF-21 secretion may be caused by different PPARα binding of saturated and unsaturated fatty acids. Whether such mechanism contributes to the fatty acid–specific effects regarding the development of metabolic disorders remains to be elucidated.
A negative correlation between fasting insulin and FGF-21 levels was recently described in diabetic subjects (6,11), while this association was reported to be positive in obese individuals (5). Given this discrepancy, the relationship between insulin and FGF-21 is unclear. Since we observed slightly higher insulin levels during LHI compared with SHI in our participants, hyperinsulinemic-euglycemic clamps were performed for an in-depth analysis of the effects of insulin on FGF-21 levels. The slight increase of FGF-21 by hyperinsulinemia supports an independent effect of insulin on the regulation of FGF-21. Given a well-known reduction of FFAs during hyperinsulinemia, this effect may have been even underestimated. These results are in line with recent data of Izumiya et al. (21), showing that activation of AKT contributes to the expression of FGF-21. Thus, hyperinsulinemia and elevated levels of FFAs might contribute together to the elevated FGF-21 levels seen in diabetic patients (5,6). Nevertheless, both in vivo and in vitro data support a direct effect of FFAs on FGF-21 expression and secretion, suggesting that the effect of FFAs seen in our lipid trial would be at least in part independent of the moderate hyperinsulinemia, which occurs in the time frame presented here compared with the control group. Most importantly, no correlation was found between the change of FGF-21 levels and the change of insulin levels during LHI and SHI, a fact that is in line with an independent effect of FFAs on FGF-21 levels.
The FFA-induced effect in liver cells seems to depend on PPARα stimulation, which is strongly supported by the in vitro data presented here. However, it should be mentioned in that context, other tissues like adipose tissue or muscle might also contribute to the FFA-induced increase in FGF-21 in vivo. We do not feel that the fact of absent FGF-21 expression in primary human myoblasts contradicts a substantial release of FGF-21 from muscle cells in vivo. Our cells were predominantly undifferentiated cells, and the degree of differentiation might contribute to the expression pattern respective of FGF-21. A potential role of muscle cells was clearly supported by the study of Izumiya et al. (17), although the relevance of muscle cell differentiation might require furthers studies.
Even if recent animal data suggested a regulation of FGF-21 also by PPARγ (12), no change of FGF-21 levels was detected during insulin-sensitizing treatment with PPARγ stimulation in the human trial in subjects with impaired glucose tolerance (Fig. 5). Although PPARγ agonists may recruit different nuclear cofactors, which may explain different biological effects, our data do not support (but also not entirely exclude) that the observed effects on FGF-21 levels during LHI were mediated in part by PPARγ stimulation.
Limitations of the present study need to be mentioned. In a recent study (3), a prolonged fasting situation was required to increase FGF-21 levels in humans. Although small effects may have been underestimated in the abovementioned study, it is noteworthy that the increase of FFAs during fasting is substantially lower in comparison to changes observed during lipid infusion. As such, although the intervention performed here is a well-established model investigating metabolic effects of hyperlipidemia (22–26), supraphysiological levels of FFAs, as observed in the present study, may have reduced the period potentially required to observe effects of more moderate FFA elevations on FGF-21. In addition, FFAs and triacylglycerols are both increased by LHI (27–29), and the respective effects are difficult to dissociate. Although the correlation between changes in FFAs and FGF-21 during LHI suggests that FFAs contribute to the effect on FGF-21, we cannot entirely exclude a role of triacylglycerols or other compounds within the applied infusions. Notably, no effect of glycerol or lecithin was detectable in HepG2 cells, suggesting that those substances have no substancial effect on FGF-21 secretion. Recent human data (6) indicate a specific role of FFAs on FGF-21 secretion by showing a positive correlation between both parameters in healthy subjects. Furthermore, Dostalova et al. (30) observed not only reduced FGF-21 levels but also lower FFAs in anorectic women compared with control subjects, while no difference in insulin, triacylglycerols, and fasting glucose was detected between anorectic and healthy women in this study. Those data do support a model of FFAs as major regulators of FGF-21 levels.
In summary, we demonstrated in vitro that FFAs stimulate FGF-21 expression and secretion in a human hepatic carcinoma cell line in a PPARα-dependent fashion. The in vivo relevance of this finding was confirmed in a controlled, randomized cross-over trial in humans. Within that trial, acutely increased levels of FGF-21 were observed. Most interestingly, hyperinsulinemia also induced a small but significant increase of FGF-21, suggesting that both FFAs and insulin regulate FGF-21, which may explain elevated levels of FGF-21 in obese and diabetic patients but offers also a potential mechanism linking regulation of FGF-21 to the switch of metabolism during starvation.
Acknowledgments
J.S. was supported by a research group (Molecular Nutrition) of the Bundesministerium für Bildung und Forschung, a graduate school (GK1208), a Heisenberg-Professorship (SP716/1-1), and a clinical research group of the Deutsche Forschungsgemeinschaft (KFO192/1). K.B., S.M., M.S., M.M., and J.W. were also supported by GK1208. S.S. was also supported by KFO192/1.
No potential conflicts of interest relevant to this article were reported.
We thank N. Huckauf and K. Sprengel for excellent technical assistance.
Footnotes
Clinical trial reg. no. NCT00473603, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB: FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627– 1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA: Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007; 5: 415– 425 [DOI] [PubMed] [Google Scholar]
- 3.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M: The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169– 174 [DOI] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ: The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007; 148: 774– 781 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A: Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246– 1253 [DOI] [PubMed] [Google Scholar]
- 6.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G: Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2008; 116: 65– 68 [DOI] [PubMed] [Google Scholar]
- 7.Mai K, Bobbert T, Reinecke F, Andres J, Maser-Gluth C, Wudy SA, Mohlig M, Weickert MO, Hartmann MF, Schulte HM, Diederich S, Pfeiffer AF, Spranger J: Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. J Clin Endocrinol Metab 2008; 93: 3900– 3906 [DOI] [PubMed] [Google Scholar]
- 8.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM: The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 2002; 51: 2968– 2974 [DOI] [PubMed] [Google Scholar]
- 9.Basu R, Pajvani UB, Rizza RA, Scherer PE: Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes 2007; 56: 2174– 2177 [DOI] [PubMed] [Google Scholar]
- 10.Blumer RM, van der Crabben SN, Stegenga ME, Tanck MW, Ackermans MT, Endert E, Van der PT, Sauerwein HP: Hyperglycemia prevents the suppressive effect of hyperinsulinemia on plasma adiponectin levels in healthy humans. Am J Physiol Endocrinol Metab 2008; 295: E613– E617 [DOI] [PubMed] [Google Scholar]
- 11.Li L, Yang G, Ning H, Yang M, Liu H, Chen W: Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract 2008; 82: 209– 213 [DOI] [PubMed] [Google Scholar]
- 12.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK: Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol 2008; 74: 403– 412 [DOI] [PubMed] [Google Scholar]
- 13.Pocock S: In Clinical Trials: A Practical Approach New York, John Wiley, 1984 [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 15.Mai K, Bobbert T, Kullmann V, Andres J, Rochlitz H, Osterhoff M, Weickert MO, Bahr V, Mohlig M, Pfeiffer AF, Diederich S, Spranger J: Free fatty acids increase androgen precursors in vivo. J Clin Endocrinol Metab 2006; 91: 1501– 1507 [DOI] [PubMed] [Google Scholar]
- 16.Mai K, Andres J, Bobbert T, Maser-Gluth C, Mohlig M, Bahr V, Pfeiffer AF, Spranger J, Diederich S: Rosiglitazone decreases 11beta-hydroxysteroid dehydrogenase type 1 in subcutaneous adipose tissue. Clin Endocrinol (Oxf) 2007; 67: 419– 425 [DOI] [PubMed] [Google Scholar]
- 17.Oishi K, Uchida D, Ishida N: Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett 2008; 582: 3639– 3642 [DOI] [PubMed] [Google Scholar]
- 18.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Ryden M: FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 2008; 582: 1725– 1730 [DOI] [PubMed] [Google Scholar]
- 19.Riserus U: Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care 2008; 11: 100– 105 [DOI] [PubMed] [Google Scholar]
- 20.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W: Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A 1993; 90: 2160– 2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K: FGF21 is an Akt-regulated myokine. FEBS Lett 2008; 582: 3805– 3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonsen S, Kjekshus JK: The effect of free fatty acids on myocardial oxygen consumption during atrial pacing and catecholamine infusion in man. Circulation 1978; 58: 484– 491 [DOI] [PubMed] [Google Scholar]
- 23.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA: Effect of fatty acids on glucose production and utilization in man. J Clin Invest 1983; 72: 1737– 1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis GF, Vranic M, Harley P, Giacca A: Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes 1997; 46: 1111– 1119 [DOI] [PubMed] [Google Scholar]
- 25.Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF: Prolonged elevation of plasma free fatty acids impairs pancreatic β-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 2000; 49: 399– 408 [DOI] [PubMed] [Google Scholar]
- 26.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P: Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003; 52: 2882– 2887 [DOI] [PubMed] [Google Scholar]
- 27.Muggeo M, Tiengo A, Fedele D, Crepaldi G: The influence of plasma triglycerides on human growth hormone response to arginine and insulin: a study in hyperlipemics and normal subjects. Horm Metab Res 1975; 7: 367– 374 [DOI] [PubMed] [Google Scholar]
- 28.Lindholm M, Eklund JO, Hamberger B, Jarnberg PO: Plasma catecholamine and free fatty acid levels during infusion of lipid emulsion in critically ill patients. Crit Care Med 1984; 12: 953– 956 [DOI] [PubMed] [Google Scholar]
- 29.Piatti PM, Monti LD, Baruffaldi L, Magni F, Paroni R, Fermo I, Costa S, Santambrogio G, Nasser R, Marchi M: Effects of an acute increase in plasma triglyceride levels on glucose metabolism in man. Metabolism 1995; 44: 883– 889 [DOI] [PubMed] [Google Scholar]
- 30.Dostalova I, Kavalkova P, Haluzikova D, Lacinova Z, Mraz M, Papezova H, Haluzik M: Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 2008; 93: 3627– 3632 [DOI] [PubMed] [Google Scholar]